Abstract
Background:
Due to advances in high-frequency ultrasound technology, it is easier to detect fine structures of skin lesions. The aim of this study was to examine the ultrasonographic features and use recurrence risk stratification to assess the diagnostic performance of pre-operative ultrasound examination of basal cell carcinoma (BCC).
Methods:
This was a retrospective study. Forty-six BCC lesions underwent pre-operative ultrasound examination using 50- and 20-MHz probes. Ultrasonographic shape, margin, internal echoes, hyper-echoic spots, posterior echoes, and depth of the lesion were evaluated and correlated with the risk of recurrence based on histological features.
Results:
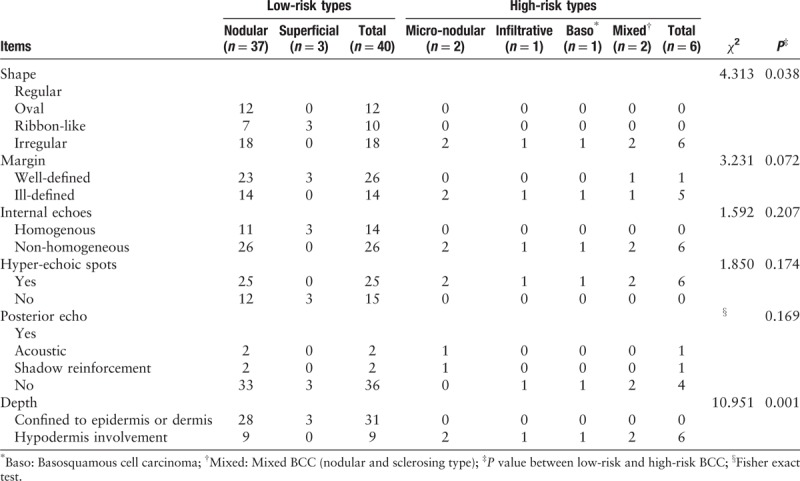
Forty-two patients had 46 skin lesions in total. The high-risk (n = 6) and low-risk (n = 40) groups exhibited considerable overlap in the ultrasonographic manifestations and no significant difference in margin (χ2 = 3.231, P = 0.072), internal echo (χ2 = 1.592, P = 0.207), or posterior echo (P = 0.169). However, high-risk BCCs tended to be irregular in shape than low-risk lesions (χ2 = 4.313, P = 0.038). Both types presented hyper-echoic spots (χ2 = 1.850, P = 0.174). Additionally, 78% of low-risk lesions were confined to the dermis (31/40), and 100% of high-risk lesions infiltrated into the sub-cutaneous tissue, resulting in a significant difference between the two groups (χ2 = 10.951, P = 0.001). Ultrasound detected sub-clinical lesions in five patients.
Conclusions:
High-frequency ultrasound can provide important information for pre-operative evaluation of risk in BCC foci and reveal hidden lesions. The technique may play a crucial role in guiding therapeutic options for BCC.
Keywords: High-frequency ultrasound, Basal cell carcinoma, Ultrasound, Skin ultrasound, Skin cancer ultrasound, Skin cancer
Introduction
Basal cell carcinoma (BCC) is a common cutaneous tumor originating from keratinocytes in the basal layer of the epidermis. It is the most common cutaneous malignancy and one of the most common cancers in humans.[1,2] BCC has characteristic clinical and dermatoscopic features, and its diagnosis depends on histopathological examination. Due to the rapid advancement of high-frequency ultrasound in recent years, fine structures of skin lesions can now be revealed. High-frequency ultrasound is used to measure the extent of BCC lesions, including their depth, which can guide therapeutic decisions.[3–5] The purpose of this study was to assess the ultrasonographic features of BCC and employ the stratification of pathological recurrence risk to evaluate the diagnostic merit of pre-operative ultrasound in BCC diagnosis.
Methods
Ethical approval
All the procedures involving humans were carried out in accordance with the ethical standards of the 2013 Declaration of Helsinki and were approved by the Medical Ethics Committee of Peking Union Medical College Hospital (No. S-K668). Informed written consent was obtained from all the patients.
Patients
We reviewed ultrasound database records for the period from June 2017 to December 2018 in the Department of Dermatology, Peking Union Medical College Hospital, which consisted of a total of 1154 ultrasound examinations of the skin. We then consecutively evaluated all ultrasound tests that had been performed before surgery in patients with a histologic diagnosis of BCC (n = 51). The cases were further selected according to the following inclusion criteria: histological confirmation of BCC performed by two or more dermatopathologists at the rank of attending physician or above; lesions were not treated by drugs or surgery within 1 month before admission. An MD300S II skin ultrasonic diagnosis system (MEDA Co., Ltd., Tianjin, China) was used to collect high-frequency skin ultrasound images with 50- and 20-MHz ultrasonic probes.
Evaluation of skin high-frequency ultrasound images
A retrospective review of BCC cases with ultrasound images was conducted. Two dermatologists trained in skin imaging and blinded to the patients’ histopathological results independently evaluated the ultrasonic features of the lesions, including the shape, margin, internal echo, hyper-echoic spots, posterior echo, and depth of lesion. If the results were different between the physicians, agreement was reached through consultation of the two dermatologists.
Histological analysis
The pathological diagnosis was obtained through surgical excision or punch biopsy. The resected specimens were immediately fixed with 10% formaldehyde solution and stained with hematoxylin and eosin. The lesions were divided into high-risk and low-risk of recurrence histological sub-types according to the National Comprehensive Cancer Network (NCCN) guidelines for clinical practice for cutaneous BCC.[2] Lesions with morpheaform, basosquamous (metatypical), sclerosing, mixed infiltrative, or micro-nodular features in any portion of the tumor were considered to have a high-risk of recurrence; in contrast, the low-risk histologic sub-type included nodular, superficial, and other non-aggressive growth patterns such as keratotic, infundibulocystic, and fibroepithelioma of Pinkus.
Statistical analysis
The mean age of patients within the high-risk and low-risk of recurrence groups was compared by a t test. Differences in ultrasonic characteristics between the two groups and between genders were analyzed by a Chi-square test. Two-sides P-values of <0.05 were considered significant. All statistical analyses were performed using SPSS statistics software 21.0 (IBM Corporation, Armonk, NY, USA).
Results
General information
In this study, 46 lesions from 42 patients were analyzed. The 42 patients included 18 males (43%) and 24 females (57%) with a median age of 61.4 years (31.0–86.0 years). The general information of patients with low-risk and high-risk BCC is shown in Table 1. The two groups displayed no significant difference in gender composition (χ2 = 0.004, P > 0.05) and the mean age (t = 1.116, P > 0.05). All of the lesions, except for one in the lower extremity and three in the trunk, were located on the face or the scalp. According to the classification criteria issued by the NCCN in 2016, the 46 skin lesions comprised 37 (80%) nodular lesions, 3 (7%) superficial lesion, 2 (4%) micro-nodular lesions, 1 (2%) infiltrative lesion, 1 (2%) basal squamous cell carcinoma lesion, and 2 (4%) mixed lesions (nodular and sclerosing mixed type, nodular and micro-nodular mixed type).
Table 1.
General information of low-risk and high-risk basal cell carcinoma.

BCC ultrasonographic features
Thirty-seven nodular BCC lesions were diagnosed histologically from 33 patients (one patient had four lesions, and another patient had two lesions). All skin lesions manifested as hypo-echoic nodules present in the skin or sub-cutaneous tissue. The ultrasonographic characteristics of the nodular BCCs [Figure 1] were detailed as follows: (i) shape: 18 lesions (49%) had an irregular shape, 12 lesions were oval nodules (32%) with a regular shape, and seven were ribbon-like lesions (19%); (ii) margin: 23 skin lesions (62%) had well-defined margins, and 14 lesions (38%) had ill-defined margins; (iii) internal echoes: 11 lesions (30%) had a homogenous internal echo, and 26 lesions (70%) had a non-homogeneous internal echo (including five harboring an internal anechoic zone); (iv) hyper-echoic spots were presented in 25 lesions (68%), and the mean hyper-echoic spot count in per lesion was 3.96 (range: 1–9); and (v) posterior echo: 33 lesions (89%) displayed no obvious posterior echo changes, two lesions (5%) showed a posterior acoustic shadowing artifact, and two lesions (5%) exhibited a posterior reinforcement artifact.
Figure 1.
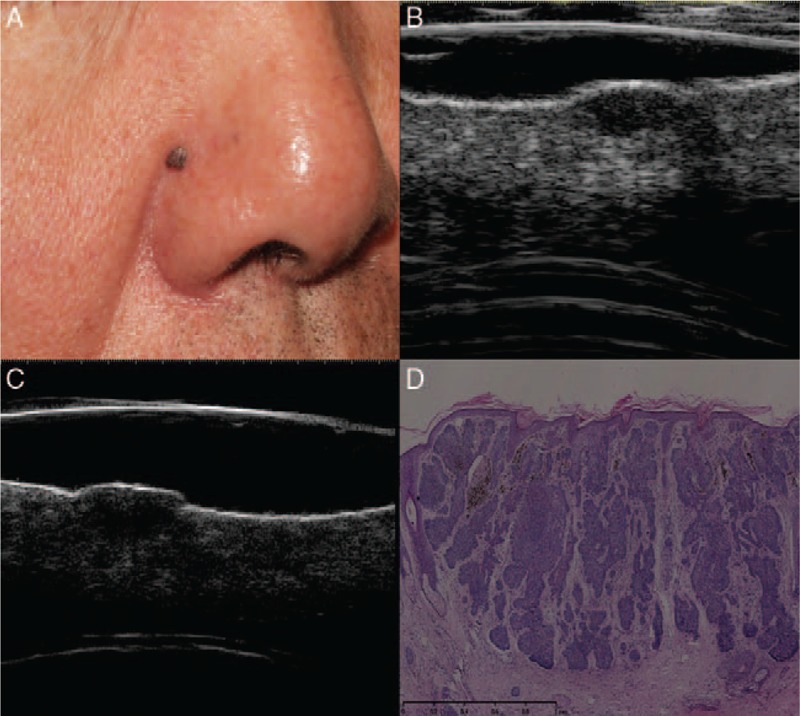
Nodular basal cell carcinoma. Clinical (A), ultrasonographic (B, C), and histologic (D) images of a nodular basal cell carcinoma lesion (low-risk type). (A) A pigmented nodule in the right nasolabial fold. Both 20 (B) and 50-MHz (C) ultrasound probes revealed a well-defined, oval-shaped, homogeneous, hypo-echoic dermal lesion with clear margins and a lack of hyper-echoic spots. (D) The histology results indicated a nodular dermal BCC (hematoxylin-eosin staining, original magnification ×200).
Superficial BCC (n = 3): three patients had lesions separately located on the trunk, face, and scalp that all presented on ultrasound as well-defined, with a homogeneous hypo-echoic ribbon-like zone, and without internal echoes and posterior acoustic artifacts. No hyper-echoic spots were detected [Figure 2].
Figure 2.
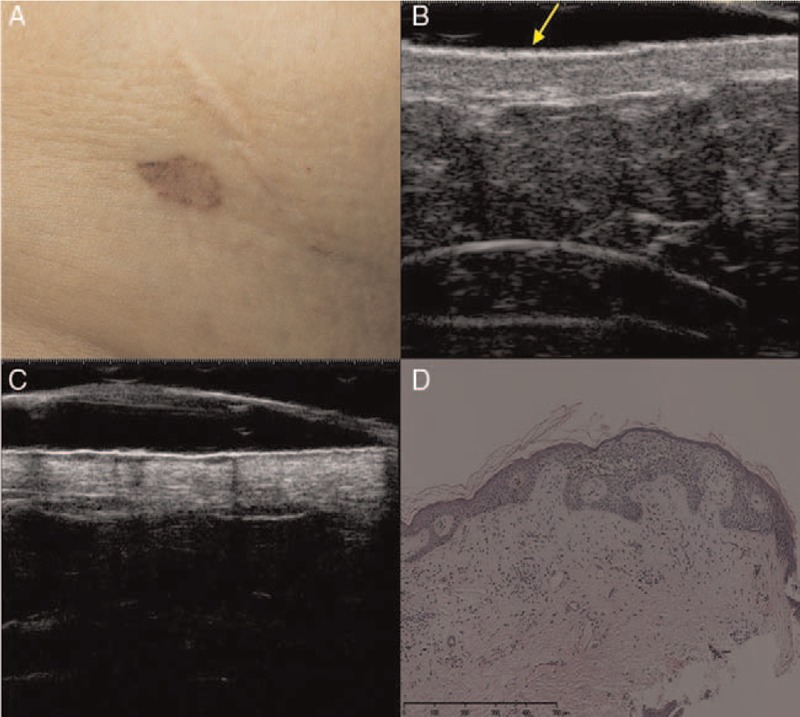
Superficial basal cell carcinoma. Clinical (A), ultrasonographic (B, C), and histological (D) images of superficial basal cell carcinoma (low-risk sub-type). (A) The clinical image shows an irregularly pigmented patch on the right abdomen. (B) A 20-MHz ultrasound examination revealed an ill-defined epidermal and upper dermal ribbon-like thickening with a slightly hypo-echoic upper dermis (arrow). (C) A 50-MHz ultrasound examination showed a well-defined, epidermal and upper dermal thickening with epidermal undulation and a homogeneous hypo-echoic ribbon-like upper dermal zone. No signs of hyper-echoic spots internal or posterior echoes were detected. (D) The lesion was confirmed histopathologically to be superficial basal cell carcinoma (hematoxylin-eosin staining, original magnification ×200).
Micro-nodular BCC (n = 2): a 74-year-old man and a 69-year-old woman both had lesions located on the tip of the nose. The lesions were characterized by ill-defined, hypo-echoic dermal nodules with internal echoes; one lesion had small anechoic cystic areas and a posterior acoustic reinforcement, and the other lesion exhibited a posterior reinforcement artifact. Both of them detected hyper-echoic spots.
Infiltrative BCC (n = 1): a 79-year-old woman had a lesion located on her nose that displayed ill-defined, irregular, hypo-echoic dermal and hypo-dermal nodules, a large number of internal hyper-echoic spots (n = 15), and no posterior acoustic artifact.
Basosquamous cell carcinoma (n = 1): a 59-year-old woman had a lesion located at the tip of the nose that exhibited ill-defined hypo-echoic dermal and hypo-dermal nodules, multiple internal hyper-echoic spots (n = 3), several internal anechoic areas, and no signs of a posterior acoustic artifact [Figure 3].
Figure 3.
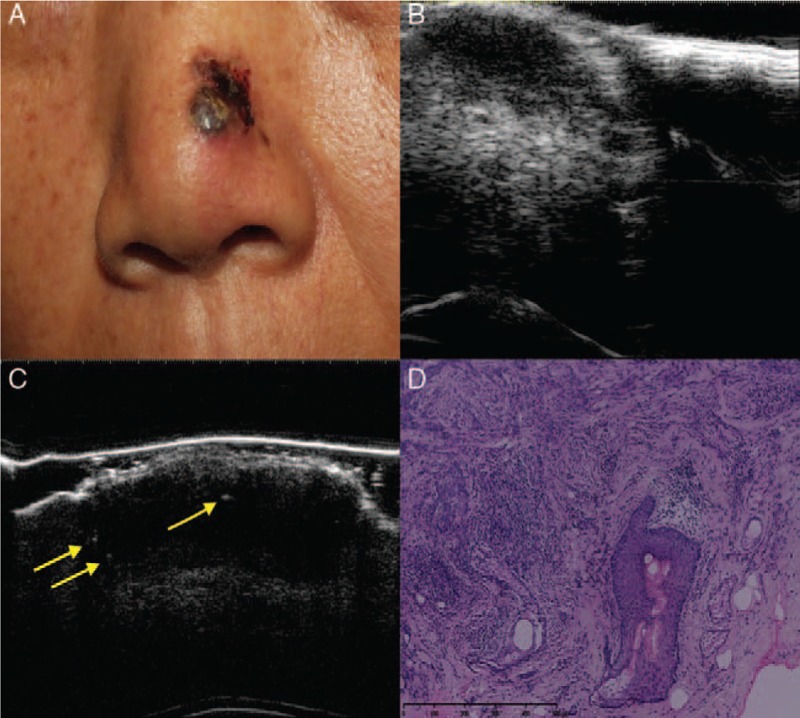
Basosquamous cell carcinoma. Clinical (A), ultrasonographic (B, C), and histologic (D) images of a basosquamous carcinoma lesion (high-risk sub-type). (A) The clinical image shows an ulcerated, pigmented, and nodular lesion on the left wing of the nose. (B) A 20-MHz ultrasound examination showed an ill-defined, irregular, hypo-echoic lesion. (C) A 50-MHz ultrasound examination showed epidermal and dermal thickening with epidermal irregularities and a hypo-echoic dermal lesion that presented three hyper-echoic spots (arrows). (D) The histology results indicated basosquamous cell carcinoma (hematoxylin-eosin staining, original magnification ×200).
Mixed BCC (nodular and sclerosing type, nodular and micro-nodular type; n = 2): a 63-year-old with a lesion located on the nose and a 58-year-old man with a lesion on the cheek presented lesions with irregular shape, ill-defined or well-defined borders, hypo-echoic dermal and hypo-dermal non-homogeneous nodules with internal echoes, multiple internal hyper-echoic spots (n = 12 and 4), and no changes in posterior echo.
Ultrasonic manifestations of BCC with high- and low-recurrence risks
The ultrasonographic features of BCC with different recurrence risk levels are described in Table 2. The two groups displayed no significant difference in ultrasonographic manifestations regarding the margin (χ2 = 3.231, P = 0.072), internal echo (χ2 = 1.592, P = 0.207), hyper-echoic spots (χ2 = 1.850, P = 0.174), or posterior echo (P = 0.169). However, high-risk BCC tended to be irregular in shape than low-risk lesions (χ2 = 4.313, P = 0.038).
Table 2.
Ultrasonic manifestations of BCC with a low- and high-recurrence risks.
Based on their depth in ultrasonographic images, the lesions extended from the epidermis to the dermis and sub-cutaneous tissue. All six high-risk BCC lesions penetrated into the sub-cutaneous tissue; among the 40 low-risk lesions, 78% were confined within the epidermis and dermis (31/40). The two groups exhibited a significant difference in lesion depth (χ2 = 10.951, P = 0.001).
Ultrasound detection of sub-clinical BCC lesions
In five patients in the study, in addition to skin lesions that were easily observed in the clinic, ultrasound detected small invisible lesions. One lesion was in a patient who had a micro-nodular BCC lesion and a sub-clinical lesion isolated from the main lesion [Figure 4]. In the other four patients, the lesions in the sub-cutaneous tissue were contiguous with the main lesion (three nodular lesions and one mixed nodular and sclerosing lesion).
Figure 4.
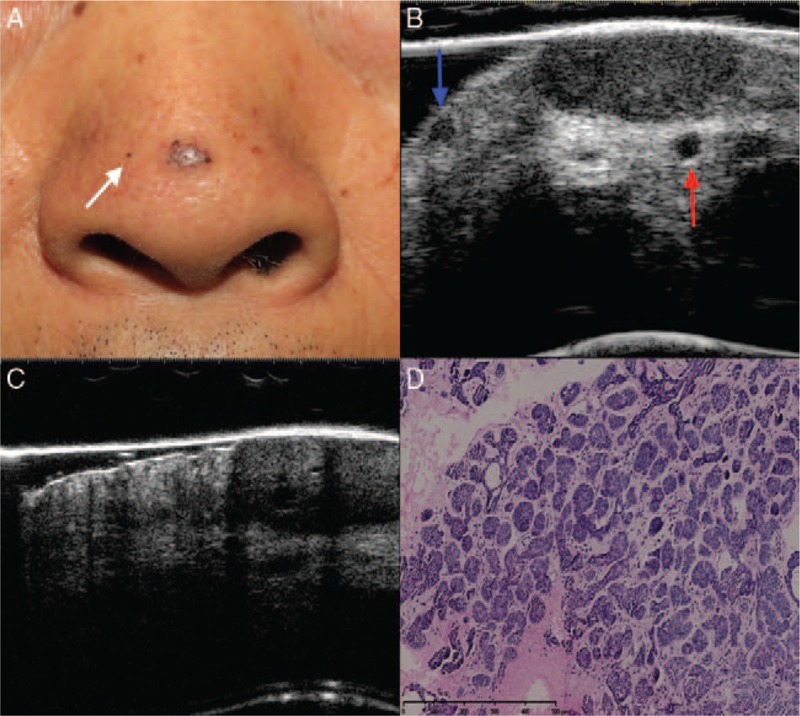
Micro-nodular basal cell carcinoma. Clinical (A), ultrasonographic (B, C), and histologic (D) images of a sub-clinical basal cell carcinoma. (A) A clinical image shows a pigmented papule at the tip of the nose with telangiectasias and a small hyper-pigmented spot (white arrow) on the right wing of the nose. (B) A 20-MHz ultrasound examination shows the main lesion and an adjacent small hypo-echoic dermal foci (blue arrow), which corresponds to the isolated hyper-pigmented lesion of the patient shown in the clinical image. The main lesion was mainly located in the dermis and slightly protruded into the sub-cutaneous tissue. An adjacent small blood vessel (red arrow) was detected underneath the lesion (cross-sectional view of the vessel). (C) An image obtained in the 50-MHz ultrasound examination shows that the lesion manifested a blurred local boundary, exhibited involvement with the sub-cutaneous tissue. (D) The histology results indicated micro-nodular basal cell carcinoma (hematoxylin-eosin staining, original magnification ×200).
Discussion
BCC is the most common cutaneous malignant tumor; it is more common in elderly individuals and is prone to occur in sun-irradiated skin regions, such as the head and face. The etiology of BCC remains unclear, but it is generally believed that the disease is related to sunlight exposure, ionizing radiation, and immunosuppression therapy. BCC generally features a low degree of malignancy, slow growth, and a good prognosis, but it can exhibit local invasiveness and cause damage to the surrounding skin tissue structure. Early diagnosis and radical removal of lesions can improve the prognosis.
BCC is generally confirmed by histopathological examination. Due to advances in high-frequency ultrasound technology, the fine structure of skin lesions can be better revealed, and the diagnostic accuracy is increased.[6–13] In this study, high-frequency ultrasound imaging was performed on 46 BCC lesions in 42 patients, and the ultrasonographic manifestations of various histopathological types and different recurrence risk levels of BCC were analyzed and compared. Our results revealed that lesions of various pathological types and the two risk levels showed tremendous overlap in ultrasonographic manifestations. This may be related to the low number of high-risk cases included in this study because high-risk BCC is rare in the Chinese population. The depth of lesions displayed by ultrasound was helpful for the differential diagnosis of lesions of different risk levels. In this group of patients, all high-risk BCC lesions involved the sub-cutaneous tissue, while 78% of low-risk lesions were located in the dermis, resulting in a significant difference between the two groups ( χ2 = 10.951, P = 0.001). Lesions with the following traits were considered to have a low-risk of recurrence: regular shape, clear boundary, homogeneous internal echo, and confinement to the epidermis and the dermis. However, lesions with the following traits should be highly suspected of being high-risk: irregular shape, blurred boundary, and deep infiltration into the sub-cutaneous tissue. In other words, these signs indicated that the tumor was highly invasive and had a higher risk of recurrence. Thus, pre-operative ultrasound can be employed to reveal lesion traits and predict the risk of recurrence, providing important information for therapeutic decision making.
Hyper-echoic spots constitute an important ultrasonographic feature of BCC lesions.[11,14,16] In this study, 76% (31/46) of BCC lesions displayed hyper-echoic spots. The underlying mechanism of this feature is yet to be established, although it is speculated to be related to several factors of BCC lesions, such as keratosis, pigmentation, and calcification. In this study, the high-risk and low-risk groups exhibited no significant difference in the occurrence of hyper-echoic spots; 25/40 of the low-risk and all of the high-risk BCC lesions had hyper-echoic spots, which was not identical to Wortsman's results. Wortsman et al reported that hyper-echoic spots were detected in all BCCs and that the number of these hyper-echoic spots can predict histologic sub-types of BCC with a high- or low-risk of recurrence. A cut-off point of seven hyper-echoic spots was used to discriminate a low versus high-risk of recurrence.[14] The number of hyper-echoic spots in high-risk BCC in this study tended to be increased. In addition, a posterior echo was found in some of these hyper-echoic spots. Hence, close attention should be paid to the ultrasonographic trait of dense hyper-echoic spots when predicting risk. We attempted to correlate the presence of hyper-echoic spots with the histological features such as the tumor nests, corneum cysts, and calcifications. However, this was not successful because ultrasound and histopathological examinations could not be performed on identical vertical planes, and the part of the lesion imaged by these two examinations may be different.
High-frequency ultrasound can explicitly present the deep structure of lesions, and important information can be obtained for therapeutic decision making.[4–6,15] Ultrasound is often used to examine the infiltration depth of BCC, and the ultrasonography-measured depth of a tumor is consistent with the corresponding pathological results.[3,5,17] In this study, a 50-MHz probe was employed for the first time to reveal hypo-echoic lesions in the deep zone of BCC tumors that were or were not connected to the corresponding main lesions. These small, deep lesions may represent the satellite foci or deep infiltrates of the primary tumor and should be completely removed during surgical resection to reduce recurrence and improve the prognosis. Hence, our results show that high-frequency ultrasound can not only indicate the infiltration depth of the skin of BCC tumors but also reveal the invasiveness of the lesions and increase visibility of deep or small lesions that could be easily overlooked. As a consequence, high-frequency ultrasound can guide the choice of treatment methods. Additional studies are needed to determine the significance of this technique for the pre-operative diagnosis of BCC.
In summary, high-frequency ultrasound exhibits promising value for the pre-operative diagnosis of BCC. The technique can reveal some important traits of BCC in great detail, such as the morphology, boundary, internal echo, and hyper-echoic spots, thereby providing important information for determining the recurrence risk of BCC lesions before surgery. High-frequency ultrasound can also reveal hidden lesions and therefore play an important role in guiding therapeutic decision making for this disease.
Funding
This work was supported by a grant from the CAMS Innovation Fund for Medical Sciences (No. 2017-I2M-3-020).
Conflicts of interest
None.
Footnotes
How to cite this article: Wang SQ, Liu J, Zhu QL, Zhao CY, Qu T, Li F, Wortsman X, Jin HZ. High-frequency ultrasound features of basal cell carcinoma and its association with histological recurrence risk. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000369
References
- 1.Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med 2015; 88:167–179. [PMC free article] [PubMed] [Google Scholar]
- 2.Bichakjian CK, Olencki T, Aasi SZ, Alam M, Andersen JS, Berg D, et al. Basal cell skin cancer, version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016; 14:574–597. doi: 10.6004/jnccn.2016.0065. [DOI] [PubMed] [Google Scholar]
- 3.Barcaui Ede O, Carvalho AC, Valiante PM, Barcaui CB. High-frequency ultrasound associated with dermoscopy in pre-operative evaluation of basal cell carcinoma. An Bras Dermatol 2014; 89:828–831. doi: 10.1590/abd1806-4841.20143176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marmur ES, Berkowitz EZ, Fuchs BS, Singer GK, Yoo JY. Use of high-frequency, high-resolution ultrasound before Mohs surgery. Dermatol Surg 2010; 36:841–847. doi: 10.1111/j.1524-4725.2010.01558.x. [DOI] [PubMed] [Google Scholar]
- 5.Pasquali P, Freites-Martinez A, Fortuno-Mar A. Ex vivo high-frequency ultrasound: a novel proposal for management of surgical margins in patients with non-melanoma skin cancer. J Am Acad Dermatol 2016; 74:1278–1280. doi: 10.1016/j.jaad.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Crişan D, Badea AF, Crişan M, Rastian I, Gheuca Solovastru L, Badea R. Integrative analysis of cutaneous skin tumours using ultrasonogaphic criteria. Preliminary results. Med Ultrason 2014; 16:285–290. doi: 10.1111/j.1524-4725.2010.01558.x. [DOI] [PubMed] [Google Scholar]
- 7.Polańska A, Dańczakpazdrowska A, Silny W, Woźniak A, Maksin K, Jenerowicz D, et al. Comparison between high-frequency ultrasonography (Dermascan C, version 3) and histopathology in atopic dermatitis. Skin Res Tech 2013; 19:432–437. doi: 10.1111/srt.12064. [DOI] [PubMed] [Google Scholar]
- 8.Mandava A, Ravuri PR, Konathan R. High-resolution ultrasound imaging of cutaneous lesions. Indian J Radiol Imaging 2013; 23:269–277. doi: 10.4103/0971-3026.120272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Zawahry MB, Abdel El-Hameed El-Cheweikh HM, Abd-El-Rahman Ramadan S, Ahmed Bassiouny D, Mohamed Fawzy M. Ultrasound biomicroscopy in the diagnosis of skin diseases. Eur J Dermatol 2007; 17:469–475. doi: 10.1684/ejd.2007.0261. [DOI] [PubMed] [Google Scholar]
- 10.Wortsman X. Ultrasound in dermatology: why, how, and when? Semin Ultrasound CT MR 2013; 34:177–195. doi: 10.1053/j.sult.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Uhara H, Hayashi K, Koga H, Saida T. Multiple hypersonographic spots in basal cell carcinoma. Dermatol Surg 2007; 33:1215–1219. doi: 10.1111/j.1524-4725.2007.33256.x. [DOI] [PubMed] [Google Scholar]
- 12.Harland CC, Kale SG, Jackson P, Mortimer PS, Bamber JC. Differentiation of common benign pigmented skin lesions from melanoma by high-resolution ultrasound. Br J Dermatol 2000; 143:281–289. doi: 10.1046/j.1365-2133.2000.03652.x. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez-Ibanez C, Blazquez-Sanchez N, Aguilar-Bernier M, Fúnez-Liébana R, Rivas-Ruiz F, de Troya-Martín M. Usefulness of high-frequency ultrasound in the classification of histologic subtypes of primary basal cell carcinoma. Actas Dermosifiliogr 2017; 108:42–51. doi: 10.1016/j.ad.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Wortsman X, Vergara P, Castro A, Saavedra D, Bobadilla F, Sazunic I. Ultrasound as predictor of histologic subtypes linked to recurrence in basal cell carcinoma of the skin. J Eur Acad Dermatol Venereol 2015; 29:702–707. doi: 10.1111/jdv.12660. [DOI] [PubMed] [Google Scholar]
- 15.Mandava A, Konathan R, Neelala K. Utility of high-resolution ultrasonography and colour Doppler in the assessment of pigmented skin lesions. Ultrasound 2012; 20:155–160. doi: 10.1258/ult.2012.012013. [Google Scholar]
- 16.Wortsman X. Sonography of facial cutaneous basal cell carcinoma: a first-line imaging technique. J Ultrasound Med 2013; 32:567–572. doi: 10.7863/jum.2013.32.4.567. [DOI] [PubMed] [Google Scholar]
- 17.Bobadilla F, Wortsman X, Munoz C, Segovia L, Espinoza M, Jemec GB. Pre-surgical high resolution ultrasound of facial basal cell carcinoma: correlation with histology. Cancer Imaging 2008; 8:163–172. doi: 10.1102/1470-7330.2008.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]


