Abstract
A CNS catecholaminergic cell line, Cath.a, was established by targeted oncogenesis in transgenic mice. Cath.a cells express neuronal properties but lack neuronal morphology. Here, we describe a variant of Cath.a, called CAD (Cath.a-differentiated), in which reversible morphological differentiation can be initiated by removal of serum or exogenously added protein from the medium. In serum- or protein-free media, CAD cells stop proliferating and extend long processes. Differentiated CAD cells can be maintained without serum or protein for at least 6 weeks. CAD cells are distinct from Cath.a cells; most significant, the original immortalizing oncogene, SV40 T antigen, was spontaneously lost. By immunostaining or immunoblotting, we show that CAD cells express neuron-specific proteins, such as class III β-tubulin, GAP-43, SNAP-25, and synaptotagmin, but not GFAP. Ultrastructurally, processes from differentiated CAD cells have abundant parallel microtubules and intermediate filaments, and bear varicosities that contain both large dense-core vesicles/granules (120–160 nm) and smaller clear vesicles (60–80 nm). Additionally, CAD cells express enzymatically active tyrosine hydroxylase and accumulatel-DOPA. CAD cells exhibit biochemical and morphological characteristics of primary neurons and provide an unique tool for studying neuronal differentiation.
Keywords: CNS cell line, catecholamine, tyrosine hydroxylase, neuronal differentiation, T antigen, microtubule, intermediate filament, synaptic vesicle
Regulation of neurogenesis within the CNS is a central focus in developmental neurobiology. Identifying factors that induce differentiation of neuronal precursors has been particularly difficult to carry out in vivo. Because of the cellular complexity of the brain, with cells of different types intermingled and differentiating at different times, molecular analyses have been limited mainly to cultured primary neurons or cell lines. Whereas primary cultures from different brain regions have been studied successfully, pure neuronal cultures are often difficult to prepare. In primary cultures, the presence of glia, which are a rich source of growth and trophic factors, complicates the search for molecules that mediate neuronal maturation. In addition, the number of primary neurons that can be obtained may be limited for studies on discrete neuronal populations. On the other hand, immortalized cell lines obviate many of these difficulties, but their differentiation is usually limited. In addition, they often express high levels of an immortalizing oncogene and are rarely of purely CNS origin.
Among the immortalized cell lines that have been studied, several can be induced to differentiate by altering growth conditions or by adding trophic factors. The neuroblastoma C-1300 cell line, derived from a spontaneous murine tumor, differentiates in low serum into neurons that are excitable, bear long processes, and express neurotransmitter-synthesizing enzymes (Augusti-Tocco and Sato, 1969;Nelson et al., 1969; Schubert et al., 1969; Seeds et al., 1970; Amano et al., 1972). P19 cells, an embryonal carcinoma cell line, can be induced to differentiate morphologically and biochemically with retinoic acid (Jones-Villeneuve et al., 1982). P19 cells form structures resembling chemical synapses (McBurney et al., 1988; Yao et al., 1995) and extend processes that are polarized into dendrites and axons. However, retinoic acid-treated P19 cells also give rise to glia and fibroblast-like cells. Similar to P19, a human teratocarcinoma cell line, NTere 2/cl.D1 (NT2) differentiates into postmitotic neurons (NT2-N) in response to retinoic acid (Pleasure and Lee, 1993; Younkin et al., 1993). NT2 cells elaborate processes that can be identified as dendrites and axons, and express a number of neuron-specific proteins including glutamate receptor channels (Pleasure et al., 1992; Younkin et al., 1993). PC12 cells are a neuroendocrine cell line derived from a rat pheochromocytoma (Greene and Tischler, 1976). Like neonatal chromaffin cells, PC12 cells differentiate into sympathetic neurons after nerve growth factor treatment and exhibit many neuronal properties (Tao-Cheng et al., 1995).
A CNS catecholaminergic cell line, Cath.a, was established from a brain tumor that arose in a transgenic mouse carrying wild-type SV40 T antigen (Tag) under the transcriptional control of the rat tyrosine hydroxylase promoter (Suri et al., 1993). In addition to making dopamine and norepinephrine, Cath.a cells express a variety of pan-neuronal markers, including neurofilaments, synaptophysin, and voltage-gated Na+, K+, and Ca2+ channels (Suri et al., 1993; Lazaroff et al., 1996). However, these cells do not bear neurites, nor can be they induced to morphologically differentiate under any conditions tested thus far.
We report here the characterization of a variant of Cath.a, called CAD, in which neuronal differentiation can be initiated by serum deprivation. The cells can be maintained in a differentiated state without any protein added to the culture media. CAD cells differ significantly from the parental Cath.a line; notable is the fact that they have lost the immortalizing oncogene. These cells express neuron-specific proteins not expressed in the parental line and synaptic vesicle proteins, and they bear processes and varicosities that resemble those of neurons. Like the parental Cath.a cell line, CAD cells express bioactive tyrosine hydroxylase. The characteristics of this cell line make it a valuable in vitro system to study neuronal differentiation.
MATERIALS AND METHODS
Cell culture. Cath.a cells were cultured as described previously (Suri et al., 1993). CAD cells were grown in DMEM/F-12 medium (DF12, catalog #12–719F; BioWhittaker, Walkersville, MD), supplemented with 8% FBS (HyClone, Logan, UT) and 1% penicillin-streptomycin (100% stocks, 10,000 U/ml penicillin G sodium and 10,000 μg/ml streptomycin sulfate in 0.85% saline, Gibco, Gaithersburg, MD) on standard tissue culture dishes in a humidified 5% C02 incubator. CAD cells were passaged every 3–4 d by pipetting cells from a confluent plate and triturating them in 5 ml of fresh medium. Cells were replated at a 1:10 dilution. To induce differentiation, CAD cells were plated in serum-containing DF12 medium, and then switched to either serum-free medium (SFM) or protein-free medium (PFM). SFM contained 20 μg/ml transferrin (Sigma, St. Louis, MO) and 50 ng/ml sodium selenite (Sigma) in DF12 medium; PFM contained 50 ng/ml sodium selenite only. Differentiated CAD cells were grown for at least 5 d in SFM or PFM before they were used for immunostaining or Western blots. Rat HTC cells, C6 glioma cells, and fibroblast cells were grown in the same medium as the CAD cells.
Southern blot. Genomic DNA was isolated by the method ofLaird et al. (1991). Ten micrograms of genomic DNA was digested withBamHI and HindIII and separated by electrophoresis through a 0.8% agarose gel as described (Maniatis et al., 1989). Restricted DNA was transferred to a GeneScreen plus nylon membrane (Dupont, Boston, MA) and probed with 32P-Tag DNA labeled by random priming.
Cell count. For the cell counts, cells were plated at 4 × 104 cells/well (Fig. 2A) or 2 × 104 cells/well (Fig. 2B) in a 6-well tissue culture plate. On day 0, after the cells attached to the plate, they were washed two times with DF12 medium, and then SFM or PFM was added to the appropriate wells. At each time point, cells were detached with trypsin-EDTA and collected by centrifugation. Cell number was determined with a hemocytometer.
Fig. 2.
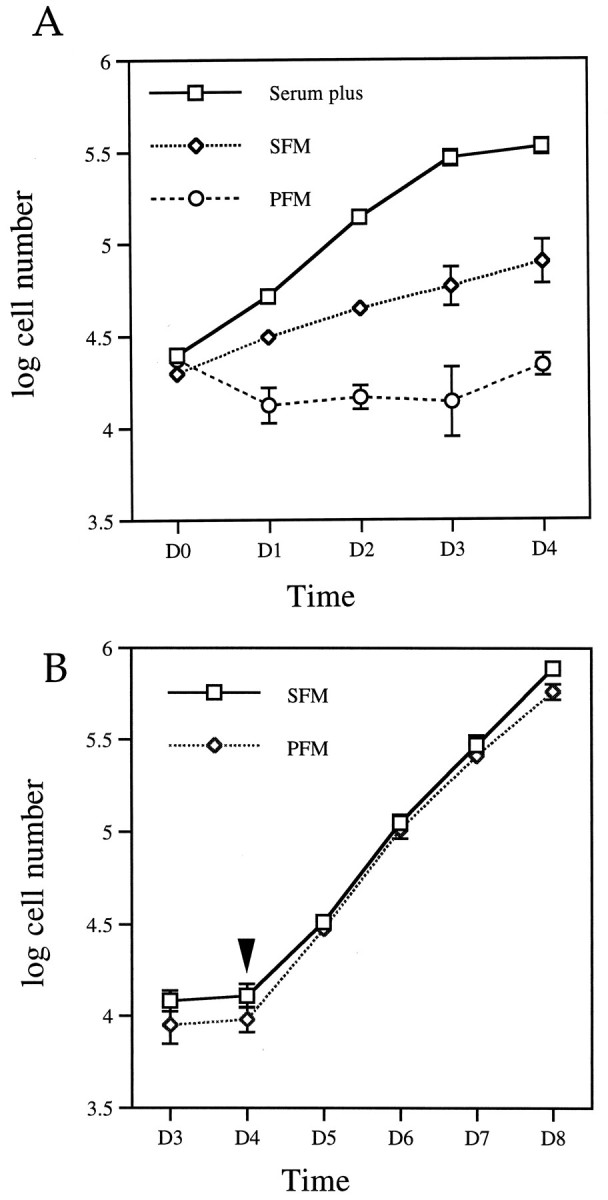
Reversible reduction of cell proliferation rate in SFM or PFM. A, Growth curve of CAD cells. Cells were grown in serum-containing medium; on day 0 (D0), they were changed into SFM or PFM or maintained in serum-containing medium for an additional 4 d. Values are the average of the cell number from four separate experiments. The cell number was obtained as described in Materials and Methods. B, Differentiated CAD cells resumed proliferation after serum addition. CAD cells were maintained in SFM or PFM for 4 d, after which FBS was added to a final concentration of 8% to the culture medium.Arrowhead indicates serum addition. Error bars indicate SEM (n = 4). Note: some of the error bars are too small to be visible.
Immunostaining. Cells were grown on glass coverslips precoated with cell-TAK (Becton Dickinson, Bedford, MA). After 4–5 d, the cells were washed once with PBS and fixed with cold methanol for 10 min. Cells were incubated in primary antibodies for 1.5 to 2 hr at room temperature. Synaptotagmin antibody was applied as undiluted hybridoma culture supernatant. For TuJ1 and TH staining, the antibodies were diluted at 1:1000 and 1:500, respectively, in PBS with 10 mg/ml BSA and 0.1% Triton X-100. Cells were washed with PBS and incubated with secondary antibody (at 1:250 in the same diluent used for the primary antibodies) conjugated either with fluorescein (Sigma) or biotin (Vector Laboratories, Burlingame, CA) for 1–2 hr at room temperature. Biotin-labeled secondary antibody was detected with streptavidin conjugated with Texas Red (Gibco, Gaithersburg, MD) used at 1:200. For controls, cells were processed the same way, except that primary antibodies were omitted from the initial incubation. No significant staining was observed with any of the secondary antibodies alone (data not shown).
Western blots. Confluent 100 mm plates of cells were washed once with sterile PBS and lysed directly on the plate with 1 ml 2% SDS, 100 μg/ml PMSF, 5 μg/ml aprotinin, and 5 μg/ml leupeptin. Lysates were sonicated for 15 sec to shear chromosomal DNA. Protein concentration was determined with BCA protein assay reagent (Pierce Chemical, Rockford, IL). Fifty micrograms of protein was loaded per lane onto an 10% acrylamide-SDS gel and transferred electrophoretically to an immobilon-P membrane (Millipore, Bedford, MA). After washing with TBS/0.05% Tween 20 (TBST), nonspecific binding was blocked by incubating the membrane for 30 min in TBST containing 5% Carnation nonfat dry milk. Primary antibodies were diluted in the same blocking solution at 1:5000 for Tag and SNAP-25 antibodies, 1:1000 for GAP-43 antibody, and 1:500 for GFAP antibody. The membrane was incubated in primary antibodies for 1 to 1.5 hr at room temperature, washed 3 times with TBST, and incubated with secondary antibodies conjugated with horseradish peroxidase at a 1:5000 dilution for 0.5 hr. The membrane was washed three times with TBST and once with TBS, and the bands were visualized by the ECL detection system (Amersham, Arlington Heights, IL).
Antibodies. Antibodies were obtained as follows: TH monoclonal antibody (mAb) from INCSTAR (Stillwater, MN); GFAP mAb from Biomedical Technologies (Stoughton, MA); GAP-43 mAb from Sigma; monoclonal SNAP-25 from Sternberger Monoclonals (Baltimore, MD). The rabbit polyclonal Tag antibody was the kind gift of Dr. Doug Hanahan (University of California at San Francisco), and the mAbs to TuJ1 and synaptotagmin (clone 48) were generously provided by Dr. Anthony Frankfurter (University of Virginia) and Dr. William Matthew (Duke University), respectively.
HPLC.l-DOPA and dopamine were assayed by HPLC with electrochemical detection. Mobile phase consisted of 10% methanol, 50 mm NaH2PO4, pH 3.6, 0.387 mm sodium octyl sulfate, and 0.1 mm EDTA. The reverse-phase column was Ultremex 3 μm, C18(Phenomenex, Torrence, CA). Plates of cells were scraped, pelleted, and sonicated in 0.5 ml 0.1 N perchloric acid. Disrupted cells were centrifuged (14,000× g, 4°C, 10 min), and the pellets were used for protein determination. Supernatant was filtered through 0.45 μm nylon centrifuge filters (Fisher Scientific, Pittsburgh, PA), and 20 μl per sample was injected onto the HPLC. The applied potential of detector was 0.7 V.
Cell pellets were dissolved in 1 N NaOH, and the protein content in each plate was determined with BCA protein assay reagent (Pierce Chemical, Rockford, IL).
Electron microscopy. Cells were grown on 60 mm tissue culture plates. Fixation was carried out at room temperature with 2.5% glutaraldehyde in 0.1 m sodium cacodylate buffer, pH 7.4, from 30 min to overnight. Cells were washed twice with buffer, postfixed with 1% osmium tetroxide, and then mordanted in 1% uranyl acetate for 30 min. After another wash, cells were dehydrated and embedded in pure Epon.
RESULTS
Reversible morphological differentiation in CAD cells
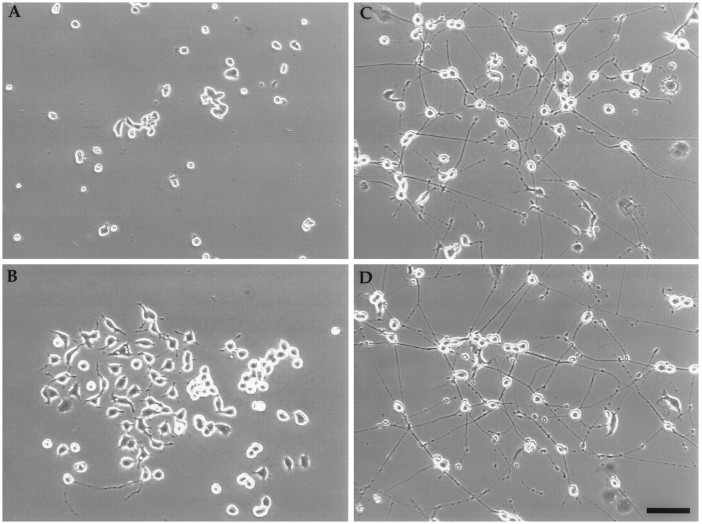
In culture, Cath.a cells are small (∼15 μm in diameter), lack processes, and grow in clumps (Fig.1A). Cells bearing short processes spontaneously appeared in cultures of Cath.a cells and were selected by two cycles of single cell cloning. Clonality was assured by visually selecting single cells in microtiter wells for each cycle of cloning. The resulting cells, named CAD (for Cath.a-differentiated), appear bigger and flatter, and grow in a more dispersed manner (Fig.1B). They also exhibit short processes similar to the initial uncloned population. When CAD cells are switched to SFM or PFM, they undergo a dramatic morphological change. Within 1 day, the cells start to put out processes. Four days after culturing in SFM or PFM, most cells have long processes with varicosities (Fig.1C,D). Under these conditions, differentiated CAD cells appear healthy despite the lack of exogenously added protein in the media. They can be maintained in this state for a minimum of 6 weeks, after which time they tend to lift off the plates as monolayers after mechanical disturbance. Detachment is probably a result of lack of appropriate substratum rather than death of the cells because the detached sheets can be triturated and the cells replated without serum. Coating of tissue culture dish with polylysine, collagen, laminin, or cell Tak did not prevent the tendency of the differentiated cells to lift off, although laminin did promote the initial outgrowth of processes (data not shown). In contrast, when the parental Cath.a cells were switched to SFM, they died.
Fig. 1.
Morphological differentiation can be induced in CAD cells. Phase-contrast photomicrograph of live cells: Cath.a cells in serum-containing medium (A); CAD cells in serum-containing medium (B), SFM (C), or PFM (D). In C and D, the cells were grown for 5 d in SFM or PFM. Note the beaded varicosities on the processes. Scale bar, 100 μm.
Because many cells stop dividing when they differentiate, the growth rate of CAD cells was assessed after differentiation. As shown in Figure 2A, in serum, the cell number doubles every 18–22 hr. Three days after plating, cell proliferation slows as the cells reached confluence. In SFM, the proliferation rate decreases three- to fourfold, and in PFM, no significant increase in cell number occurred over 4 d.
To determine whether CAD cell differentiation is reversible, serum was added back after the cells were maintained in SFM or PFM for 4 d. By time lapse videomicroscopy, we observed that more than 95% of the differentiated cells retracted their processes within 12–24 hr (data not shown). The cell count (Fig. 2B) shows that the cell number increased after serum addition, doubling every 18–22 hr. The fact that the rate of growth was the same as that of serum-grown cultures (Fig. 2A) suggests that the cells fully recover the ability to cycle. Moreover, the fact that the first doubling occurs within the first 18–22 hr suggests that virtually all of the cells reenter the cycle without a lag period. Therefore, morphological differentiation concomitant with growth arrest can be initiated in CAD cells by serum or protein deprivation, and this event is readily reversible.
SV40 T antigen oncogene is lost in CAD cells
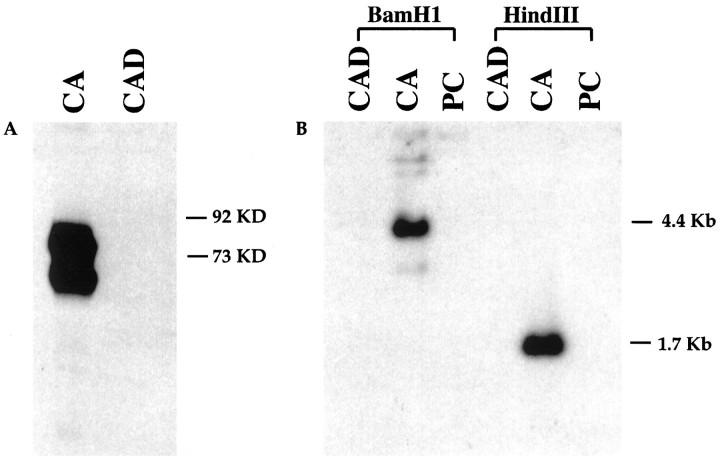
Parental Cath.a cells were immortalized by SV40 T antigen (Tag), which stimulates cell proliferation mainly by binding p53 and retinoblastoma proteins (Fanning, 1992; Bryan and Reddel, 1994). Cath.a cells express high levels of Tag, which is likely to keep the cells in the cycle and block morphological differentiation. To determine whether the CAD cells also express Tag, Western blots were performed. As shown in Figure 3A, robust Tag expression was found in Cath.a cells, but, to our surprise, no detectable Tag protein was observed in CAD cells.
Fig. 3.
SV40 T antigen (Tag) is lost in CAD cells.A, Western blot analysis with Tag-specific polyclonal antibody. Each lane contains 50 μg of protein. Tag was detected in Cath.a cells (CA) as a doublet. No Tag was detected in CAD cells. B, Southern blot analysis with a32P-labeled Tag probe. Tag is only detected in Cath.a cells, but not in CAD or PC12 cells. PC, PC12 cells;KD, kilodalton.
The presence of the Tag gene in the CAD genome was then tested by Southern blots. The original construct used to create the TH-Tag transgenic mice contains a unique BamHI restriction site and three HindIII restriction sites, such that BamHI digestion would be expected to give a fragment bigger than 3.2 kb andHindIII, a 1.7 kb fragment (see Suri et al., 1993 for the plasmid construct). As expected, in Cath.a cells, 4.4 kb and 1.7 kb Tag-containing fragments were observed with BamHI andHindIII digests, respectively (Fig. 3B). In CAD cells, no Tag-containing fragments were detected, suggesting that the Tag gene was lost in CAD cells. This result was also confirmed by PCR with genomic DNA (data not shown).
CAD cells express neuron-specific proteins
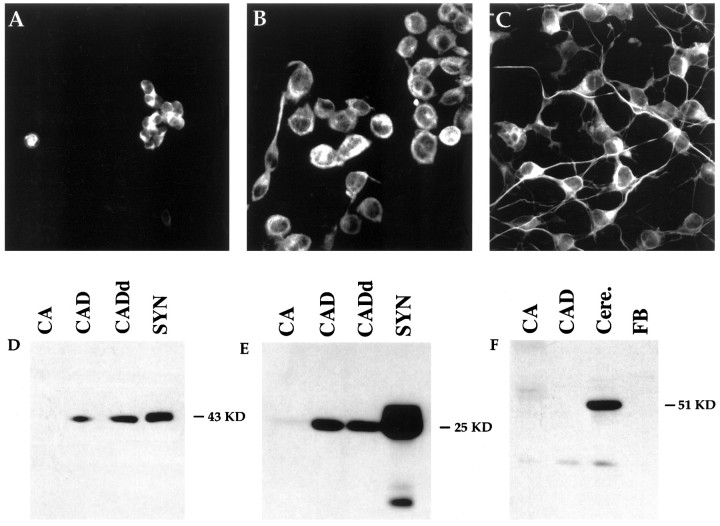
The phenotype of CAD cells was evaluated with several neuronal markers including neuronal-specific class III β-tubulin, GAP-43, and SNAP-25. Class III β-tubulin is a tubulin isotype expressed specifically in neurons (Sullivan et al., 1986) and is recognized by a mAb, TuJ1, specific for an epitope unique to the class III β-tubulin isotype. TuJ1 has been shown to label neurons specifically. By immunocytochemistry, intense cytoplasmic staining was observed in Cath.a cells, CAD cells, and differentiated CAD cells (Fig.4A–C). Note that all of the processes in differentiated CAD cells were stained evenly with the TuJ1 antibody.
Fig. 4.
CAD cells express neuron-specific proteins.A–C, Immunocytochemical staining with TuJ1 mAb against class III β-tubulin. Cath.a (A), undifferentiated CAD (B), and differentiated CAD (C) cells were all stained with TuJ1 antibody. D–F, Western blot with antibodies against GAP-43 (D), SNAP-25 (E), and GFAP (F). Fifty micrograms of Cath.a (CA), CAD, and differentiated CAD (CADd) cell protein were loaded in each lane. GAP-43 was detected in both CAD cells and rat brain synaptosome (SYN) extracts. GAP-43 protein was increased twofold after differentiation (CADd). However, no GAP-43 was detected in Cath.a cells. SNAP-25 was detected in both CAD cells and synaptosome extracts, but not in Cath.a cells (E). In F, Cath.a, CAD, and fibroblasts (FB) did not express the glial-specific protein GFAP, although extracts from mouse cerebellum (Cere.) had abundant GFAP.
GAP-43 and SNAP-25 are thought to be essential in synapse formation and in maturation of synaptic contacts (Catsicas et al., 1991, 1993; Osen-Sand et al., 1993; Lakin et al., 1995; Sabel et al., 1995). They are expressed by virtually all maturing neurons. GAP-43 is a membrane-bound protein found at high levels in the axons and growth cones of developing and regenerating neurons (Kalil and Skene, 1986;Skene et al., 1986; Strittmatter et al., 1992). SNAP-25 is a presynaptic protein that is closely associated with integral membrane proteins in presynaptic membranes, and has been found to be essential in vesicle docking and fusion (Oyler et al., 1991, 1992; Pevsner et al., 1994). To determine whether GAP-43 and SNAP-25 are expressed in CAD cells and whether they are increased after differentiation, Western blots were performed. GAP-43 was observed in serum-grown CAD cells and was increased approximately twofold after differentiation (Fig.4D). Its concentration in differentiated CAD cells was approximately one-half that observed in rat brain synaptosomes. In contrast, GAP-43 was not detected in the parental Cath.a cells (Fig.4D) or in C6 glioma cells (data not shown). SNAP-25 was also detected in CAD, but its level was not increased after differentiation (Fig. 4E). Little or no SNAP-25 was detected in Cath.a cells, suggesting that both SNAP-25 and GAP-43 proteins are increased in CAD compared with parental Cath.a cells, consistent with the neuronal morphology exhibited by CAD cells.
Because many immortalized cells exhibit a neuroglial phenotype (Schubert et al., 1974) and express both neuronal and glial proteins, we determined whether glial fibrillary acidic protein (GFAP) was expressed in CAD cells. GFAP is a 51 kDa intermediate filament protein that is expressed specifically in astrocytes (Eng, 1985). As shown in Figure 4F, GFAP was detected in mouse cerebellum, a rich source of GFAP, but not in Cath.a and CAD cells, nor in fibroblast cells.
In summary, CAD cells express neuron-specific proteins, but not a glial marker, which supports the morphological data suggesting they are neuronal in nature.
Ultrastructurally, differentiated CAD cells resemble a primary neuron
The morphological differentiation and presynaptic protein expression in CAD cells prompted us to investigate whether CAD cells have cytoskeletal and vesicular structures typical of neurons. Under electron microscopy, cellular organelles including mitochondria, endoplasmic reticulum, and abundant polyribosomes were easily identified in the cell body of differentiated CAD cells (Fig.5A). Randomly oriented microtubules could be detected in the cytoplasm. Where a process initiated, microtubules and intermediate filaments assumed an orientation parallel to the process. Except for mitochondria, organelles found in the cell body were largely excluded from the process. Figure 5B showed a typical process filled with parallel microtubules and intermediate filaments. Mitochondria, and occasionally dense-core vesicles and clear vesicles, were also observed in the processes. In the light microscope, numerous varicosities were observed along the processes and at terminals. Figure5C shows that in the electron microscope one such terminal contains two types of vesicles: dense-core vesicles or granules (arrows) with an average diameter of 120–160 nm and smaller, clear vesicles (arrowheads) with an average diameter of between 60 and 80 nm. Mitochondria and clathrin-coated vesicles were also observed in the terminals, as well as structures that resemble actin filament-rich filopodia.
Fig. 5.
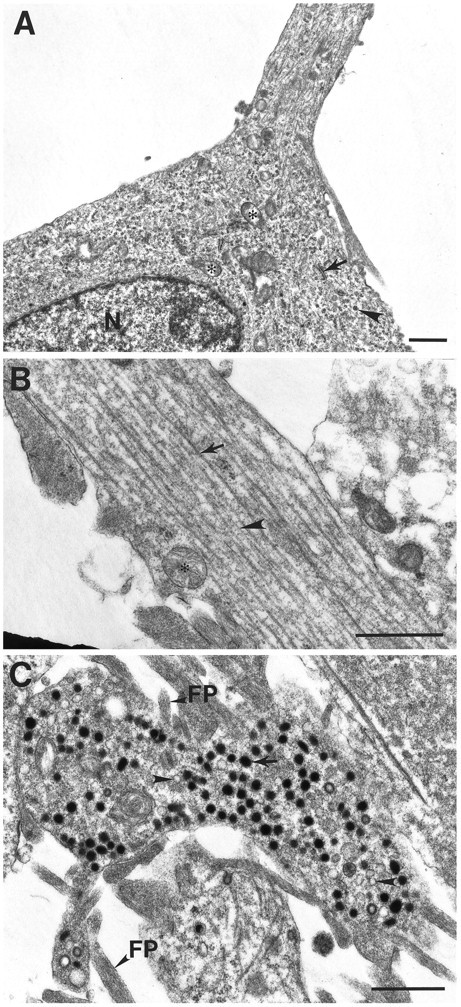
Electron micrographs of differentiated CAD cells.A, Cell body and initial segment of a process in differentiated CAD cells. The cytoplasm contains ribosomes (arrowhead), mitochondria (asterisk), rough endoplasmic reticulum (arrow), and microtubules.B, A typical process is filled with parallel microtubules (arrow) and intermediate filaments (arrowhead). C, Dense-core vesicles (arrow) and clear vesicles (arrowhead) in terminal. FP, Filopodia; N, nucleus. Scale bars, 1 μm.
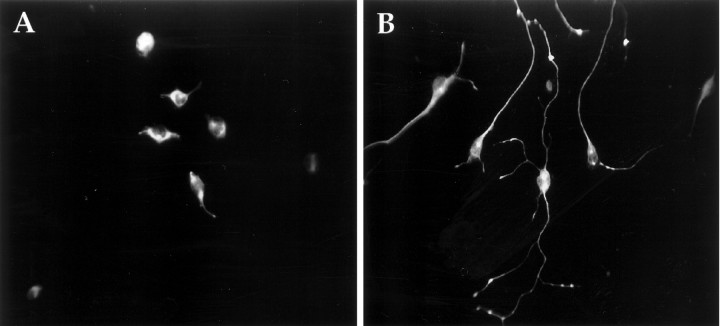
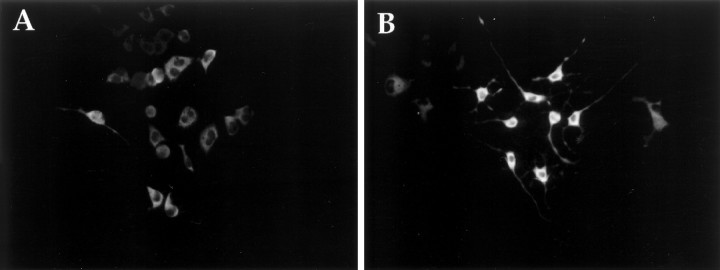
To determine whether synaptic vesicle proteins are present in terminals, we localized synaptotagmin (p65) by immunofluorescence. Synaptotagmin is an abundant synaptic vesicle protein found exclusively in the nervous system and certain neuronal-like secretory cells (Matthew et al., 1981; Perin et al., 1991; Wendland et al., 1991). Although it is generally considered a synaptic vesicle protein, it is also present on some dense-core vesicles (Walch-Solimena et al., 1993;Egger et al., 1994). A mAb against synaptotagmin stained both differentiated and undifferentiated CAD cells (Fig. 6). Intensive staining was observed in a perinuclear position, which is likely to mark the Golgi apparatus, and in terminals and varicosities. This staining pattern is characteristic for synaptotagmin (Tao-Cheng et al., 1995). Therefore, ultrastructurally and biochemically differentiated CAD cells resemble neurons.
Fig. 6.
Immunocytochemical staining of CAD cells with synaptotagmin mAb. A, CAD cells grown in serum-containing medium. B, CAD cells grown for 4 d in PFM.
CAD cells are catecholaminergic neurons
TH protein could be detected by immunohistochemistry in both the undifferentiated and differentiated CAD cells (Fig. 7). The staining was heterogeneous from cell to cell and was confined to the cytoplasm and proximal processes. Differentiated cells had slightly stronger staining, consistent with data from Western blots showing a twofold increase in TH protein after differentiation (Lazaroff et al., unpublished data).
Fig. 7.
Expression of TH in CAD cells. Immunocytochemistry with monoclonal TH antibody showing cytoplasmic TH staining in both growing CAD (A) and differentiated CAD cells (B).
To determine whether TH is enzymatically active in CAD cells, catecholamine production was measured by HPLC. Because the parental Cath.a cells have high levels of dopamine and norepinephrine (Table1; Suri et al., 1993), we expected similar results in CAD cells. Contrary to our expectation, we detected no dopamine or norepinephrine in CAD cell extracts (or in media from CAD-grown cells). Instead, we observed an accumulation of L-dihydroxyphenylalanine (l-DOPA), which is the immediate product of TH action on tyrosine. Undifferentiated CAD cells have 163.17 pmoll-DOPA/mg protein, whereas differentiated cells have 254.913 pmol/mg protein. The higher level of l-DOPA in differentiated CAD cells may reflect the higher expression of TH enzyme. Rat hepatoma cell line, HTC, was used as negative control and contained no catecholamines. In most TH+ cells, including Cath.a, DOPA decarboxylase (aromatic amino acid decarboxylase), the second enzyme in the catecholamine biosynthetic pathway, convertsl-DOPA to dopamine so rapidly that l-DOPA does not accumulate. Hence, it is likely that TH is indeed biologically active in the CAD cells, but the lack of decarboxylase activity prevents conversion of l-DOPA to dopamine.
Table 1.
l-DOPA and dopamine production by HPLC
| Cell line | l-DOPA (pmol/mg) | DA (pmol/mg) |
|---|---|---|
| Cath.a | 0 | 1998.59 ± 57.89 (3) |
| CAD | 163.17 ± 9.39 (3) | 0 |
| CADd | 254.913 ± 24.79 (3) | 0 |
| HTC | 0 | 0 |
Values are expressed as pmol/mg cell protein ± SD (n). Cells were disrupted, and the filtered cell lysate was injected onto the HPLC. The minimum detectability is 8.6 pmol forl-DOPA and 0.263 pmol for dopamine (DA).
DISCUSSION
We describe here a CNS neuronal cell line that can undergo reversible morphological differentiation. In serum-containing medium, CAD cells are morphologically undifferentiated and proliferate with a doubling time of 18–22 hr. After serum withdrawal, they cease proliferation and differentiate. Insulin and insulin-like growth factors partially sustain CAD cell proliferation in SFM (our unpublished data) and, hence, are likely to be among the factors in serum that maintain cells in the cycle. Therefore, CAD cell differentiation is likely to be blocked by proliferation rather than by differentiation inhibitors in serum. Most immortalized cells, particularly neuronal lines like PC12, ND7 (Howard et al., 1993;Lindenboim et al., 1995), and the parental Cath.a cells from which CAD cells were derived, die after serum deprivation. Some immortalized cells and primary neurons can survive for extended periods in SFM supplemented with transferrin, an iron carrier protein, and a high concentration of insulin, a growth factor, which may act as a general survival factor (Bottenstein and Sato, 1979). With few exceptions, mammalian cells need trophic factors to survive (Raff, 1992). It is surprising that differentiated CAD cells survive without any protein added to the medium for extended periods, perhaps indefinitely. However, it is likely that they may make autocrine or paracrine factors that support their own survival. Alternatively, like neurofibromin mutant cells (Vogel et al., 1995), they may have sustained a gain of function mutation in a signaling pathway, which renders them trophic factor independent.
It has been thought that the morphological differentiation of neural progenitors occurs after cells have stopped dividing. In CAD cells, serum deprivation reduced proliferation greatly, and protein deprivation stopped the increase in cell number immediately. Our preliminary data show that overexpression of the tumor suppressor protein p53 is sufficient for morphological differentiation in the presence of serum (our unpublished data). Because one of the biological activities of p53 is to arrest cell cycle progression (Oren, 1992), cell cycle arrest alone may be sufficient for CAD cell differentiation. Indeed, our preliminary data are consistent with the notion that CAD cells differentiate if they are blocked from proliferating by any number of treatments. When treated with cell cycle blockers (mimosine and aphidocolin in the presence of serum), morphological changes consistent with limited differentiation were observed. However, the processes were shorter and thicker than those in SFM, and significant toxicity was apparent at all concentrations (our unpublished data). Hence, differentiation may be the “default” pathway, which is over-ridden by proliferative signals.
Differentiation is readily reversible. When serum was added back to cultures in SFM or PFM, more than 95% of the cells retracted their processes within 12–24 hr and proliferated without a detectable lag period. Hence, the cells remain sensitive to proliferative signals unlike most terminally differentiated cells.
In general, it has been difficult to immortalize differentiated neurons with oncogenes (Noble et al., 1992). Because retroviruses can only integrate into a dividing cell, this precludes immortalization of postmitotic neurons. In addition, oncogenes often prevent differentiation. For example, activated oncogenes have been shown to arrest neuronal differentiation of retinoic acid-treated P19 cells (Boulter and Wagner, 1988). In PC12 cells, both E1A andmyc oncogenes block nerve growth factor-induced neurite growth (Maruyama et al., 1987; Bogenmann et al., 1995). Temperature-sensitive oncogenes have been used to generate cell lines in which the activity of the oncogene can be turned off by culturing cells at nonpermissive temperature. For example, a temperature sensitive SV40 Tag was used to generate cell lines in which the activity of Tag protein can be turned off by growing cells at high temperature (Chou, 1989; Eves et al., 1994; White et al., 1994). Although these cell lines differentiate at a nonpermissive temperature, it is unclear whether they can be maintained for extended periods in their differentiated state.
Although the initial immortalizing oncogene, SV40 T antigen, was lost in CAD cells, it is still immortalized. As suggested by Suri et al. (1993), it is likely that the original tumor cells, from which Cath.a and CAD were derived, required at least two “hits” for full oncogenic transformation. The primary event was the expression of the SV40 T antigen transgene, and the second was an unknown mutation that occurred stochastically over a period of 1 year or more, during which time tumors arose in vivo (Suri et al., 1993). Hence, it is likely that the second immortalizing event sustains proliferation of the CAD line in the presence of serum. The spontaneous loss of Tag in CAD cells is fortuitous because this cell line was already committed to a neuronal phenotype, and differentiation can be easily and reversibly induced.
CAD cells are quite distinct from parental Cath.a cells, which do not undergo morphological differentiation. Although both lines express biologically active tyrosine hydroxylase, CAD cells accumulatel-DOPA but lack dopamine, suggesting thatl-DOPA decarboxylase activity, which converts DOPA to dopamine, may be deficient. In contrast, Cath.a cells have high levels of dopamine and undetectable levels of l-DOPA, which is converted so rapidly to dopamine it is usually undetectable in most catecholaminergic cells. The two lines also differ in transcriptional regulation of tyrosine hydroxylase. The Cath.a cells rely almost exclusively on the CRE site at −45 for basal enhancer activity. In contrast, CAD cells rely equally on the CRE and an AP1 site at −205 (Lazaroff et al., unpublished data). This equal and additive ability of the CRE and AP1 sites to direct transcription is unlike any other catecholaminergic cell line examined to date.
CAD cells express neuronal markers such as GAP-43, synaptotagmin, and SNAP-25, which are not found in Cath.a cells. These markers are present in both proliferating and differentiated cells, although GAP-43 and TH levels are modestly increased after differentiation. At the ultrastructural level, CAD cell bodies are crowded with ribosomal profiles, their processes are rich in microtubules and intermediate filaments, and they have both dense-core and clear vesicles in their terminals. The dense-core vesicles have an average diameter of 120–160 nm, and the clear vesicles are 60–80 nm. On the basis of light microscopy, it is likely that synaptotagmin resides in these vesicles. Although synaptotagmin is generally considered a marker for small (50 nm) synaptic vesicles, it is also found in some dense-core vesicles (Walch-Solimena et al., 1993; Egger et al., 1994). Hence, synaptotagmin may be in either or both vesicular populations.
In summary, we have established a clonal CNS neuronal cell line in which morphological differentiation can be initiated and maintained by removal of serum or protein from the culture media. CAD cells express biochemical markers found in differentiated neurons and, in their differentiated state, bear processes that ultrastructurally resemble neurites, complete with dense-core and clear vesicles. These properties make this cell line unique and a valuable resource for studying neuronal differentiation.
Footnotes
This work was supported by National Institutes of Health Grants NS30590 (J.K.T.W.) and NS22675 (D.M.C.). We thank Kimberly Stark for assisting with cloning the cell line and Dr. Sara Jones for performingl-DOPA and catecholamine assays.
Correspondence should be addressed to Dr. Dona M. Chikaraishi, Department of Neurobiology, 427G Bryan Research Building, Duke University Medical Center, Durham, NC 27710.
Dr. Qi’s present address: Department of Neurobiology, Duke University Medical Center, Durham, NC 27710.
REFERENCES
- 1.Amano T, Richelson E, Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones. Proc Natl Acad Sci USA. 1972;69:258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Augusti-Tocco G, Sato G. Establishment of functional clonal lines of neurons from mouse neuroblastoma. Proc Natl Acad Sci USA. 1969;64:311–315. doi: 10.1073/pnas.64.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogenmann E, Torres M, Matsushima H. constitutive N-myc gene expression inhibits trkA mediated neuronal differentiation. Oncogene. 1995;10:1915–1925. [PubMed] [Google Scholar]
- 4.Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci USA. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulter CA, Wagner EF. The effects of v-src expression on the differentiation of embryonal carcinoma cells. Oncogene. 1988;2:207–214. [PubMed] [Google Scholar]
- 6.Bryan TM, Reddel RR. SV40-induced immortalization of human cells. Crit Rev Oncog. 1994;5:331–357. doi: 10.1615/critrevoncog.v5.i4.10. [DOI] [PubMed] [Google Scholar]
- 7.Catsicas S. Inhibition of axonal growth by SNAP-25 antisence oligonucleotides in vitro and in vivo. Nature. 1993;364:445–448. doi: 10.1038/364445a0. [DOI] [PubMed] [Google Scholar]
- 8.Catsicas S, Larhammar D, Blomqvist A, Sanna PP, Milner RJ, Wilson MC. Expression of a conserved cell type-specific protein in nerve terminals coincides with synaptogenesis. Proc Natl Acad Sci USA. 1991;88:785–789. doi: 10.1073/pnas.88.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou JY. Differentiated mammalian cell lines immortalized by temperature-sensitive tumor viruses. Mol Endocrinol. 1989;3:1511–1514. doi: 10.1210/mend-3-10-1511. [DOI] [PubMed] [Google Scholar]
- 10.Egger C, Kirchmair R, Kapelari S, Fischer-Colbrie R, Hogue-Angeletti R, Winkler H. Bovine posterior pituitary: presence of p65 (synaptotagmin), PC1, PC2 and secretoneurin in large dense core vesicles. Neuroendocrinology. 1994;59:169–75. doi: 10.1159/000126655. [DOI] [PubMed] [Google Scholar]
- 11.Eng LF. Glial fibrillary acidic protein (GFAP): the major protein of glial intermediate filaments in differentiated astrocytes. J Neuroimmunol. 1985;8:203–214. doi: 10.1016/s0165-5728(85)80063-1. [DOI] [PubMed] [Google Scholar]
- 12.Eves EM, Kwon J, Downen M, Tucker MS, Wainer BH, Rosner MR. Conditional immortalization of neuronal cells from postmitotic cultures and adult CNS. Brain Res. 1994;656:396–404. doi: 10.1016/0006-8993(94)91484-2. [DOI] [PubMed] [Google Scholar]
- 13.Fanning E. Structure and function of simian virus 40 large tumor antigen. Annu Rev Biochem. 1992;61:55–85. doi: 10.1146/annurev.bi.61.070192.000415. [DOI] [PubMed] [Google Scholar]
- 14.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howard MK, Burke LC, Maihos C, Pizzey A, Gilbert CS, Lawson D, Collins MKL, Thomas NSB, Latchman DS. Cell cycle arrest of proliferating neuronal cells by serum deprivation can result in either apoptosis or differentiation. J Neurochem. 1993;60:1783–1791. doi: 10.1111/j.1471-4159.1993.tb13404.x. [DOI] [PubMed] [Google Scholar]
- 16.Jones-Villeneuve EMV, Mcburney MW, Rogers KA, Kalnins VI. Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. J Cell Biol. 1982;94:253–262. doi: 10.1083/jcb.94.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalil K, Skene JHP. Elevated synthesis of an axonally transported protein correlates with axon outgrowth in normal and injured pyramidal tracts. J Neurosci. 1986;6:2563–2570. doi: 10.1523/JNEUROSCI.06-09-02563.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lakin ND, Morris PJ, Theil T, Sato TN, Moroy T, Wilson MC, Latchman DS. Regulation of neurite outgrowth and SNAP-25 gene expression by the Brn-3a transcription factor. J Biol Chem. 1995;270:15858–15863. doi: 10.1074/jbc.270.26.15858. [DOI] [PubMed] [Google Scholar]
- 20.Lazaroff M, Dunlap K, Chikaraishi DM. A CNS catecholaminergic cell line expresses voltage-gated currents. J Membr Biol. 1996;151:279–291. doi: 10.1007/s002329900078. [DOI] [PubMed] [Google Scholar]
- 21.Lindenboim L, Diamond R, Rothenberg E, Stein R. Apoptosis induced by serum deprivation of PC12 cells is not preceded by growth arrest and can occur at each phase of the cell cycle. Cancer Res. 1995;55:1242–1247. [PubMed] [Google Scholar]
- 22.Maniatis T, Fritsch EF, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, New York: 1989. [Google Scholar]
- 23.Maruyama K, Schiavi SC, Huse W, Johnson GL, Ruley HE. myc and E1A oncogenes alter the responses of PC12 cells to nerve growth factor and block differentiation. Oncogene. 1987;1:361–367. [PubMed] [Google Scholar]
- 24.Matthew WD, Tsavaler L, Reichardt LF. Identification of a synaptic vesicle-specific membrane protein with a wide distribution in neuronal and neurosecretory tissue. J Cell Biol. 1981;91:257–269. doi: 10.1083/jcb.91.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McBurney MW, Reuhl KR, Ally AI, Nasipuri S, Bell JC, Craig J. Differentiation and maturation of embryonal carcinoma-derived neurons in cell culture. J Neurosci. 1988;8:1063–1073. doi: 10.1523/JNEUROSCI.08-03-01063.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson P, Ruffner W, Nirenberg M. Neuronal tumor cells with excitable membranes grown in vitro. Proc Natl Acad Sci USA. 1969;64:1004–1009. doi: 10.1073/pnas.64.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noble M, Groves AK, Ataliotis P, Jat PS. From chance to choice in the generation of neural cell lines. Brain Pathol. 1992;2:39–46. [PubMed] [Google Scholar]
- 28.Oren M. p53: the ultimate tumor suppressor gene? FASEB J. 1992;6:3169–3176. doi: 10.1096/fasebj.6.13.1397838. [DOI] [PubMed] [Google Scholar]
- 29.Osen-Sand A, Catsicas M, Staple JK, Jones KA, Ayala G, Knowles J, Grenningloh G, Oyler GA, Polli JW, Higgins GA, Wilson MC, Billingsley ML. Distribution and expression of SNAP-25 immunoreactivity in rat brain, rat PC-12 cells and human SMS-KCNR neuroblastoma cells. Dev Brain Res. 1992;65:133–146. doi: 10.1016/0165-3806(92)90172-s. [DOI] [PubMed] [Google Scholar]
- 30.Oyler GA, Polli JW, Wilson MC, Billingsley ML. Developmental expression of the 25 kDa synaptosomal-associated protein (SNAP-25) in rat brain. Proc Natl Acad Sci USA. 1991;88:5247–5251. doi: 10.1073/pnas.88.12.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perin MS, Brose N, Jahn R, Sudhof TC. Domain structure of synaptotagmin (p65). J Biol Chem. 1991;266:623–629. [PubMed] [Google Scholar]
- 32.Pevsner J, Jsu SH, Braun JEA, Calakos N, Ting AE, Bennet MK, Scheller RH. Specificity and regulation of a synaptic vesicle docking complex. Neuron. 1994;13:353–361. doi: 10.1016/0896-6273(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 33.Pleasure SJ, Lee VM-Y. NTera 2 cells: a human cell line which displays characteristics expected of a human committed neuronal progenitor cell. J Neurosci Res. 1993;35:585–602. doi: 10.1002/jnr.490350603. [DOI] [PubMed] [Google Scholar]
- 34.Pleasure SJ, Page C, Lee VM-Y. Pure, postmitotic, polarized human neurons derived from NTera 2 cells provide a system for expressing exogenous proteins in terminally differentiated neurons. J Neurosci. 1992;12:1802–1815. doi: 10.1523/JNEUROSCI.12-05-01802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raff MC. Social controls on cell survival and cell death. Nature. 1992;356:397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- 36.Sabel M, Bele S, Gass P, Sommer C, Kiessling M. Developmental expression of GAP-43 and SNAP-25 in heterotopic rat cortical grafts. Neurosci Lett. 1995;189:151–154. doi: 10.1016/0304-3940(95)11478-f. [DOI] [PubMed] [Google Scholar]
- 37.Schubert D, Humphreys S, Baroni C, Cohn M. In vitro differentiation of a mouse neuroblastoma. Proc Natl Acad Sci USA. 1969;64:316–323. doi: 10.1073/pnas.64.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schubert D, Heinemann S, Carlisle W, Tarikas H, Kimes B, Patrick J, Steinbach JH, Culp W, Brandt BL. Clonal cell lines from the rat central nerous system. Nature. 1974;249:224–227. doi: 10.1038/249224a0. [DOI] [PubMed] [Google Scholar]
- 39.Seeds NW, Gilman AG, Amano T, Nirenberg MW. Regulation of axon formation by clonal lines of a neural tumor. Proc Natl Acad Sci USA. 1970;66:160–167. doi: 10.1073/pnas.66.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skene JHP, Jacobson RD, Snipes GJ, Mcguire CB, Norden JJ, Freeman JA. A protein induced during nerve growth (GAP-43) is a major component of growth-cone membranes. Science. 1986;233:783–785. doi: 10.1126/science.3738509. [DOI] [PubMed] [Google Scholar]
- 41.Strittmatter SM, Vartanian T, Fishman MC. GAP-43 as a plasticity protein in neuronal form and repair. J Neurobiol. 1992;23:507–520. doi: 10.1002/neu.480230506. [DOI] [PubMed] [Google Scholar]
- 42.Sullivan KF, Havercroft JC, Machlin PS, Cleveland DW. Sequence and expression of the chicken β5- and β4-tubulin genes define a pair of divergent β-tubulins with complementary patterns of expression. Mol Cell Biol. 1986;6:4409–4418. doi: 10.1128/mcb.6.12.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suri C, Fung BP, Tischler AS, Chikaraishi DM. Catecholaminergic cell lines from the brain and adrenal glands of tyrosine hydroxylase-SV40 T antigenic mice. J Neurosci. 1993;13:1280–1291. doi: 10.1523/JNEUROSCI.13-03-01280.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tao-Cheng JH, Dosemeci A, Bressler JP, Brightman MW, Simpson DL. Characterization of synaptic vesicles and related neuronal features in nerve growth factor and ras oncogene differentiated PC12 cells. J Neurosci Res. 1995;42:323–334. doi: 10.1002/jnr.490420306. [DOI] [PubMed] [Google Scholar]
- 45.Vogel KS, Brannan CI, Jenkins NA, Copeland NG, Parada LF. Loss of neurofibromin results in neurotrophin-independent survival of embryonic sensory and sympathetic neurons. Cell. 1995;82:733–742. doi: 10.1016/0092-8674(95)90470-0. [DOI] [PubMed] [Google Scholar]
- 46.Walch-Solimena C, Takei K, Marek KL, Midyett K, Sudhof TC, Camilli PD, Jahn R. Synaptotagmin: a membrane constituent of neuropeptide-containing large dense-core vesicles. J Neurosci. 1993;13:3895–3903. doi: 10.1523/JNEUROSCI.13-09-03895.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wendland B, Miller KG, Schilling J, Scheller RH. Differential expression of the p65 gene family. Neuron. 1991;6:993–1007. doi: 10.1016/0896-6273(91)90239-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White LA, Eaton MJ, Castro MC, Klose KJ, Globus MY-T, Shaw G, Whittemore SR. Distinct regulatory pathways contol neurofilament expression and neurotransmitter synthesis in immortalized serotonergic neurons. J Neurosci. 1994;14:6744–6753. doi: 10.1523/JNEUROSCI.14-11-06744.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao M, Bain G, Gottlieb DI. Neuronal differentiation of P19 embryonal carcinoma cells in defined media. J Neurosci Res. 1995;41:792–804. doi: 10.1002/jnr.490410610. [DOI] [PubMed] [Google Scholar]
- 50.Younkin DP, Tang C-M, Hardy M, Reddy UR, Shi Q-Y, Pleasure SJ, Lee VM-Y, Pleasure D. Inducible expression of neuronal glutamate receptor channels in the NT2 human cell line. Proc Natl Acad Sci USA. 1993;90:2174–2178. doi: 10.1073/pnas.90.6.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]