Abstract
Monoclonal antibody Cat-307 identifies a 165 kDa neuronal protein expressed in the cat visual cortex during the period of sensitivity to alterations in visual experience (Kind et al., 1994). Dark-rearing, which prolongs the sensitive period, also prolongs the expression of the Cat-307 protein. The Cat-307 protein localizes to an organelle, here called the botrysome (from the Greek botrys, cluster of grapes), that is located between the endoplasmic reticulum (ER) and Golgi apparatus. The botrysome is composed of small ring-shaped profiles with electron-dense coats. The size and morphology of the rings and their coats are similar to those described for ER to Golgi transport vesicles. Biochemically, the Cat-307 protein cofractionates with microsomes and partitions with subunits of the coatomer proteins that coat ER-to-Golgi transport vesicles. Partial amino acid sequencing reveals that the Cat-307 protein is phospholipase C-β1, the G-protein-dependent phosphodiesterase that hydrolyses phosphatidylinositol 4,5 biphosphate into inositol 1,4,5 triphosphate and diacylglycerol after the stimulation of a variety of neurotransmitter receptors at the cell surface. These results suggest a role for phospholipase C-β1 and the botrysome in developmental plasticity and provide a possible link between receptor activation at the cell surface and protein transport during neuronal development.
Keywords: Cat-307, phospholipase C, Golgi apparatus, endoplasmic reticulum, dark-rearing, protein transport, transport vesicles, critical period
Neurons in the developing cat visual cortex pass through a sensitive period, during which their physiological and anatomical properties can be modified by alterations in visual experience (Hubel and Wiesel, 1970; Olson and Freeman, 1980; Mitchell and Timney, 1984; Blakemore, 1991; Antonini and Stryker, 1993a,b). In the cat, the sensitive period for the ocular dominance organization of the visual cortex begins at 3 weeks of age, peaks at 4–5 weeks, and is primarily over by the end of the fourth postnatal month (Olson and Freeman, 1980), with some residual plasticity remaining in the extragranular layers until 10–12 months of age (Daw et al., 1992). The time course of the sensitive period can be altered by rearing animals in complete darkness. Dark-rearing from birth delays both the onset and the close of the sensitive period for cortical ocular dominance (Cynader and Mitchell, 1980; Cynader, 1983; Mower, 1991), disrupts the normal segregation of geniculocortical afferents in primary visual cortex (Swindale, 1981, 1988; Mower et al., 1985; Stryker and Harris, 1986), and disrupts the regulation of the expression of a number of neuronal proteins (Sur et al., 1988; Guimaraes et al., 1990; Reid and Daw, 1995).
Numerous neurotransmitters and their receptors have been demonstrated to participate in developmental plasticity. Both the NMDA and the metabotropic subfamilies of glutamate receptors (mGluR) play a role in synaptic plasticity in the hippocampus and the visual cortex (Kleinschmidt et al., 1987; Collingridge and Davies, 1989; Bashir and Collingridge, 1992; Bashir et al., 1993; Kato, 1993; Bolshakov and Siegelbaum, 1994; Bortolotto et al., 1994; Kirkwood and Bear, 1994,1996; Hensch and Stryker, 1996). In addition, blockade of the serotonin (5-HT) receptor can reduce the monocular deprivation-induced shift in ocular dominance (Gu and Singer, 1995), and acetylcholinergic receptors (AChR) and noradrenergic receptors may function to facilitate plastic modifications in response to activity (Kasamatsu and Pettigrew, 1976;Bear and Singer, 1986). Despite these insights into the receptors involved in synaptic plasticity, very little is known of the effector molecules that directly alter neuronal phenotype.
In an attempt to identify gene products that may play a role in the cellular mechanisms of developmental plasticity, we used a tolerization and rapid immunization strategy (Hockfield, 1987; Kind and Hockfield, 1995) to generate monoclonal antibodies to proteins that are downregulated after the close of the sensitive period in the cat visual cortex (Kind et al., 1994). One of these antibodies, Cat-307, recognizes a 165 kDa protein that is present in neurons of the cat visual cortex from birth, peaks in expression at 5 weeks of age, and is downregulated by 15 weeks of age. Here we report several findings that further implicate the Cat-307 protein in the cellular mechanisms of developmental plasticity. First, dark-rearing prevents the normal developmental downregulation in Cat-307 immunoreactivity in area 17 but not in other primary sensory cortical areas. Second, the Cat-307 protein is found within cortical neurons in association with an unusual organelle, which we have named the botrysome (from the Greekbotrys, meaning cluster of grapes). The botrysome is located between the endoplasmic reticulum (ER) and the Golgi apparatus and is composed of small vesicles that resemble ER to Golgi transport vesicles, suggesting a role in protein transport. Such a role is supported further by the observation that Cat-307 immunoreactivity copurifies with microsomes and ER to Golgi transport vesicle proteins. Third, purification and partial amino acid sequence analysis of the Cat-307 protein identify it as phospholipase C-β1 (PLC-β1), a G-protein-activated phosphodiesterase that hydrolyses phosphatidylinositol 4,5 biphosphate into the two second messengers diacylglycerol (DAG) and inositol 1,4,5 triphosphate (IP3). The association of PLC-β1 with the botrysome raises the possibility that mGluR activation at the cell surface could lead to changes in protein transport during neuronal development.
MATERIALS AND METHODS
Animals and rearing paradigms. Cats of 4, 5, and 15 weeks of age and ferrets 3–6 weeks of age were used in this study. The pattern of Cat-307 immunoreactivity on tissue sections and Western blots is identical in ferret and cat. To determine whether the expression of the Cat-307 protein was regulated by visual experience, two cats from separate litters were reared in complete darkness from birth until 15 weeks of age. Two normally reared animals served as controls. In addition, the brain from a 17-week-old dark-reared cat was generously provided by Dr. Donald Mitchell (Dalhousie University).
Histological tissue preparation. Cats were deeply anesthetized with an overdose of Nembutal and perfused through the ascending aorta with sodium PBS, pH 7.4, followed by 4% paraformaldehyde in 0.1 m sodium phosphate buffer for light microscopy and with 4% paraformaldehyde/0.075% glutaraldehyde in 0.1m sodium phosphate buffer for electron microscopy. For light microscopy, primary visual, somatosensory, and auditory cortices and the dorsal lateral geniculate nucleus (dLGN) were dissected, equilibrated in 30% sucrose, and frozen sectioned at 50 μm. The sections were reacted for Cat-307 as described previously (Kind et al., 1994). For electron microscopy, sections of the visual cortex were cut on a vibratome at 50 μm and reacted with the Cat-307 antibody. The DAB reaction product was intensified with 1% osmium tetroxide in 0.1m phosphate buffer for 0.5–2 hr. Sections used for electron microscopy were subsequently dehydrated into propylene oxide and embedded in either Epon or Durcupan (Polysciences, Warrington, PA). Ultrathin sections were counterstained with lead citrate and uranyl acetate and viewed on a Jeol-JEM 1010 electron microscope. For colloidal gold immunohistochemistry, the sections were incubated in Cat-307 antibody overnight at room temperature and then incubated in a 1:50 dilution of goat anti-mouse IgM antibody coupled to a 1 nm gold particle (Amersham, Arlington Heights, IL). The reaction was intensified using a silver enhancement kit (Amersham).
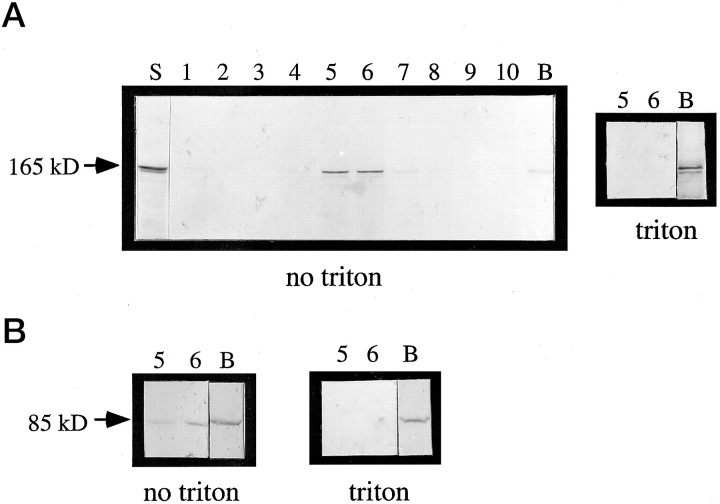
Microsome preparation. Microsomal fractions were prepared from one 4-week-old cat cortex and six 5-week-old ferret cortices. Cortices were homogenized in a 10 mm HEPES buffer containing 320 mm sucrose solution, 1 mm dTT, 2 mm EGTA, and protease inhibitors (10 mmleupeptin, 2 mm ε-amino-n-caproic acid). The homogenate (H) was subjected to a series of centrifugations to separate different organelles based on their mass. The crude H was first centrifuged for 10 min at 600 rpm on a JA 18.1 rotor to remove nuclei and unbroken cells. The resulting supernatant was then centrifuged at 11,500 rpm on a JA 18.1 rotor for 30 min to pellet Golgi and mitochondria. The supernatant from this fraction was subsequently centrifuged at 30,000 rpm on a SW41 rotor to pellet microsomes. The microsomal fraction was resuspended in 1.5 m sucrose in HEPES buffer containing 1 mm dTT, 2 mm EGTA, and protease inhibitors (10 mm leupeptin, 2 mmε-amino-n-caproic acid). The microsomes were overlaid with 1.2m and 0.8 m sucrose in HEPES buffer (with additions as above) and centrifuged at 30,000 rpm on a SW41 rotor for 20 hr. Eleven 1 ml fractions were collected per gradient, and 10 μl of each fraction was analyzed by SDS-PAGE, as described previously (Kind et al., 1994). Under these conditions, microsomes migrate to the interface between the 1.2 and 0.8 m sucrose, corresponding to fractions 5 and 6. In the Triton-treated condition, all of the above sucrose solutions contained 1% Triton X-100.
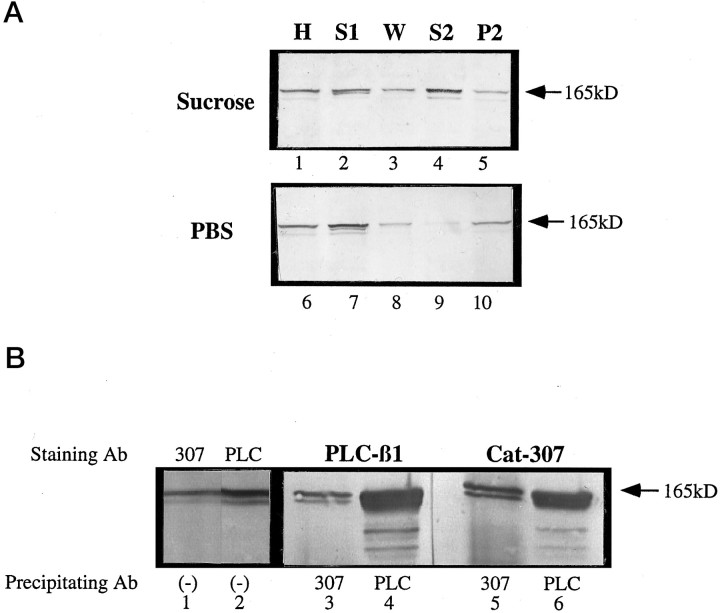
Purification of the Cat-307 protein. The Cat-307 protein was purified from ferret cortex. The first step of the Cat-307 purification relied on the mechanism by which Cat-307 associates with membranes. Three-week-old ferret cortex was homogenized in 10 mm HEPES buffer containing 320 mm sucrose, 1 mm dTT, 2 mm EGTA, and protease inhibitors (10 mmleupeptin, 2 mm ε-amino-n-caproic acid). The H was centrifuged at 30,000 rpm in a SW41 swinging bucket rotor and separated into supernatant (S1) and pellet (P1) fractions. The P1 fraction was resuspended in PBS containing protease inhibitors and recentrifuged at 100,000 × g for 1 hr and separated into supernatant (S2) and pellet (P2) fractions. Equal volumes of each fraction were subjected to SDS-PAGE analysis and immunoblotted for Cat-307 immunoreactivity. The supernatant fraction (S2) from samples in which the cortex was homogenized in 320 mm sucrose contained much higher levels of Cat-307 immunoreactivity than did the S2 fraction from samples in which the cortex was initially homogenized in PBS. Measurements of integrated density confirmed the qualitative assessment, with densities of 945 in the S2 fraction of the sucrose H and 168 in the S2 fraction of the PBS H (see Fig.6A).
Fig. 6.
The Cat-307 protein cofractionates with microsomal membranes and with β-COP. A crude microsomal fraction prepared by differential centrifugation (Malhotra et al., 1989) contains the Cat-307 protein (A, lane S). The microsomal fraction was then further purified in a step sucrose gradient (A, lanes 1-10,B). A, Western blot analysis shows that the protein recognized by Cat-307 comigrates on a step sucrose density gradient with microsomes (no triton, fractions 5 and 6). Little immunoreactivity is detected at the bottom of the gradient (B, no triton). However, after treatment of the microsomes with Triton X-100 before centrifugation, the Cat-307 protein is retained in the bottom fraction of the gradient (B,triton). Triton X-100 solubilizes microsomal membranes; thus, the lack of floatation of the Cat-307 protein in the presence of Triton X-100 indicates that the floatation in the gradient in the absence of Triton X-100 is attributable to an association of the protein with intact microsomal membranes. B, Western blot analyses of β-COP shows an identical fractionation pattern to that observed for the Cat-307 protein; that is, in the absence of Triton X-100, immunoreactivity for β-COP is seen in fractions 5 and 6, thereby confirming the association of the Cat-307 protein with the microsomal membrane fractions. Furthermore, pretreatment of the microsomal membranes with 1% Triton X-100 prevents the floatation of β-COP in the sucrose density gradient; thus, immunoreactivity for β-COP is no longer detected in fractions 5 and 6 but is seen exclusively at the bottom of the gradient.
The Cat-307 protein was purified further and concentrated by subjecting the S2 fraction to ammonium sulfate (NH3SO4) precipitation. NH3SO4 (20%) is ineffective in precipitating the Cat-307 protein. The S2 fraction was first subjected to 20% NH3SO4 precipitation, and the precipitated proteins were discarded. The sample was subsequently subjected to 35% NH3SO4, a concentration that is effective in precipitating the majority of the Cat-307 protein, and the pellet fraction was resuspended in 50 mm TBS, pH 7.2, and dialyzed against the same buffer. The resulting sample was subjected to two rounds of FPLC on a monoQ column. The first column was eluted with a 50m Tris-buffered 0 m to 2 m NaCl gradient, and the fractions containing Cat-307 immunoreactivity were pooled. The pooled fractions again were subjected to FPLC using a monoQ column and eluted using a 50 mm Tris-buffered 0m to 800 mm NaCl gradient. The fractions containing Cat-307 immunoreactivity were pooled and concentrated by TCA precipitation according to standard methods. The resulting samples were subjected to SDS-PAGE analysis, and the gels were either stained with Coomassie blue or transferred to nitrocellulose and Western blotted for Cat-307 immunoreactivity. Under these conditions, the Cat-307 immunoreactive band was clearly distinguishable from any other protein. Coomassie-stained bands corresponding to the Cat-307 immunoreactive protein were excised from the gel, digested with trypsin, and subjected to N-terminal amino acid sequence analysis by the William Keck Foundation Biotechnology Resource Laboratory at Yale University.
Immunoprecipitations. Cat-307 (an IgM) and anti-PLC-β1 (an IgG; Upstate Biotechnology, Lake Placid, NY) were adsorbed to goat anti-mouse IgM or IgG agarose beads (Sigma, St. Louis, MO), respectively, by incubation at 4°C overnight. The beads were washed with TBS and incubated with a soluble fraction of 5-week-old cat visual cortex. The bound antigens were eluted by boiling in SDS-PAGE sample buffer containing 5% β-mercaptoethanol for 5 min. The samples were then subjected to SDS-PAGE analysis, transferred to nitrocellulose, and immunostained for either Cat-307 or PLC-β1 immunoreactivity.
RESULTS
Monoclonal antibody Cat-307 identifies a 165 kDa protein that is present in the cat visual cortex from birth, increases until 5 weeks of age, and is subsequently downregulated in the cortex at 15 weeks. In the 5-week-old cat, the Cat-307 protein is expressed in all cortical areas examined but is absent from all cortical areas in the adult. Cat-307 immunoreactivity is seen as small, intensely stained dots in neurons; based on the fine structure of these dots (see below), they have been named botrysomes, from the Greek botrys, meaning cluster of grapes. Cat-307 immunoreactive botrysomes are seen in neurons, but not in glial cells, and are present in all layers of the cortical plate, in both nonpyramidal and pyramidal neurons, and in GABAergic and non-GABAergic neurons (Kind et al., 1994).
Expression of the Cat-307 protein is prolonged by dark-rearing
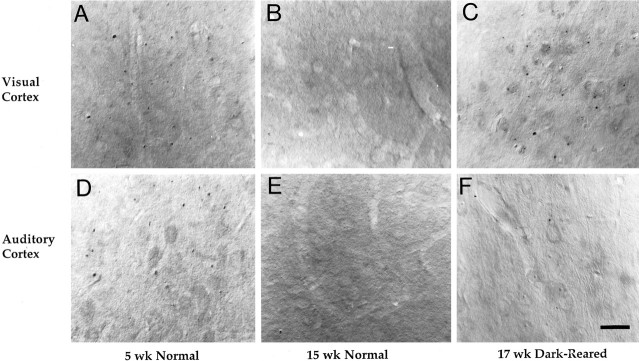
We have demonstrated previously that Cat-307 immunoreactive botrysomes are present throughout area 17 of 5-week-old, but not 15-week-old, cats (Kind et al., 1994). The regulation of the Cat-307 protein over the course of development suggested that its expression might correlate with periods of developmental plasticity. To pursue this possibility, Cat-307 immunoreactivity was examined in the cortex of dark-reared cats. Cats were raised in total darkness from birth to 4 months of age, an age at which Cat-307 can no longer be detected in the visual cortex of normally reared animals. Previous reports have demonstrated that the sensitive period, during which the physiological properties of neurons of the visual cortex can be altered by monocular deprivation, is prolonged by rearing animals in complete darkness (Cynader, 1983; Mower et al., 1985; Mower, 1991). Dark-rearing to 4 months of age attenuated the normal downregulation of the Cat-307 protein in the cat visual cortex (Fig. 1), but not in any other cortical area examined. As shown in Figure 1, numerous Cat-307-immunoreactive botrysomes were observed in the visual cortex (Fig. 1E), but not in the auditory cortex (Fig.1F), of dark-reared animals. Cat-307 immunoreactive botrysomes were not observed in the visual (Fig.1C) or auditory (Fig. 1D) cortex of age-matched, normally reared animals. The pattern of Cat-307 staining in area 17 of dark-reared animals was qualitatively similar to that observed in young kittens (compare Fig. 1, E withA and B) and was seen in both of the animals examined. Cat-307 immunoreactive botrysomes were present in neurons throughout area 17. Botrysomes were also found in all layers of the visual cortex of dark-reared animals, with a distribution essentially indistinguishable from that seen in 5-week-old animals.
Fig. 1.
Dark-rearing prevents the normal downregulation of the Cat-307 protein in primary visual, but not in primary auditory, cortex. Sections through layers IV and V of primary visual (A, B, C) and primary auditory (D, E, F) cortex from a 5-week-old normally reared (A,D), a 15-week-old normally reared (B,E), and a 17-week-old dark-reared (C,F) cat immunoreacted with Cat-307. Immunoreactive botrysomes are present in both the visual (A) and the auditory (D) cortices in the 5-week-old cat but absent from both areas by 15 weeks of age (B,E). Botrysomes are present in the visual cortex (C) after dark-rearing, but are not present in the auditory cortex (F) of dark-reared animals. Immunoreactive botrysomes can be found in all layers of area 17 of dark-reared animals in a pattern that is very similar to that observed in 5-week-old normal animals. Scale bar, 30 μm.
Cat-307 immunoreactivity is located in the somatodendritic domain of cortical neurons
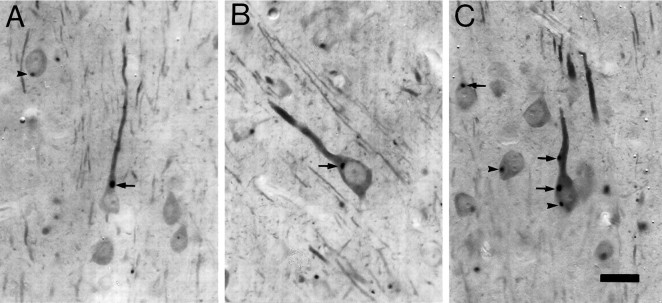
Light microscopic analysis of Cat-307-labeled cortical sections showed that Cat-307 immunoreactive botrysomes were not randomly distributed throughout the cell body, but resided near the proximal portions of dendrites (Fig. 2). Many neurons appeared to have only one botrysome, but up to three botrysomes were seen within a single neuron. The intraneuronal distribution of the botrysomes in the 5-week-old cat visual cortex was more closely examined using antibody-stained sections that were post-fixed with osmium tetroxide. This procedure not only intensified Cat-307 immunoreactivity, but also permitted the visualization of neuronal cell bodies and dendrites. In 100 neurons in which at least a single dendrite and one Cat-307-positive botrysome could be identified, 77 botrysomes were located either at the base of, or within, the proximal part of a dendrite. The remaining 23 botrysomes did not appear to be associated with a visible dendrite, but most of these were located in the basal portion of the cell body and thus may have been close to basal dendrites that were not within the plane of section. Therefore, the majority of botrysomes were located in or near dendrites.
Fig. 2.
Cat-307 identifies a cytoplasmic structure in neurons that is often located near a major dendrite.A–C, Three layer V neurons containing Cat-307 immunoreactive botrysomes. In 50-μm-thick sections, Cat-307 immunoreactive profiles are observed in the cytoplasm of neurons and are most often found within, or just below, the apical dendrites of pyramidal cells. A, Two botrysomes, one located at the base of the large apical dendrite of a layer V pyramidal neuron (arrow) and the other in the basal portion of a neuron (arrowhead). The apical dendrite of the pyramidal neuron (arrow) contains a small botrysome farther from the cell body. B, A layer V pyramidal cell with a botrysome located just below the apical dendrite and very close to the nucleus.C, A large layer V pyramidal cell containing three immunoreactive botrysomes within its cell body and apical dendrite. Two of the botrysomes are associated with the apical dendrite (arrows), one located within and the other at the base of the dendrite. The third is located in the basal portion of the cell body (arrowhead). Such a basally located botrysome would have been classified as not associated with a dendrite; however, the basal dendrites of the cell are out of the plane of section, and this botrysome may also be at the base of a dendrite. The largest botrysomes are seen in layer V neurons, where they can reach a diameter of 4 μm. The diameter of botrysomes varies; even within a single cell, botrysome size does not correlate with neuronal size, although the smallest botrysomes are usually found within dendrites. Scale bar, 20 μm.
Cat-307 identifies a novel cortical organelle, the botrysome, associated with the ER and Golgi complex
The unusual appearance and location of Cat-307 immunoreactivity at the light microscopic level prompted us to examine the subcellular localization of the antigen by immunoelectron microscopy (Fig.3). Cat-307 identified an organelle, the botrysome, that has not been described previously in cortical neurons. Botrysomes appeared as compact, ovoid structures with an immunohistochemically dense central core and less dense flanking regions (Fig.3B–D). The organelle had no limiting membrane. A large conglomeration of ring-shaped profiles surrounded the core. The size of the organelle ranged from 1 to 4μm along its long axis; however, there was no obvious correlation between neuronal size or type and botrysome size. Serial reconstructions of single botrysomes demonstrated the Cat-307-labeled structure to be located between the ER and the cis-Golgi (Fig. 4). To date, four botrysomes have been partially reconstructed and all showed a close association with both the ER and Golgi apparatus. Whereas the botrysome was always seen juxtaposed to both the ER and Golgi apparatus, the Golgi network was observed to be far more widely distributed and was not always associated with botrysomes. Figure 5 shows an example of three cortical neurons, only one of which contained a botrysome. A large pyramidal cell that did not contain a botrysome contained several Golgi complexes in the cell body that extended into the proximal portion of the apical dendrite.
Fig. 3.
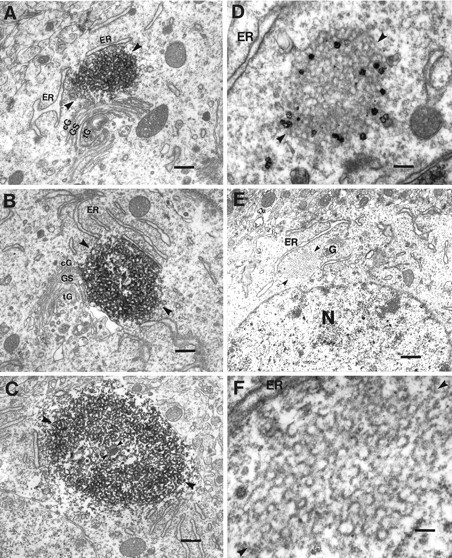
Cat-307 identifies a neuronal organelle, the botrysome, that is located between the ER and thecis-Golgi and that can be identified independently of Cat-307 immunoreactivity. A, B, Electron micrographs showing two examples of Cat-307 immunoreactivity in botrysomes located between the ER (ER) and thecis-Golgi (cG). Cat-307 immunoreactivity has never been observed within the Golgi stack (GS) or on the trans face of the Golgi (tG). The immunoreactive botrysome (arrowheads) is a compact, ovoid structure that is a conglomeration of small, ring-shaped profiles. The DAB reaction product, however, prevents a detailed analysis of the substructure of the organelle. Scale bars: A,B, 350 nm. C, Another example of an immunoreactive botrysome (large arrowheads) showing that the internal structure is not uniform (see also B). The center of the botrysome has a dense core (small arrowheads) with less dense flanking regions (asterisks). The ring-shaped elements surround the core and are the major components of the botrysome. Although the example inC does not demonstrate an association with the Golgi complex, three-dimensional reconstructions of single botrysomes suggest that all botrysomes are associated with the Golgi complex. Scale bar, 350 nm. D, Electron micrographs of Cat-307 immunoreactivity visualized with a gold-conjugated secondary antibody enhanced with silver demonstrate that the immunoreactive botrysome (arrowheads) is an organelle that can be identified independent of HRP histochemistry. Scale bar, 140 nm. E,F, Low- (E) and high-power (F) electron micrographs of a botrysome (arrowheads in E) that was deep in the section, inaccessible to the gold-conjugated antibody. (In tissue in which an HRP-conjugated secondary antibody was used, we have never seen an unlabeled botrysome.) The low-power micrograph (E) clearly demonstrates the location of the botrysome between the Golgi apparatus and the ER. Higher magnification (F) of this unlabeled botrysome confirms it to be a conglomeration of small ring-shaped profiles. The diameter of an individual ring is ∼70 nm, similar to the size of COP-coated vesicles. The rings have a moderately electron dense wall with a clear center. Not all of the rings are complete; many are c-shaped. The rings are covered with an electron-dense material with a thickness of 15 nm, similar to the protein coat of transport vesicles between the ER and Golgi apparatus. Scale bars: E, 650 nm; F, 100 nm.
Fig. 4.
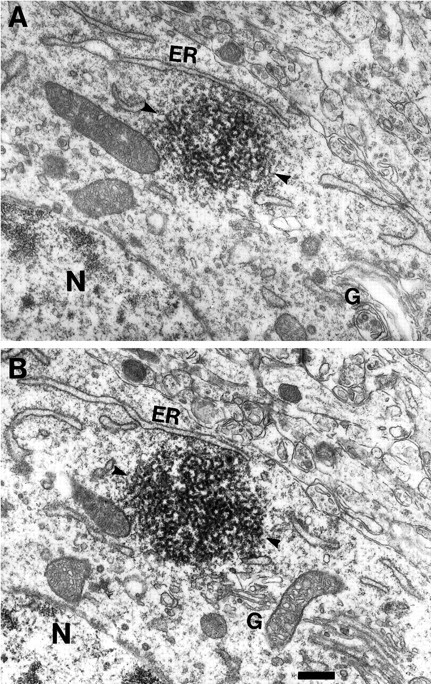
The botrysome is always associated with the ER and Golgi complex. Sections from a serial reconstruction of a single botrysome (arrowheads). In A, the botrysome is not closely associated with the Golgi complex (G); however, the micrograph in B of a subsequent section clearly demonstrates the close approximation of the botrysome and the Golgi apparatus. Scale bar, 200 nm. We have partially reconstructed four botrysomes, all of which were associated with both the ER (ER) and Golgi apparatus. Scale bar, 800 nm.
Fig. 5.
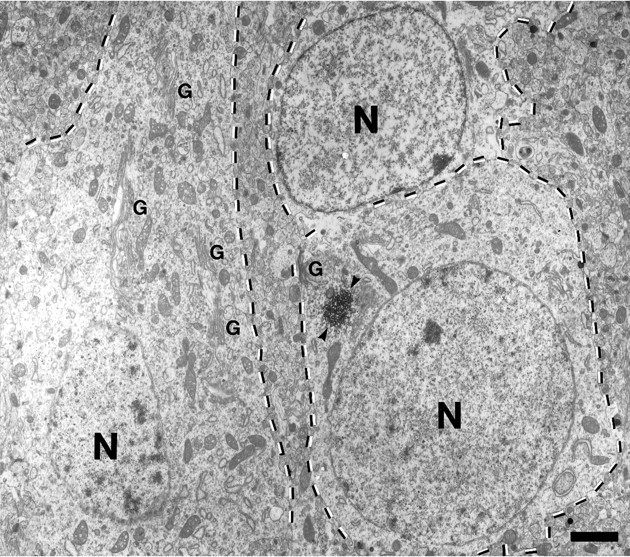
The Golgi apparatus is not always associated with the botrysome. Low-power electron micrograph of three neurons (outlined in dotted lines) in layer V of kitten visual cortex. One neuron contains a Cat-307 immunoreactive botrysome (arrowheads) labeled with HRP, located adjacent to a Golgi apparatus (G). The adjacent neuron contains numerous Golgi apparati but no botrysomes. Golgi apparati are much more numerous than botrysomes and can be seen throughout the cell body. The botrysome appears to be associated with a specialized region of the Golgi apparatus located near the base of dendrites. Scale bar, 3 μm.
To reveal the ultrastructure of the botrysome without the distortions in membrane density that attend HRP labeling, a colloidal gold conjugated secondary antibody was also used to visualize Cat-307 immunoreactivity (Fig. 3E). Cat-307 immunoreactivity visualized with a gold-conjugated antibody identified the same organelle that was identified with an HRP-conjugated antibody. The gold conjugate allowed for better visualization of the organelle and permitted the identification of the unstained organelle in deep regions of the tissue into which the gold-conjugated secondary antibody could not penetrate (Fig. 3E,F). No unlabeled botrysomes were observed in HRP-labeled tissue or in the superficial regions of gold-labeled sections. In addition, gold immunohistochemistry confirmed our initial observations from HRP-stained sections that the botrysome was comprised of many small, ring-shaped elements. Not all of the ring-shaped subunits were complete circles; many were crescent-shaped or otherwise incomplete circles (Fig. 3F). The rings had a moderately electron-dense wall with a clear center. The size of individual rings was ∼70 nm. In addition, the rings appeared to be coated with an electron-dense material that was ∼15 nm in thickness.
The Cat-307 protein cofractionates with microsomes
The position of the botrysome between the ER and Golgi apparatus, as well as the similarity in structure of the small vesicles of the botrysome to transport vesicles, suggested an association with microsomes. To determine whether the Cat-307 protein is associated with microsomal membranes, we isolated microsomes and associated proteins by subcellular fractionation using differential centrifugation (Fig.6). This method has been used previously to demonstrate a membrane association for a group of proteins, called coatomers or COPs, that coat transport vesicles between the ER and Golgi apparatus (Malhotra et al., 1989). The microsomes and their associated proteins were then purified further by floatation in a step sucrose gradient.
The Cat-307 protein fractionated with the microsomes (Fig.6A, no triton) and behaved identically to members of both the COP I (Fig. 6B, no triton) and the COP II (data not shown) protein complexes. To ensure that the migration of the Cat-307 protein within the step sucrose gradient was not simply attributable to the formation of macromolecular complexes that could also float in the sucrose gradient, the microsomes were pretreated with 1% Triton X-100 to disrupt membranes (Fig.6A, triton). The Cat-307 protein did not float in the step sucrose gradient after membranes had been extracted with detergent. As a control for extraction of membrane components, we also assayed the behavior of β-COP, a subunit of the COP I complex (Fig. 6B), which similarly did not float in the step sucrose gradient after detergent treatment (Fig. 6B,triton).
Cat-307 recognizes PLC-β1
The Cat-307 protein was purified and subjected to amino acid sequence analysis. The first step of the purification relied on the fact that the Cat-307 protein is a noncovalently linked, peripheral membrane protein with an association dependent on osmolarity (Fig.7A), and final purification was achieved by FPLC and SDS-PAGE. Two tryptic peptides (8 and 17 amino acids in length) were sequenced and showed 100% identity with PLC-β1 isolated from bovine (Suh et al., 1988) and rat brain (Katan et al., 1988). Four isoforms of PLC-β (PLCβ-1–4) have been isolated. The four isoforms share two highly conserved regions, called the X domain and the Y domain. One of the sequenced peptides was located in the conserved Y domain (representing amino acids 552–560), and the other was located in a less conserved region closer to the C terminal, representing amino acids 1016–1028. The identity between the Cat-307 protein and PLC-β1 was confirmed by immunoprecipitation (Fig. 7B). Supernatant fractions from 5-week-old cat visual cortex were immunoprecipitated with either Cat-307 or a mixture of monoclonal antibodies specific for bovine PLC-β1. The Cat-307 antibody immunoprecipitated a doublet at 150 and 165 kDa that was recognized by both Cat-307 (Fig.7B, lane 5) and antibodies to PLC-β1 (Fig.7B, lane 6). Furthermore, antibodies to PLC-β1 immunoprecipitated a doublet with the same electrophoretic mobility that was also recognized by antibodies to both PLC-β1 (Fig. 7B, lane 4) and Cat-307 (Fig. 7B, lane 3). Together, these data indicate that the Cat-307 protein is PLC-β1.
Fig. 7.
The Cat-307 protein is PLC-β1. A, Cortical tissue was homogenized in either 320 mm sucrose (lanes 1-5) or PBS (lanes 6–10). The homogenates (H, lanes 1 and 6) were spun at 100,000 ×g for 1 hr and separated into supernatant (S1, lanes 2 and 7) and pellet fractions. The pellet fraction was washed in an equal volume of the appropriate buffer and spun at 100,000 × g. The supernatant fraction was removed (W, lanes 3 and 8) and the pellet rehomogenized in an equal volume of PBS. The H was allowed to sit for 20–30 min at 4°C and was then centrifuged for 1 hr. The supernatant (S2,lanes 4 and 9) and pellet (P2, lanes 5 and 10) fractions were collected, and all fractions were subjected to Western blot analysis for the Cat-307 protein. The large amount of Cat-307 protein in the S2 fraction of the sucrose homogenates compared with the PBS homogenates indicates that the Cat-307 protein is associated with the microsomal membranes in a noncovalent manner that is dependent on the osmolarity of the homogenizing solution. This type of membrane association is characteristic of peripheral membrane proteins and is identical to that shown previously for the COPs. The Cat-307 protein was further concentrated and purified from the S2 fraction by ammonium sulfate precipitation and FPLC using a monoQ column (data not shown). Amino acid sequence analysis identified the Cat-307 protein as PLC-β1. B, Immunoblot of purified Cat-307 protein stained with antibodies to Cat-307 (lane 1) or PLC-β1 (lane 2). One lane of the purified protein was transferred to nitrocellulose, cut in half, and stained for the two antibodies. Cat-307 and antibody to PLC-β1 each identifies an exactly comigrating protein doublet. Proteins immunoprecipitated with either PLC-β1 (lanes 4 and 6) or Cat-307 (lanes 3 and 5) are immunoreactive for both Cat-307 (lanes 5 and6) and PLC-β1 (lanes 3 and4). Each antibody immunoprecipitates a protein recognized by the other antibody, indicating that the Cat-307 protein is PLC-β1.
DISCUSSION
The cellular and molecular mechanisms underlying the ability of immature neurons to undergo alterations in morphology in response to activity are beginning to be elucidated. We reported previously the isolation of the Cat-307 monoclonal antibody, which identifies a neuronal protein that is present in the 5-week-old kitten visual cortex, but is absent from the 15-week-old visual cortex (Kind et al., 1994). The possibility that this protein may participate in cellular mechanisms used during developmental neuronal plasticity is raised by the observation that the normal reduction in Cat-307 protein expression over the course of development is attenuated by dark-rearing. The subcellular localization of the Cat-307 protein to an intermediate compartment-like structure, the botrysome, and its identification as PLC-β1, a phosphodiesterase that hydrolyzes phosphatidylinositol 4,5 biphosphate (PIP2) into IP3 and DAG, together suggest that receptor activation at the cell surface during periods of developmental plasticity may lead to alterations in protein transport and, ultimately, to changes in neuronal morphology.
The expression of the Cat-307 protein is regulated by visual experience
The development of many of the physiological and anatomical properties of visual cortical neurons is known to be dependent on the nature of an animal’s early visual experience. Dark-rearing from birth disrupts the normal development of orientation and direction-selective cells (Buisseret and Imbert, 1976; Czepita et al., 1994). Furthermore, dark-rearing attenuates geniculocortical segregation in layer IV of primary visual cortex (Swindale, 1981, 1988; Mower et al., 1985;Stryker and Harris, 1986); and prevents the normal age-dependent decline in ocular dominance plasticity to monocular deprivation in the visual cortex, but not in the dorsal lateral geniculate nucleus. In addition, dark-rearing has been shown to extend the period of ocular dominance plasticity in response to monocular deprivation. Dark-rearing from birth to 15–17 weeks of age prolonged the expression of the Cat-307 protein in the visual cortex, but not in nonvisual cortical areas, strongly suggesting a role for the protein in developmental plasticity.
The association of Cat-307 immunoreactivity with dendrites suggests that the Cat-307 protein may play a role in dendritic plasticity. Dendritic development can be affected by visual experience, as evidenced by studies showing that dark-rearing prevents the normal formation of laterally extending dendrites (Coleman and Riesen, 1968) and decreases the length and complexity of laterally projecting dendrites (Reid and Daw, 1995). The time course of dendritic plasticity after dark-rearing has not been examined; however, the location and expression of Cat-307 immunoreactivity indicate that dendritic fields of neurons in all cortical layers may remain plastic after dark-rearing from birth to 4 months of age.
Localization of PLC-β1 to the botrysome suggests a role in protein trafficking
Immunoelectron microscopic analysis of Cat-307-labeled neurons revealed a neuronal organelle whose location, between the ER and Golgi apparatus, is suggestive of a role in protein transport between these structures. Studies over the last several years have described many features of the molecular machinery of ER to Golgi trafficking. Two groups of proteins, called COPs, that coat transport vesicles and mediate vesicle budding from donor membranes have been described (Malhotra et al., 1989; Waters et al., 1991; Rothman, 1994). Newly synthesized proteins are packaged into COP I- and COP II-coated vesicles that transport the proteins from the ER to thecis-Golgi for additional post-translational modifications (Rothman, 1994). The morphological and biochemical properties of the botrysome are very similar to those of transport vesicles. The small, ring-shaped profiles that make up the botrysome are 70 nm in diameter, corresponding closely in size to ER-to-Golgi transport vesicles (Rothman, 1994). In addition, the rings have an electron-dense coat with a thickness of 15 nm, very similar in thickness to the coated vesicles between the ER and Golgi apparatus. The location of the botrysome suggests that it may be a specialized form of the intermediate compartment; however, the large size and general appearance of the organelle is unlike any previous descriptions for the intermediate compartment.
Although the small rings of the botrysome are similar in size and overall structure to transport vesicles, they do not exhibit a trilaminar structure typical of coated vesicles. The cofractionation of Cat-307 immunoreactivity with a microsomal preparation previously used to purify COP I (Malhotra et al., 1989) definitively demonstrates an association of the Cat-307 protein with microsomal membranes. Like the COPs, the association of the Cat-307 protein with membranes is dependent on the osmolarity of the homogenization solution. These data indicate that the Cat-307 protein is a peripheral membrane protein of transport-like vesicles that is noncovalently associated with membranes.
To our knowledge, a structure like the botrysome has only been observed previously in the unipolar brush cell of the adult cerebellum of cats (Mugnaini, 1972) and rats (Mugnaini et al., 1994); these anatomical studies did not include any biochemical or developmental analysis of the organelle. Interestingly, the botrysome is similar to a structure found in neurons of the hypothalamus and is referred to as a nematosome (Leranth et al., 1991). The nematosomes appear in hypothalamic neurons of ovariectomized animals that have been treated with estrogen, a procedure that induces synaptic rearrangements on the dendrites of these neurons (Naftolin et al., 1985; Leranth et al., 1991). It will be interesting to determine whether the Cat-307 antibody recognizes the nematosomes of hypothalamic neurons and whether the organelle in the unipolar brush cell also reflects a capacity for synaptic plasticity.
It is difficult to know with certainty whether the normal loss of Cat-307 immunoreactivity with age represents a disappearance of the botrysome itself or simply a loss of the protein from an organelle that is present at all ages; however, several observations make the former a more likely possibility. First, we have observed the botrysome in unstained tissue from kitten, but not from adult, visual cortex. Second, although it may be surprising that this organelle has not been described previously outside of the cerebellum, it is more understandable if it is restricted to young neurons. Most of the detailed electron microscopic studies of neuronal structure in the visual cortex have been carried out on tissue from mature animals, whereas those studies done in young animals have been largely restricted to the examination of synapses.
The Cat-307 protein is PLC-β1
Amino acid sequence analysis of purified Cat-307 protein revealed 100% identity to PLC-β1 over the 25 amino acids obtained. Cross-immunoprecipitation experiments using Cat-307 and antibodies to PLC-β1 confirmed that the protein identified by Cat-307 is PLC-β1. PLC-β1 is a phosphodiesterase that when activated by a receptor-stimulated G-protein, Gq/11, hydrolyzes PIP2 into the two second messenger molecules, DAG and IP3. DAG in turn activates protein kinase C, and IP3 binds to receptors on the ER causing the release of Ca2+ into the cytoplasm (Rhee, 1994). PIP2 hydrolysis takes place in cortical neurons in response to stimulation of several neurotransmitter receptors (Sarri et al., 1995), including the group I mGluRs (mGluR1 and mGluR5), the 5-HT2 (Peroutka et al., 1994), and the muscarinic ACh receptor (mAChR) (Carter et al., 1990; Bernstein et al., 1992; Zhou et al., 1994); however, only the mGluRs and the mAChR have been demonstrated to specifically stimulate PLC-β1. In addition, in cat visual cortical neurons, IP3 turnover after mGluR, but not after mAChR, stimulation is maximal at the peak of the critical period and declines with age, leading to the conclusion that the group I mGluRs and the IP3 second messenger pathway play a role in developmental plasticity (Dudek and Bear, 1989).
A role for mGluRs in several forms of synaptic plasticity has been suggested by studies in both the hippocampus and the cerebral cortex. Most notably, the mGluRs appear to play a critical role in the establishment of long-term depression in both the hippocampus and the visual cortex. Blockade of the mGluRs prevents the induction of long-term depression in cortical neurons in the mouse visual cortex, but does not affect ocular dominance plasticity to monocular deprivation in the cat visual cortex (Hensch and Stryker, 1996). Whereas the group I mGluRs undergo some degree of downregulation over the course of development, the level of expression in the adult cortex remains relatively high (Reid et al., 1995). In contrast, the expression of PLC-β1 in the botrysome parallels neuronal plasticity, with maximal expression during the peak of neuronal plasticity and a subsequent decrease, mimicking the developmental regulation of mGluR-stimulated IP3 turnover (Dudek and Bear, 1989). Regulation of PLC-β1 expression could play a role in the regulation of IP3 turnover.
Both the 5-HT2 receptors and the NMDA receptor are also known to affect PLC activity (Dixon et al., 1994; Peroutka et al., 1994). 5-HT2 receptors activate PLC directly through heterotrimeric G-proteins, although the isotype of PLC that is activated in response to 5-HT2 stimulation has yet to be determined. NMDA receptor stimulation can also lead to IP3 accumulation in mouse and monkey cortical slices, presumably also through PLC activation (Dixon et al., 1994). The activity of PLC is known to be dependent on the level of free calcium; therefore, NMDA receptor activation could influence PLC activity by increasing the level of calcium in the cytoplasm.
Phosphoinositides and inositolphosphates also are likely to participate in protein trafficking (De Camilli et al., 1996). For example, PIP2 can promote, whereas IP3 can inhibit, vesicle budding. PLC-β1 could, by controlling the levels of PIP2 and IP3, regulate vesicle trafficking. PLC-β1 has been detected in coated vesicle preparations from bovine brain (Litosch et al., 1993; Martin et al., 1995). This observation agrees well with our ultrastructural and biochemical characterization of the botrysome and PLC-β1. The botrysome has an ovoid shape and is comprised of smaller ring-shaped profiles that are very similar in size and shape to the ER to Golgi transport vesicles. Furthermore, Cat-307 copurifies with members of both the COP I and COP II in microsomal preparations from both cat and ferret cortices.
PLC-β1, therefore, may serve a dual role in developing cortical neurons. First, the classical role of PLC-β1 is as a signaling molecule in response to neurotransmitter stimulation. Second, our observations suggest that PLC-β1 may also regulate protein trafficking between the ER and Golgi apparatus by controlling the levels of phosphoinositides and inositol polyphosphates. In support of this hypothesis, heterotrimeric G-proteins have been demonstrated to play a major role in protein transport (Schwaninger et al., 1992; Bauerfeind and Huttner, 1993), including regulating apical versus basal transport in epithelial cells (Pimplikar and Simons, 1993; Le Gall et al., 1995). Furthermore, it has been suggested that G-proteins can translocate from the cell surface to intracellular organelles (Ibarrondo et al., 1995), and both Gq and Gi3proteins have been demonstrated in the Golgi apparatus (Wilson et al., 1994; Denker et al., 1996). This potential dual role for PLC-β1 in both signal transduction and protein trafficking raises exciting possibilities for the elucidation of the mechanisms by which receptor stimulation at the cell surface leads to alterations in neuronal morphology during critical periods in development.
Footnotes
This work was supported by National Institutes of Health Grants EY06511 (S.H.) and EY06606 (P.K.). We thank Drs. Pietro DeCamilli, Ira Mellmann, and David Sheff for helpful advice in the course of this work, and Dr. Donald Mitchell for providing brain tissue. We also thank members of the Hockfield Laboratory for critical readings of this manuscript.
Correspondence should be addressed to Dr. Peter Kind, Section of Neurobiology, Yale University School of Medicine, 333 Cedar Street, New Haven, CT 06511.
REFERENCES
- 1.Antonini A, Stryker MP. Rapid remodeling of axonal arbors in the visual cortex. Science. 1993a;260:1819–1821. doi: 10.1126/science.8511592. [DOI] [PubMed] [Google Scholar]
- 2.Antonini A, Stryker MP. Development of individual geniculocortical arbors in cat striate cortex and effects of binocular impulse blockade. J Neurosci. 1993b;13:3549–3573. doi: 10.1523/JNEUROSCI.13-08-03549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bashir ZI, Collingridge GL. Synaptic plasticity: long-term potentiation in the hippocampus. Curr Opin Neurobiol. 1992;2:328–335. doi: 10.1016/0959-4388(92)90124-4. [DOI] [PubMed] [Google Scholar]
- 4.Bashir ZI, Bortolotto ZA, Davies CH, Berretta N, Irving AJ, Seal AJ, Henley JM, Jane DE, Watkins JC, Collingridge GL. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature. 1993;363:347–350. doi: 10.1038/363347a0. [DOI] [PubMed] [Google Scholar]
- 5.Bauerfiend FR, Huttner WB. Biogenesis of constitutive secretory vesicles, secretory granules and synaptic vesicles. Curr Opin Cell Biol. 1993;5:628–635. doi: 10.1016/0955-0674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- 6.Bear MF, Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986;320:172–176. doi: 10.1038/320172a0. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein G, Blank JL, Smrcka AV, Higashijima T, Sternweis PC, Exton JH, Ross EM. Reconstitution of agonist-stimulated phosphatidyl-inositol 4,5-biphosphate hydrolysis using purified m1 muscarinic receptor, Gq11 and phospholipase C-β1. J Biol Chem. 1992;267:8081–8088. [PubMed] [Google Scholar]
- 8.Blakemore C. Sensitive and vulnerable periods in the development of the visual system. The childhood environment and adult disease: Ciba Foundation Symposium (Brock GR, Whelan J, eds), pp 129–154. Wiley; New York: 1991. [DOI] [PubMed] [Google Scholar]
- 9.Bolshakov VY, Siegelbaum SA. Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science. 1994;264:1148–1152. doi: 10.1126/science.7909958. [DOI] [PubMed] [Google Scholar]
- 10.Bortolotto ZA, Bashir ZI, Davies CH, Collingridge GL. A molecular switch activated by metabotropic glutamate receptors regulates induction of long-term potentiation. Nature. 1994;368:740–743. doi: 10.1038/368740a0. [DOI] [PubMed] [Google Scholar]
- 11.Buisseret P, Imbert M. Visual cortical cells: their developmental properties in normal and dark reared kittens. J Physiol (Lond) 1976;255:511–525. doi: 10.1113/jphysiol.1976.sp011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter HR, Wallace MA, Fain JN. Purification and characterization of PLC-βm, a muscarinic cholinergic regulated phospholipase C from rabbit brain membranes. Biochim Biophys Acta. 1990;1054:119–128. doi: 10.1016/0167-4889(90)90213-w. [DOI] [PubMed] [Google Scholar]
- 13.Coleman PD, Riesen AH. Environmental effects on cortical dendritic fields. I. Rearing in the dark. J Anat. 1968;102:363–374. [PMC free article] [PubMed] [Google Scholar]
- 14.Collingridge GL, Davies SN. NMDA receptors and long-term potentiation in the hippocampus. In: Watkins JC, Collingridge GL, editors. The NMDA receptor. Oxford UP; Oxford: 1989. p. 242. [Google Scholar]
- 15.Cynader M. Prolonged sensitivity to monocular deprivation in dark-reared cats: effects of age and visual exposure. Dev Brain Res. 1983;8:155–164. doi: 10.1016/0165-3806(83)90002-0. [DOI] [PubMed] [Google Scholar]
- 16.Cynader M, Mitchell DE. Prolonged sensitivity to monocular deprivation in dark-reared cats. J Neurophysiol. 1980;43:1026–1040. doi: 10.1152/jn.1980.43.4.1026. [DOI] [PubMed] [Google Scholar]
- 17.Czepita D, Reid SN, Daw NW. Effects of longer periods of dark rearing on NMDA receptors in cat visual cortex. J Neurophysiol. 1994;72:1220–1226. doi: 10.1152/jn.1994.72.3.1220. [DOI] [PubMed] [Google Scholar]
- 18.Daw NW, Fox K, Sato H, Czepita D. Critical period for monocular deprivation in the cat visual cortex. J Neurophysiol. 1992;67:197–202. doi: 10.1152/jn.1992.67.1.197. [DOI] [PubMed] [Google Scholar]
- 19.De Camilli P, Emr SD, McPherson PS, Novick P. Phosphoinositides as regulators in membrane traffic. Science. 1996;271:1533–1539. doi: 10.1126/science.271.5255.1533. [DOI] [PubMed] [Google Scholar]
- 20.Denker SP, McCaffery JM, Palade GE, Insel PA, Farquhar MG. Differential distribution of alpha subunits and beta gamma subunits of heterotrimeric G proteins on Golgi membranes of the exocrine pancreas. J Cell Biol. 1996;133:1027–1040. doi: 10.1083/jcb.133.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixon JF, Los GV, Hokin LE. Lithium stimulates glutamate “release” and inositol 1,4,5-triphosphate accumulation via activation of the N-methyl-d-aspartate receptor in monkey and mouse cerebral cortical slices. Proc Natl Acad Sci USA. 1994;91:8358–8362. doi: 10.1073/pnas.91.18.8358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dudek S, Bear MF. A biomedical correlate of the critical period for synaptic modification in the visual cortex. Science. 1989;246:673–675. doi: 10.1126/science.2573152. [DOI] [PubMed] [Google Scholar]
- 23.Gu Q, Singer W. Involvement of serotonin in developmental plasticity of kitten visual cortex. Eur J Neurosci. 1995;7:1146–1153. doi: 10.1111/j.1460-9568.1995.tb01104.x. [DOI] [PubMed] [Google Scholar]
- 24.Guimaraes A, Zaremba S, Hockfield S. Molecular and morphological changes in the cat lateral geniculate nucleus and visual cortex induced by visual deprivation are revealed by monoclonal antibodies. J Neurosci. 1990;10:3014–3024. doi: 10.1523/JNEUROSCI.10-09-03014.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hensch TK, Stryker MP. Ocular dominance plasticity under metabotropic glutamate receptor blockade. Science. 1996;272:554–557. doi: 10.1126/science.272.5261.554. [DOI] [PubMed] [Google Scholar]
- 26.Hockfield S. A map to a unique cerebellar neuron generated by immunosuppression and rapid immunization. Science. 1987;237:67–70. doi: 10.1126/science.3603010. [DOI] [PubMed] [Google Scholar]
- 27.Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol (Lond) 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibarrondo J, Joubert D, Dufour MN, Cohen-Solal A, Homburger V, Jard S, Guillon G. Close association of the α subunits of Gq and G11 G proteins with actin filaments in WRK1 cells: relation to G protein-mediated phospholipase C activation. Proc Natl Acad Sci USA. 1995;92:8413–8417. doi: 10.1073/pnas.92.18.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasamatsu T, Pettigrew JD. Depletion of brain catecholamines: failure of ocular dominance shift after monocular occlusion in kittens. Science. 1976;194:206–209. doi: 10.1126/science.959850. [DOI] [PubMed] [Google Scholar]
- 30.Katan M, Kriz RW, Totty N, Philp R, Meldrum E, Aldalpe RA, Knopf JL, Parker PJ. Determination of the primary structure of PLC-154 demonstrates diversity of phosphoinositide-specific phospholipase C activities. Cell. 1988;54:171–177. doi: 10.1016/0092-8674(88)90549-1. [DOI] [PubMed] [Google Scholar]
- 31.Kato N. Dependence of long-term depression on postsynaptic metabotropic glutamate receptors in visual cortex. Proc Natl Acad Sci USA. 1993;90:3650–3654. doi: 10.1073/pnas.90.8.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kind PC, Hockfield S. Neonatal tolerization and rapid immunization: an immunological subtraction technique for the generation of monoclonal antibodies. Neurosci Proc. 1995;4:1–13. [Google Scholar]
- 33.Kind PC, Blakemore C, Fryer H, Hockfield S. Identification of proteins downregulated during the postnatal development of the cat visual cortex. Cereb Cortex. 1994;4:361–375. doi: 10.1093/cercor/4.4.361. [DOI] [PubMed] [Google Scholar]
- 34.Kirkwood A, Bear MF. Hebbian synapses in visual cortex. J Neurosci. 1994;14:1634–1645. doi: 10.1523/JNEUROSCI.14-03-01634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirkwood A, Bear MF. Experience-dependent modification of synaptic plasticity in visual cortex. Nature. 1996;381:526–528. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- 36.Kleinschmidt A, Bear MF, Singer W. Blockade of “NMDA” receptors disrupts experience-dependent plasticity of kitten striate cortex. Science. 1987;238:355–358. doi: 10.1126/science.2443978. [DOI] [PubMed] [Google Scholar]
- 37.Le Gall AH, Yeaman C, Muesch A, Rodriquez-Boulan E. Epithelial cell polarity: new perspectives. Semin Nephrol. 1995;15:272–284. [PubMed] [Google Scholar]
- 38.Leranth C, Shanabrough M, Naftolin F. Estrogen induces ultrastructural changes in progesterone receptor-containing GABA neurons of the primate hypothalamus. Neuroendocrinology. 1991;54:571–579. doi: 10.1159/000125962. [DOI] [PubMed] [Google Scholar]
- 39.Litosch I, Sulkholutskaya I, Weng C. G protein-mediated inhibition of phospholipase C activity in a solubilized membrane preparation. J Biol Chem. 1993;268:8692–8697. [PubMed] [Google Scholar]
- 40.Malhotra V, Serafini T, Orci L, Shepherd JC, Rothman JE. Purification of a novel class of coated vesicles mediating biosynthetic protein transport through the Golgi stack. Cell. 1989;58:329–336. doi: 10.1016/0092-8674(89)90847-7. [DOI] [PubMed] [Google Scholar]
- 41.Martin M, Sanz JM, Ros M, Cubero A. Metabotropic glutamate receptor analogues inhibit p[NH]ppG-stimulated phospholipase C activity in bovine brain coated vesicles: involvement of a pertussis toxin-sensitive G-protein. Biochem J. 1995;307:851–857. doi: 10.1042/bj3070851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell DE, Timney B. Postnatal development of function in the mammalian visual system. In: Darian-Smith I, editor. Handbook of physiology, Sec I, The nervous system. American Physiological Society; Bethesda, MD: 1984. pp. 507–555. [Google Scholar]
- 43.Mower GD. The effect of dark rearing on the time course of the critical period in cat visual cortex. Dev Brain Res. 1991;58:151–158. doi: 10.1016/0165-3806(91)90001-y. [DOI] [PubMed] [Google Scholar]
- 44.Mower GD, Caplan CJ, Christen WG, Duffy FH. Dark rearing prolongs physiological but not anatomical plasticity of the cat visual cortex. J Comp Neurol. 1985;235:448–466. doi: 10.1002/cne.902350404. [DOI] [PubMed] [Google Scholar]
- 45.Mugnaini E. The histology and cytology of the cerebellar cortex. In: Larsell O, Jansen J, editors. The comparative anatomy and histology of the cerebellum: the human cerebellum, cerebellar connections and cerebellar cortex. University of Minnesota; Minneapolis: 1972. pp. 201–265. [Google Scholar]
- 46.Mugnaini E, Floris A, Wright-Goss M. Extraordinary synapses of the unipolar brush cell: an electron microscopic study in the rat cerebellum. Synapse. 1994;16:284–311. doi: 10.1002/syn.890160406. [DOI] [PubMed] [Google Scholar]
- 47.Naftolin F, Bruhlmann-Papazyan M, Baetens D, Garcia-Segura LM. Neurons with whorl bodies have increased numbers of synapses. Brain Res. 1985;329:289–293. doi: 10.1016/0006-8993(85)90536-0. [DOI] [PubMed] [Google Scholar]
- 48.Olson CR, Freeman RD. Profile of the sensitive period for monocular deprivation in kittens. Exp Brain Res. 1980;39:17–21. doi: 10.1007/BF00237065. [DOI] [PubMed] [Google Scholar]
- 49.Peroutka SJ, Elliott JM, Flanigan TP, Newberry NR, Zetterstrom T, Leslie RA. 5-Hydroxytryptamine receptor subtypes in vertebrates and invertebrates. Neurochem Int. 1994;25:503–532. doi: 10.1016/0197-0186(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 50.Pimplikar SW, Simons K. Role of heterotrimeric G proteins in polarized membrane transport. J Cell Sci Suppl. 1993;17:27–32. doi: 10.1242/jcs.1993.supplement_17.5. [DOI] [PubMed] [Google Scholar]
- 51.Reid SNM, Daw NW. Dark-rearing changes dendritic microtubule-associated protein-2 (MAP2) but not subplate neurons in cat visual cortex. J Comp Neurol. 1995;359:38–47. doi: 10.1002/cne.903590104. [DOI] [PubMed] [Google Scholar]
- 52.Reid SNM, Romano C, Hughes T, Daw NW. Immunohistochemical study of two phosphoinositide-linked metabotropic glutamate receptors (mGLuR1a and mGLuR5) in the cat visual cortex before, during, and after the peak of the critical period for eye-specific connections. J Comp Neurol. 1995;355:470–477. doi: 10.1002/cne.903550311. [DOI] [PubMed] [Google Scholar]
- 53.Rhee SG. Regulation of phosphoinositide-specific phospholipase C by G protein. In: Liscovitch M, editor. Signal-activated phospholipases. Landes; Austin, TX: 1994. pp. 1–12. [Google Scholar]
- 54.Rothman JE. Mechanisms of intracellular transport. Nature. 1994;372:55–62. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 55.Sarri E, Picatoste F, Claro E. Neurotransmitter-specific profiles of inositol phosphates in rat brain cortex: relation to the mode of receptor activation of phosphoinositide phospholipase C. J Pharmacol Exp Ther. 1995;272:77–84. [PubMed] [Google Scholar]
- 56.Schwaninger R, Plutner H, Bokoch GM, Balch WE. Multiple GTP-binding proteins regulate vesicular transport from the ER to Golgi membranes. J Cell Biol. 1992;119:1077–1096. doi: 10.1083/jcb.119.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stryker MP, Harris WA. Binocular impulse blockade prevents the formation of ocular dominance in cat visual cortex. J Neurosci. 1986;6:2117–2133. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suh PG, Ryo SH, Moon KH, Suh WS, Rhee SG. Cloning and sequence of multiple forms of phospholipase C. Cell. 1988;54:161–169. doi: 10.1016/0092-8674(88)90548-x. [DOI] [PubMed] [Google Scholar]
- 59.Sur M, Frost DO, Hockfield S. Expression of a surface-associated antigen on Y-cells in the cat lateral geniculate nucleus is regulated by visual experience. J Neurosci. 1988;8:874–882. doi: 10.1523/JNEUROSCI.08-03-00874.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swindale NV. Absence of ocular dominance patches in dark reared cats. Nature. 1981;290:332–333. doi: 10.1038/290332a0. [DOI] [PubMed] [Google Scholar]
- 61.Swindale NV. Role of visual experience in promoting segregation of eye dominance patches in the visual cortex of the cat. J Comp Neurol. 1988;267:472–488. doi: 10.1002/cne.902670403. [DOI] [PubMed] [Google Scholar]
- 62.Waters MG, Serafini T, Rothman JE. “Coatomer”: a cytolsolic protein complex containing subunits of non-clathrin-coated Golgi transport vesicles. Nature. 1991;349:248–251. doi: 10.1038/349248a0. [DOI] [PubMed] [Google Scholar]
- 63.Wilson BS, Komuro M, Farquhar MG. Cellular variations in heterotrimeric G protein localization and expression in rat pituitary. Endocrinology. 1994;134:233–244. doi: 10.1210/endo.134.1.8275939. [DOI] [PubMed] [Google Scholar]
- 64.Zhou C, Akhtar RA, Abdel-Latif AA. Identification of phosphoinositide-specific PLC-β1 and GTP-binding protein, Gqa, in bovine iris sphincter membranes: characteristics of the phospholipase and its coupling to cholinergic muscarinic receptors. Exp Eye Res. 1994;59:377–384. doi: 10.1006/exer.1994.1121. [DOI] [PubMed] [Google Scholar]