Abstract
Background:
With the publication of Sepsis-3 definition, epidemiological data based on Sepsis-3 definition from middle-income countries including China are scarce, which prohibits understanding of the disease burden of this newly defined syndrome in these settings. The purpose of this study was to describe incidence and outcome of Sepsis-3 in Yuetan sub-district of Beijing and to estimate the incidence rate of Sepsis-3 in China.
Methods:
The medical records of all adult residents hospitalized from July 1, 2012 to June 30, 2014 in Yuetan sub-district of Beijing were reviewed. Patients with sepsis-3 and severe sepsis/septic shock were identified. The incidence rates and mortality rate of sepsis-3 and sepsis/septic shock were calculated, incidence rates and in-hospital mortality rates were normalized to the population distribution in the 2010 National Census. Population incidence rate and case fatality rate between sexes were compared with the Z test, as the data conformed to Poisson distribution.
Results:
Of the 21,191 hospitalized patients, 935 patients were diagnosed with Sepsis-3, and 498 cases met severe sepsis/septic shock criteria. The crude annual incidence rate of Sepsis-3 in Yuetan sub-district was 363 cases per 100,000 population, corresponding to standardized incidence rates of 236 cases per 100,000 population per year, respectively. The overall case fatality rate of Sepsis-3 was 32.0%, the crude population mortality rates of Sepsis-3 was 116 cases per 100,000 population per year, the standardized mortality rate was 67 cases per 100,000 population per year, corresponding to a speculative extrapolation of 700,437 deaths in China. The incidence rate and mortality rate of Sepsis-3 were significantly higher in males, elderly people, and patients with more comorbidities. The 62.1% of patients with Sepsis-3 had community-acquired infections, compared with 75.3% of infected patients without Sepsis-3 (P < 0.001). The most common infection in patients with Sepsis-3 was lower respiratory tract infection. When compared with patients with Sepsis-3, patients diagnosed as severe sepsis/septic shock were more likely to have higher case fatality rate (53.4% vs. 32.0%, P < 0.001)
Conclusions:
This study found the standardized incidence rate of 236 cases per 100,000 person-year for Sepsis-3, which was more common in males and elderly population. This corresponded to about 2.5 million new cases of Sepsis-3 per year, resulting in more than 700,000 deaths in China.
Clinical trial registration:
NCT02285257, https://clinicaltrials.gov/ct2/show/record/NCT02285257.
Keywords: Sepsis-3, Severe sepsis, Incidence, Mortality
Introduction
Sepsis, defined as systemic inflammatory response syndrome (SIRS) induced by infection in 1991,[1] is the leading cause of death among critically ill patients in intensive care unit (ICU). Due to multiple factors such as aging population, progress in peri-operative care, advances in invasive diagnostic and therapeutic procedures, and widespread use of chemotherapy and immunosuppression therapy, the morbidity and mortality of sepsis have steadily increased worldwide.[2–4] A systematic review estimated the incidence rate of 437 cases per 100,000 person-years for sepsis in high-income countries/regions in the last decade, with case fatality rate of 17%.[5] A tentative extrapolation from these data suggested a global estimate of 31.5 million sepsis cases, with potentially 5.3 million deaths annually.[5]
After more than two decades of widespread use of the original sepsis definition in both clinical practice and research, it is now well understood that both pro- and anti-inflammatory responses are involved in the pathogenesis of sepsis.[6] Moreover, SIRS criteria are too sensitive and insufficiently specific to identify some severe infected patients.[7,8] In 2001, definitions of sepsis and septic shock were revised,[9] incorporating the concept and diagnostic criteria of organ damage. However, owing to its complexity, the revised sepsis definition had not been widely applied in clinical practice. In 2016, the Third International Consensus Definitions for Sepsis and Septic Shock Task Force redefined sepsis (Sepsis-3)[10] as a “life-threatening organ dysfunction caused by a dysregulated host response to infection.” Organ dysfunction was defined as an acute increase in total sequential (sepsis-related) organ failure assessment (SOFA) score ≥2 points consequent to the infection.
With the publication of Sepsis-3 definition, multiple studies have reported prevalence of Sepsis-3 among ICU patients, hospitalized patients, and community-dwelling adults.[11,12] However, epidemiological data based on Sepsis-3 definition from middle-income countries including China are scarce, which prohibits understanding of the disease burden of this newly defined syndrome in these settings.
Based on a retrospective study of all hospitalized adult citizens in a sub-district in Beijing during 2-year study period, we speculated the national incidence rate of sepsis as 461 cases per 100,000 person-year, with case fatality rate of 20.6%.[13] As the definition of sepsis changes, the population incidence of sepsis may also require an updated evaluation. In the current study, we estimated the incidence and mortality rates of sepsis in a secondary analysis of the above database using Sepsis-3 definition.
Methods
Ethical approval
This study was approved by the ethics committee of Peking Union Medical College Hospital and informed consent was waived. This study was registered at ClinicalTrials.gov, with registration number as NCT02285257.
Patients and study design
The methods of this study have been described in detail previously.[13] In brief, this study was carried out in Yuetan sub-district of Beijing, China from July 1, 2012 to June 30, 2014. All adults (≥18 years) hospitalized during the study period were identified with the use of the hospital discharge database of Beijing Public Health Information System. All available medical records of enrolled patients were manually reviewed independently by any two of three investigators with more than 5 years of working experience in ICU. Any disagreement was resolved by discussion among the three investigators, and then among the steering committee (XM, YA, and BD) if consensus could not be reached.
Retrieved data included demographic data; admission category (medical, elective surgery, or emergency surgery); comorbidities[14]; and hospital death. Derived from the above data, severity of underlying illness was assessed by McCabe and Jackson classification,[15] while chronic organ dysfunction or immunosuppression was defined based on the criteria in acute physiology and chronic health evaluation II score.[16] In addition, body mass index (BMI) was calculated based on the height and weight on hospital admission.
For patients with infection, we collected data about source of infection and relevant microbiological information, data about SOFA score[17] were also collected.
For the purpose of this study, infection was diagnosed based on clinical manifestations, laboratory tests, and radiographic findings, including microbiologically documented (with definite positive results of microbial culture of body fluids or blood) and clinically documented (with no definite positive culture results but with imaging or pathological evidence of clinical infection) infections.[18] Severe sepsis and septic shock were diagnosed according to the American College of Chest Physicians (ACCP)/Society of Critical Care Medicine (SCCM) consensus definition,[1] whereas Sepsis-3 was diagnosed according to the Third International Consensus Definitions for Sepsis and Septic Shock Task Force,[10] defined as life-threatening organ dysfunction caused by a dysregulated host response to infection, with infection-related organ dysfunction identified as an acute change in total SOFA score ≥2 points in patients with pre-existing organ dysfunction, or total SOFA score ≥2 points in patients not known to have pre-existing organ dysfunction. Patients who developed Sepsis-3 before hospital admission or during hospital stay were both categorized as patients with sepsis. Patients who were readmitted into hospitals during the same study period were regarded as new patients. Only the first incidence of sepsis was counted during this same period of hospitalization. For clinical outcome of sepsis patients, we reported both mortality rate (the number of patients who died from sepsis in the entire population) and case fatality rate (the proportion of patients who died from sepsis among all septic patients).
Statistical analysis
Continuous variables were presented as median (Q1, Q3), and compared using the Wilcoxon rank-sum test. Categorical variables were presented as a percentage of the group from which they were derived, and compared by the use of Chi-square test or Fisher exact test. Incidence rates and in-hospital mortality rates were normalized to the population distribution in the 2010 National Census.[19] The 95% confidence interval (CI) was calculated.[20] Population incidence rate and hospital case fatality rate between sexes were compared with the Z test, as the data conformed to Poisson distribution. All comparisons were unpaired and all tests of significance were two-tailed. A P < 0.05 was considered as statistically significant. All statistical analyses were performed using SPSS version 17.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Patient enrollment
During two years study period, a total of 22,552 adult residents in Yuetan sub-district were hospitalized. We were unable to review the medical records of 1361 patients, due to refusal by the hospital (n = 1084) and missing records (n = 277). Therefore, the medical records of 21,191 patients were manually reviewed and included in the final analysis.
Of the 21,191 adult patients enrolled in this study, median age was 66 (49, 78) years, 9431 patients (44.5%) were male, and median Charlson comorbidity index was 1 (0, 2).
Characteristics of patients with Sepsis-3
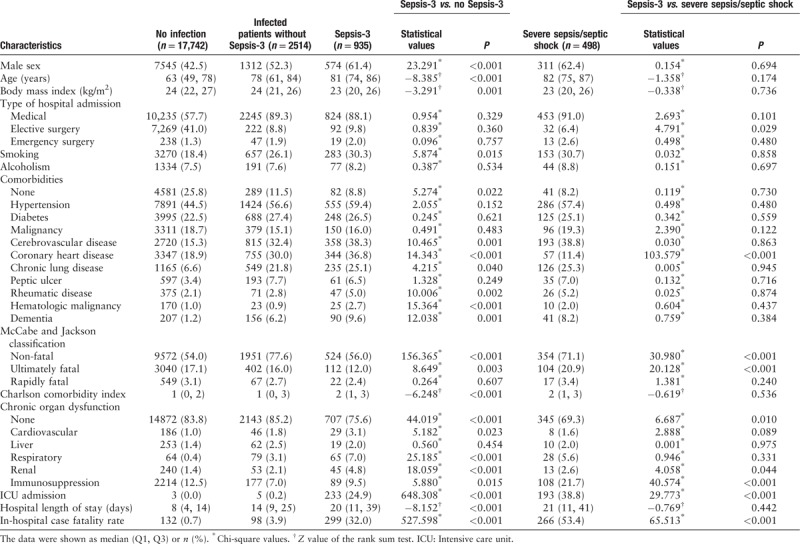
A total of 3449 patients with infection were identified, among whom 935 cases (27.1%) met Sepsis-3 criteria (median age: 81 [74, 86] years), and 498 cases (14.4%) met severe sepsis/septic shock criteria (median age: 82 [75, 87] years). When compared with patients suffering from non-septic infection, patients with Sepsis-3 were more likely to be male and older, and have more comorbidities (including cerebrovascular disease, coronary heart disease, chronic lung disease, rheumatic disease, hematologic malignancy, and dementia) and lower BMI [Table 1].
Table 1.
Characteristics of patients with non-infection, infected patients without Sepsis-3, and Sepsis-3 (N = 21,191).
The 62.1% of patients with Sepsis-3 had community-acquired infections, compared with 75.3% of infected patients without Sepsis-3 (P < 0.001). The most common infection in patients with Sepsis-3 was lower respiratory tract infection, followed by intra-abdominal infection and urogenital tract infection [Supplemental Tables 1 and 2]. A total of 433 bacteria were isolated from 314 patients with sepsis (33.6%), with Acinetobacter baumannii as the most common pathogen, followed by Pseudomonas aeruginosa, Klebsiella pneumoniae, methicillin-resistant Staphylococcus aureus, and Escherichia coli [Supplemental Table 2].
Patients with Sepsis-3 had worse clinical outcome than infected patients without Sepsis-3, as suggested by higher hospital case fatality rate (32.0% vs. 3.9%, P < 0.001) and longer hospital length of stay (20 [11, 39] days vs. 14 [9, 25] days, P < 0.001).
When compared with patients with Sepsis-3, patients diagnosed as severe sepsis/septic shock based on ACCP/SCCM consensus definition were more likely to have fewer elective surgeries (6.4% vs. 9.8%, P = 0.029), more immunosuppression (21.7% vs. 9.5%, P < 0.001), ultimately fatal diseases (20.9% vs. 12.0%, P < 0.001), and ICU admissions (38.8% vs. 24.9%, P < 0.001), as well as higher case fatality rate (53.4% vs. 32.0%, P < 0.001) [Table 1].
Incidence and mortality rates of Sepsis-3
The crude annual incidence rate of Sepsis-3 was 363 cases per 100,000 population, corresponding to 4.4 (95% CI: 4.1–4.7) cases per 100 hospital admissions. After adjustment for age and sex, the standardized incidence rate was 236 cases per 100,000 population. Generalization of the above-standardized incidence rate to the whole country produced a national estimate of 2,487,949 new cases of Sepsis-3 per year [Table 2].
Table 2.
Crude and standardized incidence rates and mortality rates of Sepsis-3.
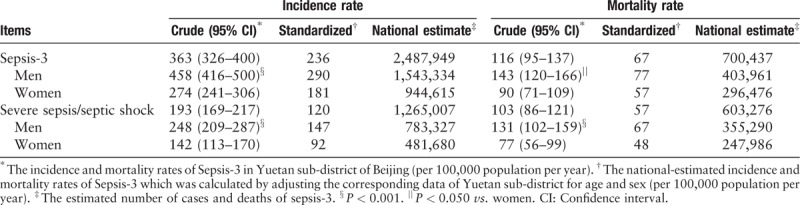
The crude incidence rate of Sepsis-3 exhibited significant sex difference, with 458 and 274 cases per 100,000 population per year in men and women, respectively [Figure 1 and Table 2]. The incidence rate of Sepsis-3 steadily increased with age, from 20 cases per 100,000 population <50 years to 895 cases per 100,000 population 50 to 89 years, and to 10,305 cases per 100,000 population ≥90 years [Figure 1]. In addition, the incidence rate of Sepsis-3 exhibited significant seasonal variation, being highest in winter (December, January, and February) and lowest in autumn (September, October, and November) [Supplemental Table 3].
Figure 1.
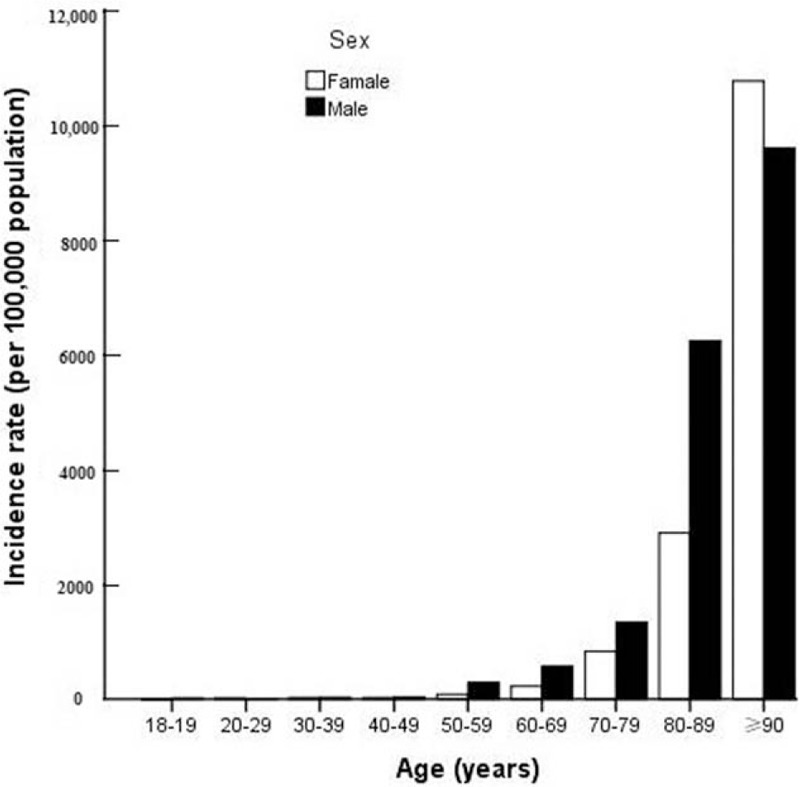
Age-specific incidence rate of Sepsis-3 by sex.
The crude population mortality rate of Sepsis-3 was 116 cases per 100,000 population per year, corresponding to a speculative extrapolation of 700,437 deaths in China. The mortality rate was significantly higher in men than women [Table 2], while case fatality rate showed no sex difference [Figure 2]. Case fatality rate for patients with Sepsis-3 significantly increased with age (from 11.1% in patients <50 years to 40.7% in those ≥90 years) [Figure 2].
Figure 2.
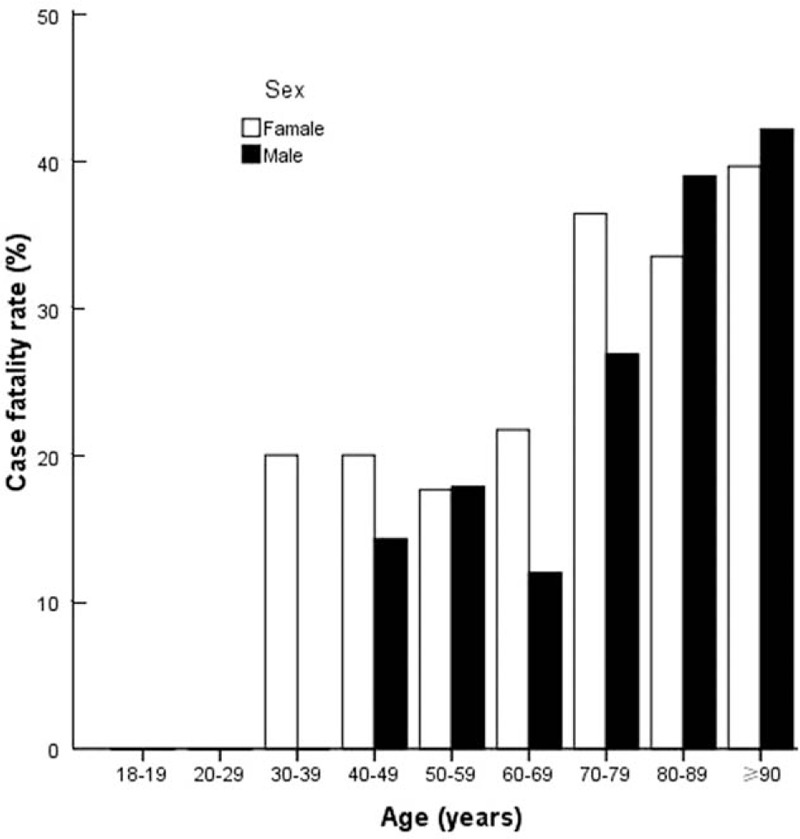
Age-specific fatality rate of Sepsis-3 by sex.
Discussion
In a secondary analysis of a database of 21,191 hospitalized patients who were residents in a sub-district of Beijing, we reported standardized incidence and mortality rate of Sepsis-3 as 236 and 67 cases per 100,000 population per year, respectively, corresponding to approximately 2.5 million new cases of Sepsis-3 and 700,437 deaths every year. Moreover, men had a significantly higher incidence and mortality rates of sepsis than women, despite a similar case fatality rate. In addition, patients meeting Sepsis-3 criteria were less severe than traditional severe sepsis/septic shock.
Clinical characteristics of septic patients in our cohort were consistent with previous studies.[12,21] For example, Donnelly et al[12] also reported that patients with Sepsis-3 were more likely to be male and older, to have more comorbidities, lower BMI, and higher case fatality rate. Likewise, lower respiratory tract infection had been consistently reported as the most common infections in sepsis, despite significant variation in the prevalence and ranking of other infections, such as urinary tract infection and intra-abdominal infection.[3,22–24] In addition, Gram-negative bacilli had been reported as the most common pathogens of sepsis,[2,23,25–27] which might be associated with higher case fatality rate than Gram-positive infections.[3]
There have been very few studies of population-based epidemiology of Sepsis-3, which reported much higher incidence rates than our study. Mellhammar et al[28] performed a retrospective chart review of 482 adult patients (≥18 years) in two regions in Sweden who started to receive intra-venous antibiotics on four dates which were evenly distributed over the year of 2015. A total of 109 patients met Sepsis-3 criteria, corresponding to an annual incidence of 780 cases of Sepsis-3 per 100,000 population. In addition, in a retrospective analysis of data from 30,239 American adults aged ≥45 years, Donnelly et al reported an incidence of 580 cases per 100,000 person-years for Sepsis-3.[12] Apart from different demographics, socioeconomic characteristics, and case mix among these studies, other potential reasons for conflicting results between these studies and ours might be related to different methodology, such as study population (≥18 years[28]vs. ≥45 years[12]), study dates (randomly selected dates[28]vs. whole year[12]), time window for data extraction (entire hospitalization[28]vs. first 28 h after admission[12]), and descriptive variables (crude[12,28]vs. standardized incidence). Moreover, it is noteworthy that Donnelly et al[12] and Mellhammar et al[28] also reported an incidence rate of 820 cases per 100,000 person-years for Sepsis-1, and 687 cases per 100,000 person-years for traditional severe sepsis, respectively. Both were much higher than those in the study by Fleischmann et al, which reported the incidence rates of 437 for sepsis and 270 for severe sepsis per 100,000 person-years.[5] In comparison, based on the same database as in the current study, we estimated the population-based annual incidence of 461 cases per 100,000 population for Sepsis-1.[13]
Sex-specific differences in sepsis epidemiology have been extensively studied in experimental and clinical studies. Since the first report by McGowan et al,[29] it has been consistently reported by large epidemiologic studies that incidence of sepsis was 20% to 28% higher in men than women.[2,30] Similar to our previous study of Sepsis-1,[13] we also noticed a significantly higher standardized incidence of Sepsis-3 among men than women (290 vs. 181 cases per 100,000 person-years) in the current study. Such disparities in the incidence of sepsis are likely to be explained by a variety of factors, including demographics, comorbidities, high-risk behaviors (such as smoking), infection source, different microbes, sex hormones, and differential immune response to infection.[31] Despite agreement over the higher incidence of sepsis in men, there is controversy regarding whether this translates into a higher case fatality rate.[2,32–36] In the current study, case fatality rates did not differ significantly according to sex. However, the higher incidence of sepsis in men resulted in more than 1/3 increase of the number of hospital deaths related to sepsis.
Changes in the definition of sepsis might exert a remarkable impact on future clinical trials. The diagnostic criteria of Sepsis-3 might capture patients with less severity of illness, such as mild thrombocytopenia (1 point for <150 × 109/L) and/or hypoxemia (1 point for PaO2/FiO2 300–400 mmHg), which was not regarded as organ dysfunction based on diagnostic criteria of severe sepsis.[1,10] As a result, in the current study, the new Sepsis-3 criteria identified a group of patients with less severe clinical syndrome than traditional severe sepsis/septic shock, as suggested by more elective surgeries, fewer comorbidities (including immunocompromise and ultimately fatal comorbidities) and fewer ICU admissions, as well as lower case fatality rates. This was consistent with that of Williams et al,[37] which reported a significantly lower 30-day case fatality rate of Sepsis-3 than traditional severe sepsis (11.4% vs. 13.6%). Sample size calculations might be affected by enrolling septic patients with lower case fatality rate. For example, a sample size of 1232 patients with Sepsis-3, compared with 1388 patients with traditional severe sepsis/septic shock, would provide the study with 80% power to detect an absolute between-group difference of 7.5 percentage points in 28-day case fatality rate,[38] with a two-sided P < 0.05 indicating statistical significance. Although the impact of such a reduction of sample size on patient recruitment rate remains to be elucidated, it was noteworthy that enrollment of less severe septic patients in clinical trials might lead to more negative results,[39] since the efficacy of anti-inflammatory therapies during sepsis was dependent on the risk of death.[40]
The projected national estimates of Sepsis-3 burden merited cautious interpretation. In the United States, remarkable variations in economic development and availability of medical service had been associated with significant geographic differences in both incidence and mortality rates of sepsis.[41,42] As the capital city of China, data from a sub-district of Beijing could not be generalized to other provinces. Therefore, our findings with regards to standardized incidence and mortality rates of sepsis required validation by further prospective, large-scale cohort studies.
Our study had some strengths. This was the population-based epidemiological study of hospitalized patients with Sepsis-3 in the mainland of China. All hospitalized patients who were residents of Yuetan sub-district were identified through the hospital discharge database of Beijing Public Health Information System, and cases of sepsis were diagnosed based on manual review of individual medical record. This approach, although labor-intensive, might provide accurate diagnosis of sepsis while avoiding the unreliability of administrative data.[43]
Our study was also subject to some limitations. First, this was a retrospective study which was not originally designed for the purpose to illustrate the demographic characteristics of Sepsis-3. Second, we did not report epidemiology of septic shock because lactate level was seldom measured in general wards whereas only one out of four patients with Sepsis-3 was admitted to ICUs. Third, the national estimates of Sepsis-3 epidemiology required cautious interpretation and future validation.
In conclusions, in a secondary analysis of a population-based database of hospitalized residents in a sub-district in Beijing, we reported the standardized incidence rate of 236 cases per 100,000 person-years for Sepsis-3, which was more common in males and elderly population. This corresponded to about 2.5 million new cases of Sepsis-3 per year, resulting in more than 700,000 deaths, indicating the national burden of this devastating clinical syndrome which merited further studies to improve better health care policy, rational allocation of resources, and funding for sepsis research.
Funding
This study was supported by grants from the Capital Clinical Application Research from the Science and Technology Commission of Beijing (No. Z1311017002213112), and the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (No. 2016-I2M-1-014).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Tian HC, Zhou JF, Weng L, Hu XY, Peng JM, Wang CY, Jiang W, Du XP, Xi XM, An YZ, Duan ML, Du B; for China Critical Care Clinical Trials Group (CCCCTG). Epidemiology of Sepsis-3 in a sub-district of Beijing: secondary analysis of a population-based database. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000392
Hong-Cheng Tian and Jian-Fang Zhou contributed equally to this work.
References
- 1.Bone R, Balk R, Cerra F, Dellinger R, Fein A, Knaus W, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003; 348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence 2014; 5:4–11. doi: 10.4161/viru.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreu Ballester JC, Ballester F, Almela Quilis A, Colomer Rubio E, Peñarroja Otero C. Epidemiology of sepsis in the Valencian Community (Spain), 1995–2004. Infect Control Hosp Epidemiol 2008; 29:630–634. doi: 10.1086/589583. [DOI] [PubMed] [Google Scholar]
- 5.Fleischmann C, Scherag A, Adhikari NKJ, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Am J Respir Crit Care Med 2016; 193:259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 6.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 2013; 13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Churpek MM, Zadravecz FJ, Winslow C, Churpek MM, Zadravecz FJ, Winslow C, et al. Incidence and prognostic value of the systemic inflammatory response syndrome and organ dysfunctions in ward patients. Am J Respir Crit Care Med 2015; 192:958–964. doi: 10.1164/rccm.201502-0275OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaukonen K-M, Bailey M, Pilcher D, Cooper J, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med 2015; 372:1629–1638. doi: 10.1056/NEJMoa1415236. [DOI] [PubMed] [Google Scholar]
- 9.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Intensive Care Med 2003; 31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.38. [Google Scholar]
- 10.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shankar-Hari M, Harrison DA, Rubenfeld GD, Rowan K. Epidemiology of sepsis and septic shock in critical care units: comparison between sepsis-2 and sepsis-3 populations using a national critical care database. Br J Anaesth 2017; 119:626–636. doi: 10.1093/bja/aex234. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly JP, Safford MM, Shapiro NI, Baddley JW, Wang HE. Application of the third international consensus definitions for sepsis (sepsis-3) classification: a retrospective population-based cohort study. Lancet Infect Dis 2017; 17:661–670. doi: 10.1016/S1473-3099(17)30117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou J, Tian H, Du X, Xi X, An Y, Duan M, et al. Population-based epidemiology of sepsis in a subdistrict of Beijing. Crit Care Med 2017; 45:1168–1176. doi: 10.1097/CCM.0000000000002414. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.McCabe WR, Jackson GG. Gram-negative bacteremia. I: etiology and ecology. Arch Intern Med 1962; 110:847–855. doi: 10.1001/archinte.1962.03620240029006. [Google Scholar]
- 16.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13:818–829. doi: 10.1097/00003246-198608000-00028. [PubMed] [Google Scholar]
- 17.Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 1998; 26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control 1988; 16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 19.Population Census Office Under the State Council and Department of Population and Employment Statistics NBoS. Tabulation on the 2010 Population Census of People's Republic of China. 2012; Beijing: China Statistics Press, 16-111. [Google Scholar]
- 20.Daniel WW. Biostatistics: A Foundation for Analysis in the Health Sciences. 7th ed.New York: Wiley; 1999. [Google Scholar]
- 21.Abe T, Ogura H, Kushimoto S, Shiraishi A, Sugiyama T, Deshpande GA, et al. Variations in infection sites and mortality rates among patients in intensive care units with severe sepsis and septic shock in Japan. J Intensive Care 2019; 7:28.doi: 10.1186/s40560-019-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baykara N, Akalin H, Arslantaş MK, Hanci V, Çağlayan Ç, Kahveci F, et al. Epidemiology of sepsis in intensive care units in Turkey: a multicenter, point-prevalence study. Crit Care 2018; 22:93.doi: 10.1186/s13054-018-2013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, Qian C, Zhao M, Yu X, Kang Y, Ma X, et al. Epidemiology and outcome of severe sepsis and septic shock in intensive care units in mainland China. PLoS One 2014; 9:e107181.doi: 10.1371/journal.pone.0107181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen J, Cristofaro P, Carlet J, Opal S. New method of classifying infections in critically ill patients. Crit Care Med 2004; 32:1510–1526. doi: 10.1097/01.CCM.0000129973.13104.2D. [DOI] [PubMed] [Google Scholar]
- 25.Finfer S, Bellomo R, Lipman J, French C, Dobb G, Myburgh J. Adult-population incidence of severe sepsis in Australian and New Zealand intensive care units. Intensive Care Med 2004; 30:589–596. doi: 10.1007/s00134-004-2157-0. [DOI] [PubMed] [Google Scholar]
- 26.Brun-Buisson C, Doyon F, Carlet J. French Bacteremia-Sepsis Study Group. Bacteremia and severe sepsis in adults: a multicenter prospective survey in ICUs and wards of 24 hospitals. Am J Respir Crit Care Med 1996; 154:617–624. doi: 10.1164/ajrccm.154.3.8810595. [DOI] [PubMed] [Google Scholar]
- 27.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis occurrence in acutely ill patients investigators sepsis in European intensive care units: results of the SOAP study. Crit Care Med 2006; 34:344–353. doi: 10.1097/01.CCM.0000194725.48928.3A. [DOI] [PubMed] [Google Scholar]
- 28.Mellhammar L, Wullt S, Lindberg Å, Lanbeck P, Christensson B, Linder A. Sepsis incidence: a population-based study. Open Forum Infect Dis 2016; 3:ofw207.doi: 10.1093/ofid/ofw207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGowan JE, Jr, Barnes MW, Finland M. Bacteremia at Boston City Hospital: occurrence and mortality during 12 selected years (1935-1972), with special reference to hospital-acquired cases. J Infect Dis 1975; 132:316–335. doi: 10.1093/infdis/132.3.316. [DOI] [PubMed] [Google Scholar]
- 30.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, Martin GS. The role of infection and comorbidity: factors that influence disparities in sepsis. Crit Care Med 2006; 34:2576–2582. doi: 10.1097/01.CCM.0000239114.50519.0E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schröder J, Kahlke V, Staubach K, Zabel P, Stüber F. Gender differences in human sepsis. Arch Surg 1998; 133:1200–1205. doi: 10.1001/archsurg.133.11.1200. [DOI] [PubMed] [Google Scholar]
- 33.Adrie C, Azoulay E, Francais A, Clec’h C, Darques L, Schwebel C, et al. Influence of gender on the outcome of severe sepsis: a reappraisal. Chest 2007; 132:1786–1793. doi: 10.1378/chest.07-0420. [DOI] [PubMed] [Google Scholar]
- 34.Pietropaoli A, Glance LG, Oakes D, Fisher SG. Gender differences in mortality in patients with severe sepsis and septic shock. Gend Med 2010; 7:422–437. doi: 10.1016/j.genm.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakr Y, Elia C, Mascia L, Barberis B, Cardellino S, Livigni S, et al. The influence of gender on the epidemiology and outcome from severe sepsis. Crit Care 2013; 17:R50.doi: 10.1186/cc12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colbert JF, Traystman RJ, Poisson SN, Herson PS, Ginde AA. Sex-related differences in the risk of hospital-acquired sepsis and pneumonia post acute ischemic stroke. J Stroke Cerebrovasc Dis 2016; 25:2399–2404. doi: 10.1016/j.jstrokecerebrovasdis.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams JM, Greenslade JH, McKenzie JV, Chu K, Brown AFT, Lipman J. Systemic inflammatory response syndrome, quick sequential organ function assessment and organ dysfunction: insights from a prospective database of ED patients with infection. Chest 2017; 151:586–596. doi: 10.1016/j.chest.2016.10.057. [DOI] [PubMed] [Google Scholar]
- 38.Caironi P, Tognoni G, Masson S, Fumagalli R, Pesenti A, Romero M, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med 2014; 370:14120–21421. doi: 10.1056/NEJMoa1305727. [DOI] [PubMed] [Google Scholar]
- 39.Du B, Weng L. Systemic inflammatory response syndrome, sequential organ failure assessment, and quick sequential organ failure assessment: more pieces needed in the puzzle. J Thorac Dis 2017; 9:452–454. doi: 10.21037/jtd.2017.02.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eichacker PQ, Parent C, Kalil A, Esposito C, Cui X, Banks SM, et al. Risk and the efficacy of antiinflammatory agents: retrospective and confirmatory studies of sepsis. Am J Respir Crit Care Med 2002; 166:1197–1205. doi: 10.1164/rccm.200204-302OC. [DOI] [PubMed] [Google Scholar]
- 41.Xavier Moore J, Donnelly JP, Griffin R, Safford MM, Howard G, Baddley J, et al. Community characteristics and regional variations in sepsis. Int J Epidemiol 2017; 46:1607–1617. doi: 10.1093/ije/dyx099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodwin AJ, Nadig NR, McElligott JT, Simpson KN, Ford DW. Where you live matters: the impact of place of residence on severe sepsis incidence and mortality. Chest 2016; 150:829–836. doi: 10.1016/j.chest.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rhee C, Jentzsch MS, Kadri SS, Seymour CW, Angus DC, Murphy DJ, et al. Variation in identifying sepsis and organ dysfunction using administrative versus electronic clinical data and impact on hospital outcome comparisons. Crit Care Med 2019; 47:493–500. doi: 10.1097/CCM.0000000000003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


