To the Editor: Cosmetics (non-prescription skin care products) use-induced adverse events could present as skin erythema, papules, pruritus, dryness, desquamation, and abnormal discoloration. Non-medicated cosmetics use is more and more common in the China, but little is known about the characteristics of cosmetic-related adverse events. In this study, we assessed the clinical features, involved products, path-testing rate, and patient's education level distributions in cosmetic-related adverse events in southern China.
This study involved 341 subjects who were diagnosed with contact dermatitis induced by cosmetics in the Third Affiliated Hospital of Sun Yat-sen University in Guangzhou from January 2015 to December 2017. Subjects’ general information, use of cosmetics, and the occurrence of adverse events were recorded using registration forms.
The Medical Ethical Committee of the Third Affiliated Hospital of Sun Yat-sen University approved the study protocol (No. [2010]1-6) and informed consent form. Subjects provided informed written consents.
Evaluation criteria of adverse reactions were followed the guidelines of the implementation of diagnostic standards and principles of cosmetic dermatology issue published by National Health Commission of China. The diagnostic criteria also included: there was a clear history of contacting cosmetics before the onset of disease; skin damage occurred originally at the location where cosmetics were applied; disease progression was related to the amount of cosmetics used and the use frequency; there was evidence from laboratory test results. Skin problems caused by non-cosmetic factors were excluded.
After patients had stopped the systemic antihistamines treatment for 3 days and/or glucocorticoids or immunosuppressants treatment for 2 weeks, patch test was done with individual suspected cosmetics. In the patch test, cosmetics was applied on subjects using The T.R.U.E.TEST® patch test system (True Test Technology Inc., Mineral Wells, TX, USA) for 48 h. Suspect cosmetics was placed in direct contact with the skin, on the upper back, within small disks (True Test Technology Inc.). Adhesive tape was used to fix them in place, and the test sites were marked. The patches were left in place for 48 h, during which time the subjects were not allowed to wash the area or play vigorous sports. After 48 h, patches were removed and an initial reading taken 1 h later. Twenty-four, 48, and 72 h after removing, additional readings were carried out.
The reaction was read as: negative (−); doubtful reaction (±): faint macular erythema only; weak (non-vesicular) positive reaction (+): erythema, infiltration, possibly papules; strong (vesicular) positive reaction (++): erythema, infiltration, papules, vesicle; extreme positive reaction (+++): bullous reaction; irritant reaction (IR) of different types: pustules as well as patchy follicular or homogeneous erythema without infiltrations are usually signs of irritation and do not indicate allergy.
Majority (75.7%) of the 341 patients (333 females and 8 males, aged from 12 to 75 years) were between 21 and 40 years old. About 70.4% patients had accepted college education including 143 post-graduates (41.9%) and 97 undergraduates (28.5%). And 38.7% patients (n = 132) had a history of allergy: 61 were sensitive to cosmetics; 71 were sensitive to other contact substances.
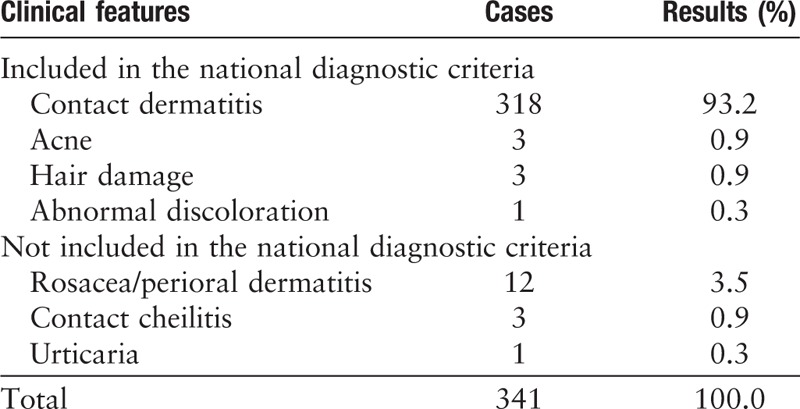
A total of 325 cases of adverse events were identified using the National Diagnostic Criteria of China, while 16 adverse events were diagnosed relating to cosmetics usage but not described in National Diagnostic Criteria by dermatologists [Table 1]. The most common reported was contact dermatitis (93.3%, n = 318), followed by rosacea and perioral dermatitis (3.5%, n = 12). The appearance of acne, hair damage, and contacting cheilitis was 3 (0.9%, n = 3). No disputes caused by cosmetics were reported.
Table 1.
Clinical presentation of cosmetic use-induced adverse events.
A total of 646 cosmetics were reported. About 372 products were made in China and 261 products were imported. The occurrence of cosmetic-related adverse events was associated with mixing of different types of cosmetics together (31.4%, n = 107). Among these, mixing products from the same brand-induced adverse events were 34.6% (n = 37), while mixing products from the different brand-induced adverse events were 65.4% (n = 70) [Supplementary Table 1].
Patch test using suspected cosmetics was performed in 71 patients with contact dermatitis. There were 41 positive cases (57.7%), 13 cases with suspected positive results, and 17 negative cases.
Compared with the previous data in China, we found the rate of cosmetic use-related adverse events was decreased in 2017 than in 2007 (200 cases)[1] and 2008 (240 cases).[2] We observed that the case of cosmetics use-related adverse events was increased in high-level educated group (41.9% post-graduates and 28.5% undergraduates). Persons with better education are more aware of skin health and beauty in China, therefore, they are more likely to become cosmetic consumers.
Clinical manifestation of adverse reactions caused by cosmetics was various and should be considered comprehensively in practical application. Cornell et al[3] analyzed 166 total adverse events and reported to the Food and Drug Administration for cosmetics and personal care products marketed to newborns and infants from 2004 to 2016 and found that the majority of reports indicated rash or other skin reaction. Contact dermatitis was the most common found adverse events in this study, which was consistent with the findings of previous studies in China.[4,5]
The history of cosmetic product usage was an important predictor for adverse event occurrence. Bilal et al[6] assessed the prevalence and determinants of cosmetic-related adverse events among Jigjiga Town residents and found that the occurrence of cosmetic-related adverse events was significantly associated with the number of cosmetics used per day, the frequency of use, mixing of different types of cosmetics together, and mixing of cosmetics with water or saliva. This implies the need to pay attention to the history of cosmetic product usage in dermatologist's diagnosis process.
Our data also showed that the cases of adverse reaction induced by domestic cosmetics were higher than that of imported cosmetics. Besides considering the factor of domestic cosmetics using rate was higher in our country, we also proposed that the supervision of domestic cosmetics still needs to be improved in China.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 8167120875, 81673085), Pearl River Nova Program of Guangzhou, China (No. 201610010152), and Meizhou Science and Technology Program (No. 171102172051439).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Yang SL, Zheng Y, Zhang YQ, Ye CX, Yi JL, Liu YF, Lai W. Cosmetics use-related adverse events. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000371
References
- 1.Xie SX, Yang SL, Ye ZZ, Guang L, Lai W, Wan MJ, et al. Analysis of the clinical features of 200 patients with cosmetics dermatoses in Guangzhou in 2007. Skin STD J Neurol 2009; 16:284–286. doi: 10.3969/j.issn.1674-8468.2009.05.004. [Google Scholar]
- 2.Xie SX, Yang SL, Guan L, Ou FX, Lai W, Wan MJ, et al. Analysis on the monitoring results of cosmetic dermatology in Guangzhou in 2008 (in Chinese). Chin J Nat Med 2009; 11:443–445. [Google Scholar]
- 3.Cornell E, Kwa M, Paller AS, Xu S. Adverse events reported to the Food and Drug Administration from 2004 to 2016 for cosmetics and personal care products marketed to newborns and infants. Pediatr Dermatol 2018; 35:225–229. doi: 10.1111/pde.13419. [DOI] [PubMed] [Google Scholar]
- 4.Long HL, Yang LN, Li JH. Monitoring and analysis of the adverse reactions of cosmetics in one province in 2016. Central South Pharmacy 2017; 15:860–864. doi: 10.7539/j.issn.1672-2981.2017.06.038. [Google Scholar]
- 5.Yang SL, Xie SX, Yin SC, Ou FX, Zhang YQ, Lai W. Analysis of patch-testing results in 127 patients with facial dermatitis (in Chinese). Chin J Health Inspection 2016; 26:2456–2458. [Google Scholar]
- 6.Bilal AI, Tilahun Z, Osman ED, Mulugeta A, Shekabdulahi M, Berhe DF. Cosmetics use-related adverse events and determinants among Jigjiga town residents, eastern Ethiopia. Dermatol Ther (Heidelb) 2017; 7:143–153. doi: 10.1007/s13555-016-0157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


