Abstract
Background:
Compared with Caucasians, unique demographic and clinical features have been reported in Chinese patients with malignant melanoma, but similar comparative studies of melanocytic nevi (MN) are lacking. This study examined the clinical and dermoscopic features of MN in surgically treated Chinese cases.
Methods:
Clinical data and dermoscopic findings from 1046 cases of MN were collected and analyzed. Cases were treated from January 1 to December 31, 2014 at the Department of Dermatology and Venerology, Peking University First Hospital. The association between nevi location and histologic subtypes was examined with Chi-squared test and univariate logistic regression. Chi-squared test was also used to analyze the proportion of globular patterns across different body sites, and proportion of parallel furrow patterns across different histologic subtypes.
Results:
The majority of the nevi were from female patients, irrespective of location. The range of age at the time of nevi onset was from 0 (birth) to 79 years. There were 381 (36.4%, 381/1046) congenital nevi; of these 81.6% (311/381) were present at birth. Nevi appeared before 30 years of age in 83.2% (870/1046) of the cases. Median values of length growth rate in congenital and acquired MN were 2.0 and 1.6, respectively. Median values of length growth rates in four age groups (0–9, 10–19, 20–29, and ≥30 years) of congenital nevi were 2.2, 2.0, 2.4, and 2.0, respectively. In acral nevi, which often need to be differentiated from acral lentiginous melanoma, 50.2% (109/217) were junctional (odds ratio [OR]; 95% confidence interval [CI]: 91.572 [52.210–160.959], P < 0.05). Acral location was also associated with a higher likelihood of compound nevi subtype (OR [95% CI]: 14.468 [8.981–23.306], P < 0.05). The globular (59.4%, 354/596) and pseudonetwork (48.8%, 291/596) dermoscopic patterns were often seen in the head and neck region. In areas other than head and neck and acral regions, the globular pattern was the commonest pattern (34.8%, 71/204) regardless of age. Parallel furrow pattern occurred in 46.0% (87/189) of acral MN, followed by fibrillar pattern (21.7%, 41/189).
Conclusion:
Unique clinical and dermoscopic features exist in Chinese patients with MN compared with observations reported in other population.
Keywords: Nevus, Melanoma, Dermoscopy
Introduction
Melanocytic nevi (MN) are common and have been the most frequently surgically treated neoplasm at the Department of Dermatology and Venerology, Peking University First Hospital. They can be classified as congenital or acquired. Congenital MN are present at birth or appear within 2 years of birth.[1] Acquired MN are more common, and appear throughout childhood and adulthood with peaks in the third decade.[2] Histologically, MN are classified into junctional, intradermal, and compound nevi according to the location of the nests of melanocytes. Different histologic subtypes have varying risks of developing into malignant melanoma (MM).[3]
Dermoscopy enables noninvasive visualization of structures invisible to the naked eye. For decades, dermoscopy has proven useful in the differential diagnosis of early stage melanoma and benign nevi.[4–6]
Unique demographic and clinical features have been reported in MM of Chinese patients. Compared with Caucasians, acral MM has been reported to be more prevalent than other subtypes in Asians.[7,8] However, reports of the general features of MN in Chinese are lacking. To fill this gap of knowledge, we retrospectively reviewed a series of MN removed from Chinese patients over a period of 12 months. Our findings may help dermatologists understand the unique features of MN in the Chinese population, and aid in the clinical and dermoscopic assessment of MN and their differentiation from MM.
Methods
Ethical approval
The study was approved by the Ethical Committee of Peking University First Hospital (No. 2019-184), with exception from informed consent. The study was performed in accordance with the established guidelines and regulations.
Study population and data collection
This retrospective study included 1046 cases of MN from 956 patients who underwent surgery at the Department of Dermatology and Venerology, Peking University First Hospital, during the period of January 1 to December 31, 2014.
Clinical and demographic data were collected from chart review; these included age, gender, anatomic location of lesion, brief history, disease duration, date of diagnosis, and MN histology. Because of the retrospective nature of this study, some data items were not available in all cases; these missing data were not included in relevant analyses. Length of lesion at onset was estimated by the patient, and length at presentation was measured by the physician before surgery; length growth rate ([length at presentation/length at onset] − 1) was then calculated to examine the growth of lesions. Of the 956 patients, 136 were randomly selected and surveyed for their motivation to seek surgical removal. Clinical and dermoscopic images were available for all nevi, and were reviewed by three experienced dermatologists and dermoscopists.
Statistical analysis
The descriptive analysis included calculations of various proportions (gender, location, and histologic subtypes among others). The association between nevi location and histologic subtypes was examined with Chi-squared test and univariate logistic regression. Chi-squared test was also used to analyze the proportion of globular patterns across different body sites, and the proportion of parallel furrow patterns in different histologic subtypes. Statistical analyses were performed using SPSS statistical package 22.0 (IBM SPSS Inc., Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
Results
Clinical and histologic features
The general characteristics of the patients and nevi are presented in Table 1. MNs were divided into three groups by location: head and neck, acral sites (palms, soles, and nails), and others (including trunk and nonglabrous extremities). The commonest location was head and neck. Regarding reasons for seeking excision, concerns about malignant transformation and cosmetic concerns were the most prevalent. The majority of the nevi were asymptomatic. There were 381 congenital nevi; 81.6% (311/381) were present at birth; 78.2% (298/381) were less than 1.5 cm in size; the head and neck region was the commonest location for congenital nevi. The majority of the nevi were from female patients, irrespective of location [Figure 1].
Table 1.
General characteristics of the patients and nevi.
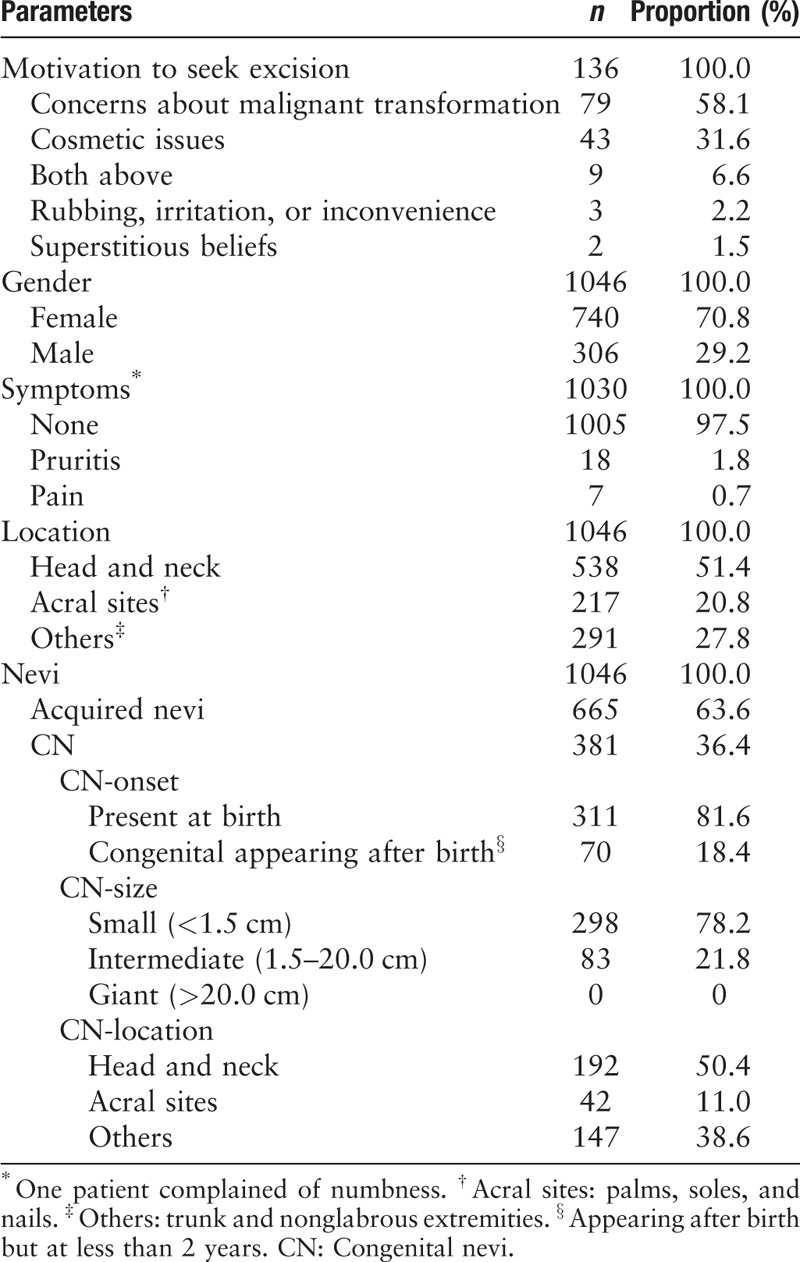
Figure 1.
Gender differences according to body sites. Irrespective of location, the majority of nevi were from female patients. Others: trunk and nonglabrous extremities.
Patient age at the time of the first appearance of their MN ranged from 0 to 79 years. The majority of acquired nevi appeared in the second through fourth decades of life.
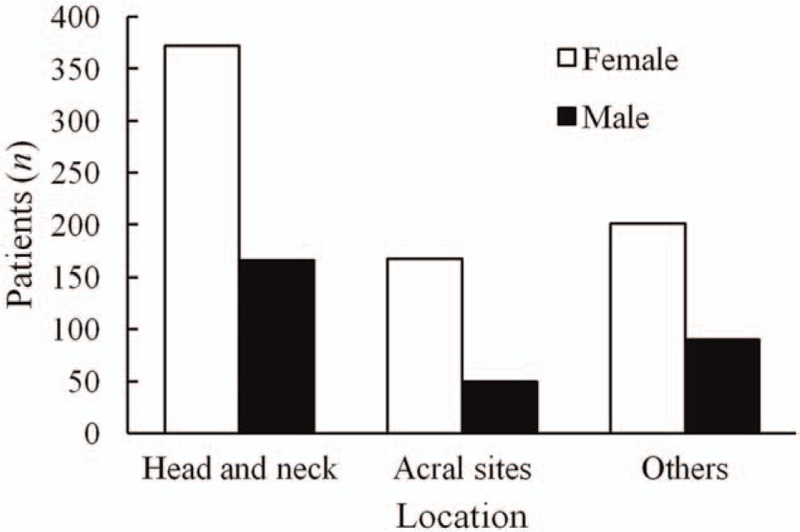
Length growth rate of congenital and acquired MN were shown in Figure 2. The median length growth rate of congenital and acquired MN were 2.0 and 1.6, respectively. To further study the relationship between length growth rate of congenital MN and age, we divided these patients into four age groups: 0 to 9, 10 to 19, 20 to 29, and ≥30 years; median values of length growth rate of each age group were 2.2, 2.0, 2.4, and 2.0, respectively.
Figure 2.
Length growth rate of congenital melanocytic nevi (A) and acquired melanocytic nevi (B). The median value of length growth rate of congenital melanocytic nevi and acquired melanocytic nevi was 2.0 and 1.6, respectively.
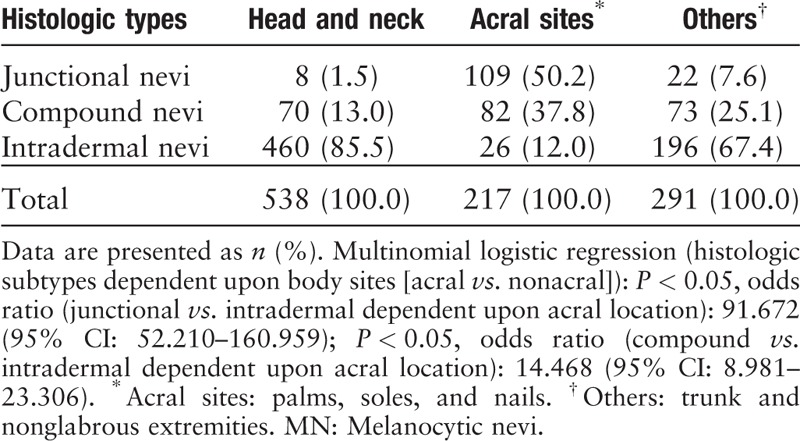
The histologic subtypes of the excised nevi and the correlations between histologic subtype and body location are shown in Table 2. The most commonly excised nevi were intradermal nevi. Junctional nevi were less common, but ranked first in number in nevi removed from acral sites, which was confirmed with univariate multinomial logistic regression (odd ratio [OR], 95% confidence interval [CI]: 91.572 [52.210–160.959], P < 0.05). Acral location was also associated with a higher likelihood of compound nevi subtype (OR [95% CI]: 14.468 [8.981–23.306], P < 0.05).
Table 2.
Histologic subtype and body site of 1046 MN.
Dermoscopic features
The globular pattern was the most frequent component in nonacral nevi [Table 3, and Figure 3A], regardless of age [Table 4]. In acral MN, parallel furrow pattern and fibrillar pattern were commonest. The pseudonet work pattern was the second commonest on the head and neck [Figure 3B]. On other sites, globular and homogeneous patterns were also common. A pure reticular pattern was only seen in 6.9% (14/204) of patients.
Table 3.
Ten most common dermoscopic patterns in MN on different body sites.
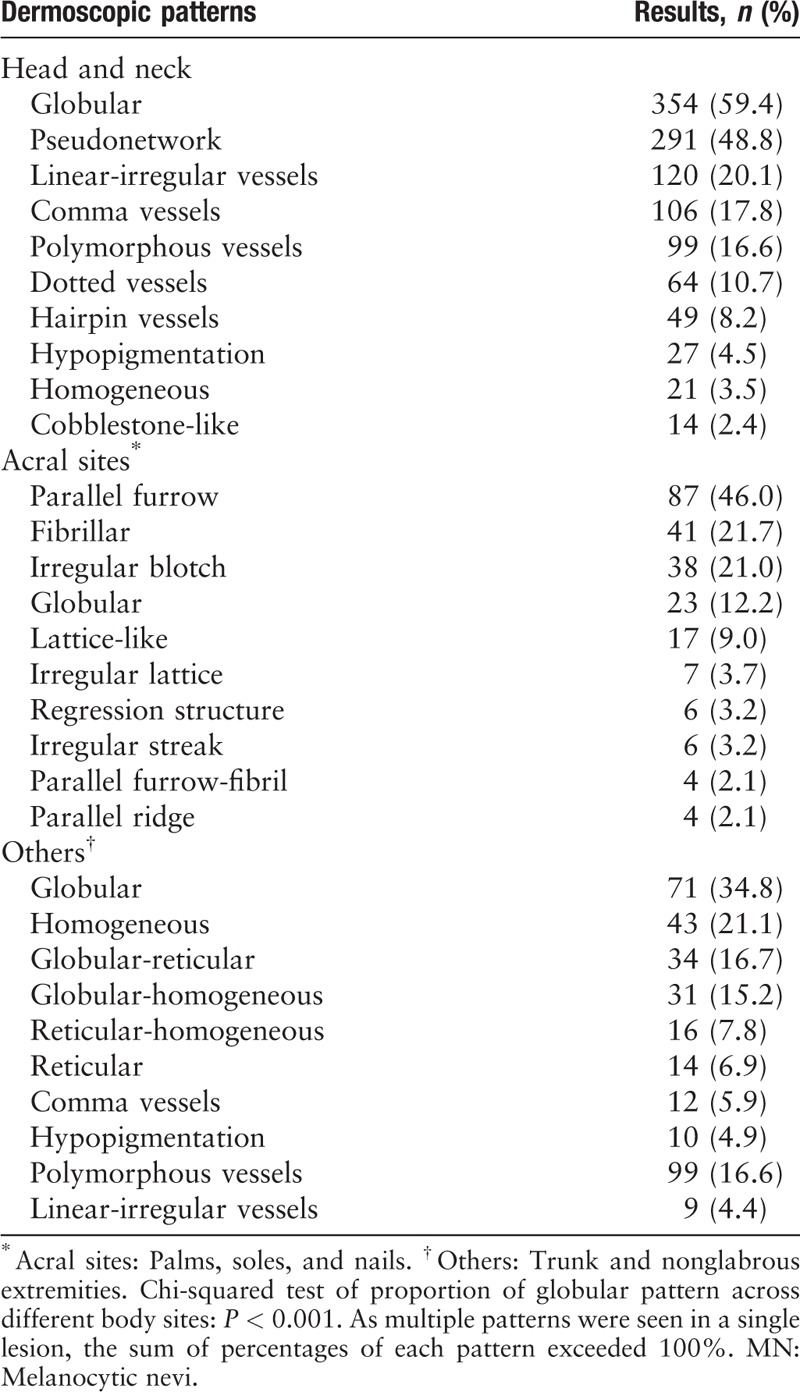
Figure 3.
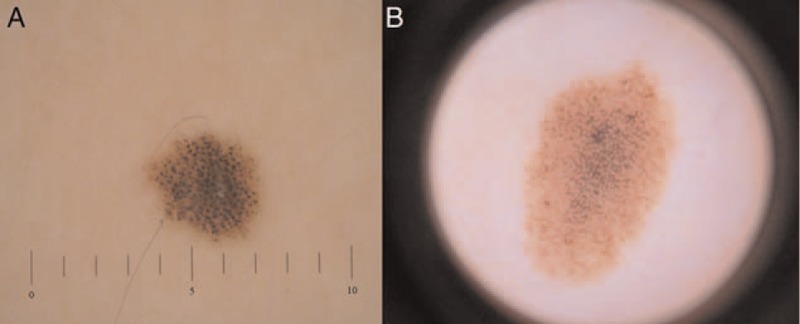
Two cases of dermoscopic features. (A) Globular pattern on the trunk of a girl and (B) pseudonetwork pattern on the face of another girl (original magnification ×20).
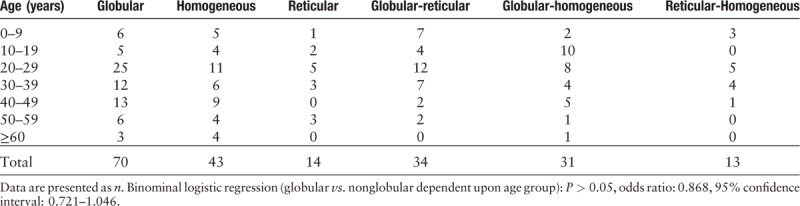
Table 4.
Patterns of nevi in other sites (trunk and nonglabrous extremities) according to age.
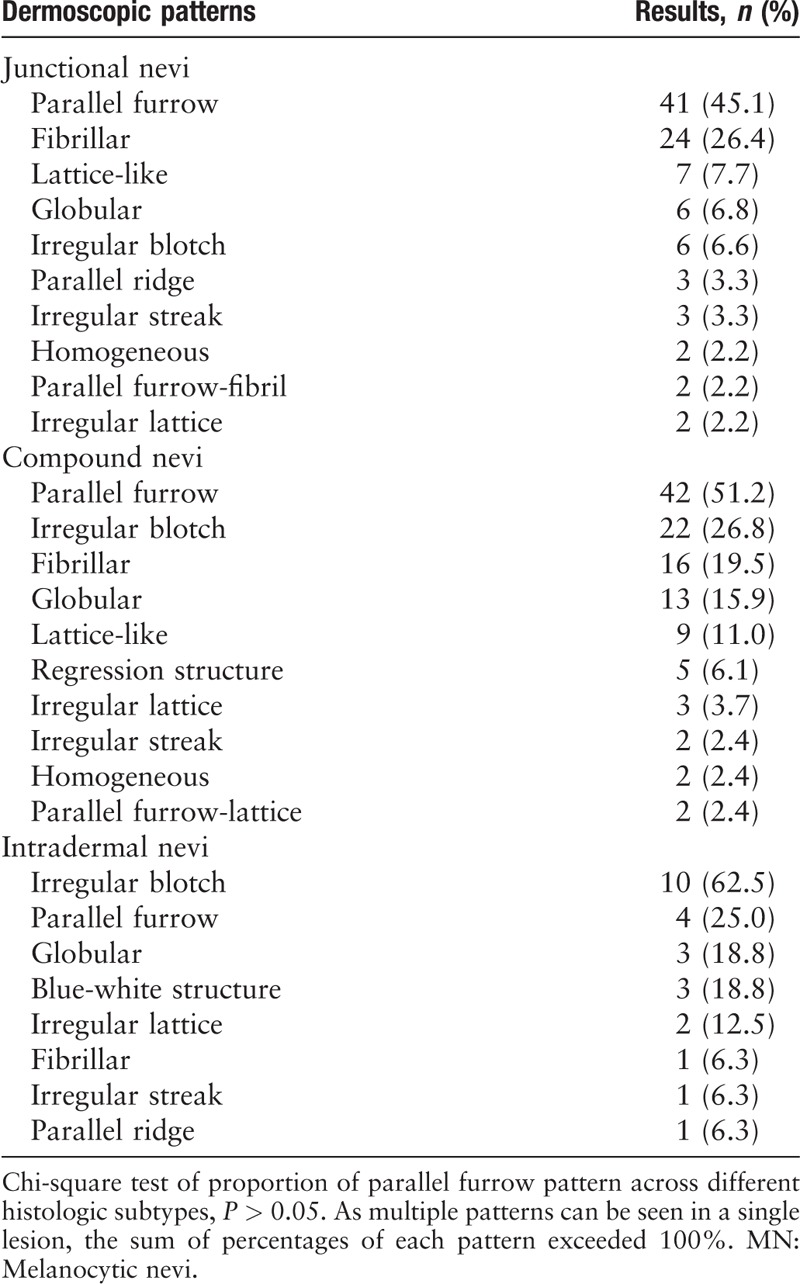
In the acral region, parallel pattern was common in both junctional and compound MN, while irregular blotch pattern was frequently seen in nevi with dermal components and was the commonest pattern in intradermal nevi [Table 5].
Table 5.
Ten most common dermoscopic patterns of different histopathologic subtypes in MN on acral sites.
Discussion
This report describes the clinical, histologic, and dermoscopic features of nevi surgically removed from a series of patients in an academic dermatology center in China.
More than half of the patients required excision of their nevi out of malignant concerns without any suspicious malignant signs. This identified a public education gap for identifying suspicious melanocytic lesions and a need to promote patient education regarding MN and risk of melanoma, including visible signs of malignant transformation.
A female predominance was noted in our study, irrespective of nevus location. This may indicate a stronger concern not only with their appearance, but also with general skin health.
The prevalence of MN is related to age, race, and perhaps genetic and environmental factors. A few nevi are present at birth and in early childhood. The number then increases, reaching a peak during the third decade of life; thereafter, nevi tend to degenerate with increasing age. There is a period of particularly rapid development during puberty.[2,9] In the study, 54.7% (573/1046) of the patients developed nevi before 10 years of age, 70.2% (734/1046) before 20, and 83.2% (870/1046) before 30; this was in accordance with prior studies.
The incidence of MN on the palms and soles, nail beds and conjunctivae are correlated with race; nevi on these surfaces are more common in Africans and Asians than in Caucasians.[2] In our Chinese series, most resected nevi were located on nonacral regions (79.2%, 829/1046) rather than on the palms and soles (20.8%, 217/1046). The high percentage of head and neck nevi being resected might be due to the visibility of nevi in these sites, triggering aesthetic or health concerns.
Of the 381 congenital nevi, 81.6% (311/381) were present at birth, and 18.4% (70/381) appeared during the first 2 years after birth. Parents often became worried after noticing obvious growth of these nevi in their children. As shown in Figure 2A, by the time surgical consultation is sought, 66.5% (212/319) congenital MN have increased by 0 to 3 times in length, 26.6% (85/319) by 4 to 10 times, and 2.8% (9/319) by more than 10 times (median value = 2.0). Furthermore, median values of length growth rate of the four age groups suggested that most congenital MN increased by 2.2 times in length between age 0 to 9 years, and then stayed relatively stable; this results in decreased ratio of nevus area/body surface area as the patient grow, which, in association with increased patient compliance, suggest that unless there is reasonable suspicion of malignancy, small and medium-sized nevi presented during 0 to 9 years of age be removed when the patient grows older. In large and giant congenital nevi, early removal might be beneficial due to elasticity of the skin and ease of closure.
According to Figure 2B, 82.7% (477/577) acquired MN have increased by 0 to 3 times in length, 6.1% (35/577) by 4 to 10 times, and 1.0% (6/577) by more than 10 times (median = 1.6) by the time surgery is considered. These observations regarding the trends of growth may provide patients an anticipation of the future sizes of their nevi, thus better informing decisions concerning nevi removal, especially in regards of cosmetic concerns.
Nevi of the head and neck and trunk were predominantly intradermal. Junctional nevi were more prevalent on acral sites, but more than half of all removed acral nevi were not junctional.
A previous study showed that facial nevi typically showed a pseudonetwork pattern intermingled by hairs.[10] In our series, globular and pseudonetwork patterns were most frequently seen in MN of head and neck. In other locations (areas excluding acral region and head and neck), globular pattern was still the commonest pattern (34.8%, 71/204), with homogeneous pattern (21.1%, 43/204) ranking second. The globular pattern and its variants were the commonest pattern irrespective of age, a finding that differs from observations in Caucasian patients where it decreases with age and become uncommon after the age of 30 years.[1,11] Scope et al[12] suggested that the presence of globular nevi in adults is concerning because such occurrence is unusual. The significance of our findings is that, in Chinese skin, the presence of a globular pattern in an older individual may be less alarming and less likely to trigger closer scrutiny or a biopsy.
Polymorphous blood vessel pattern has been incorporated in some dermoscopic algorithms designed to differentiate benign from malignant melanocytic lesions, with the presence of it being ominous.[13,14] In our series it was revealed that this pattern was not uncommon in benign lesions. In fact, 33.7% (58/172) of the nevi had three or more blood vessel patterns, although this pattern was seen nearly exclusively in dermal nevi. Vessel patterns were more commonly seen in MN located on head and neck than on other body sites [Table 3].
The present study was limited by the selection bias inherent in limiting findings to excised nevi. This bias may limit the ability to extrapolate our findings to nevi in Chinese skin generally. For example, it is possible that globular nevi were more likely to create patient anxiety than reticular nevi, resulting in a higher proportion of globular nevi in this study. Secondly, the length at onset of nevus was estimated by the patient, which may result in bias and imprecise data. In addition, this is a single center study that cannot necessarily represent the patterns expected in the whole population of China.
In conclusion, this report describes the clinical, histologic, and dermoscopic features of 1046 MN in a single-center series of Chinese patients. Most of these features are similar to those reported in Caucasian patients, but differences of potential clinical significance exist. Awareness of these differences might prove valuable when screening ethnically diverse groups of patients with melanocytic lesions.
Funding
The work was supported by grants from Clinical Characteristics and Application Research of Capital, Beijing Municipal Science and Technology Commission (No. Z121107001012162), and the National Natural Science Foundation of China (No. 81572675).
Conflicts of interest
None.
Footnotes
How to cite this article: Li QX, Swanson DL, Tu P, Yang SX, Li H. Clinical and dermoscopic features of surgically treated melanocytic nevi: a retrospective study of 1046 cases. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000416
References
- 1.Kincannon J, Boutzale C. The physiology of pigmented nevi. Pediatrics 1999; 104 (Pt 2):1042–1045. [PubMed] [Google Scholar]
- 2.Whiteman DC, Pavan WJ, Bastian BC. The melanomas: a synthesis of epidemiological, clinical, histopathological, genetic, and biological aspects, supporting distinct subtypes, causal pathways, and cells of origin. Pigment Cell Melanoma Res 2011; 24:879–897. doi: 10.1111/j.1755-148X.2011.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carli P, Massi D, Santucci M, Biggeri A, Giannotti B. Cutaneous melanoma histologically associated with a nevus and melanoma de novo have a different profile of risk: results from a case-control study. J Am Acad Dermatol 1999; 40:549–557. doi: 10.1016/S0190-9622(99)70436-6. [DOI] [PubMed] [Google Scholar]
- 4.Carrera C, Marchetti MA, Dusza SW, Argenziano G, Braun RP, Halpern AC, et al. Validity and reliability of dermoscopic criteria used to differentiate nevi from melanoma: a web-based international dermoscopy society study. JAMA Dermatol 2016; 152:798–806. doi: 10.1001/jamadermatol.2016.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tognetti L, Cevenini G, Moscarella E, Cinotti E, Farnetani F, Mahlvey J, et al. An integrated clinical-dermoscopic risk scoring system for the differentiation between early melanoma and atypical nevi: the iDScore. J Eur Acad Dermatol Venereol 2018; 32:2162–2170. doi: 10.1111/jdv.15106. [DOI] [PubMed] [Google Scholar]
- 6.Tognetti L, Cinotti E, Moscarella E, Farnetani F, Malvehy J, Lallas A, et al. Impact of clinical and personal data in the dermoscopic differentiation between early melanoma and atypical nevi. Dermatol Pract Concept 2018; 8:324–327. doi: 10.5826/dpc.0804a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradford PT, Goldstein AM, McMaster ML, Tucker MA. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986–2005. Arch Dermatol 2009; 145:427–434. doi: 10.1001/archdermatol.2008.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of california cancer registry data, 1988–93. Cancer Causes Control 1997; 8:246–252. doi: 10.1023/A:1018432632528. [DOI] [PubMed] [Google Scholar]
- 9.Maize JC, Foster G. Age-related changes in melanocytic naevi. Clin Exp Dermatol 1979; 4:49–58. doi: 10.1111/j.1365-2230.1979.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 10.Argenziano G, Soyer HP, Chimenti S, Talamini R, Corona R, Sera F, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. J Am Acad Dermatol 2003; 48:679–693. doi: 10.1067/mjd.2003.281. [DOI] [PubMed] [Google Scholar]
- 11.Zalaudek I, Grinschgl S, Argenziano G, Marghoob AA, Blum A, Richtig E, et al. Age-related prevalence of dermoscopy patterns in acquired melanocytic naevi. Br J Dermatol 2006; 154:299–304. doi: 10.1111/j.1365-2133.2005.06973.x. [DOI] [PubMed] [Google Scholar]
- 12.Scope A, Marchetti MA, Marghoob AA, Dusza SW, Geller AC, Satagopan JM, et al. The study of nevi in children: Principles learned and implications for melanoma diagnosis. J Am Acad Dermatol 2016; 75:813–823. doi: 10.1016/j.jaad.2016.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henning JS, Dusza SW, Wang SQ, Marghoob AA, Rabinovitz HS, Polsky D, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol 2007; 56:45–52. doi: 10.1016/j.jaad.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Argenziano G, Catricala C, Ardigo M, Buccini P, De Simone P, Eibenschutz L, et al. Seven-point checklist of dermoscopy revisited. Br J Dermatol 2011; 164:785–790. doi: 10.1111/j.1365-2133.2010.10194.x. [DOI] [PubMed] [Google Scholar]


