Abstract
Early experience can affect nervous system development in both vertebrate and invertebrate animals. We have now demonstrated that visual stimulation modifies the size of the optic lobes in the laboratory fruitfly Drosophila melanogaster. Monocular deprivation (painting over one eye) decreases the aggregate volume of the lamina, medulla, and lobula plate by up to 6%. The laminae of control flies kept in complete darkness showed a more robust volume difference that could be as much as 30%. An electron microscopy study revealed that the changes in the lamina are largely attributable to an increase in the terminals of the photoreceptor cell axons. The volume of the lamina increases during the first 24 hr after emergence, and it grows more in the light than in darkness. When flies are kept in the dark for the first 12 hr of their adult life and are then brought back to light for the next 3.5 days, the lamina is almost as small as in flies raised for 4 d in constant darkness. Twelve hour dark shifts at a later time are less effective. This finding suggests a critical period for lamina development during day 1 of the adult. The lamina depends on visual stimulation to maintain its size during the first 5 d after emergence. Dark-rearing for 1 d or more at any stage during that period decreases its volume to the level of flies raised in constant darkness. A lamina that is once reduced in size seems not to recover.
Keywords: Drosophila melanogaster, visual system, optic lobe development, structural plasticity, critical period, dark-rearing
Evidence is growing that the nervous systems of higher and lower animals share basic characteristics (Goodman, 1995; Halder, 1995; Reichert, 1996). The ability to learn and remember, for example, was considered a hallmark of vertebrates for two thirds of this century but is now known to be possessed by invertebrate animals. Indeed, they are now widely exploited to uncover the molecular and cellular basis of learning (Menzel, 1983; Kandel et al., 1987;Heisenberg, 1989).
Like learning and memory, for a long time, experience-dependent plasticity in the brain has been ascribed exclusively to higher vertebrates. For example, rearing rats in different social environments results in chemical and anatomical changes in the brain (Bennett et al., 1964). The weight of the cerebral cortex increased when the animals were kept in a complex environment for several weeks. Subsequently, changes at the cellular level were described in the brains of monkeys; under enriched conditions, the Purkinje cells of the cerebellum developed larger and more complex dendritic trees than in the deprived controls (Floeter and Greenough, 1979). Finally, raising cats in restricted visual environments produced specific morphological changes in cells in the visual cortex (Tieman and Hirsch, 1982; Hirsch, 1985).
The role of experience in the development of the adult brain has also been recognized in invertebrates. First, Drosophila melanogaster flies reared under enriched conditions have more Kenyon cell fibers in the peduncle of the mushroom body than do their deprived siblings (Technau, 1984; Balling et al., 1987). In pairs of flies, the volume of the mushroom body calyx depends on the sex of the partner (Heisenberg et al., 1995). Second, in honey bees, significant volume changes in the calyces could be observed at the time of the first reconnaissance flight of the worker (Withers et al., 1993, 1995;Durst et al., 1994; Fahrbach and Robinson, 1995). Third, in the flyMusca domestica, dark-rearing during the first 5 d after emergence altered light and contrast sensitivity (Deimel and Kral, 1992). A potential anatomical correlate has been reported by Kral and Meinertzhagen (1989), who found that during the first 4 d of adulthood, the number of the L2 feedback synaptic profiles in the lamina are changed as a consequence of rearing in different light regimes.
In the present study, we investigate the structural plasticity of the optic lobes of Drosophila melanogaster in response to differential exposure to light during adulthood. As a metric of the changes occurring, we have measured the volume of the four neuropil regions in the optic lobes, lamina, medulla, lobula, and lobula plate. We have tried to identify which neuropils are affected and how their volume develops in the light and in darkness. We tried to identify some of the cell types that contribute to the volume changes and to determine a critical period for the changes during which the volume of the optic lobe is particularly sensitive to the light regime. Finally, we have examined whether during that period, the neuropil requires light to grow and maintain its size. The availability inDrosophila of a large collection of mutants with known molecular defects enables us to start studying some of the underlying mechanisms.
MATERIALS AND METHODS
Animals and rearing conditions
Unless stated otherwise, Drosophila melanogasterflies of the wild-type stock Canton S (WT CS) were used. They were allowed to lay eggs overnight on a Petri dish containing 5% sucrose and 3% agar to which some live brewer’s yeast had been added. The next morning, batches of 200 eggs were transferred to each of several 200 ml vials containing 40 ml standard medium (corn meal, molasses, and no fresh yeast) and a filter paper. Cultures were kept in an incubator at 25°C and 60% relative humidity on a 16:8 light/dark (LD) cycle. On day 9, new flies eclosed and were collected either within 1 hr for the critical period and development of the lamina experiments, or within 3 hr for all other experiments.
Flies were anesthetized on ice, sexed, and assigned to one of the following three experimental groups: (1) constant light (LL), (2) LD, as described above, or (3) constant darkness (DD). Flies were kept in groups of 10–20 animals of the same sex. DD flies were transferred to light-proof boxes that permitted air to flow through and then, as a control, were placed in the same room with LD or LL flies. For LD flies, the incubator was the same as that used for preadult stages. LL flies were maintained in a separate room at 25°C and 40–60% humidity. Lighting was provided by full-spectrum fluorescent lights (40 W at ∼40 cm distance, color 25, Universal-Weiβ, Osram, Berlin, Germany) that flickered at 20 kHz. Flies were reared under these conditions for between 1 hr and 6 days, but in the standard experiment, 4-d-old flies were used. Each experimental group consisted of 10–20 flies. In experiments with monocularly deprived flies, one eye (either left or right) was painted with an opaque, black, water-soluble paint (Deka-Lack number 318; black) within 1 hr after eclosion. The pigment layer reduced the light flux in a photometer (Lunasix 3, Gossen) to ∼0.1%. In all experiments except for the development of the lamina, flies were collected in the morning between 9:30 and 11:00 A.M.
Mass histology. Collected flies were processed for mass histology (Ashburner, 1989). Paraffin sections were inspected by fluorescence microscopy, and volumes of the neuropil regions were evaluated by planimetric measurement of their autofluorescent profiles (Heisenberg et al., 1995). Flies that underwent different treatments or mutant and wild-type flies were arranged in random order in collars (Heisenberg and Böhl, 1979) so that brain sizes could be evaluated without knowledge of the rearing conditions or genotype of the respective flies. We carefully followed our standard protocol in an effort to keep the larval-rearing conditions and histological treatment as constant as possible, but still found considerable variation among different experiments in the volume of the optic lobes. Therefore, we included our standard 4 d visual deprivation experiment with wild-type CS flies as part of the design of each experiment to verify that our manipulations and techniques remained effective.
Electron microscopy (EM). Male Canton S flies were raised in a 12:12 LD cycle during their larval stages and subsequently kept in LL and DD conditions during adulthood. At day 4, they were anesthetized and fixed using a cacodylate-buffered glutaraldehyde/paraformaldehyde primary fixative, followed by osmication and embedment in PolyBed (Meinertzhagen, 1997). Tangential sections of the lamina were cut on a Reichert Ultracut S microtome to expose cross-sections of the unit cartridges of the lamina neuropil (Fig. 1).
Fig. 1.

A cross-section of the lamina close to its proximal margin demonstrates the basic organization of a cartridge. Six photoreceptors (R) surround two monopolar cells in the middle (L1, L2). The cartridge in turn is surrounded by epithelial glial cells (g). Magnification, 4860×. Scale bar, 1.0 μm.
Profiles of lamina cartridges were sampled at two depths: (1) distally, just beneath the basement membrane of the eye, where the first cartridges were contained in semithin sections stained with toluidine blue, and (2) close to its proximal margin, just distal to the external chiasm connecting lamina and medulla. At the appropriate location, 80 nm ultrathin sections were collected on Formvar-coated slot grids and stained for EM for 5–8 min in saturated uranyl acetate in 50% ethanol, followed by 2–20 min in Reynolds’ lead citrate (Reynolds, 1963). Laminae of 19 flies were sectioned, some both distally and proximally. In total, for each depth and rearing condition, 7–8 eyes were sectioned and analyzed by EM.
Electron micrographs of cartridges and surrounding glial cells were taken on 35 mm film at an original magnification of 2260×. Negative micrographs were viewed on a light table, and a closed-circuit television camera in an overhead enlarger stand was used to provide a 570 × 485 pixel image of the micrograph. Images were captured into an IBM computer with frame-grabbing software and transferred to a Macintosh computer. The areas and perimeters of cartridge cross-sections and their component axon profiles were then measured with morphometric software (National Institutes of Health Image 1.44). Each cartridge cross-section (Fig. 1) comprised the identified profiles of two large monopolar cells, L1 and L2, at the cartridge axis, surrounded by six photoreceptor terminals (R1–R6) and was invested by three epithelial glial cells (Meinertzhagen and O’Neil, 1991). To estimate the cross-sectional area of the photoreceptor terminals, the combined areas of the axon profiles of L1 and L2 were measured and subtracted from the area of the cartridge cross-section. To obtain an approximate estimate of the size of the epithelial glia separating the cartridges, the total area of several cartridges and their surrounding tissue was measured. By subtracting the area of the cartridges and then dividing the remainder by the number of cartridges, we arrived at a value for the area of the glial cell profiles per cartridge. Between 30 and 100 cartridges were analyzed for each fly and depth, and >1000 cartridges were inspected in total.
Immunohistochemistry. Antihistamine immunoactivity was applied in one experiment to identify the medulla endings of the two photoreceptor axons of the central cells in the ommatidium, R7 and R8. The techniques used for immunostaining are described in detail elsewhere (e.g., Buchner et al., 1993). In brief, after fixation, the fly heads were frozen and sectioned at 10 μm thickness on a cryostat, incubated with an antiserum against histamine (dilution 1:1000 in PBS, PAN19C, Incstar, Stillwater, MA) and stained with diaminobenzidine as the chromogen.
Statistical analyses. Data for all experiments were analyzed using the t test, Mann–Whitney U test (MW), ANOVA and Kruskal–Wallis (KW) routines of INSTAT, and the MANOVA routine of STATISTICA (StatSoft, Tulsa, OK). All tests were two-tailed.
RESULTS
Optic lobe neuropil structures are differentially affected by the light regime
To investigate whether visual experience during adulthood may ultimately have lasting effects on the structure of the optic lobe neuropils processing that input, newly eclosed flies were monocularly deprived. They were then kept for 4 d in an LD cycle. To control for possible effects of the paint or the painting procedure on the neuropils beneath the occluded eye, a second group of flies was kept in DD.
Of the four neuropil regions (lamina, medulla, lobula, and lobula plate), three showed a significant increase in volume beneath the open eye (Fig. 2A). During the course of this study, we conducted 10 experiments (each with ∼10–20 animals) in which flies were monocularly deprived. In total, the lamina underneath the open eye was significantly increased (p < 0.00001, t test) and ∼5% larger than beneath the painted eye. Similar results could be observed for the medulla (p < 0.005, t test) and lobula plate (p < 0.0005, ttest) but, surprisingly, not for the lobula (p> 0.1, t test). We did not find such effects for the DD control flies. This indicates that the paint itself had no negative effect on the size of the neuropils. Thus, the differences between the two sides observed in the light must be ascribed to the differential visual input the flies received during the first 4 d of adult life. Because there were no obvious sex differences in the effects of the light regime (data in Fig. 2A are pooled), we continued to use only males for the rest of the experiments.
Fig. 2.
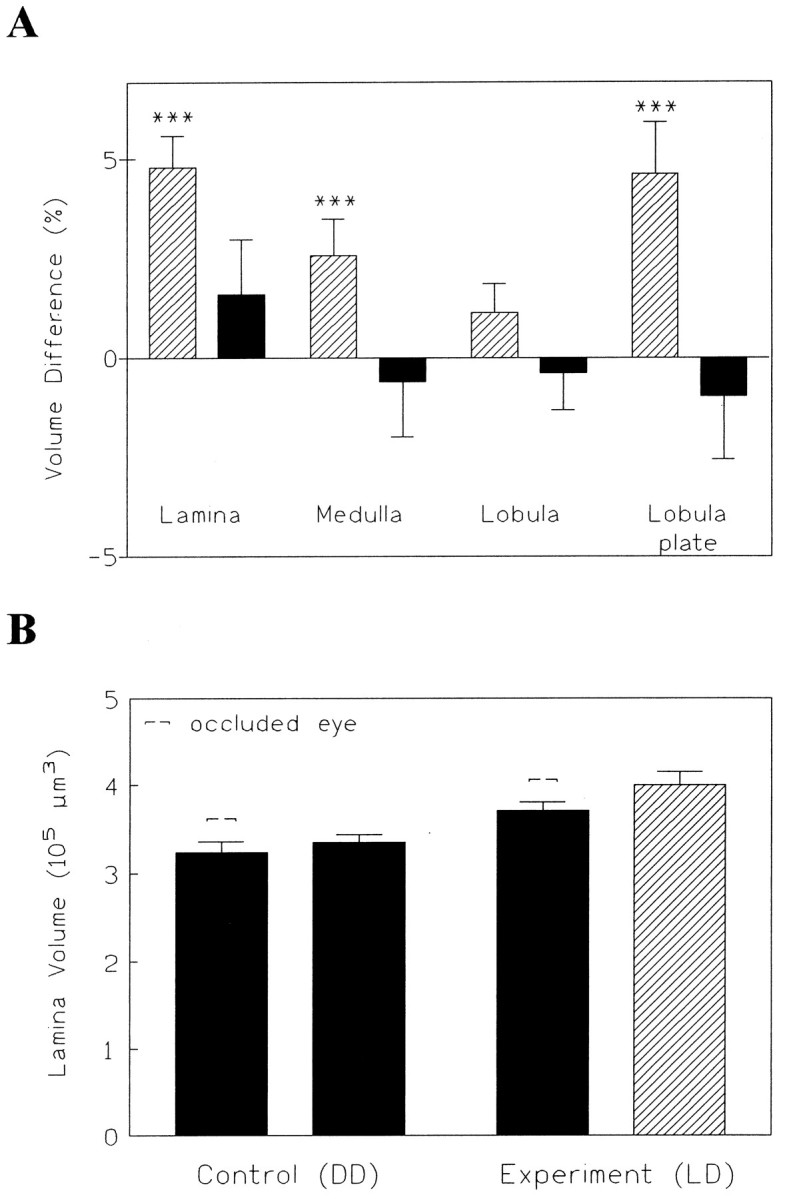
Effects of monocular deprivation and rearing in darkness. A, Effects of painting one eye (monocular deprivation) on the optic lobes. Except for the lobula, the neuropils underneath the open eye had a larger volume than under the occluded eye if the flies were kept in LD conditions (striped bars;n = 130). As a control for the effects of painting, some of the monocularly painted flies were reared in DD (solid bars; n = 80). Thus, the painting itself did not reduce neuropil volume. B, Rearing in DD (n = 24) gave a more obvious volume difference than monocular deprivation in the LD cycle (n = 37).
The flies kept in DD, originally considered to be the control group, unexpectedly exhibited even more robust effects of visual deprivation during the course of our study than the monocularly blindfolded flies in the LD cycle. Whereas the difference between the occluded and nonoccluded eye in the LD cycle was only small, the difference between the nonoccluded eye in LD and the eyes in DD flies was up to 25% for the lamina (KW = 29.8, p < 0.0001) (Fig.2B). Painting one eye does not reduce the light flux in this eye to zero (see Materials and Methods). Because scattered light may also enter through the head capsule, the light reaching the rhabdomeres is probably not below 1% of the normal level. What the paint does, however, is destroy the optics of the eye, because it alters the air–cornea interface. Therefore, no spatial contrast will be transmitted by this eye. The fact that in the lamina, darkness is much more effective than monocular occlusion seems to indicate that the effects are largely attributable to light intensity and not to pattern contrast, visual motion, or other more advanced visual functions. In the medulla, on the other hand, differences between LD and DD flies never exceeded 6%, and only comparisons between occluded eye and open eye revealed significant effects. The medulla may, therefore, be less directly dependent on light intensity but rather on higher visual processing.
Taken together, these results indicate that the lamina, medulla, and lobula plate were changed in volume as a function of the visual input the flies received during adult life. When animals raised under DD and LD conditions were compared, as opposed to comparing the occluded and nonoccluded sides in the same animal, the changes of the lamina and lobula plate, but not of the medulla, were most prominent. Differences between LL and DD flies exceeded those between LD and DD flies (see Fig. 9). Therefore, we used LL flies in most of the following experiments.
Fig. 9.
A, Effects of a 12 hr dark shift at different ages after emergence. Dark-rearing during the first 12 hr (B) leaves the lamina as small as that found after 4 d in darkness (G) but similar dark shifts at later ages exert smaller effects (groupsC–E). Dark-rearing during the last 12 hr (F), i.e., during the subjective night of the animals, reduces size even less (nA = 11,nB = 10, nC = 11,nD = 10, nE = 10,nF = 10, nG = 11). B, A reduction in volume compared with LL flies (n = 14) could represent a more natural response, because flies kept in an LD light regime (n = 26) have a lamina size that is intermediate between LL and DD flies (n = 22).
Next, we wanted to know specifically which cells in the lamina change because of light and whether these changes can account for the volume differences observed. To examine this question, cell sizes in the lamina were measured in LL and DD flies using EM. Tangential sections through the lamina close to both its distal and its proximal margins were compared in the two groups of animals. Approximately two-thirds of the lamina cross-sectional area (and thus of its volume) was contributed by the profiles of photoreceptor terminals and monopolar cells, one third by those of glia. Surprisingly, changes in the size of these fractions were more pronounced in the distal than the proximal part of the lamina. For example, at the distal level, the combined area of cartridges and surrounding glia cells was 28.5% (MW = 9,p < 0.05) larger for LL flies than for DD flies, whereas proximally the difference (3%) was not significant (MW = 23, p > 0.5) (Table 1). With a linear interpolation between the distal and proximal measurements, the resulting difference in volume would be 15.7% [(28.5% + 3%/2)]. This concurs nicely with the data we obtained in the volumetric study (e.g., Fig. 2). Therefore, we conclude that in the lamina, the photoreceptor terminals account for most (>90%) of the volume changes we observed. Nevertheless, changes in L1/L2 monopolar cells were quite robust as well, and even though their contribution to the overall volume change was much smaller, their relative change between the two groups of flies even exceeded that of the six photoreceptors.
Table 1.
Effects of visual deprivation (DD) and light exposure (LL) on identified cells or cell types in the lamina. LL flies have significantly larger cells than DD flies at the distal margin of the lamina but not at the proximal margin. The resulting overall volume decrease would be 15.8%. Cross-sectional area of cell profile(s), mean ± SD (μm2), n = 7-8.
| DD flies | LL flies | Δ (%) | Significance | |
|---|---|---|---|---|
| Proximal | ||||
| Cartridge size | 11.5 ± 0.9 | 13.2 ± 3.1 | 12.7 | p > 0.1 NS |
| Photoreceptors | 10.8 ± 0.2 | 12.2 ± 2.8 | 11.4 | p > 0.1 NS |
| Monopolar cells (L1 + L2) | 0.75 ± 0.2 | 1.0 ± 0.3 | 22.4 | p > 0.1 NS |
| Glia cells | 4.8 ± 0.6 | 3.6 ± 0.7 | −24.7 | p < 0.005 |
| Sum (cartridge + glia) | 16.3 ± 1 | 16.8 ± 3.5 | 3.0 | p > 0.1 NS |
| Distal | ||||
| Cartridge size | 13.5 ± 1.6 | 19.8 ± 4.3 | 31.6 | p < 0.005 |
| Photoreceptors | 12.8 ± 1.5 | 18.7 ± 4.1 | 31.6 | p < 0.005 |
| Monopolar cells (L1 + L2) | 0.74 ± 0.2 | 1.18 ± 0.2 | 37.3 | p < 0.001 |
| Glia cells | 6.1 ± 1.6 | 7.7 ± 1.9 | 20.5 | p > 0.1 NS |
| Sum (cartridge + glia) | 19.6 ± 2.9 | 27.5 ± 5.6 | 28.5 | p < 0.005 |
A comparison between the data from the distal and proximal part of the lamina revealed another feature of these experience-dependent changes. Not only was the size of the cartridges under the influence of the light regime but also their shape. Whereas in DD flies the difference between distal and proximal cross-sections was only 17%, it was 39% in LL flies. In other words, the cartridges were more cylindrical in DD flies but were more conical in LL flies (Fig.3A). Considering that the lamina neuropil is cup-shaped (i.e., a fraction of the mantle of a sphere), the radius of this cup should be about three times as large in DD flies than it is in LL flies (∼70 vs 210 mμ) (Fig. 3B). This gross anatomical difference between LL and DD flies is quite apparent in light-microscopical preparations.
Fig. 3.

A, Electron micrographs of lamina cross-sections demonstrating the volume increase and shape modification in the light. Distally, LL flies had significantly larger cartridges than DD flies. No significant difference was found at an ∼20 μm more proximal level. Magnification, 2260×; scale bar, 1.0 μm.B, Scale drawing to illustrate the changes in the shape of the lamina resulting from the differential growth effects in light (left) and darkness (right). Note that the calculated radius (r) of the sphere is three times larger in DD than in LL flies. The calculation is based on the data in Table 1, assuming a distance of 20 μm between the distal and proximal levels.
In the next experiment, we examined whether changes in the photoreceptor terminals of R7 and R8 could account for the volume changes observed in the medulla. If so, the effects should have been confined to the distal medulla where the axons of R7 and R8 terminate. The distal part of the medulla (strata 1–6) (Fischbach and Dittrich, 1989) was visualized using antihistamine antibodies that selectively labeled photoreceptor axons and terminals (Fig. 4). As noted above, volume changes in the medulla could be seen only in experiments with monocular deprivation, and this regime was therefore used again. We measured (1) the immunostained distal strata of the medulla (containing the terminals of R7/R8); (2) the proximal part (not stained); and (3) the medulla as a whole. (As an internal control for the deprivation effects, the lamina was also measured.) Consistent with the previous data, the light-dependent changes in the medulla were significant only for the distal strata (p < 0.05, t test), contributing a slightly higher difference to the overall volume change in the medulla than the proximal strata. Thus, changes in photoreceptor terminals seem at least likely to contribute to the volume changes in the medulla, although it also seems that, as in lamina, they are accompanied by changes in the interneurons.
Fig. 4.
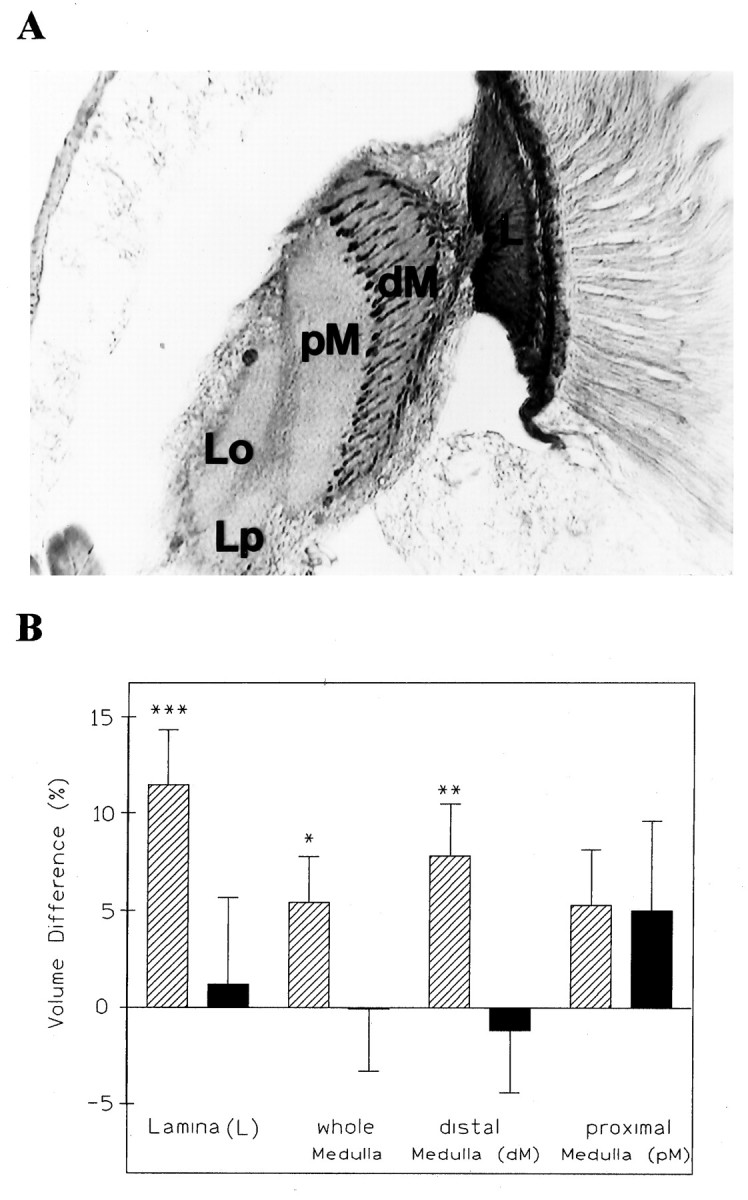
A, Antihistamine immunoreactivity of a horizontal section of a fly’s head showing the optic neuropils. The terminals of photoreceptor axons from R7 and R8 are heavily stained, allowing the subdivision of the medulla in a distal (dM) and proximal (pM) portion. L, Lamina;Lo, lobula; Lp, lobula plate.B, Volume changes resulting from monocular deprivation are found in the lamina and in the entire medulla and its distal part in LD flies (n = 24), suggesting that the photoreceptor terminals in the distal part contribute more to the overall effect in the medulla than its proximal layers. In DD flies (n = 16), no such effects could be found.
Light exerts its effect through the compound eyes
Does the light have its effect as regular visual input using the well-known phototransduction cascade in retinula cells, or are we dealing with a yet obscure photosensitive process somewhere in the organism? Are the volume changes in neurons and photoreceptors attributable to hormonal regulation? In Drosophila, some of these questions can be answered using mutants.
We studied the effect of the light regime on the volume of the lamina in the blind mutant norpAP24, which is defective in phospholipase C, an essential step of the phototransduction process in the compound eye. No volume difference between LD and DD flies was detected (Fig. 5A). The lamina stayed small and was, in fact, as small as the one in dark-reared CS control flies (r = 4.2, p < 0.05, MANOVA controlling for genotype and rearing condition).
Fig. 5.
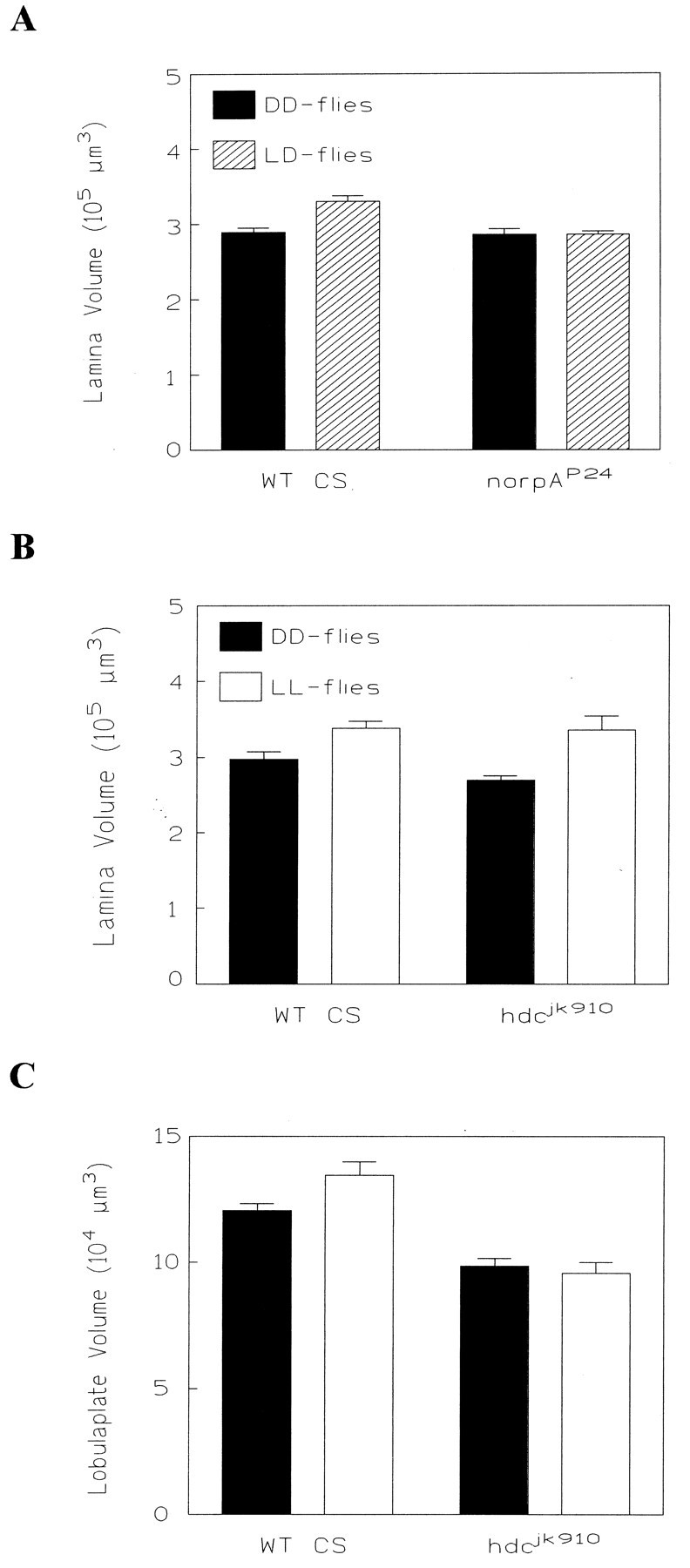
Effects of rearing in different light regimes for wild-type CS flies and the mutants norpAP24and hdcjk910. A, ThenorpAP24 mutant (nDD = 17; nLD = 13) lacks volume changes in its lamina. The laminae of both groups innorpAP24 are as small as the lamina of DD flies in wild-type CS (nDD = 22;nLD = 26). B, In contrast, the lamina in both wild-type CS andhdcjk910 shows a significant difference between flies reared in DD and those reared in LL (n = 12 for all groups). C, In the lobula plate, hdcjk910 fails to show a difference between DD and LL flies. Compared with wild-type CS, the lobula plates in both groups are significantly reduced (n = 12; the same animals were analyzed as inB).
There is considerable evidence that histamine is the neurotransmitter between fly photoreceptors and the large monopolar cells in the lamina (Hardie, 1987). Histamine release (Sarthy, 1991) and photoreceptor histamine immunoreactivity (Pollack and Hofbauer, 1991) both are reported in Drosophila. In the histamine-null mutanthistidine decarboxylasejk910(hdcjk910), this synaptic transmission is blocked (Melzig et al., 1996). We kept parallel cultures of adult mutant and wild-type flies for 4 d in either permanent light or DD. For the lamina, a 20% difference could be found between LL and DD flies in the mutant and the wild-type control (for both:p < 0.05, t test) (Fig. 5B), suggesting once more that the photoreceptors are responsible for most of the volume changes in the lamina. For the the lobula plate, we found a significant difference between LL and DD flies only for the wild type (p < 0.05, t test). In the mutant, no significant difference was detected (p > 0.5, t test) (Fig. 5C) as would be expected from a fly with a block in the first visual synapse. Interestingly, the lobula plate of hdcjk910 flies from both rearing conditions was significantly smaller than it was in wild-type flies (F = 21.9, p < 0.0001, ANOVA), although the laminae had been of similar size. This may indicate that a block in synaptic transmission suppresses electrical activity central to the lamina and that this reduced activity interferes with the development of the lobula plate (Fig.6B).
Fig. 6.

Development of the lamina and lobula plate in wild-type CS flies during the first 48 hr after emergence.A, The lamina enlarges in both groups during the first 24 hr but more so in LL flies. The first significant difference between DD and LL flies was at 12 hr after emergence (nLL = 20(1hr), 10(3hr), 10(6hr), 11(9hr), 8(12hr), 6(24hr), 9(48hr);nDD = 20(1hr), 10(3hr), 9(6hr), 8(12hr), 10(24hr), 11(48hr)). B, The lobula plate develops during the first 24 hr as well, and the first significant difference between DD and LL flies appears at 9 hr after emergence (same animals as in A).
Photoreceptors R1–R6 not only maintain synaptic input to monopolar cells in the lamina but also, reciprocally, receive synaptic input from monopolar cells including L2 (Meinertzhagen and O’Neil, 1991). In principle, this feedback mechanism could be responsible for the differences in volume of R1–R6 between flies reared under LL and those reared under DD conditions. The result with the mutanthdcjk910 seems to exclude this hypothesis, however. When transmission to L1 and L2 at the afferent synapse is blocked, L2 can provide no signal back to the photoreceptor terminals. Yet, the volume change still persists. Hence, the L2→R synapse does not mediate the structural plasticity of the photoreceptors.
Effect of light regime on development of the lamina and lobula plate in the adult fly
So far, we have shown that visual deprivation affects the volume of several neuropil regions in the optic lobes. Do these volume changes, however, result from an increase in LL flies or from a decrease in DD flies? In other words, is visual input necessary for the optic lobes to grow and mature, or does it start large at eclosion and diminish without light?
To distinguish between these two possibilities, we reared flies under the two conditions (LL and DD) and sacrificed them for histology at different times after eclosion. Irrespective of the light conditions, the lamina and lobula plate increased in size during that period. In the first 6 hr, the light regime had no significant effect, and the lamina and lobula plate in both groups both had a small volume (Fig.6A,B). Thereafter, in LL flies the volume of the two neuropils increased rapidly (for the lamina:F = 10, p < 0.0005; for the lobula plate: F = 3.6, p < 0.005; ANOVA), whereas in DD flies it stayed relatively small (lamina:F = 2.1, p > 0.05; lobula plate:F = 0.9, p > 0.1; ANOVA). At 9 hr after eclosion, the first significant difference between LL and DD flies appeared in the lobula plate (MW = 89, p < 0.05), and at 12 hr after eclosion, we found a significant difference (p < 0.05, t test) of ∼15% between the two experimental groups in the lamina. Thus, within only 12 hr after emergence, the lamina and lobula plate both developed an increased volume in the group of light-reared flies and stayed relatively constant thereafter.
Critical period for the lamina development
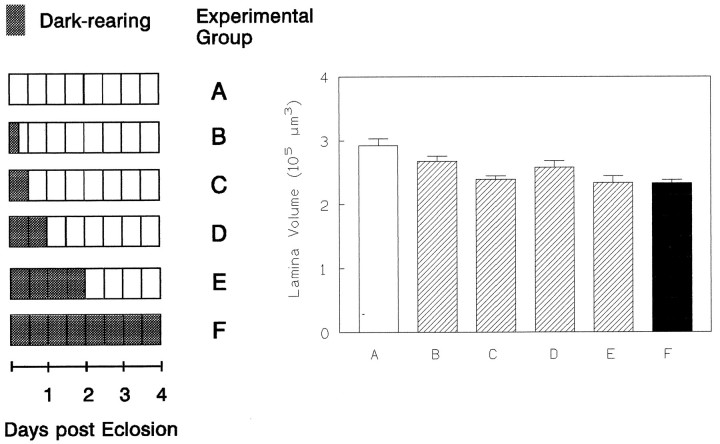
We wondered whether the sensitivity of the lamina to the light regime extends over the entire 4 d or is confined to a shorter period. In an initial experiment, we applied periods of dark-rearing of increasing duration from the beginning of the fly’s adult life. One group of flies was dark-reared for the first 6 hr after eclosion, a second for the first 12 hr, a third for 24 hr, and a fourth for 2 d. After this period, all flies were brought back to LL conditions and were kept there for the remainder of the 4 d period. They were then sectioned together with a control group of 4-d-old LL and DD flies. Surprisingly, the lamina of the flies that were in darkness for only the first 6 hr after eclosion was already significantly smaller than that of the LL controls (KW = 8.9, p < 0.05) (Fig. 7B). This is particularly surprising, given that immediately after the 6 hr period of darkness or light, no effect on lamina size was yet apparent (Fig. 6). After an initial dark period of 12 hr followed by 3.5 d of light, the lamina was as small as in DD flies. Consistent with this finding, in all other groups the lamina was about as small. Therefore, we consider the first 6–12 hr of adult life to be especially important for the development of the lamina. If flies received no visual stimulation during this time, subsequent rearing in light fails to lead to a full recovery of lamina volume.
Fig. 7.
The effects of rearing in darkness at different times after emergence suggest a critical period for the development of the lamina. Dark-rearing during the first 6 hr after emergence (B) prevented the lamina from increasing to the size seen in controls (A). Any other period of dark-rearing (C–E) resulted in a significant volume difference compared with the LL control group (A) (nA = 17, nB = 19, nC = 11, nD = 15, nE = 16, nF = 25).
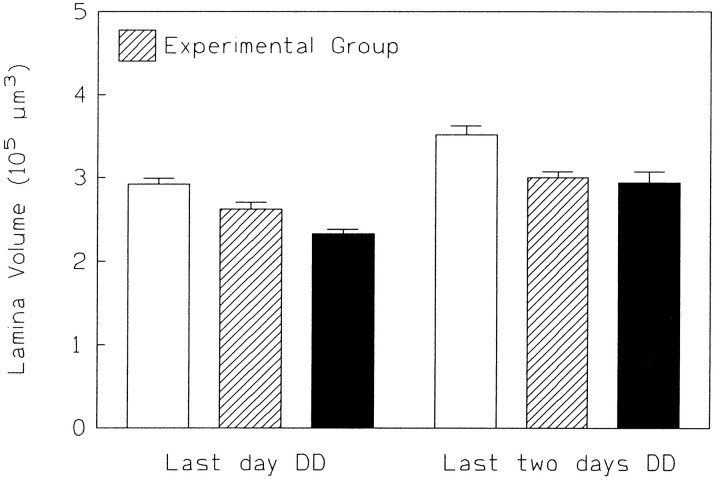
Do flies lose their responsiveness toward visual deprivation at later stages of their adult life? To test this question, LL flies were shifted to DD only during the last 24 or 48 hr of the 4 d period. The volume of the lamina decreased in both cases significantly (final 24 hr: p < 0.05, t test; final 2 d:p < 0.01, t test) suggesting that the flies’ visual system remained sensitive to light deprivation during the first 4 d after eclosion (Fig. 8), which is a considerable portion of their adult life (Bouletreau, 1978).
Fig. 8.
Rearing in darkness during the last 2 d (right-hand experimental group: n = 17; control:LL4 days: n = 12,DD4 days: n = 17) or even for only the last day (left-hand experimental group:n = 16; control: LL4 days = 17; DD4 days = 25) significantly reduces the volume of the lamina in 4-d-old flies. Whereas a dark shift of only 1 d leaves the lamina intermediate in volume between that in LL flies (open bars) and that in DD flies (solid bars), 2 d of darkness reduce the size to the volume found in DD controls. The data came from two independent experiments so that the absolute size of the laminae varies considerably.
We examined next whether the susceptibility to dark-rearing decreased with age. LL flies were placed into the dark for 12 hr at different times during the 4 d rearing time. Although at any time, the period of darkness had volume-decreasing effects, its effects were most pronounced on the first day after eclosion (compared with 4-d-old LL flies: p < 0.001, t test). The same treatment at day 2, 3, or 4 decreased the lamina‘s volume in comparison with that in LL flies, but the lamina was still significantly larger than that of flies dark-shifted on day 1 (lamina of flies from day 1 tested against the combined data of the laminae from flies of days 2–4: p < 0.05, t test) (Fig. 9).
All flies used in this study were grown as larvae and pupae in a 16:8 hr LD cycle. Thus, the animals represented in Figure 9A, panel B, had just moved out of their subjective night for ∼2 hr when they were shifted back to darkness during their subjective day. Also in panels C–E, the flies were dark-shifted during their subjective day. If flies were allowed to stay in the normal 16:8 hr cycle for the entire 4 d period (LD flies), their lamina was intermediate in size between LL and DD flies (Fig.9B). In Figure 9A, panel F, the lamina volume for flies is shown that were dark-shifted on day 4 as inE, but during their subjective night, right before the histology. Their lamina was slightly (but not significantly) larger than that of the flies dark-shifted during their subjective day (panelsC–E) and similar in size to that of LD flies.
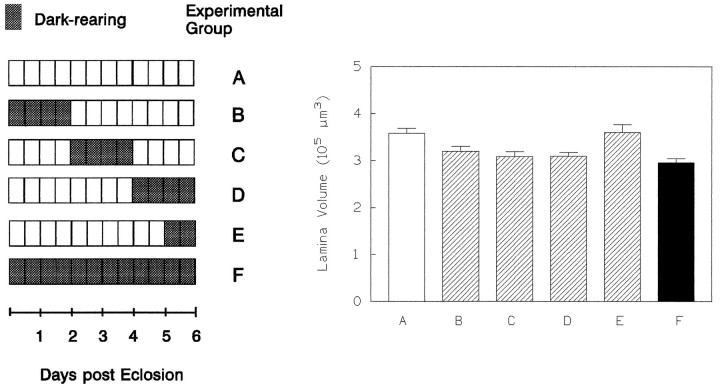
Thus far, our results show that sensitivity to light deprivation extends at least to day 4 (Figs. 8, 9). In an additional experiment, flies were kept for 2 additional days and then dark-shifted for the last 24 or 48 hr (Fig. 10, panelsD,E). A 2 d dark shift still had a volume-reducing effect (p < 0.005,t test). A 1 d shift after day 5, however, left the lamina as large as in the LL controls of the same age (p > 0.5, t test). This finding suggests that the lamina becomes gradually less sensitive to light deprivation after day 4.
Fig. 10.
Effects of extended periods of darkness in 6-d-old flies indicate that the lamina becomes less responsive compared with earlier ages (compare Figs. 7 and 9). Dark-rearing between days 2–4 (C) and 4–6 (D) reduces the lamina’s volume to that seen in DD control flies (F), whereas a dark shift during just the last day (E) no longer affects lamina volume. As before, dark-rearing for the first 2 d (B) leaves the lamina as small as in DD flies (F) (nA = 10, nB = 10, nC = 8, nD = 13, nE = 10, nF = 7).
In the flies reared for 6 d, dark shifts were also applied during earlier 2 d periods (Fig. 10, panelsB,C). The reduction in lamina volume was approximately as large as in DD flies. Therefore, it seems that a lamina that is once decreased in size because of extensive light deprivation may remain small for the rest of the fly’s life. An increase in lamina volume, on the other hand, seems to be possible only during the first 24 hr.
Taken together, these results show that visual stimulation during adulthood is necessary for the growth and, therefore, development of the lamina and other parts of the optic lobe. The first 24 hr after eclosion seem to play an important role for this development. If a fly does not receive visual input during that time, the lamina remains as small as at eclosion and cannot recover in the future. During the first 5 d of adult life, the maintenance of lamina volume depends on visual stimulation. Extensive dark periods during that time result in an irreversible decrease in lamina volume and a return toward the size of the lamina in a newly eclosed fly.
The cAMP cascade is not involved in lamina and lobula plate plasticity
In an earlier study of structural plasticity in theDrosophila brain (Balling et al., 1987), the cAMP signaling cascade has been implicated in the regulation of fiber number in the peduncle of the mushroom body. Therefore, we investigated two mutants that are thought to affect cAMP signaling at different levels (dunce1 and rutabaga1) and a gene for a neuropeptide that may also be involved in cAMP regulation (amnesiac1) (Feany and Quinn, 1995). All three mutations are known to interfere with behavioral plasticity (Davis, 1996). Interestingly, irrespective of the genotype, the lamina showed a difference of up to 30% between light- and dark-reared flies (for all mutants and the wild-type control: p < 0.05,t tests) (Fig. 11A). Thus, the cAMP cascade does not regulate the volume changes in the photoreceptor terminals that dominate the structural plasticity in the lamina. Neither does it regulate the plasticity in the size of the lobula plate (for all mutants: p < 0.05, ttest; in this particular case, the difference for the wild-type control is not significant: p > 0.1, t test) (Fig.11B). The lack of influence of these mutations on neuropil volume changes endorses the finding that immunohistochemically detected levels of expression of Dnc and Rut in the optic lobes are both low (Nighorn et al., 1991; Han et al., 1992) and suggests that the mechanisms regulating structural plasticity in the mushroom bodies and the optic lobes are based on molecular and, perhaps therefore, cellular mechanisms that are quite different.
Fig. 11.
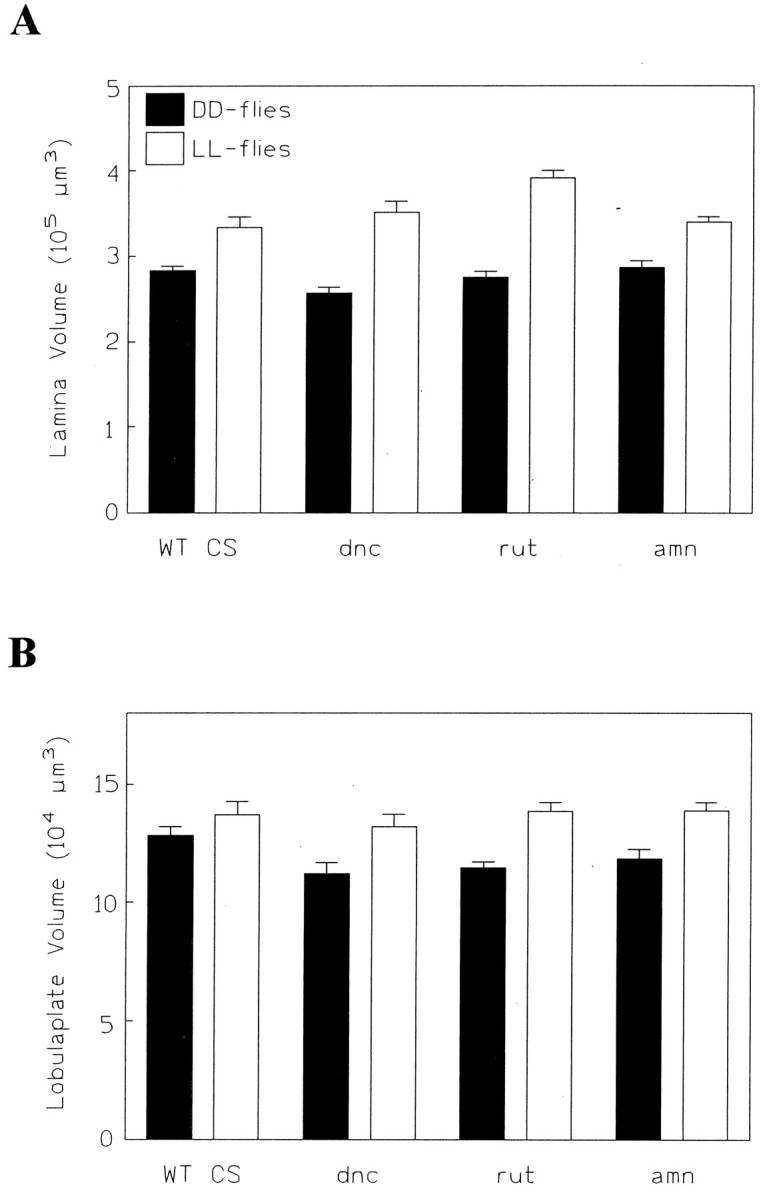
Effects of rearing in different light regimes in wild-type CS (nDD = 15,nLL = 13) and in three learning and memory mutants [dunce1(dnc;nDD = 13, nLL = 14), rutabaga1 (rut;nDD = 13, nLL = 13), amnesiac (amn; nDD = 12,nLL = 11)]. A, In all three mutants, the lamina exhibits a significant difference between DD and LL flies, ranging from 15 to 30%. B, In the lobula plate, the three mutants exhibit significant volume differences between DD and LL flies, of from 15 to 20%. In this experiment, however, the corresponding difference in the wild-type CS control was not quite significant.
DISCUSSION
Our overall findings indicate that the neuropil volume grows during the first days after eclosion in a way that is sensitive to visual experience during the first 24 hr, but with the volume increases being sustained only if visual stimulation is also received during the first 4 d of adult life. In previous studies of structural plasticity in Drosophila (Technau, 1984; Balling et al., 1987; Heisenberg et al., 1995), complex environmental stimuli, questionable procedures of sensory deprivation, and long exposure times had been used. Light, the stimulus used here, is, on the other hand, an easily quantifiable and manipulatable stimulus, and deprivation periods as short as 6 hr are effective. In addition to the ease of controlling the stimulus, the cellular mechanisms of plasticity are also accessible. The fly’s optic lobe is structurally well characterized (Strausfeld and Nässel, 1980). Most important, the lamina, which shows the clearest evidence of volumetric plasticity, has a cellular organization that is one of the most thoroughly analyzed of any neuropils (Fischbach and Dittrich, 1989).
Cellular analysis
These advantages allow us to assign the volume changes in the lamina to certain cell types, the photoreceptors, and the large monopolar cells. Surprisingly, the differences in lamina volume are accompanied by distinct differences in cellular shape. The shape change exhibited between cartridges in 4-d-old DD flies and LL flies leads to a different curvature of the entire lamina neuropil. The difference is so pronounced that it can be seen by light microscopy in LL and DD flies prepared for mass histology, a feature that could eventually be used to select for mutants.
Our EM study attributes >90% of the volume changes in the lamina to the R1–R6 terminals of the photoreceptors. This is certainly an overestimate insofar as the profiles of the dendritic spines of L1 and L2, as well as of the third monopolar cell L3, and the remaining lamina interneurons have all been included within the volume of the receptor terminals, which as a first approximation was derived simply by subtracting the summed cross-sectional areas of L1 and L2 from that of the cartridge. An earlier planimetric study (Hauser-Holschuh, 1975) gives an aggregate cross-sectional area for R1–R6 of 5.70 μm2, only 49% of the cartridge cross-sectional area, whereas the summed profile areas of R1–R6 and L1–L3 are 73% of the cartridge cross-sectional area.
The increased size of the R1–R6 terminals in the lamina has not been separately documented, but it can, for instance, be seen directly in Figure 3B. This conclusion is, moreover, supported by results from the two mutants norpAP24 andhdcjk910. The finding that a block in phototransduction suppresses the structural plasticity in the lamina suggests that it is the light-evoked response of the photoreceptors in the compound eye that triggers their cell-autonomous growth in the lamina. This coincides with the result obtained using the mutanthdcjk910, which blocks the final step in the synthesis of the photoreceptor transmitter histamine. Histamine is released (Sarthy, 1991) at sites of transmission on L1 and L2 (Meinertzhagen and O’Neil, 1991). Although the mutation fully suppresses transmission at the R→L synapses (Melzig et al., 1996), it fails to abolish the structural plasticity in the lamina. We assume that in these mutants L1 and L2, which receive no signals from the photoreceptor terminals, do not contribute to the volume changes that persist between DD and LL, thereby implicating R1–R6.
Critical periods
We have described a critical period for the effects of light-rearing on volumetric plasticity in the optic lobe. Only during the first day of imaginal life is the lamina ready to grow to its full size and only then provided that light is available. At least for the first 5 d, on the other hand, the lamina stays sensitive to light deprivation. It should be emphasized that thus far, these periods apply clearly to the photoreceptors only because it is the photoreceptor endings that dominate the lamina volume. The lamina monopolar cells and the lobula plate may have other time windows for their sensitivity to visual stimulation. The exact duration of the critical period does, however, coincide with other phenomena in the development of the fly’s visual system (Kral and Meinertzhagen, 1989; Deimel and Kral, 1992).Heisenberg et al. (1995) have shown that various parts of the brain including the optic lobe are plastic in 8- to 16-d-old flies. In their experiments, social and olfactory deprivation, as well as confinement to a small space, was the differentiating stimuli. Most likely, various processes are at work modifying different brain cells differently during adult life.
Our experiments provide a first cue that this is indeed the case. In a preliminary attempt to examine the molecular basis for structural plasticity, cAMP seems to have no major effect in the photoreceptors and lobula plate, in contrast to the peduncle of the mushroom body, in which it seems to be an important regulator of fiber number (Balling et al., 1987). A tentative mechanism for the neuropile volume changes invokes ion pumps on the membranes of surrounding epithelial glial cells to explain comparable, daily recurring changes in the volume of L1 and L2 (Meinertzhagen and Pyza, 1996).
Critical periods are thought to reflect continuing developmental processes. Analysis of reporter gene expression in enhancer-trap lines in Drosophila reveals that the genetic network of changing spatial expression patterns does not abruptly stop when the fly emerges from the pupal case. For instance, Blake et al. (1995) showed that the level of expression increases in some genes in the antennae of adultDrosophila, decreases with age in another group of genes, and has a peak 4–5 d after emergence in a third group of genes. A similar situation may be assumed for the optic lobe and brain.
Circadian adaptations
In the present study, we have described the volumetric growth of the lamina and lobula plate of flies that had emerged at the onset of their subjective day. For these animals, the critical period appears to be the first 12 hr after emergence. What happens to flies that emerge later in the day or even at the onset of night? It was always possible that they would have a smaller lamina than their siblings that eclosed at an earlier time in the LD cycle. Preliminary data suggest, however, that the critical period in these flies is simply delayed and that the light deprivation occurring during the subjective night has less severe consequences than a dark shift during the subjective day (see also Fig.9A, panel F).
The involvement of a circadian rhythm in the regulation of both the number of lamina synapses and the axon diameters of L1 and L2 is demonstrated in Musca (Pyza and Meinertzhagen, 1993, 1995a;Meinertzhagen and Pyza, 1996). The axons of L1 and L2 undergo daily size oscillations, with the largest volume in the morning. This rhythm persists even when the flies are brought to LL or DD conditions (Pyza and Meinertzhagen, 1995a), arguing that such changes are circadian. The changes in axon diameter are accompanied by variations in the number of the L2→R feedback synapses, which are more numerous at night than during day (Pyza and Meinertzhagen, 1993). In Drosophila, both features are abolished by the circadian mutantper0 (Pyza and Meinertzhagen, 1995b) endorsing the circadian basis for such changes. It is not clear whether the volume of the entire lamina also exhibits daily oscillations, superimposed on the volumetric changes seen in this study.
Functional significance
Our investigation shows that flies need visual stimulation early on in adult life for their optic lobes to fully grow. Of course, such volumetric growth may simply reflect the osmotic shifts secondary to the resetting of ion pumps. Nevertheless, it is tempting to speculate that this growth is accompanied by maturation and that with the volume changes the neural processing in these neuropils adapts to the properties of the particular environment or to the specific needs of the individual, in a way comparable, for example, to growth in the mammalian visual cortex (Greenough and Volmar, 1973). Morphometric studies on individual identified cells will be necessary to invoke such parallels.
Experience-dependent lasting modifications of visual behavior are well documented in flies. Associative changes in visual pattern and color preferences have been described in Drosophila (Menne and Spatz, 1977; Wolf and Heisenberg, 1991) and other flies (Fukushi, 1989; Troje, 1993). Moreover, evidence has been presented inDrosophila (Hirsch et al., 1990) as well as in the flyBoettcherisca (Mimura, 1986, 1987) that rearing in different light regimes affects visual orientation behavior. Recently, this finding has been extended to courtship (Hirsch and Tompkins, 1994;Hirsch et al., 1995), in which it has been shown that females copulate faster with males that are raised in the same light regime, underlining the evolutionary significance of experience-dependent behavioral modifications (Barth et al., 1997). These and the present observations invite a more detailed comparison of structural, physiological, and behavioral changes with genetic basis during the critical period of the fly’s visual system.
Footnotes
This research was supported by a grant from the German Science Foundation (DGF) and a short-term fellowship from the Boehringer Ingelheim Foundation to M.B. for his work in electron microscopy in Canada. The EM work for this project also was supported by National Institutes of Health Grant EY-03592 to I.A.M. H.V.B.H. was supported by a grant from the Whitehall Foundation, and M.H. was supported by Grant He986 of the DGF. Dieter Dudaczek helped with the sectioning on the cryostat. We thank M. Reif for critically reading this manuscript and for many hours of fruitful discussion. This work was initiated in part by Eric Hernady and Adrian Glasser in the laboratory of H.V.B.H.
Correspondence should be addressed to Martin Barth, Theodor-Boveri Institut für Biowissenschaften, 97074 Würzburg, Germany.
REFERENCES
- 1.Ashburner M. Drosophila—a laboratory handbook, pp 192–194. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1989. [Google Scholar]
- 2.Balling A, Technau GM, Heisenberg M. Are the structural changes in adult Drosophila mushroom bodies memory traces? Studies on biochemical learning mutants. J Neurogenet. 1987;4:65–73. [PubMed] [Google Scholar]
- 3.Barth M, Hirsch HVB, Heisenberg M (1997) Rearing in different light regimes affects courtship behavior in Drosophila melanogaster. Anim Behav, in press.
- 4.Bennett EL, Diamond MC, Krech D, Rosenzweig MR. Chemical and anatomical plasticity of brain. Science. 1964;146:610–619. doi: 10.1126/science.146.3644.610. [DOI] [PubMed] [Google Scholar]
- 5.Blake KL, Rogina B, Centurion A, Helfand SL. Changes in gene expression during post-eclosional development in the olfactory system of Drosophila melanogaster. Mech Dev. 1995;52:179–185. doi: 10.1016/0925-4773(95)00398-k. [DOI] [PubMed] [Google Scholar]
- 6.Buchner E, Buchner S, Burg MG, Hofbauer A, Pak WL, Pollack I. Histamine is a major mechanosensory neurotransmitter candidate in Drosophila melanogaster. Cell Tissue Res. 1993;273:119–125. doi: 10.1007/BF00304618. [DOI] [PubMed] [Google Scholar]
- 7.Bouletreau J. Ovarian activity and reproductive potential in a natural population of Drosophila melanogaster. Oecologia (Berlin) 1978;35:319–342. doi: 10.1007/BF00345140. [DOI] [PubMed] [Google Scholar]
- 8.Davis RL. Physiology and biochemistry of Drosophila learning mutants. Physiol Rev. 1996;76:299–317. doi: 10.1152/physrev.1996.76.2.299. [DOI] [PubMed] [Google Scholar]
- 9.Deimel E, Kral K. Long-term sensitivity adjustment of the compound eyes of the housefly Musca domestica during early adult life. J Insect Physiol. 1992;38:425–430. [Google Scholar]
- 10.Durst C, Eichmüller S, Menzel R. Development and experience lead to increased volume of subcompartments of the honey bee mushroom body. Behav Neural Biol. 1994;62:259–263. doi: 10.1016/s0163-1047(05)80025-1. [DOI] [PubMed] [Google Scholar]
- 11.Fahrbach SE, Robinson GE. Behavioral development in the honey bee: toward the study of learning under natural conditions. Learn Memory. 1995;2:199–224. doi: 10.1101/lm.2.5.199. [DOI] [PubMed] [Google Scholar]
- 12.Feany MB, Quinn WG. A neuropeptide gene defined by the Drosophila memory mutant amnesiac. Science. 1995;268:869–873. doi: 10.1126/science.7754370. [DOI] [PubMed] [Google Scholar]
- 13.Fischbach K-F, Dittrich APM. The optic lobe of Drosophila melanogaster. I. A Golgi analysis of wild-type structure. Cell Tissue Res. 1989;258:441–475. [Google Scholar]
- 14.Floeter MK, Greenough WT. Cerebellar plasticity: modification of Purkinje cell structure by differential rearing in monkeys. Science. 1979;206:227–229. doi: 10.1126/science.113873. [DOI] [PubMed] [Google Scholar]
- 15.Fukushi T. Learning and discrimination of coloured papers in the walking blowfly, Lucilia cuprina. J Comp Physiol [A] 1989;166:57–64. doi: 10.1007/BF00190210. [DOI] [PubMed] [Google Scholar]
- 16.Goodman CS. The likeness of being: phylogenetically conserved molecular mechanisms of growth cone guidance. Cell. 1995;78:353–356. doi: 10.1016/0092-8674(94)90413-8. [DOI] [PubMed] [Google Scholar]
- 17.Greenough WT, Volmar FR. Pattern of dendritic branching in occipital cortec of rats reared in complex environments. Exp Neurol. 1973;40:491–504. doi: 10.1016/0014-4886(73)90090-3. [DOI] [PubMed] [Google Scholar]
- 18.Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 1995;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- 19.Han PL, Levin LR, Reed RR, Davis RL. Preferential expression of the Drosophila rutabaga gene in mushroom bodies, neural centers for learning in insects. Neuron. 1992;9:619–627. doi: 10.1016/0896-6273(92)90026-a. [DOI] [PubMed] [Google Scholar]
- 20.Hardie RC. Is histamine a neurotransmitter in insect photoreceptors? J Comp Physiol [A] 1987;161:201–213. doi: 10.1007/BF00615241. [DOI] [PubMed] [Google Scholar]
- 21.Hauser-Holschuh H (1975) Vergleichende quantitative Untersuchungen an den Sehganglien der Fliege Musca domesticaund Drosophila melanogaster. PhD thesis, Eberhard-Karls-Universität.
- 22.Heisenberg M. Genetic approach to learning and memory (mnemogenetics) in Drosophila melanogaster. In: Rahmann, editor. Fundamentals of memory formation: neuronal plasticity and brain function. Fischer; Stuttgart, Germany: 1989. pp. 3–45. [Google Scholar]
- 23.Heisenberg M, Böhl K. Isolation of anatomical brain mutants of Drosophila by histological means. Z Naturforsch. 1979;34c:143–147. [Google Scholar]
- 24.Heisenberg M, Heusipp M, Wanke T. Structural plasticity in the Drosophila brain. J Neurosci. 1995;15:1951–1960. doi: 10.1523/JNEUROSCI.15-03-01951.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsch HVB. The tunable seer: activity-dependent development of vision. In: Blass EM, editor. Handbook of behavioral neurobiology. Plenum; New York: 1985. pp. 237–295. [Google Scholar]
- 26.Hirsch HVB, Tompkins L. The flexible fly: experience-dependent development of complex behaviors of Drosophila melanogaster. J Exp Biol. 1994;195:1–18. doi: 10.1242/jeb.195.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Hirsch HVB, Potter D, Zawierucha D, Choudhri T, Glasser A, Murphey RK, Byers D. Rearing in darkness changes visually-guided choice behavior in Drosophila. Visual Neurosci. 1990;5:281–289. doi: 10.1017/s0952523800000353. [DOI] [PubMed] [Google Scholar]
- 28.Hirsch HVB, Barth M, Luo S, Sambaziotis H, Huber M, Possidente D, Ghiradella H, Tompkins L. Early visual experience affects mate choice in Drosophila melanogaster. Anim Behav. 1995;50:1211–1217. [Google Scholar]
- 29.Kandel ER, Klein M, Hochner B, Shuster M, Siegelbaum SA, Hawkins RD, Glanzman DL, Castellucci VF, Abrams TW. Synaptic modulation and learning: new insights into synaptic transmission from the study of behavior. In: Edelman GM, Gall WE, Cowand WM, editors. Synaptic function. Wiley; New York: 1987. pp. 471–518. [Google Scholar]
- 30.Kral K, Meinertzhagen I. Anatomical plasticity of synapsis in the lamina of the optic lobe of the fly. Philos Trans R Soc Lond [B] 1989;323:155–183. doi: 10.1098/rstb.1989.0004. [DOI] [PubMed] [Google Scholar]
- 31.Menne D, Spatz HC. Colour vision in Drosophila melanogaster. J Comp Physiol [A] 1977;114:301–312. [Google Scholar]
- 32.Meinertzhagen IA (1997) Ultrastructure and quantification of synapses in the insect central nervous system. J Neurosci Methods, in press. [DOI] [PubMed]
- 33.Meinertzhagen IA, O’Neil SD. Synaptic organization of columnar elements in the lamina of the wild type Drosophila melanogaster. J Comp Neurol. 1991;305:232–263. doi: 10.1002/cne.903050206. [DOI] [PubMed] [Google Scholar]
- 34.Meinertzhagen IA, Pyza E. Daily rhythms in cells of the fly’s optic lobe: taking time out from the circadian clock. Trends Neurosci. 1996;19:285–291. doi: 10.1016/S0166-2236(96)10033-3. [DOI] [PubMed] [Google Scholar]
- 35.Melzig J, Buchner S, Wiebel F, Wolf R, Burg M, Pak WL, Buchner E. Genetic depletion of histamine from the nervous system of Drosophila melanogaster eliminates specific visual and mechanosensory behaviour. J Comp Physiol [A] 1996;179:763–773. doi: 10.1007/BF00207355. [DOI] [PubMed] [Google Scholar]
- 36.Menzel R. Neurobiology of learning and memory: the honeybee as a model system. Naturwissenschaften. 1983;70:504–511. doi: 10.1007/BF00394056. [DOI] [PubMed] [Google Scholar]
- 37.Mimura K. Development of visual pattern discrimination in the fly depends on light experience. Science. 1986;232:83–85. doi: 10.1126/science.232.4746.83. [DOI] [PubMed] [Google Scholar]
- 38.Mimura K. Persistence and extinction of the effect of visual pattern deprivation in the fly. Exp Biol. 1987;46:155–162. [PubMed] [Google Scholar]
- 39.Nighorn A, Healy MJ, Davis RL. The cyclic AMP phosphodiesterase encoded by the Drosophila dunce gene is concentrated in the mushroom body neuropil. Neuron. 1991;6:455–467. doi: 10.1016/0896-6273(91)90253-v. [DOI] [PubMed] [Google Scholar]
- 40.Pollack I, Hofbauer A. Histamine-like immunoreactivity in the visual system and brain of Drosophila melanogaster. Cell Tissue Res. 1991;266:391–398. doi: 10.1007/BF00318195. [DOI] [PubMed] [Google Scholar]
- 41.Pyza E, Meinertzhagen IA. Daily and circadian rhythms of synaptic frequency in the first visual neuropil of the housefly’s (Musca domestica L.) optic lobe. Proc R Soc Lond [B] 1993;254:97–105. doi: 10.1098/rspb.1993.0133. [DOI] [PubMed] [Google Scholar]
- 42.Pyza E, Meinertzhagen IA. Monopolar cell axons in the first optic neuropil of the housefly, Musca domestica L., undergo daily fluctuations in diameter that have a circadian basis. J Neurosci. 1995a;15:407–418. doi: 10.1523/JNEUROSCI.15-01-00407.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pyza E, Meinertzhagen IA. Day/night size changes in lamina cells are influenced by the period gene in Drosophila. Soc Neurosci Abstr. 1995b;21:408. [Google Scholar]
- 44.Reichert H (1996) The making of a brain: developmental, genetic, and evolutionary insights in insects. In: Brain and evolution, Vol 1 (Elsner N, Schnitzler H-U, eds), pp 41–63. 24th Göttingen Neurobiology Conference, Göttingen, Germany. Stuttgart: Thieme.
- 45.Reynolds ES. The use of lead citrate at high PH as an electron opaque stain in electron microscopy. J Cell Biol. 1963;17:208. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarthy PV. Histamine: a neurotransmitter candidate for Drosophila photoreceptors. J Neurochem. 1991;57:1757–1768. doi: 10.1111/j.1471-4159.1991.tb06378.x. [DOI] [PubMed] [Google Scholar]
- 47.Strausfeld NJ, Nässel DR. Neuroarchitectures serving compound eyes of crustacea and insects. In: Autrum H, editor. Handbook of sensory physiology, Vol VII/6B, Comparative physiology and evolution of vision in invertebrates. Springer; Berlin: 1980. pp. 1–132. [Google Scholar]
- 48.Technau G. Fiber number in the mushroom bodies of adult Drosophila melanogaster depends on age, sex and experience. J Neurogenet. 1984;1:113–126. doi: 10.3109/01677068409107077. [DOI] [PubMed] [Google Scholar]
- 49.Tieman SB, Hirsch HVB. Exposure to lines of only one orientation modifies dendritic morphology of cells in the visual cortex of the cat. J Comp Neurol. 1982;211:353–362. doi: 10.1002/cne.902110403. [DOI] [PubMed] [Google Scholar]
- 50.Troje N. Spectral categories in the learning behaviour of blowflies. Z Naturforsch. 1993;48c:96–104. [Google Scholar]
- 51.Withers GS, Fahrbach SE, Robinson GE. Selective neuroanatomical plasticity and division of labour in the honeybee. Nature. 1993;364:238–240. doi: 10.1038/364238a0. [DOI] [PubMed] [Google Scholar]
- 52.Withers GD, Fahrbach SE, Robinson GE. Effects of experience and juvenile hormone on the organization of mushroom bodies of honey bees. J Neurobiol. 1995;26:130–144. doi: 10.1002/neu.480260111. [DOI] [PubMed] [Google Scholar]
- 53.Wolf R, Heisenberg M. Basic organization of operant behavior as revealed in Drosophila flight orientation. J Comp Physiol [A] 1991;169:699–705. doi: 10.1007/BF00194898. [DOI] [PubMed] [Google Scholar]