Abstract
Cerebellar granule cells express six GABAA receptor subunits abundantly (α1, α6, β2, β3, γ2, and δ) and assemble various pentameric receptor subtypes with unknown subunit compositions; however, the rules guiding receptor subunit assembly are unclear. Here, removal of intact α6 protein from cerebellar granule cells allowed perturbations in other subunit levels to be studied. Exon 8 of the mouse α6 subunit gene was disrupted by homologous recombination. In α6 −/− granule cells, the δ subunit was selectively degraded as seen by immunoprecipitation, immunocytochemistry, and immunoblot analysis with δ subunit-specific antibodies. The δ subunit mRNA was present at wild-type levels in the mutant granule cells, indicating a post-translational loss of the δ subunit. These results provide genetic evidence for a specific association between the α6 and δ subunits. Because in α6 −/− neurons the remaining α1, β2/3, and γ2 subunits cannot rescue the δ subunit, certain potential subunit combinations may not be found in wild-type cells.
Keywords: GABAA receptor, α6 subunit, granule cell, cerebellum, homologous recombination, gene targeting, transgenic mice, knockout mice, ligand-gated ion channel, subunit sorting, subunit assembly, internal ribosome entry site, dicistronic mRNA, muscimol, SR95531, Ro 15-4513, flunitrazepam
In vertebrate brains, GABAA receptors are the principal mediators of inhibitory synaptic transmission. They are agonist-gated anion channels formed of pentameric assemblies of subunits arranged around an aqueous pore (Seeburg et al., 1990;Sieghart, 1995; Stephenson, 1995; Tyndale et al., 1995; McKernan and Whiting, 1996). The subunit genes (α1–6, β1–3, γ1–3, and δ) are differentially transcribed, and the polypeptides are assembled in many possible combinations depending on cell type (Persohn et al., 1992; Wisden et al., 1992; Fritschy and Möhler, 1995). There are several important unresolved issues. Why is this receptor heterogeneity needed for synaptic function? What is the subunit composition of native subtypes of receptor? Are there rules guiding which subunits assemble with each other?
The cerebellum is an excellent brain area for investigating these questions. Its clearly defined circuitry allows an almost complete account of which cerebellar cell types express which GABAAreceptor subunit genes (Wisden et al., 1996). For example, cerebellar granule cells express six subunit genes abundantly (α1, α6, β2, β3, γ2, and δ), and so they probably have several distinct GABAAreceptor subtypes of unknown subunit stoichiometry. As for the canonical muscle nicotinic acetylcholine receptor subunits (Verrall and Hall, 1992; Green and Claudio, 1993; Kreienkamp et al., 1995), there is likely to be selective discrimination between GABAAsubunits; however, assembling a neuronal receptor requires solving an extensive combinatorial element. To form a native receptor, subunits have to recognize and distinguish their neighbors. The assembly pathways used by granule cells to sort the six principal subunits into different receptor subtypes are not known. Granule cells receive a single GABAergic input from Golgi interneurons onto their distal dendrites. At this synapse, the GABAA receptor subtypes might be colocalized and intermingled (Nusser et al., 1995, 1996), and to date the α1, α6, β2/3, and γ2 subunits have been demonstrated to be present in the synaptic junction (Nusser et al., 1995, 1996; Somogyi et al., 1996).
Despite the comparative simplicity of the system, the receptor subunit composition of granule cell GABAA receptors is controversial. Current views accommodate α1β2/3γ2, α6β2/3γ2, α1α6β2/3γ2, α1α6β2/3γ2δ, α1β2/3γ2δ, and α6β2/3δ combinations (Korpi and Lüddens, 1993; Mertens et al., 1993; Caruncho and Costa, 1994;Khan et al., 1994, 1996; Quirk et al., 1994; Caruncho et al., 1995;Korpi et al., 1995; Pollard et al., 1995). The evidence for such combinations is derived from antibody-based data and correlation of pharmacological fingerprints of native binding sites, with binding profiles of subunits expressed in cell lines. Here we have targeted the α6 subunit gene by homologous recombination techniques. Removal of α6 protein from cerebellar granule cells allowed perturbations in other subunit levels to be studied and provided genetic evidence for a specific association between the α6 and δ subunits. Our results begin to reveal the rules guiding receptor subunit assembly.
MATERIALS AND METHODS
Generation of mutant mice
The replacement vector for homologous recombination, designed for positive–negative selection (Mansour et al., 1988), was generated from a 6 kb mouse 129 strain α6 subunit gene fragment (Jones et al., 1996), comprising part of exon 4 through to the middle of intron 8. This fragment was subcloned into pBluescript (Stratagene, La Jolla, CA) (see Fig. 1A). Into this plasmid, a BamHI cassette (TAG3IRESlacZpAMC1neopA) (Nehls et al., 1996) was inserted between the AflII and NcoI sites located in exon 8, after the site ends were modified by adding BamHI adaptors. This cassette, designed to report target gene expression and to provide a dominant marker to select for insertion into the gene, consisted of stop codons (TAG) in all three frames, followed by an internal ribosome entry site (IRES) linked to a lacZ reading frame and SV40 polyadenylation sequences. It also contained a neomycin resistance gene under independent transcriptional control (Nehls et al., 1996). The IRES-lacZ cassette was inserted with the lacZ coding sequence in the same transcriptional orientation as the α6 gene coding sequences. For negative selection, aXhoI–SalI fragment containing two MC1tk gene head-to-tail repeats (Smith et al., 1995) was placed in the targeting vector polylinker appending the longer homology arm (see Fig.1A). The vector was linearized with SalI and electroporated into 129 strain-derived embryonic stem (ES) cells (ES line “CCB,” kindly supplied by Drs. W. Colledge and M. Evans, Wellcome/CRC, University of Cambridge).
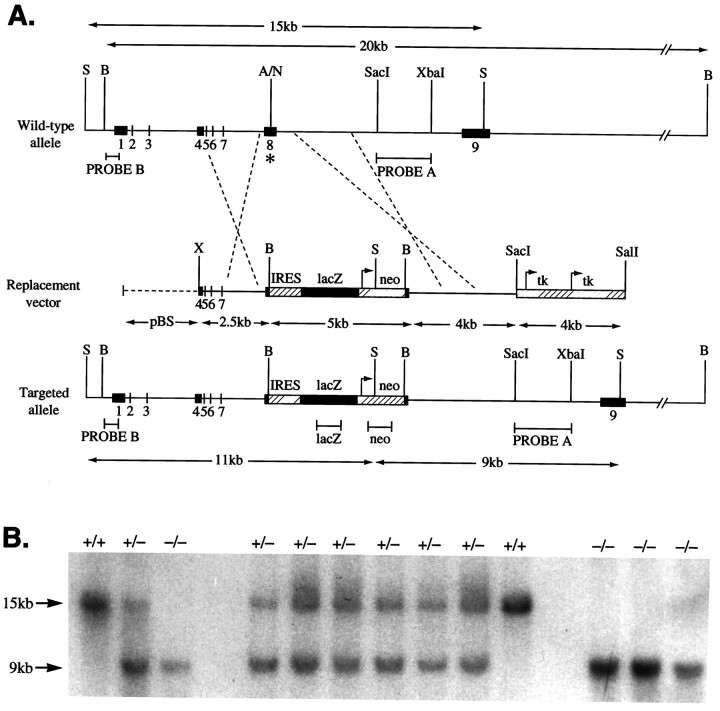
Fig. 1.
GABAA receptor α6subunit gene disruption by homologous recombination. A, Wild-type α6 gene, targeting (replacement) vector, and the disrupted α6 gene structures. Numbersindicate exons. On the Replacement vector, thebroken line indicates pBluescript (pBS) sequences. On the wild-type allele, theasterisk marks exon 8 where the IRES lacZ/neo cassette was inserted. Only relevant restriction sites are shown. A, AflII;B, BamHI; N,NcoI; S, SphI;X, XhoI. Arrows mark theneo and tk gene promoter sites and direction of transcription. The lacZ coding sequence orientation is the same as the α6 gene, thus permitting its translation from the IRES sequence (striped box) to be initiated on the mRNA derived from the α6 promoter. Expected restriction fragment lengths diagnostic for homologous recombination and the probes used to detect these are marked by double-headed arrows andhorizontal bars, respectively. B, Confirmation of α6 mutant allele germline transmission. Biopsy tail DNA samples were digested with SphI, electrophoresed, and Southern-blotted. The membrane was probed withPROBE A (3′ flanking). Wild-type (+/+) individuals give a 15 kb band, the homozygous null (−/−) animals give a 9 kb band, and heterozygotes (+/−) give both bands.
Transfected ES cells were grown on G418r primary embryonic fibroblast feeder cells, in medium supplemented with leukemia inhibitory factor (Life Technologies, Paisley, UK) and selected in G418 (Life Technologies) and FIAU (Bristol-Myers, Hounslow, UK) (Mansour et al., 1988; Smith et al., 1995). Genomic DNA was isolated from individual colonies, digested with SphI, Southern-blotted, and probed with an intron 8-derived SacI–XbaI restriction fragment (see PROBE A in Fig.1A). The addition of the neomycin gene creates an additional SphI site in the α6 gene locus, thus enabling the discrimination between wild-type (15 kb) and null (9 kb) alleles (see Fig. 1A,B). Confirmation of correct targeting events was established with restriction fragment probes from the lacZ and neo genes (marked on Fig. 1A) (data not shown) and probe B (the complete sequence of probe B, which comprises the promoter and 5′ untranslated region, is deposited in the EMBL database, accession number X97475; Jones et al., 1996) (see Fig.1A). Additional diagnostic restriction digests of targeted genomic DNA used BamHI (see Fig.1A).
Male chimeras derived from targeted cells were mated with wild-type C57BL/6. Mutation germ-line transmission was determined by Southern blot analysis of agouti progeny tail DNA (see Fig.1B). Heterozygotes were intercrossed to generate a homozygous α6 −/− line.
β-Galactosidase staining
Mice were transcardially perfused with 4% paraformaldehyde (PFA) in PBS. Brains were removed, post-fixed for 1 hr in 4% PFA, and then equilibrated at 4°C in PBS containing 30% sucrose. Sections (40 μm) were cut on a sliding microtome and incubated free-floating in 5-bromo-4-chloro-3-indolyl-β-galactoside (X-Gal) (Bonnerot and Nicolas, 1993). After X-Gal staining, some sections were counterstained with neutral red (Sigma, Poole, UK), allowing non-lacZ-expressing cells to be visualized. Alternatively, whole brains (see Fig. 3C) were immersed in the X-Gal staining reagent.
Fig. 3.
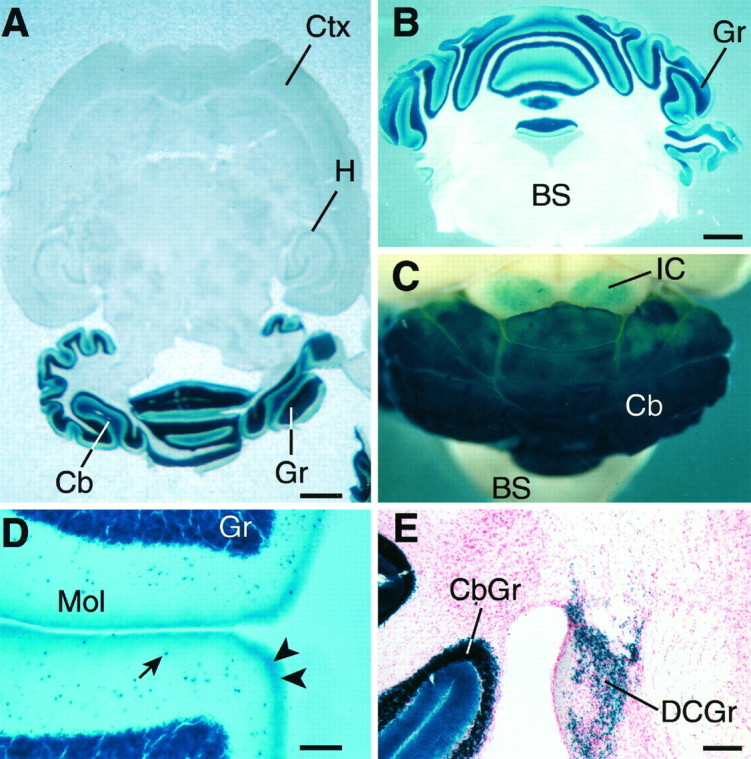
LacZ expression driven from the α6gene locus in α6 −/− adult mouse brains illustrated in horizontal (A), coronal (B), and whole-mount views (C); blue colorationindicates lacZ activity. A and B show the confined expression of the α6 gene to the cerebellar granule cell layer; C shows the expression in the dorsal regions of the inferior colliculi; D shows higher-power view of α6 gene expression in the molecular layer of the cerebellum. The arrow indicates an example of the numerous lacZ positive cells in the molecular layer. These are probably nonmigrated granule cells. The arrowheads mark putative parallel fiber staining; E, α6 gene expression in the dorsal cochlear nucleus granule cells.BS, Brainstem; Cb, cerebellum;CbGr, cerebellar granule cell layer; Ctx, neocortex; Gr, cerebellar granule cells;DCGr, dorsal cochlear nucleus granule cells;H, hippocampus; IC, inferior colliculi;Mol, cerebellar molecular layer. Scale bars:A, 1.3 mm; B, C, 1 mm;D, 150 μm; E, 300 μm.
Staining of cultured granule cells. Cells on coverslips (see Granule Cell Culture and Electrophysiological Analysis) were washed in PBS and fixed in ice-cold 2% PFA/0.2% glutaraldehyde in PBS for 5 min. The coverslips were washed in PBS, incubated with X-Gal solution at 37°C overnight, and counterstained with neutral red.
Antibodies
α1-specific antibodies.α1(1–9), an N-terminal-specific antibody (Zezula and Sieghart, 1991); α1-N, affinity-purified, raised against rat N terminus residues 1–14 (S. Pollard and F. A. Stephenson, unpublished data). α1(328–382)/α1L was prepared as described (Mossier et al., 1994). Rabbits were immunized with an MBP-α1(328–382)-7His fusion protein, and the antibodies were purified with a GST-α1(328–382)-7His fusion protein. This antibody precipitates GABAA receptors and is selective for the α1 subunit (R. Pelz and W. Sieghart, unpublished data).
α6-specific antibodies. α6-N (Batch R54XV), affinity-purified polyclonal, was raised to bovine α6 subunit N-terminal residues 1–16 (Thompson et al., 1992); α6(429–434) batch P24, affinity-purified rabbit polyclonal antibody, was raised to rat α6 subunit residues 429–434 (Tögel et al., 1994); α6-C, affinity-purified rabbit polyclonal, was directed against the C-terminus sequence CSKDTMEVSSTVE (S. Pollard and F. A. Stephenson, unpublished data).
δ-specific antibodies. δ(318–400), rabbit polyclonal was raised against the rat cytoplasmic loop sequence between TM3 and TM4 (Quirk et al., 1995); δ(1–44) (rabbit R7) polyclonal was prepared by immunizing with an MBP-δ(1–44)-7His fusion protein and purifying by affinity chromatography, as described (Mossier et al., 1994; R. Pelz and W. Sieghart, unpublished data). This antibody is specific for the δ subunit and does not precipitate α1β3γ2 receptors (R. Pelz and W. Sieghart, unpublished data).
Immunocytochemistry
Five α6 −/− and five +/+ mice were transcardially perfused with 4% PFA, 0.05% glutaraldehyde, and ∼0.2% picric acid for 7–17 min. After perfusion the brains were washed in 0.1 m phosphate buffer. Preembedding immunocytochemistry was carried out on 70-μm-thick vibratome sections (Somogyi et al., 1989). Floating sections were incubated in 20% normal goat serum (NGS) diluted in Tris-buffered saline (TBS), pH 7.4, for 1 hr. The purified antibodies were diluted in TBS containing 1% NGS. After they were washed, the sections were incubated for 2 hr in biotinylated goat anti-rabbit IgG (diluted 1:50 in 1% NGS containing TBS), followed by incubation in avidin–biotinylated horseradish peroxidase complex (diluted 1:100; Vector Laboratories, Peterborough, UK) for 90 min. Peroxidase enzyme reaction was with 3,3′-diaminobenzidine tetrahydrochloride as chromogen and H2O2 as oxidant. In some cases, Triton X-100 (0.1–0.3%) was added to the TBS throughout the experiment. The antibody concentrations used for immunocytochemistry were δ(1–44)R7, 0.7–2.2 μg/ml; α6-N, 1.5–3.0 μg/ml.
For controls, selective labeling could not be detected when the primary antibodies were either omitted or replaced by 5% normal rabbit serum. No immunoreactivity was obtained when the antibodies were preincubated with the appropriate peptides used for immunization (Nusser et al., 1996).
Ligand autoradiography
The procedures were slightly modified from Olsen et al. (1990)and Wong et al. (1996). Cryostat sections (14 μm) from frozen nonfixed adult mouse brains were preincubated in 50 mmTris-HCl, pH 7.4, and 120 mm NaCl for 15 min at 0°C, except for the GABA site assays when 0.31 m Tris-citrate solution, pH 7.1, was used. Incubations with ligands used fresh buffers of composition identical to those used for preincubation. For the benzodiazepine (BZ) site, [3H]Ro 15-4513 (5 nm, Du Pont de Nemours, NEN Division, Dreieich, Germany) was used with and without 100 μm diazepam (Orion, Espoo, Finland) for a 60 min incubation at 0°C, followed by three 30 sec washes, a dip in distilled water, and rapid drying. The same conditions and washes were used for the GABA site, with [3H]muscimol (20 nm, Amersham, Buckinghamshire, UK) and [3H]SR 95531 (20 nm, Du Pont), except that the incubation time was 30 min. The sections were washed three times for 15 sec in 10 mm Tris-HCl, pH 7.4, followed by dipping in distilled water and air drying. Sections were exposed to Hyperfilm-3H (Amersham) for 1–6 weeks. The images were produced by scanning the films. The nonspecific binding components to BZ and GABA sites were defined in the presence of 10 μmRo 15-1788 (Hoffmann-La Roche, Basel, Switzerland) and 100 μm GABA, respectively.
Radioligand binding
Radioligand binding on membranes prepared from individual mouse cerebella was as described previously (Quirk et al., 1994). Membranes prepared from each animal were used for saturation binding with [3H]Ro 15-1788 (0.1–17.0 nm), [3H]zolpidem (1–30 nm), and [3H]Ro 15-4513 (0.8–60.0 nm) displaced with Ro 15-4513 (10 μm) to define the total number of BZ binding sites, or with flunitrazepam (1 μm) to define binding to diazepam-sensitive sites only. Saturation binding with [3H]muscimol (2–45 nm) used 1 mmGABA to determine nonspecific levels. All assays used eight concentrations of ligand, with total and nonspecific binding measured in duplicate with 30–80 μg of protein/assay tube.Bmax and Kd values were determined by nonlinear least-squares fit of the saturation curves using the data analysis software RS1 (Bolt, Beranek and Newman, Cambridge MA).
Immunoprecipitation analysis
Immunoprecipitation of GABAA receptors solubilized from individual mouse cerebella used antibody δ(318–400) bound to protein A-Sepharose as described previously (Quirk et al., 1994, 1995). [3H]muscimol binding to the solubilized receptor was measured after gel filtration through Sephadex G-25 to remove any remaining endogenous GABA.
PAGE and immunoblotting
Membranes from individual +/+ and −/− cerebella were prepared, and equal amounts of protein per slot were subjected to SDS-PAGE in 10% polyacrylamide gels and immunoblotted. For the α1(1–14), α6(1–16), and α6-C antibodies, the ECL Western blotting system (Amersham) was used for detection (Pollard et al., 1995). ECL blots were quantitated by normalizing with an anti-neuron specific enolase (NSE) antibody (Sigma) and then probing with α1(1–14) (S. Pollard and F. A. Stephenson, unpublished data). Multiple exposures were taken for both anti-NSE and α1(1–14) immunoreactivity and quantitated using a Molecular Dynamics Personal Quantitator. For the δ(1–44)R7 and α1(328–382) antibodies, membranes were incubated with digoxygenin-labeled antibodies and were then treated with anti-digoxygenin-alkaline phosphatase Fab fragments (Boehringer Mannheim, Mannheim, Germany). Proteins were detected by fluorescence using the CSPD substrate (Tropix). Blots were quantitated by densitometry of Kodak X-Omat S films with the DocuGel 2000i gel documentation system using the RFLPscan software (MWG-biotech).
Granule cell culture and electrophysiological analysis
Cell culture. Cerebellar granule cells, attached to matrigel-coated coverslips, were cultured from postnatal day 5 (P5) mice as described for rat cells (Randall and Tsien, 1995). Minimal essential medium was supplemented with glucose (5 mg/ml), transferrin (100 μg/ml), insulin (5 mg/ml), glutamine (0.3 mg/ml), and 10% fetal calf serum. After 2 d, the cells were fed with media that was supplemented further with 4 μm cytosine arabinoside, and they were then fed every 5 d by a 50% replacement of the culture media. Electrophysiological measurements were made after 14–17 din vitro.
Electrophysiology
Recordings were from single, visually identified, granule cells using both outside-out patches and whole cells pulled away from the underlying cell-attachment substrate. No differences were observed between data from patches and whole cells, and results from both data sets were therefore pooled. A piezoelectrically driven theta tube-based application system delivered 120 msec pulses of GABA. Concentration jumps from control to agonist and vice versa took place within ∼1 msec. Five 120 msec 20 μm GABA pulses were applied at 0.1 Hz before and during the application of 1 μmflunitrazepam (Sigma). Recovery from the actions of flunitrazepam were studied with 20 additional GABA applications. Data were filtered at 2 kHz and sampled directly to computer at 10 kHz under control of the pClamp software suite. Because of the presence of some application-to-application variability in the current peak amplitude generated by GABA, an arbitrary threshold was set, with a 15% increase in the GABA response considered to be a potentiation above the baseline variability.
In situ hybridization
In situ hybridization with 35S-labeled oligonucleotide probes was as described (Wisden and Morris, 1994). The oligonucleotide sequences used were α4: 5′-TTCTGGACAGAAACCATCTTCGCCACATGCCATACTTTAAGCCTGT-3′ (EMBL accession number L08493) and δ: 5′-AGCAGCTGAGAGGGAGAAAAGGACGATGGCGTTCCTCACATCCAT-3′ (EMBL accession number M60596)
Behavioral observations
The animals (n = 23 for both +/+ and −/− lines, from two generations) were observed in their normal activities. Open field explorative activity was determined, under artificial lighting, in a round area (diameter 83 cm) divided into 19 segments. The mice were in the area for the first time. Their behavior was recorded for 5 min with a video recorder, and the behavioral parameters (number of segment crossings with all four feet and number of rearings) were scored blindly afterward. The number of fecal boli was counted before the area was cleaned for the next animal. The ability of the mice to learn to climb up onto a thin horizontal wire while initially hanging from their forepaws was tested in three trials during 1 d. Their ability to learn to stay on an accelerating rotating rod (Rotamex, Columbus Instruments, Columbus, OH) for 180 sec was tested during daily sessions. The initial session was 3 min on a nonmoving rod. On subsequent sessions, the mice were placed on the stationary rod, and the rotation speed was then set at 5 rpm and increased to 15 rpm over a 180 sec interval.
RESULTS
Creation of a mouse line with no GABAA receptor α6 subunit protein: mapping α6 expression with a dicistronic RNA encoding lacZ
Homologous recombination in embryonic stem cells was used to create a 129/Sv × C57BL/6 mouse line in which the α6subunit gene was disrupted at exon 8 (Fig.1A,B) (see Materials and Methods). The mutation, located just after the TM2 (channel lining) region, consisted of an insertion of stop codons in all three reading frames, an IRES linked to a β-galactosidase (lacZ) open reading frame and SV40 polyadenylation site, and finally a neomycin resistance gene expressed from its own promoter (Mountford et al., 1994; Nehls et al., 1996) (Fig. 1A). Translation of the α6subunit mRNA from the mutant allele should terminate just after the TM2 region, resulting in a 300 amino acid protein designated α6M2. The stop codon-IRES insertion generates a dicistronic mRNA in which β-galactosidase protein translation is then linked to an α6 gene expression, i.e., lacZ expression is under α6 transcriptional regulatory element control.
Homozygous mutant mice had no overt defects and could breed normally (see Behavioral Characterization of α6 −/− Mice). By the criteria of Nissl staining, the size, folding of folia, and histological appearance of the cerebellum in α6 −/− animals were completely normal. Immunocytochemistry with the α6 N-terminal-specific antibody α6-N (Thompson et al., 1992), however, demonstrated a total loss of α6-specific immunoreactivity from the cerebellar granule cell layer of −/− mice (Fig. 2A,B). Using both N- and C-terminal α6 subunit-specific antibodies (Thompson et al., 1992; S. Pollard and F. A. Stephenson, unpublished data), a complete loss of the 57 kDa immunoreactive α6 band was also seen on Western blots of cerebellar protein extracts isolated from −/− animals (Fig. 2C). Identical results were found with an additional C-terminal anti-peptide antibody α6(429–434) (R. Pelz and W. Sieghart, data not shown). Long exposure times of blots probed with the N-terminal antibody failed to show any α6-specific degradation products in the −/− samples (data not shown), indicating that α6M2 is not a stable entity. In a γ2subunit gene knockout study, which similarly used an exon 8 disruption, a truncated γ2 form was also not detectable (Günther et al., 1995).
Fig. 2.
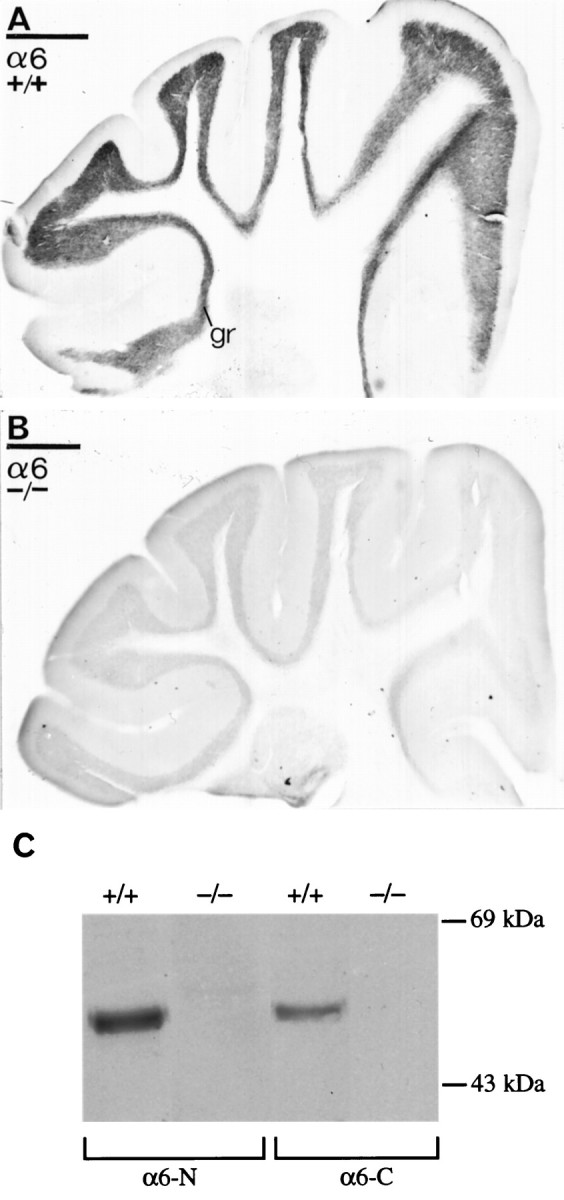
Immunodetection of the α6 subunit of the GABAA receptor in α6 +/+ (A, C) or α6 −/− (B, C) cerebella as visualized with either light microscopic immunoperoxidase reactions (A, B) or immunoblotting (C). A, B, An intense immunoreactivity for the α6subunits in the granule cell layer (gr) disappeared in α6 −/− mice (B). The sections and one immunoblot were reacted with the same N-terminal domain-specific antibody. Scale bars: A, B, 500 μm.C, The 57 kDa α6 protein is absent in α6 −/− cerebella, as shown with either α6-N, an N-terminal-specific antibody, or α6-C, a C-terminal-specific antibody to the α6 subunit.
We mapped α6 gene expression in adult −/− animals using β-galactosidase staining (Fig. 3). An intense blue coloration was seen in the cerebellar granule cell layer (Fig.3A,B). The reaction product started to appear within the first 5 min of incubating the sections at room temperature in X-Gal and was fully developed within 30 min, thus demonstrating the extremely high level of α6 locus expression. There were numerous small blue cells in the cerebellar molecular layer. These are probably ectopic granule cells (Fig. 3D) (cf. Thompson et al., 1992;Gao and Fritschy, 1995; Gutiérrez et al., 1996). Strong, blue coloration was also seen along the molecular layer outer edge, probably corresponding to β-galactosidase enzyme transported into granule cell axons, the parallel fibers. As expected, there were many blue granule cells in the dorsal cochlear nuclei (Fig. 3E) (Varecka et al., 1994). With little exception, the rest of the brain showed no detectable staining (Fig. 3A). Unexpectedly, however, many cells in the inferior colliculi dorsal regions stained blue (Fig.3C), and there were other minor cell populations in the substantia nigra and thalamus (geniculate nuclei) with faint but consistent blue staining (data not shown). These populations of stained cells were not seen in wild-type animals, and thus were not attributable to endogenous β-galactosidase-like activity.
α6 subunit expression has not been noted previously in the inferior colliculi, substantia nigra, or thalamus by in situ hybridization or immunoreactivity, although a rat α6 gene proximal promoter fragment consistently drives lacZ expression in the inferior colliculi of transgenic mice (Jones et al., 1996). The reasons for the lack of detection in previous studies could include low α6 mRNA and protein levels. Alternatively, α6 may be part of presynaptic receptors transported to distant axon terminals outside the inferior colliculi and so may escape detection in the inferior colliculi nucleus itself. The long half-life of β-galactosidase in mammalian tissue contributes to the extreme sensitivity of the lacZ reporter method. Over time, low levels of transcription from the α6-lacZ hybrid gene will lead to accumulating amounts of β-galactosidase. These results make clear the usefulness of tracking gene expression using dicistronic-based reporters (Mountford et al., 1994; Nehls et al., 1996).
Pharmacological characterization of α6 −/− cerebellar granule cells: BZ sensitivity
The α6 protein absence was established further by pharmacological analysis. GABAA receptors containing the α6 subunit are insensitive to most types of BZs, such as diazepam or flunitrazepam (Lüddens et al., 1990; Hadingham et al., 1996). Ro 15-4513, however, is a BZ that binds to all subtypes of GABAA receptor with αxβγ2subunit combinations, including those containing the α6subunit (Lüddens et al., 1990; Sieghart, 1995). Thus, a diagnostic assay for α6 in cerebellar granule cells is the high level of [3H]Ro 15-4513 binding on granule cell membranes that is insensitive to full BZ agonists such as diazepam (Sieghart et al., 1987; Malminiemi and Korpi, 1989; Lüddens et al., 1990; Turner et al., 1991). Normally, more than half of the [3H]Ro 15-4513 binding in the granule cell layer is diazepam insensitive (DIS) but can be displaced by micromolar concentrations of the BZ antagonist flumazenil (also known as Ro 15-1788). This profile is thought to be attributable to the abundant expression of α6β2/3γ2receptors on granule cells (Lüddens et al., 1990; Korpi and Lüddens, 1993; Korpi et al., 1993; for review, see Wisden et al., 1996). In the α6 −/− animals, DIS binding over the cerebellar granule cell layer is completely absent (Fig.4A), whereas in wild-type brains [3H]Ro 15-4513 still binds over the granule cell layer even in the presence of 100 μm diazepam (Fig.4A). From binding studies using isolated cerebellar membranes, the contribution that the α6 subunit makes to the number of total cerebellar Ro 15-4513 binding sites was estimated to be ∼40% (see Evaluation of α1 Subunit Levels; also see Table 1).
Fig. 4.
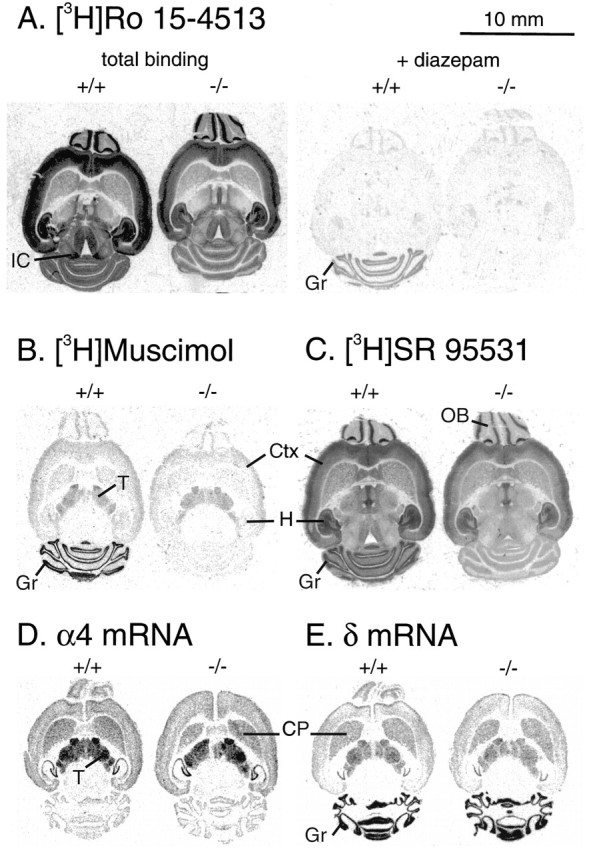
Autoradiographic analysis of GABAAreceptor binding sites in wild-type (+/+) and α6 −/− mice. A, Benzodiazepine sites labeled by 5 nm [3H]R0 15-4513 showing total and diazepam-insensitive binding in the presence of 100 μmdiazepam. B, GABAA receptor sites labeled by 20 nm [3H]muscimol, showing total binding. The nonspecific signal in the presence of 100 μm GABA was at the film background level. C, GABAAreceptor sites labeled by 20 nm [3H]SR 95531, showing total binding. The nonspecific binding signal in the presence of 100 μm GABA was similar in wild-type and −/− brains (data not shown). Similar distinct pharmacological profiles were observed between the wild-type and α6 −/− brains in each of seven pairs of adult mice studied. D, E,In situ hybridization x-ray film autoradiographs of adult mouse brains hybridized with α4 (D) and δ-specific (E) 35S-labeled oligonucleotide probes. Wild-type (+/+) brains are on theleft; α6 −/− brains are on theright. No differences can be seen in subunit mRNA levels between +/+ and −/− brains. Note also the very similar pattern of α4 and δ gene expression in the forebrain/thalamus regions, and the correlation with the distribution of [3H]muscimol (B). Cbgr, Cerebellar granule cells; CP, caudate-putamen;Ctx, cerebral cortex; Gr, cerebellar granule cell layer; H, hippocampus; IC, inferior colliculus; OB, olfactory bulb;T, thalamus.
Table 1.
Determination of Bz binding in α6 −/− mice
| Ligand | +/+ | −/− | ||
|---|---|---|---|---|
| Kd | Bmax | Kd | Bmax | |
| Ro 15-1788 | 0.91 ± 0.16 | 1222 ± 144 | 0.84 ± 0.17 | 1021 ± 141 |
| Ro 15-4513 total sites | 9.4 ± 2.0 | 2106 ± 203 | 6.3 ± 1.5* | 1159 ± 1071-160 |
| DS | 5.9 ± 0.8 | 1199 ± 114 | 5.7 ± 0.5 | 1088 ± 174 |
| Zolpidem | 17.9 ± 4 | 1155 ± 152 | 19.6 ± 4.8 | 993 ± 161 |
| Muscimol | 6.3 ± 0.8 | 2568 ± 326 | 6.2 ± 1.7 | 661 ± 234 |
Data shown are the mean ± SEM of cerebellar membranes prepared independently from six animals. Saturation analysis used eight concentrations of ligand in duplicate for each animal.Bmax (fmol/mg protein) andKd (nm) values were determined by nonlinear least-squares fit of the saturation curves using the data analysis software RS1.
Significantly different from +/+ (p < 0.05);
F1-160: significantly different from +/+ (p < 0.005). DS, Diazepam sensitive.
The α6 subunit has a closely related homolog, the α4 subunit, which is expressed in certain forebrain areas such as the thalamus (Wisden et al., 1991, 1992). The recombinant α4 subunit in an α4βxγ2 configuration displays a pharmacological profile identical to that of α6βxγ2 receptors, and α4 mRNA is found at low levels in cerebellar granule cells of adult rats (Wisden et al., 1991; Laurie et al., 1992). Thus we looked to see whether there had been a compensatory change in α4 expression in the cerebellum of α6 −/− mice (Fig. 4D); however, consistent with the absence of DIS binding in α6 −/− animals, there was no upregulation of α4 mRNA in −/− cerebella (Fig.4D).
The BZ sensitivity of GABAA receptors in α6 −/− cerebellar granule cells was investigated directly using electrophysiology on cultured granule cells isolated from P5 animals. After 14–17 d in vitro, we tested the effects of BZ agonist flunitrazepam (1 μm) coapplication on the current amplitude generated by 20 μm GABA. Results of a typical culture are shown in Figure 5. Examination of wild-type cells revealed a heterogeneous response: flunitrazepam-induced potentiation of the GABA response took place in approximately half (20 of 36) of the cells tested (Fig. 5C,top row). In those cells with flunitrazepam-potentiated GABA responses, the average potentiation was 58 ± 7%. In contrast, in age-matched cultures derived from α6 −/− cerebella, 16 of 18 cells tested had flunitrazepam-induced potentiations of their GABA responses. The average potentiation was 62 ± 7% (Fig.5C).
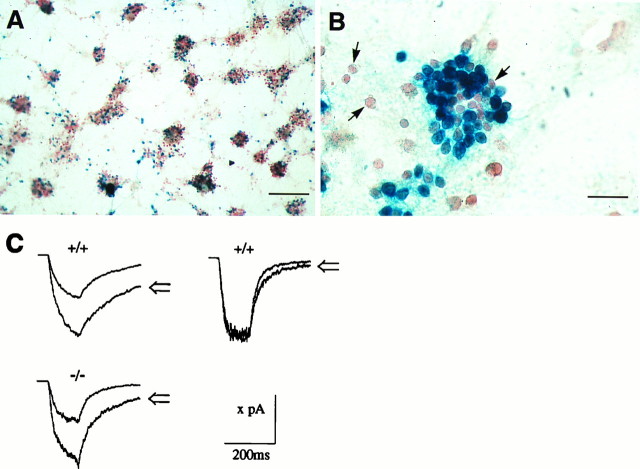
Fig. 5.
Electrophysiological characterization of GABAA receptors in cerebellar granule cells from wild-type and α6 −/− cells. Photomicrographs in Aand B show typical examples of lacZ-expressing cerebellar granule cells, isolated from P5 α6 −/− mouse cerebella, and cultured for 3 weeks. A is a low-magnification view, showing the mosaic of blue(lacZ-positive) cells scattered throughout the culture. Both isolated and clustered blue cells can be seen. Within any given cluster, not all the cells are blue and therefore are not expressing the α6 gene. The cells have been counterstained with neutral red. All electrophysiological recordings were from isolated cells. Arrows inB show examples of non-lacZ-expressing cells. Scale bars: A, 200 μm; B, 30 μm.C, Example responses to 120 msec applications of 20 μm GABA alone, and 20 μm GABA with 1 μm flunitrazepam (open arrows). Thetop row shows an example of wild-type cells (+/+) with GABAA receptors that responded to flunitrazepam (left trace) or were insensitive to flunitrazepam (right trace). The bottom row shows a typical GABAA response from an α6 −/− cell and the associated flunitrazepam potentiation. From left to right and top to bottom, the valuex on the scale bar corresponds to 200, 230, and 170 pA, respectively. The traces were averages of three to five consecutive records.
To examine a possible reason for the heterogeneity of the GABAA receptor response, the extent of gene expression from the α6 locus in cultured α6 −/−granule cells was analyzed with β-galactosidase histochemistry. At 3 weeks in culture, numerous cells strongly stained dark blue after incubation with X-Gal (Fig. 5A,B); however, there were many adjacent “granule-like” cells that either contained just a few blue particles or were completely unstained (Fig. 5B). This applied both to isolated cells and to cells in large clusters. There was no obvious correlation between the location of cells (isolated or in clusters) and lacZ expression. This mosaic of blue cells is evidence that at least in culture, not all granule cells or granule-like cells express the α6 gene (cf. Santi et al., 1994), and may explain the heterogeneous nature of the BZ potentiation seen in our cultures.
Selective δ subunit protein loss from cerebellar granule cells of α6 −/− mice
A key and controversial question for cerebellar granule cell GABAA receptors has been which subunits co-assemblein vivo (Wisden et al., 1996). To examine one aspect of this, we immunoprecipitated deoxycholate-solubilized cerebellar GABAA receptors from α6 −/− mice with a δ-specific polyclonal antiserum, δ(318–400), raised against the putative intracellular loop domain between TM3 and TM4 (Quirk et al., 1994, 1995). In wild-type and α6 +/− cerebella, the δ(318–400) antiserum precipitated the same number of muscimol binding sites (Fig. 6A). By this assay, the δ protein was also present in both pure wild-type 129/Sv and pure C57BL/6 cerebella (data not shown). In contrast, immunoprecipitation of δ-containing receptors from α6−/− cerebella was greatly reduced (Fig. 6A). These data were extended by Western blot analysis of membrane protein samples isolated from individual +/+ and α6 −/− cerebella. With use of a δ-subunit-specific antibody, δ(1–44)R7, raised against the N terminus (R. Pelz and W. Sieghart, unpublished data), α6 −/− samples showed a dramatic reduction in the 53 ± 1 kDa δ subunit band intensity to 25 ± 8% of +/+ levels (Fig. 6B). The residual δ protein in the α6 −/− tissue had the same molecular weight as that in wild-type tissue (Fig. 6B).
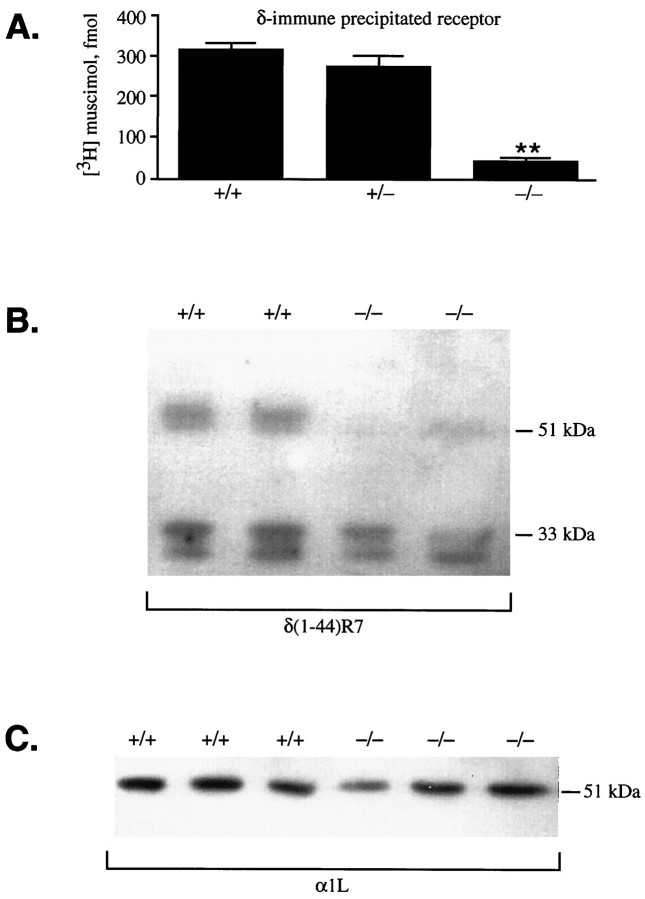
Fig. 6.
Immunoprecipitation and immunoblot analysis of GABAA receptor δ subunit levels in wild-type and α6 −/− cerebella. A, After cerebellar GABAA receptors were solubilized in Triton X-100/deoxycholate, the number of [3H]muscimol binding sites immunoprecipitated by the δ(318–400) antiserum from +/+, +/−, and −/− cerebella was determined (n = 10–14).B, Immunoblot analysis: the marked δ subunit reduction in α6 −/− cerebella detected with the δ(1–44)R7 antiserum. The identity of the low molecular weight doublet (33 and 31 kDa) seen in all samples is unknown. C, A 51 kDa α1 immunoreactive band is present in both −/− and +/+ cerebellar samples as detected with the α1(328–382)/α1L antibody.
Immunocytochemistry with the δ(1–44)R7 antibody clearly supported the Western blot and immunoprecipitation data (Fig. 7). Light microscopic immunocytochemistry with this antibody revealed a very intense cerebellar granule cell layer staining in wild-type animals (Fig. 7A), similar to that reported earlier using a different δ-specific antibody (Benke et al., 1991; Gao and Fritschy, 1995). The immunoreactivity originated mainly from staining of the glomeruli, and granule cell bodies were only weakly outlined (Fig.7C). The glomeruli appeared as dark rings of labeled granule cell dendrites surrounding pale centers representing the unstained mossy fiber terminals (Fig. 7C). In contrast to the wild-type animals, in α6 −/− mice the granule cell layer immunostaining for the δ subunit was virtually absent (Fig.7B). In particular, no immunoreactivity could be detected in the glomeruli (Fig. 7D), suggesting that α6−/− granule cell dendrites contain either no δ subunit protein or an undetectably low level. Electron microscopic examination of the immunoreactivity for the δ subunit in the granule cell layer further confirmed the lack of δ subunit immunoreaction in α6−/− granule cells (not shown). Therefore, the residual δ subunit immunoreactivity seen on Western blots may represent a level of protein undetectable by immunocytochemistry under our conditions, or it could come from cell types other than granule cells, because whole cerebella were used to prepare the protein extracts.
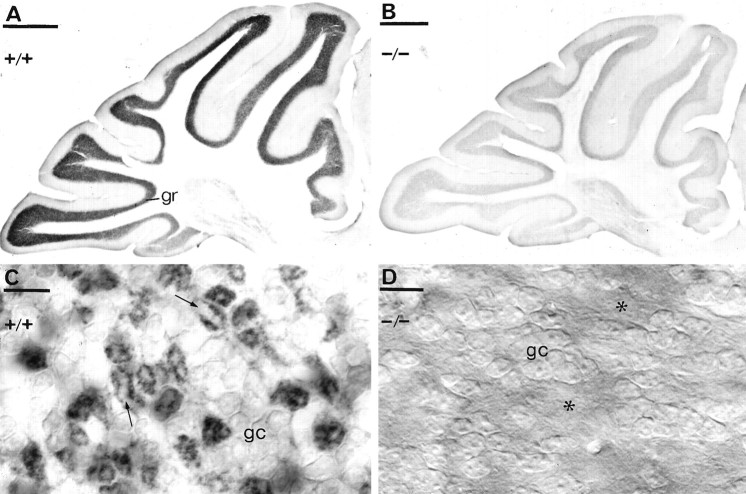
Fig. 7.
Immunodetection of the δ subunit of the GABAA receptor in α6 +/+ (A,C) or α6 −/− (B, D) cerebella, using a polyclonal antibody δ R7 and immunoperoxidase reaction. The granule cell layer showed intense immunoreactivity in α6 +/+ animals but almost no staining was observed in the α6 −/− mouse. C, At higher magnification, it is evident that the δ subunit is localized mainly in the glomeruli, granule cell bodies (gc) being only weakly outlined. The glomeruli appear as dark rings of granule cell dendrites surrounding a pale center (arrows) representing the unstained mossy fiber terminal. D, In the α6 −/− mice, both the granule cell bodies (gc) and the glomeruli (asterisks) are immunonegative for the δ subunit. C andD were photographed using DIC optics. Scale bars:A, B, 500 μm; C, D, 10 μm.
The δ subunit loss occurs post-translationally
One possibility to explain the loss of δ protein from α6 −/− granule cells is through a change in regulation at the mRNA level; however, the δ subunit mRNA level in the cerebellar granule cells was at normal levels when examined by in situ hybridization (Fig. 4E). High levels of δ mRNA were seen in both wild-type and −/− granule cells. δ mRNA expression was also examined in both pure 129/Sv and C57BL/6 strain wild-type animals and found not to differ (not shown). This result suggests that the loss of δ subunit from the α6 −/− cerebellar granule cells occurs post-translationally.
[3H]Muscimol and [3H]SR95531, two ligands that mark out α6-and δ-containing GABAA receptors
[3H]Muscimol and [3H]SR95531 are ligands that highlight restricted GABA molecule conformations (Sieghart, 1995). In particular, [3H]muscimol is the classic GABAA ligand and has been used extensively for mapping GABAA receptors in the brain (Palacios et al., 1980; Olsen et al., 1990). We used [3H]muscimol and [3H]SR95531 to autoradiographically probe the remaining GABAA receptors in the δ subunit-deficient/α6 −/− cerebellar granule cell layer. A clear cut, but completely unanticipated, pharmacological feature was revealed: the selective and extensive loss of high-affinity [3H]muscimol (Fig. 4B) and [3H]SR95531 (Fig. 4C) binding from the granule cells. Binding over the cerebellar molecular layer with these ligands remained unchanged, as did the levels of binding in the forebrain, e.g., normal levels of [3H]muscimol binding remain over the thalamus of −/− animals (Fig. 4B). The decrease in [3H]muscimol binding seen by autoradiography in α6 −/− individuals was further quantified by studying [3H]muscimol binding to membranes from whole cerebella (Table 1). The level of high-affinity [3H]muscimol sites was reduced to ∼25% of that found in control animals (Table 1). No significant reduction in [3H]muscimol binding was seen in +/− animals (data not shown). Saturation analysis revealed no change in the observed Kd values for [3H]muscimol in −/− animals (Kdis ∼6 nm; Table 1). The residual binding is likely to come from sites within the molecular layer, the granule cell layer, and the deep cerebellar nuclei, all of which contain an α1β2/3γ2 component. Under autoradiographic conditions, however, [3H]muscimol does not highlight these α1-containing receptors in the cerebellum. Rather, [3H]muscimol and [3H]SR95531 seem to selectively highlight α6δ-containing receptors.
Evaluation of α1 subunit levels in α6−/− and δ-deficient cerebella
The α1 protein is expected to account for the majority of the remaining α subunits in the cerebellum of α6 −/− mice (Sieghart, 1995; McKernan and Whiting, 1996; Wisden et al., 1996). To examine whether there was any concomitant change in the α1-receptor population in the α6 −/− cerebella, the portion of α1subunits complexed with the β2/3 and γ2subunits was measured using three different ligands targeting the BZ binding site. These assays are not likely to measure any α1 subunits that are complexed with the δ but not the γ2 subunits, e.g., α1β2/3δ, because the GABA responses of these complexes cannot be modulated by BZs (Saxena and Macdonald, 1994).
Full saturation analysis was carried out on cerebellar membranes to determine the Kd and Bmaxvalues for [3H]Ro 15-1788 binding, diazepam-sensitive [3H]Ro 15-4513 binding, and [3H]zolpidem binding (Table 1). All three ligands identified approximately the same number of binding sites in the cerebellar membranes (∼990–1160 fmol/mg protein). There was no statistically significant difference between the number of binding sites for any ligand between the α6 +/+ and δ-deficient/α6 null groups, although the trend was always toward a reduced number of sites in the −/− animals. Total [3H]Ro 15-4513 binding sites (both diazepam-sensitive and -insensitive components, with the nonspecific binding being defined in the presence of 10 μm Ro 15-4513), however, were decreased by 44% in the −/− cerebella (Table1), with a minor change in the affinity Kdconstant. This figure is in line with the 30–40% contribution that the α6β2/3γ2 component has been reported to make to the total Ro 15-4513 binding sites in the cerebellum (Sieghart et al., 1987; Turner et al., 1991; Korpi et al., 1993; Quirk et al., 1994).
These binding results suggest that the amount of total α1 protein complexed with β2/3 and γ2 in the cerebellum is essentially unchanged between δ-deficient/α6 null and wild-type animals. Furthermore, immunocytochemistry with a polyclonal α1-specific antibody (P16) showed no overt change in granule cell layer immunostaining at the light microscopic level in α6 −/− cerebella compared with wild-type tissue (data not shown); however, this method may not pick out small changes in subunit levels. In fact, immunoblotting with α1-specific antibodies did show a downward trend in α1 protein levels between δ-deficient/α6 null and +/+ cerebellar samples (Fig.6C). In −/− animals, a small reduction with high variability was seen in the α1 51 kDa band intensity, as determined by densitometric measurements. This was observed independently with three different α1-specific antibodies: α1(1–14) (S. Pollard and F. A. Stephenson, unpublished data), α1(1–9) (Zezula and Sieghart, 1991), and α1(328–382) (R. Pelz and W. Sieghart, unpublished data) (see Materials and Methods).
Behavioral characterization of α6null/δ-deficient mice
The total loss of α6 from the cerebellum and the associated severe reduction of δ subunit levels might be expected to have consequences for nervous system function in the mutant mice. The cerebellum integrates sensory input needed for maintaining balance and orientation, has a prominent role in the refinement of motor action, and may also participate in motor memory storage (Raymond et al., 1996). We looked for evidence of cerebellar-associated motor deficits in the −/− mice. In terms of simple observable behavior, mutant mice seem indistinguishable from wild-type littermates. The adult α6 null/δ-deficient mice are active and agile, whether they be in the cage or roaming freely, and exhibit spontaneous activity, such as walking upside down on the ceiling of their cages. In an open field test, mutant mice showed as much exploratory activity as individuals with normal levels of α6 and δ proteins (Table 2). Mutant as well as wild-type mice learned the horizontal wire task (data not shown; see Materials and Methods). Both the wild-type and α6 null/δ-deficient mice reached the rotating rod test learning criterion (Table 2). With these tests, we found no evidence to suggest any form of ataxia associated with cerebellar dysfunction. Additionally, a detailed behavioral analysis on an independently generated α6 −/− mouse line, where the same exon was disrupted by insertion of a neo gene (exon 8,NcoI site) in a 129xC57BL/6 background, showed no abnormalities in motor behaviors (Gregg E. Homanics, Department of Anesthesiology, University of Pittsburgh, personal communication).
Table 2.
Open field activity and rotating rod learning of wild-type and α6 −/− mice
| n | Open field activity/5 min | ||||||
|---|---|---|---|---|---|---|---|
| Number of crossings | Number of fecal boli | Number of rearings | |||||
| +/+ | 10 | 96 ± 5 | 3.3 ± 0.7 | 6.7 ± 1.5 | |||
| −/− | 10 | 84 ± 18 | 3.2 ± 0.5 | 5.2 ± 1.1 | |||
| n | Rotarod performance: seconds on the rod per 180 sec/(percent of animals staying for 180 sec) | ||||||
|---|---|---|---|---|---|---|---|
| Trial 1 | Trial 2 | Trial 3 | Trial 4 | Trial 5 | Trial 6 | ||
| +/+ | 15 | 114 ± 11 (20%) | 147 ± 9 (40%) | 160 ± 11 (67%) | 179 ± 1 (87%) | 179 ± 1 (87%) | 180 ± 0 (100%) |
| −/− | 14 | 144 ± 11 (43%) | 148 ± 12 (57%) | 167 ± 8 (79%) | 176 ± 3 (86%) | 176 ± 4 (93%) | 180 ± 0 (100%) |
Results are given as means ± SEM. No statistically significant differences (p < 0.05; Student’s ttest and repeated measures ANOVA). The Rotarod learning test was repeated with another batch of mice with similar results.
DISCUSSION
A mouse line lacking functional GABAA receptor α6 subunit protein has been generated. Because of the restricted α6 gene expression profile, this mutation was expected to principally affect the cerebellum. Furthermore, in the cerebellum, the granule cell δ subunit protein level was markedly reduced relative to wild-type levels. Thus these mice effectively harbor a region-specific double subunit knock-out, and the GABAA receptor complexity on granule cells is reduced to receptors largely containing just α1, β2/3, and γ2 subunits. Two issues are discussed: the significance of multiple α subunits and defined assembly pathways for receptor subunits.
GABAA receptor α subunit heterogeneity
Surprisingly, in spite of a large loss of granule cell GABAA receptors, the α6 null/δ-deficient mice are not grossly impaired in motor skills. This lack of phenotype under laboratory conditions was not anticipated from α6gene comparative studies. Both the conservation of peptide sequence in the N-terminal domain and a granule cell-specific expression pattern in the cerebellum of fish, birds, rodents, and humans imply that there has been a continual selection for the α6 protein (Bahn et al., 1996; Hadingham et al., 1996).
In the α6 null/δ-deficient mice, physiological changes in granule cell GABAA receptors are expected, but these have not obviously impaired cerebellar function. Removal of two of the six subunits from granule cells will still leave functional receptors with α1β2/3γ2 subunit combinations. Nevertheless, substitution of different α subunits in an αxβxγ2 complex may influence the inhibitory postsynaptic current kinetics (Gingrich et al., 1995; Tia et al., 1996). Synaptic transmission at GABAergic synapses is generated by millisecond pulses of 0.5–1 mmGABA (reviewed by Mody et al., 1994). Under these conditions, recombinant α1β2/3γ2 and α6β2/3γ2 receptors do behave differently, with the α6-containing receptors having a slower deactivation rate (Tia et al., 1996). The physiological role of the δ subunit remains obscure (Shivers et al., 1989). During long applications of micromolar GABA, δ-subunits slow the acute macroscopic desensitization rate of recombinant GABAAreceptors (Saxena and Macdonald, 1994); however, this property has not been studied using fast, brief GABA application.
Selective subunit partnerships
The α6 −/− mouse has provided insight into GABAA receptor subunit assembly pathways in neurons. The α6 protein derived from the targeted gene should terminate just after TM2. An analogous example has been studied for the mouse muscle nicotinic receptor δ subunit. When truncated just after TM2 (δM2) and co-expressed with wild-type nicotinic α, β, γ, and δ subunits in COS cells, δM2 interferes with receptor assembly (Verrall and Hall, 1992). Similarly, the truncated GABAAα6 protein (α6M2) may prevent α6δ-containing receptors from reaching the granule cell surface. The association of α6M2 and δ may inhibit mature receptor expression by forming specific complexes in the endoplasmic reticulum that are not permissive for further receptor assembly and/or trafficking. These will be retained and degraded (Verrall and Hall, 1992; Connolly et al., 1996). As for the nicotinic acetylcholine and glycine receptors (Verrall and Hall, 1992; Kuhse et al., 1993; Sumikawa and Nishizaki, 1994; Kreienkamp et al., 1995), the information needed for specific assembly of the GABAAreceptor α6 and δ proteins is likely to be in their N-terminal domains, because the N-terminal domain of α6is sufficient to block δ expression. Because they interact as an assembly intermediate, α6 and δ probably occur adjacent to each other in the mature receptor subunit ring.
There are several other scenarios. The α6M2 polypeptide could be degraded before pairing with the δ subunit. Because the δ subunit is not efficiently incorporated with other subunits, this might in turn be degraded. Alternatively, if no α6 protein is present, the δ mRNA might be translated inefficiently, implying that α6 protein levels feed back to regulate the translation of δ mRNA. Although this is an interesting possibility, there is no known mechanism.
Our results seem to confirm the antibody-based data suggesting that in vivo, δ predominantly assembles with α6 and not α1 (Caruncho and Costa, 1994;Quirk et al., 1994; Caruncho et al., 1995). Nevertheless, from the genetic results alone, an α6 and δ interaction may be simply the first step allowing other subunits such as α1to subsequently join the complex. Thus, both α1α6βδ or even α1α6βγ2δ might existin vivo (Mertens et al., 1993; Pollard et al., 1995; R. Pelz and W. Sieghart, unpublished observations); however, because α1, β2/3, and γ2 subunits cannot rescue the δ subunit in an α6 −/− background, we predict that the α1βxδ and α1βxγ2δ combinations will not be found to any great extent in vivo.
In recombinant systems [Xenopus oocytes, human embryonic kidney (HEK) 293 cells, and mouse L929 fibroblast cells], the situation is different. The δ subunit can assemble to form functional receptors with either α1 or α6 as α1βxδ, α6βxδ, and possibly α1βxγ2δ complexes, with the exact β subunit used having little influence (Saxena and Macdonald, 1994, 1996; Ducic et al., 1995; Krishek et al., 1996). Thus there may be unique architectural editing or chaperone mechanisms present in granule neurons that are not found in Xenopus oocytes or HEK cells. Alternatively, the subunits may differ slightly in affinity for each other. In a recombinant system, the large amounts of protein present may allow many combinations to assemble, even if they have nonoptimal association parameters. The results presented here demonstrate the importance of studying subunit assembly pathways in the brain.
[3H]Muscimol as a selective autoradiographic probe for α4, α6, and δ subunit associations
It has been suggested that under autoradiographic binding conditions the GABAA site ligands [3H]muscimol and [3H]SR 95531 highlight a subpopulation of receptors in native membranes (Olsen et al., 1990). In a wide range of vertebrates, a hallmark of GABAA sites in the CNS is the high levels of [3H]muscimol binding over the cerebellar granule cell layer (Palacios et al., 1980; Schmitz et al., 1988; Olsen et al., 1990; for review, see Wisden et al., 1996). A striking feature of our study was the almost total loss of high-affinity [3H]muscimol and [3H]SR95531 binding from the granule cell layer of α6null/δ-deficient cerebella (Fig. 4B,C), suggesting that the α6 and/or δ subunits are responsible for these ligand profiles. From recombinant data, the α6βxδ subunit combination is insensitive to BZs (Saxena and Macdonald, 1996) and sensitive to GABA (EC50 in the low micromolar range) but gives small currents (Saxena and Macdonald, 1994, 1996; Ducic et al., 1995). These properties would be consistent with the pharmacology of the cerebellar δ-containing receptors immunoprecipitated with a δ-specific antibody: high muscimol affinity and no BZ binding (Quirk et al., 1994).
Despite the absence of autoradiographic signal in −/− cerebella, muscimol is still an effective agonist of GABAA receptors on cultured α6 −/− granule cells (J. Mellor and A. D. Randall, unpublished observations), although electrophysiological assays most likely use the low-affinity site. The remaining α1β2/3γ2 receptors in α6 −/− granule cells should have aKd for [3H]muscimol of ∼5 nm (Lüddens et al., 1990) and could be expected to bind [3H]muscimol, but this is not the case under autoradiographic assay conditions. Similarly, in the inferior colliculi of wild-type and −/− animals, given the high concentration of α1β2γ2 receptors present (Persohn et al., 1992; Wisden et al., 1992; Fritschy and Möhler, 1995), it is difficult to explain the virtual absence of [3H]muscimol binding sites in autoradiography (Fig.4B). There may be some factor specifically associated with α1β2/3γ2 receptors on native membranes that prevents high-affinity [3H]muscimol binding.
As pointed out previously (Shivers et al., 1989; Laurie et al., 1992), both δ subunit mRNA and protein closely parallel the distribution and abundance of [3H] muscimol binding (Fig.4B,E), e.g., highest in cerebellar granule cells, followed by the thalamus, and then caudate-putamen, neocortex, and dentate gyrus (Shivers et al., 1989; Olsen et al., 1990; Benke et al., 1991). In the forebrain, α4 subunit mRNA also largely follows δ subunit distribution (compare Fig. 4, B andD; and see Wisden et al., 1992). The α4 and α6 subunits are closely related and form a subgroup set apart from the other α subunits (Seeburg et al., 1990; Ortells and Lunt, 1995; Tyndale et al., 1995). The total evidence suggests strongly that α4δ forms part of a GABAA receptor that is an α6δ combination homolog. A phylogenetic clock comparison calculated that α4 and α6are the oldest α subunits and that the δ subunit is the oldest of all GABAA receptor subunit genes (Ortells and Lunt, 1995). Therefore, a selective interaction of α4 and α6 with δ might represent an early vertebrate GABAA receptor subtype.
In conclusion, we have provided evidence for a specific association between the α6 and δ subunits in granule cell GABAA receptors. It seems likely that similar assembly rules exist for other brain heteromeric ligand-gated channels, e.g., the neuronal nicotinic acetylcholine receptor (Vernallis et al., 1993) and ionotropic glutamate receptors.
These authors made equally important contributions to different aspects of this work.
S.B. holds a European Community Human Capital and Mobility Fellowship (category 20). A.J.H.S. was supported by the Association for International Cancer Research. This work was supported in part by EC Grant BIO4-CT96-0585 to P.S. and W.S. We thank Terry Rabbitts, Theresa Langford, and Gareth King (Laboratory of Molecular Biology, Cambridge) for providing invaluable support and guidance with transgenic mice; Grayson Richards (F. Hoffman-LaRoche, Basel) for supplying Ro 15-1788; and Pirkko Johansson, Maija Sarviharju, and Antti Turhala (Department of Alcohol Research, National Public Health Institute, Helsinki) and Frances Emms (Merck Sharp and Dohme, Harlow) for technical help with behavior, autoradiography, and ligand binding. We are grateful to Gregg E. Homanics (Department of Anesthesiology, University of Pittsburgh) for communicating data on his α6 knock-out mouse line before publication. Hilmar Bading, Louise Tierney, Stephen Hunt, Trevor Smart, and Nigel Unwin provided helpful comments on this manuscript.
Correspondence should be addressed to W. Wisden, Medical Research Council Laboratory of Molecular Biology, Medical Research Council Centre, Hills Road, Cambridge, CB2 2QH, UK.
REFERENCES
- 1.Bahn S, Harvey RJ, Darlison MG, Wisden W. Conservation of γ aminobutyric acid type A receptor α6 subunit gene expression in cerebellar granule cells. J Neurochem. 1996;66:1810–1818. doi: 10.1046/j.1471-4159.1996.66051810.x. [DOI] [PubMed] [Google Scholar]
- 2.Benke D, Mertens S, Trzecia A, Gillessen D, Möhler H. Identification and immunohistochemical mapping of GABAA receptor subtypes containing the δ subunit in rat brain. FEBS Lett. 1991;283:145–149. doi: 10.1016/0014-5793(91)80573-l. [DOI] [PubMed] [Google Scholar]
- 3.Bonnerot C, Nicolas J-F. Application of LacZ gene fusions to postimplantation development. Methods Enzymol. 1993;225:451–469. doi: 10.1016/0076-6879(93)25031-v. [DOI] [PubMed] [Google Scholar]
- 4.Caruncho HJ, Costa E. Double-immunolabelling study of GABAA receptor subunits in label-fracture replicas of cultured rat cerebellar granule cells. Receptors Channels. 1994;2:143–153. [PubMed] [Google Scholar]
- 5.Caruncho HJ, Puia G, Möhler H, Costa E. The density and distribution of six GABAA receptor subunits in primary cultures of rat cerebellar granule cells. Neuroscience. 1995;67:583–593. doi: 10.1016/0306-4522(95)00065-q. [DOI] [PubMed] [Google Scholar]
- 6.Connolly CN, Krishek BJ, McDonald BJ, Smart TG, Moss SJ. Assembly and cell surface expression of heteromeric and homomeric γ-aminobutyric acid type A receptors. J Biol Chem. 1996;271:89–96. doi: 10.1074/jbc.271.1.89. [DOI] [PubMed] [Google Scholar]
- 7.Ducic I, Caruncho HJ, Zhu WJ, Vicini S, Costa E. γ-aminobutyric acid gating of Cl− channels in recombinant GABAA receptors. J Pharmacol Exp Ther. 1995;272:438–445. [PubMed] [Google Scholar]
- 8.Fritschy J-M, Möhler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 9.Gao B, Fritschy J-M. Cerebellar granule cells in vitro recapitulate the in vivo pattern of GABAA-receptor subunit expression. Dev Brain Res. 1995;88:1–16. doi: 10.1016/0165-3806(95)00062-i. [DOI] [PubMed] [Google Scholar]
- 10.Gingrich KJ, Roberts WA, Kass RS. Dependence of the GABAA receptor gating kinetics on the α-subunit isoform: implications for structure-function relations and synaptic transmission. J Physiol (Lond) 1995;489:529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green WN, Claudio T. Acetylcholine receptor assembly: subunit folding and oligomerization occur sequentially. Cell. 1993;74:57–69. doi: 10.1016/0092-8674(93)90294-z. [DOI] [PubMed] [Google Scholar]
- 12.Günther U, Benson J, Benke D, Fritschy J-M, Reyes G, Knoflach F, Crestini F, Aguzzi A, Arigoni M, Lang Y, Bluethmann H, Möhler H, Lüscher B. Benzodiazepine-insensitive mice generated by targeted disruption of the γ2 subunit gene of γ-aminobutyric acid type A receptors. Proc Natl Acad Sci USA. 1995;92:7749–7753. doi: 10.1073/pnas.92.17.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutiérrez A, Khan ZU, De Blas AL. Immunocytochemical localization of the α6 subunit of the γ-aminobutyric acidA receptor in the rat nervous system. J Comp Neurol. 1996;365:504–510. doi: 10.1002/(SICI)1096-9861(19960212)365:3<504::AID-CNE12>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 14.Hadingham KL, Garrett EM, Wafford KA, Bain C, Heavens RP, Sirinathsinghji DJS, Whiting PJ. Cloning of cDNAs encoding the human γ-aminobutyric acid type A receptor α6 subunit and characterization of the pharmacology of α6-containing receptors. Mol Pharmacol. 1996;49:253–259. [PubMed] [Google Scholar]
- 15.Jones A, Bahn S, Grant AL, Köhler M, Wisden W. Characterization of a cerebellar granule cell-specific gene encoding the γ-aminobutyric acid type A receptor α6 subunit. J Neurochem. 1996;67:907–916. doi: 10.1046/j.1471-4159.1996.67030907.x. [DOI] [PubMed] [Google Scholar]
- 16.Khan ZU, Gutiérrez A, De Blas AL. The subunit composition of a GABAA/benzodiazepine receptor from rat cerebellum. J Neurochem. 1994;63:371–374. doi: 10.1046/j.1471-4159.1994.63010371.x. [DOI] [PubMed] [Google Scholar]
- 17.Khan ZU, Gutiérrez A, De Blas AL. The α1 and α6 subunits can coexist in the same cerebellar GABAA receptor maintaining their individual benzodiazepine-binding specificities. J Neurochem. 1996;66:685–691. doi: 10.1046/j.1471-4159.1996.66020685.x. [DOI] [PubMed] [Google Scholar]
- 18.Korpi ER, Lüddens H. Regional γ-aminobutyric acid sensitivity of t-butylbicyclophosphoro[35S]thionate binding depends on γ-aminobutyric acidA receptor α subunit. Mol Pharmacol. 1993;44:87–92. [PubMed] [Google Scholar]
- 19.Korpi ER, Uusi-Oukari M, Kaivola J. Postnatal development of diazepam-insensitive [3H]Ro 15-4513 binding sites. Neuroscience. 1993;53:483–488. doi: 10.1016/0306-4522(93)90212-x. [DOI] [PubMed] [Google Scholar]
- 20.Korpi ER, Kuner T, Seeburg PH, Lüddens H. Selective antagonist for the cerebellar granule cell-specific γ-aminobutyric acid type A receptor. Mol Pharmacol. 1995;47:283–289. [PubMed] [Google Scholar]
- 21.Kreienkamp H-J, Maeda RK, Sine SM, Taylor P. Intersubunit contacts governing assembly of the mammalian nicotinic acetylcholine receptor. Neuron. 1995;14:635–644. doi: 10.1016/0896-6273(95)90320-8. [DOI] [PubMed] [Google Scholar]
- 22.Krishek BJ, Amato A, Connolly CN, Moss SJ, Smart TG. Proton sensitivity of the GABAA receptor is associated with the receptor subunit composition. J Physiol (Lond) 1996;492:431–443. doi: 10.1113/jphysiol.1996.sp021319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuhse J, Laube B, Magalei D, Betz H. Assembly of the inhibitory glycine receptor: identification of amino acid sequence motifs governing subunit stoichiometry. Neuron. 1993;11:1049–1056. doi: 10.1016/0896-6273(93)90218-g. [DOI] [PubMed] [Google Scholar]
- 24.Laurie DJ, Seeburg PH, Wisden W. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci. 1992;12:1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lüddens H, Pritchett DB, Köhler M, Killisch I, Keinänen K, Monyer H, Sprengel R, Seeburg PH. Cerebellar GABAA receptor selective for behavioural alcohol antagonist. Nature. 1990;346:648–651. doi: 10.1038/346648a0. [DOI] [PubMed] [Google Scholar]
- 26.Malminiemi O, Korpi ER. Diazepam-insensitive [3H]Ro 15-4513 binding in intact cultured cerebellar granule cells. Eur J Pharmacol. 1989;169:53–60. doi: 10.1016/0014-2999(89)90816-9. [DOI] [PubMed] [Google Scholar]
- 27.Mansour SL, Thomas KR, Capecchi MR. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988;336:348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- 28.McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- 29.Mertens S, Benke D, Möhler H. GABAA receptor populations with novel subunit combinations and drug-binding profiles identified in brain by α5- and δ-subunit-specific immunoprecipitation. J Biol Chem. 1993;268:5965–5973. [PubMed] [Google Scholar]
- 30.Mody I, De Koninck Y, Otis TS, Soltez I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994;17:517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 31.Mossier B, Tögel M, Fuchs K, Sieghart W. Immunoaffinity purification of γ-aminobutyric acidA (GABAA) receptors containing γ1-subunits: evidence for the presence of a single type of γ-subunit in GABAA receptors. J Biol Chem. 1994;269:25777–25782. [PubMed] [Google Scholar]
- 32.Mountford P, Zevnik B, Düwel A, Nichols J, Li M, Dani C, Robertson M, Chambers I, Smith A. Discistronic targeting constructs: reporters and modifiers of mammalian gene expression. Proc Natl Acad Sci USA. 1994;91:4303–4307. doi: 10.1073/pnas.91.10.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nehls M, Kyewski B, Messerle M, Waldschütz R, Schüddekopf K, Smith AJH, Boehm T. Two genetically separable steps in the differentiation of thymic epithelium. Science. 1996;272:886–889. doi: 10.1126/science.272.5263.886. [DOI] [PubMed] [Google Scholar]
- 34.Nusser Z, Roberts JDB, Baude A, Richards JG, Somogyi P. Relative densities of synaptic and extrasynaptic GABAA receptors on cerebellar granule cells as determined by a quantitative immunogold method. J Neurosci. 1995;15:2948–2960. doi: 10.1523/JNEUROSCI.15-04-02948.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nusser Z, Sieghart W, Stephenson FA, Somogyi P. The α6 subunit of the GABAA receptor is concentrated in both inhibitory and excitatory synapses on cerebellar granule cells. J Neurosci. 1996;16:103–114. doi: 10.1523/JNEUROSCI.16-01-00103.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olsen RW, McCabe RT, Wamsley JK. GABAA receptor subtypes: autoradiographic comparison of GABA, benzodiazepine, and convulsant binding sites in the rat central nervous system. J Chem Neuroanat. 1990;3:59–76. [PubMed] [Google Scholar]
- 37.Ortells MO, Lunt GG. Evolutionary history of the ligand-gated ion-channel superfamily of receptors. Trends Neurosci. 1995;18:121–127. doi: 10.1016/0166-2236(95)93887-4. [DOI] [PubMed] [Google Scholar]
- 38.Palacios JM, Young WS, Kuhar MJ. Autoradiographic localization of γ-aminobutyric acid (GABA) receptors in rat cerebellum. Proc Natl Acad Sci USA. 1980;77:670–679. doi: 10.1073/pnas.77.1.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Persohn E, Malherbe P, Richards JG. Comparative molecular neuroanatomy of cloned GABAA receptor subunits in the rat CNS. J Comp Neurol. 1992;326:193–216. doi: 10.1002/cne.903260204. [DOI] [PubMed] [Google Scholar]
- 40.Pollard S, Thompson CL, Stephenson FA. Quantitative characterization of α6 and α1α6 subunit-containing native γ-aminobutyric acidA receptors of adult rat cerebellum demonstrates two α subunits per receptor oligomer. J Biol Chem. 1995;270:21285–21290. doi: 10.1074/jbc.270.36.21285. [DOI] [PubMed] [Google Scholar]
- 41.Quirk K, Gillard NP, Ragan CI, Whiting PJ, McKernan RM. Model of subunit composition of γ-aminobutyric acid A receptor subtypes expressed in rat cerebellum with respect to their α and γ/δ subunits. J Biol Chem. 1994;269:16020–16028. [PubMed] [Google Scholar]
- 42.Quirk K, Whiting PJ, Ragan CI, McKernan RM. Characterization of δ-containing GABAA receptors from the rat brain. Eur J Pharmacol. 1995;290:175–181. doi: 10.1016/0922-4106(95)00061-5. [DOI] [PubMed] [Google Scholar]
- 43.Randall AD, Tsien RW. Pharmacological dissection of multiple classes of Ca2+ channel currents in rat cerebellar granule cells. J Neurosci. 1995;15:2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raymond JL, Lisberger SG, Mauk MD. The cerebellum: a neuronal learning machine? Science. 1996;272:1126–1130. doi: 10.1126/science.272.5265.1126. [DOI] [PubMed] [Google Scholar]
- 45.Santi MR, Vicini S, Eldadah B, Neale JH. Analysis by polymerase chain reaction of α1 and α6 GABAA receptor subunit mRNAs in individual cerebellar neurons after whole-cell recordings. J Neurochem. 1994;63:2357–2360. doi: 10.1046/j.1471-4159.1994.63062357.x. [DOI] [PubMed] [Google Scholar]
- 46.Saxena NC, Macdonald RL. Assembly of GABAA receptor subunits: role of the δ subunit. J Neurosci. 1994;14:7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saxena NC, Macdonald RL. Properties of putative cerebellar γ-aminobutyric acidA receptor isoforms. Mol Pharmacol. 1996;49:567–579. [PubMed] [Google Scholar]
- 48.Schmitz E, Reichelt R, Walkowiak W, Richards JG, Hebebrand J. A comparative phylogenetic study of the distribution of cerebellar GABAA/benzodiazepine receptors using radioligands and monoclonal antibodies. Brain Res. 1988;473:314–320. doi: 10.1016/0006-8993(88)90860-8. [DOI] [PubMed] [Google Scholar]
- 49.Seeburg PH, Wisden W, Verdoorn TA, Pritchett DB, Werner P, Herb A, Lüddens H, Sprengel R, Sakmann B. The GABAA receptor family: molecular and functional diversity. Cold Spring Harb Symp Quant Biol. 1990;55:29–40. doi: 10.1101/sqb.1990.055.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Shivers BD, Killisch I, Sprengel R, Sontheimer H, Köhler M, Schofield PR, Seeburg PH. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron. 1989;3:327–337. doi: 10.1016/0896-6273(89)90257-2. [DOI] [PubMed] [Google Scholar]
- 51.Sieghart W. Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 52.Sieghart W, Eichinger A, Richards JG, Möhler H. Photoaffinity labelling of benzodiazepine receptor proteins with the partial inverse agonist [3H]Ro 15-4513: a biochemical and autoradiographic study. J Neurochem. 1987;48:46–52. doi: 10.1111/j.1471-4159.1987.tb13125.x. [DOI] [PubMed] [Google Scholar]
- 53.Smith AJH, De Sousa MA, Kwabi-Addo B, Heppell-Parton A, Impey H, Rabbitts P. A site-directed chromosomal translocation induced in embryonic stem cells by Cre-loxP recombination. Nature Genet. 1995;9:376–385. doi: 10.1038/ng0495-376. [DOI] [PubMed] [Google Scholar]
- 54.Somogyi P, Takagi H, Richards JG, Möhler H. Subcellular localization of benzodiazepine/GABAA receptors in the cerebellum of rat, cat, and monkey using monoclonal antibodies. J Neurosci. 1989;9:2197–2209. doi: 10.1523/JNEUROSCI.09-06-02197.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Somogyi P, Fritschy J-M, Benke D, Roberts JDB, Sieghart W (1996) The γ2 subunit of the GABAA receptor is concentrated in synaptic junctions containing the α1 and β2/3 subunits in hippocampus, cerebellum and globus pallidus. Neuropharmacology, in press. [DOI] [PubMed]
- 56.Stephenson FA. The GABAA receptors. Biochem J. 1995;310:1–9. doi: 10.1042/bj3100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sumikawa K, Nishizaki T. The amino acid residues 1–128 in the α subunit of the nicotinic acetylcholine receptor contain assembly signals. Mol Brain Res. 1994;25:257–264. doi: 10.1016/0169-328x(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 58.Thompson CL, Bodewitz G, Stephenson FA, Turner JD. Mapping of GABAA receptor α5 and α6 subunit-like immunoreactivity in rat brain. Neurosci Lett. 1992;144:53–56. doi: 10.1016/0304-3940(92)90714-i. [DOI] [PubMed] [Google Scholar]
- 59.Tia S, Wang JF, Kotchabhakdi N, Vicini S. Developmental changes of inhibitory synaptic currents in cerebellar granule neurons: role of GABAA receptor α6 subunit. J Neurosci. 1996;16:3630–3640. doi: 10.1523/JNEUROSCI.16-11-03630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tögel M, Mossier B, Fuchs K, Sieghart W. γ-Aminobutyric acidA receptors displaying association of γ3-subunits with β2/3 and different α-subunits exhibit unique pharmacological properties. J Biol Chem. 1994;269:12993–12998. [PubMed] [Google Scholar]
- 61.Turner DM, Sapp DW, Olsen RW. The benzodiazepine/alcohol antagonist Ro 15-4513: binding to a GABAA receptor subtype that is insensitive to diazepam. J Pharmacol Exp Ther. 1991;257:1236–1242. [PubMed] [Google Scholar]
- 62.Tyndale RF, Olsen RW, Tobin AJ. GABAA receptors. In: North RA, editor. Handbook of receptors and channels: ligand- and voltage-gated ion channels. CRC; Boca Raton, FL: 1995. pp. 265–290. [Google Scholar]
- 63.Varecka L, Wu C-H, Rotter A, Frostholm A. GABAA/benzodiazepine receptor α6 subunit mRNA in granule cells of the cerebellar cortex and cochlear nuclei: expression in developing and mutant mice. J Comp Neurol. 1994;339:341–352. doi: 10.1002/cne.903390304. [DOI] [PubMed] [Google Scholar]
- 64.Vernallis AB, Conroy WG, Berg DG. Neurons assemble acetylcholine receptors with as many as three kinds of subunits while maintaining subunit segregation among receptor subtypes. Neuron. 1993;10:451–464. doi: 10.1016/0896-6273(93)90333-m. [DOI] [PubMed] [Google Scholar]
- 65.Verrall S, Hall ZW. The N-terminal domains of acetylcholine receptor subunits contain recognition signals for the initial steps of receptor assembly. Cell. 1992;68:23–31. doi: 10.1016/0092-8674(92)90203-o. [DOI] [PubMed] [Google Scholar]
- 66.Wisden W, Morris BJ. In situ hybridization with synthetic oligonucleotide probes. In: Wisden W, Morris BJ, editors. In situ hybridization protocols for the brain. Academic; London: 1994. pp. 9–34. [Google Scholar]
- 67.Wisden W, Herb A, Wieland H, Keinänen K, Lüddens H, Seeburg PH. Cloning, pharmacological characteristics and expression pattern of the rat GABAA receptor α4 subunit. FEBS Lett. 1991;289:227–230. doi: 10.1016/0014-5793(91)81076-k. [DOI] [PubMed] [Google Scholar]
- 68.Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wisden W, Korpi ER, Bahn S. The cerebellum: a model system for studying GABAA receptor diversity. Neuropharmacology. 1996;35:1139–1160. doi: 10.1016/s0028-3908(96)00076-7. [DOI] [PubMed] [Google Scholar]
- 70.Wong G, Sarviharju M, Toropainen M, Matecka D, Korpi ER. Pharmacological actions of subtype-selective and novel GABAergic ligands in rat lines with differential sensitivity to ethanol. Pharmacol Biochem Behav. 1996;53:723–730. doi: 10.1016/0091-3057(95)02076-4. [DOI] [PubMed] [Google Scholar]
- 71.Zezula J, Sieghart W. Isolation of type I and type II GABAA-benzodiazepine receptors by immunoaffinity chromatography. FEBS Lett. 1991;284:15–18. doi: 10.1016/0014-5793(91)80750-w. [DOI] [PubMed] [Google Scholar]