Abstract
Background:
Both cortical and cortical-subcortical (cortex-involved) lesions are typically associated with embolic stroke, of which atrial fibrillation (AF) is the common cause. The aim of this study was to find out the associations between cortex-involved stroke, vascular risk factors, and the subtypes (discovery time and duration) of AF.
Methods:
This was an imaging study of the China Atrial Fibrillation Screening in Acute Ischemic Stroke Patients (CRIST) trial. Between October 2013 and June 2015, 1511 acute ischemic stroke or transient ischemic attack (TIA) patients within 7 days after stroke onset at 20 Chinese hospitals were enrolled in this prospective, multicenter cohort, cross-sectional study. The final analysis of this sub-study included 243 patients with AF with required magnetic resonance imaging (MRI) sequences. AF was diagnosed by 6-day Holter monitoring and classified by duration of 24 h. Two stroke specialists blinded to the clinical information reviewed MRI (diffusion-weighted MRI). The third stroke specialists, also blinded to the clinical information, assessed the conflicts. Adjusted large artery atherosclerosis as confounding factor, the associations between cortex-involved lesions, vascular risk factors, and the subtype of AF were evaluated by univariate and multivariate regression analyses.
Results:
Of 243 acute ischemic stroke patients with AF, 190 were known AF and 53 were newly detected AF. There were 28 patients with AF persistent >24 h and 25 persistent ≤24 h in newly detected AF. Patients with newly detected AF were likely to have a fewer history of stroke or TIA (16.98% vs. 36.31%, P = 0.008) and lower fasting blood glucose (5.91 ± 1.83 mmol/L vs. 6.75 ± 3.83 mmol/L, P = 0.030) than patients with known AF. Among these 243 patients, 102 (41.98%) patients were with cortex-involved lesions. Cortex-involved lesions were significantly related to newly detected AF persistent >24 h (odds ratio [OR]: 4.517, 95% confidence interval [CI]: 1.490–13.696, P = 0.008), proteinuria (OR: 3.431, 95% CI: 1.530–7.692, P = 0.021), and glycosylated hemoglobin (OR: 0.632, 95% CI: 0.464–0.861, P = 0.004).
Conclusions:
Compared to previously known AF, newly detected AF persistent >24 h was associated with cortex-involved ischemic stroke.
Clinical trial registration:
NCT02156765, https://clinicaltrials.gov/ct2/show/record/NCT02156765
Keywords: Atrial fibrillation, Ischemic stroke, Prolonged electrocardiograph monitoring, Magnetic resonance imaging
Introduction
Diagnosis of atrial fibrillation (AF) after stroke is essential for secondary prevention of stroke. Previous studies demonstrated that oral anticoagulation is superior to aspirin for stroke prevention in patients with AF.[1,2] With prolonged cardiac monitoring by a variety of techniques, AF is newly detected in nearly a quarter of patients with stroke.[3] The China Atrial Fibrillation Screening in Acute Ischemic Stroke Patients (CRIST) trial, which recruited consecutive 1556 patients with ischemic and transient ischemic attack (TIA) within 7 days from October 2013 to June 2015, showed that prolonged monitoring evaluation (6 days) could increase AF detection to about 20% in China, of these 4.4% were newly detected AF.[4] However, the available evidence demonstrated no significant benefit of oral anticoagulation for stroke prevention in such patients.[5–7] Although AF is a well-known risk factor for cardioembolic stroke, it is not always directly responsible for the embolism.[8,9] No consensus has been reached about the embolization risk for different duration of AF.[3] It is crucial to evaluate whether the exact etiology of the stroke is related to the presence of AF.
Neuroimaging may help distinguish the cause of stroke. Cardioembolic stroke was associated with the presence of cortex-involved lesions with territorial distribution or confluent lesion (>15 mm) with additional lesions involving multiple vascular territories.[10,11]
In this imaging study of China Atrial Fibrillation Screening in Acute Ischemic Stroke Patients (CRIST) trial, we aimed to investigate the associations between the cortex-involved lesion on diffusion-weighted image (DWI), which indirectly suggests embolism mechanism of the index event, and the characteristics of AF (before or after stroke, persistent more or less than 24 h).
Methods
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Beijing Tiantan Hospital. Informed written consent was obtained from all subjects or their next of kin if the consent from the patient was unavailable.
Study population
This was an imaging study of the CRIST trial. Between October 2013 and June 2015, 1511 acute ischemic stroke or TIA patients within 7 days after stroke onset at 20 Chinese hospitals were enrolled in this prospective, multicenter cohort, cross-sectional study. Initially, a total of 1556 patients were included in this study. Among them, 305 patients were diagnosed with AF by 6-day Holter monitoring. Those who were with required magnetic resonance imaging sequences (n = 243) were enrolled in our study [Figure 1].
Figure 1.
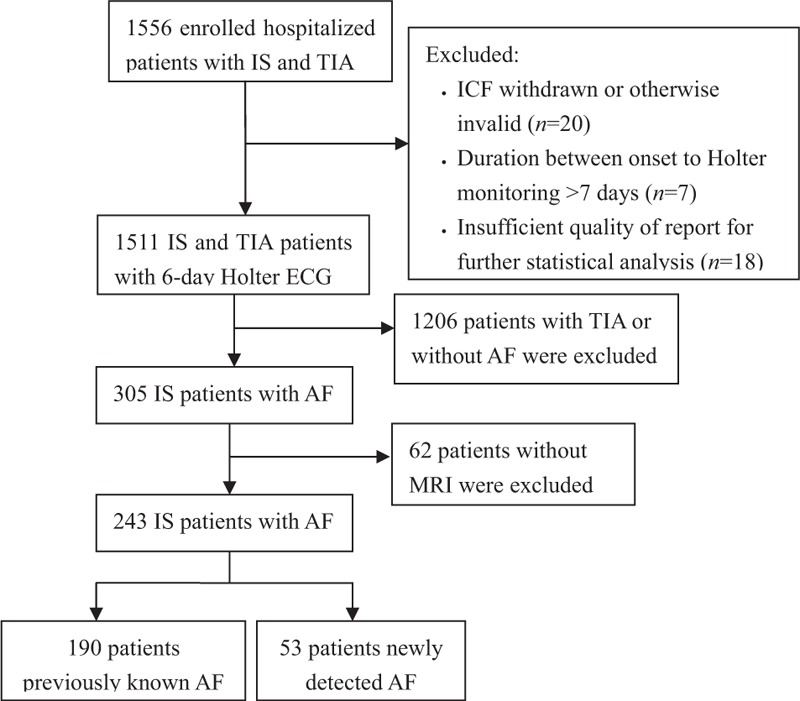
Flow chart of the study. IS: Ischemic stroke; TIA: Transient ischemic attack; ICF: Informed consent forms; ECG: Electrocardiograph; AF: Atrial fibrillation; MRI: Magnetic resonance imaging.
AF was diagnosed as ≥1 period of absolute arrhythmia without detectable P-waves (episodes >30 s duration interpreted by Holter and episodes <30 s required manual review of all possible AF events).[12] Previously known AF was diagnosed according to the medical history reported by the patients and the prior available medical records.
Clinical information
Clinical information such as demographic information, stroke risk factors, detailed medical history, and treatments were assessed as previously described.[4]
The 6-day Holter monitoring initiated within 7 days after the index event using a commercially available 3-lead monitor device (iHolter, Yocaly Information Science & Technology Co., Ltd., Jinan, Shandong, China). Two investigators from central core laboratory analyzed the electrocardiograph (ECG) recordings blindly using dedicated analysis software (DoctorClient, Software version 1.5.0.16, Yocaly Information Science & Technology Co., Ltd.). All ECG recordings with suspected AF were subsequently evaluated by another independent observer. The first time and longest duration of bursts of AF for each patient were also recorded.
MRI was performed using a 1.5-T or a 3.0-T system. The cortex-involved lesion was defined as cortical or cortical-subcortical territorial hyperintense lesions.[13] Infratentorial cortex-involved lesion was defined as a lesion involving the cerebellar cortex. Two stroke specialists blinded to the clinical information reviewed the image; any disagreements were resolved by a third stroke specialist.
Statistical analysis
Categorical and range variables were reported as absolute number and frequencies (%), and continuous variables were reported as mean ± standard deviation (SD) for normal distribution data and median (Q1, Q3) for abnormal distribution data. Intergroup differences were assessed using the Chi-squared test for categorical variables, and Student's t test or Kruskal-Wallis test for continuous variables. Adjusted large artery atherosclerosis as confounding factor, the associations between cortex-involved lesions, vascular risk factors, and the subtype of AF were evaluated by univariate and multivariate regression analyses. In multivariate logistic regression, the odds ratios (ORs) and 95% confidence intervals (CIs) were used to calculate the probability values. A probability value of ≤0.05 was considered statistically significant. Statistical analysis was performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
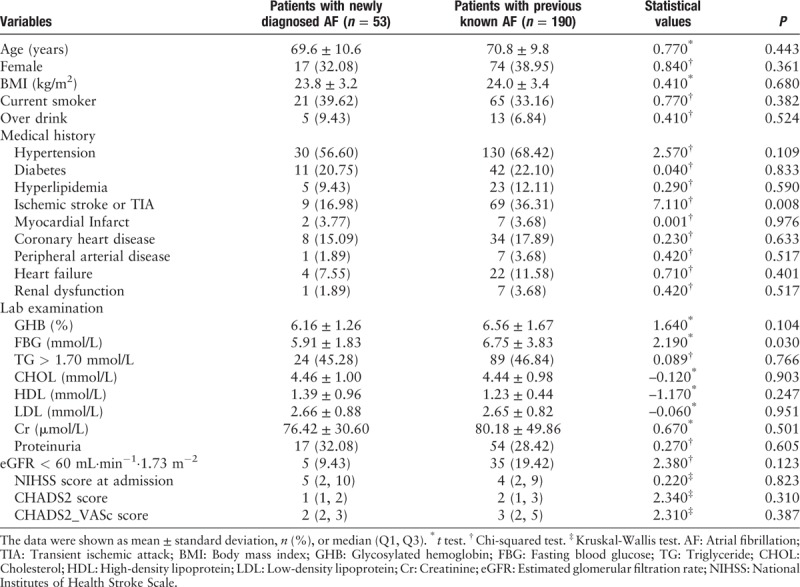
Among 243 acute ischemic stroke patients with AF, 190 (78.19%) were known AF and 53 (21.81%) were newly detected AF. There were 28 (52.83%) with AF persistent >24 h and 25 (47.17%) persistent ≤24 h in newly detected AF [Figure 1]. Patients with newly detected AF were likely to have a fewer history of stroke or TIA (16.98% vs. 36.31%, P = 0.008) and lower fasting blood glucose (5.91 ± 1.83 mmol/L vs. 6.75 ± 3.83 mmol/L, P = 0.030) than patients with known AF; other characteristics on baseline were similar between two groups (Table 1).
Table 1.
Clinical features of 243 acute ischemic stroke patients with AF.
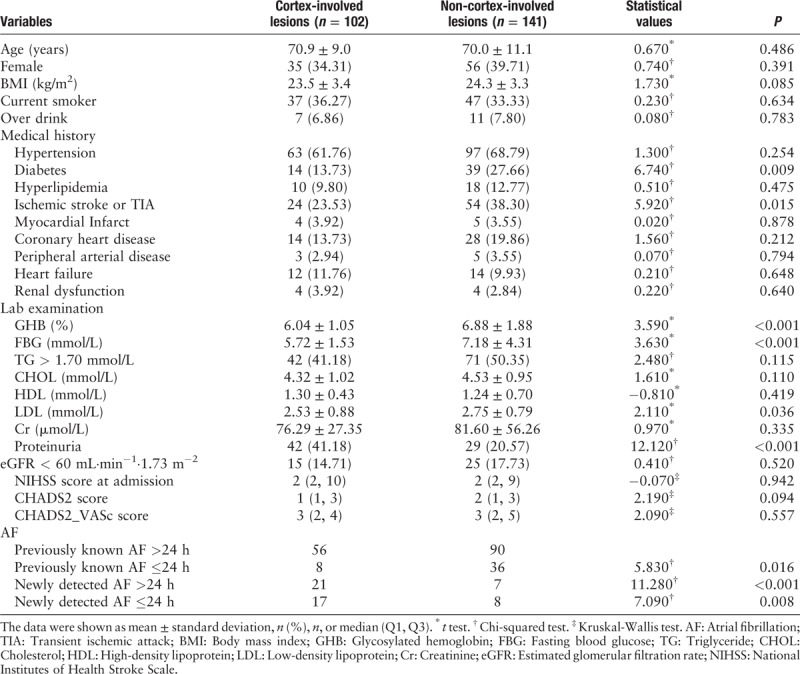
Comparison of demographic information, stroke risk factors, detailed medical history, and lab results between cortex-involved lesions and non-cortex-involved lesions are presented in Table 2. Among these 243 patients, 102 (41.98%) patients were with cortex-involved lesions. In these 102 patients with cortex-involved lesion, 38 (37.25%) were newly detected AF and further divided by duration of AF: 21 with AF persistent >24 h and 17 with AF ≤24 h. Compared with patients with non-cortex involved lesions, patients with cortex-involved lesions had more newly detected AF, either persistent >24 h (P < 0.001) or ≤24 h (P = 0.008). According to univariate regression analysis, patients with cortex-involved lesions presented more frequently with proteinuria (41.18% vs. 20.57%, P < 0.001), and less frequently with history of diabetes (13.73% vs. 27.66%, P = 0.009) and ischemic stroke or TIA (23.53% vs. 38.30%, P = 0.015). Patients with non-cortex-involved lesions had higher glycosylated hemoglobin (6.88 ± 1.88% vs. 6.04 ± 1.05%, P < 0.001), fasting blood glucose (7.18 ± 4.31 mmol/L vs. 5.72 ± 1.53 mmol/L, P < 0.001), and low-density lipoprotein (2.75 ± 0.79 mmol/L vs. 2.53 ± 0.88 mmol/L, P = 0.036).
Table 2.
Clinical features of patients with and without cortex-involved lesions.
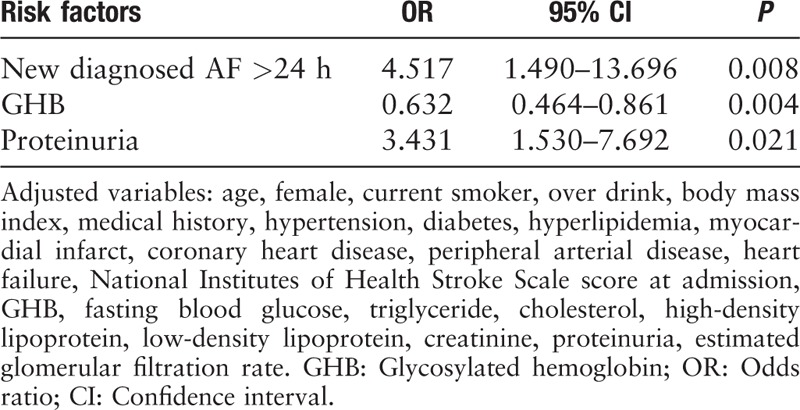
We conducted the multivariate logistic regression analysis for cortex-involved lesions compared to non-cortex-involved lesions after adjusted confounding factor. Cortex-involved lesions were significantly related to newly detected AF persistent >24 h (OR: 4.517, 95% CI: 1.490–13.696, P = 0.008), proteinuria (OR: 3.431, 95% CI: 1.530–7.692, P = 0.021), and glycosylated hemoglobin (OR: 0.632, 95% CI: 0.464–0.861, P = 0.004; Table 3). The value of C statistic was 0.759.
Table 3.
Multiple regression analysis of cortex-involved infarcts compared to non-cortex-involved lesions.
Discussion
A major finding of this study was that compared to known AF, newly detected AF, particularly those persistent >24 h, were significantly related to cortex-involved lesions, which exactly resulted in the latest stroke attack. AF is a widely recognized healthcare challenge with increasing prevalence across the world.[14,15] The development of continuous long-term monitoring improved detection of AF.[3,5,16,17] It is still a question that newly detected AF itself does cause embolism or rather is a marker of cardioembolic risk.[8,9] This doubt has raised concern about the actual clinical impact of newly detected AF in terms of death or dependence after stroke, and how it should be treated or prevented. The role of AF duration on stroke risk, however, is controversial. A pooled analysis confirmed the significant increased risk of stroke or TIA for AF ≥1 h compared with AF <1 h (HR: 1.89–2.09).[18] In a recently published analysis from the Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial (ASSERT), the increased stroke risk due to newly detected subclinical AF was seen clearly only among those patients whose longest episode of AF was at least 24 h in duration.[19] However, according to Ontario Stroke Registry, newly detected AF was related to a lower risk of ischemic stroke recurrence than known AF (7.0% vs. 9.6%).[20] All of these findings called into question that to what extent newly detected AF after stroke is the cause or a consequence.
Imaging pattern has received more and more attention to support etiologic classification[13,21] and predict recurrence after ischemic stroke or TIA.[22–24] Territorial distribution of the infarcts involving cortex or subcortex was suggested to indicate cardioembolic stroke mechanism. The cardioembolic stroke infarctions were usually in both anterior and posterior circulation or bilateral circulation of different age.[11] According to the previous report, patients with newly detected AF had reduced risk of cardioembolic stroke compared to the patients with known AF (OR: 0.11, 95% CI: 0.03–0.36, P < 0.001),[25] which studied defined cardioembolic stroke as cortex-involved territorial lesion without relevant large artery diseases or multiple non-contiguous lesions in bilateral hemispheres or both anterior and posterior circulations. In our study, only 14 patients complied with the second standards above. We only chose cortex-involved lesions to analysis to reduce heterogeneity. It was worth noting that we only analyzed DWI lesions which represented the new attack of this stroke. We have found that newly detected AF persistent >24 h was more likely associated with new cortex-involved lesions. We could not provide information on the potential role of cortex damage (such as insular) in the development of newly detected AF, which may have been attributable to the small sample size. Although we could not elucidated the exact neurogenic pathophysiology in stroke patients with newly detected AF, we might provide some clues that newly detected AF after stroke or TIA may be causally linked to the occurrence of new lesions in some cases.
We also found that cortex-involved lesions were significantly related to proteinuria and glycosylated hemoglobin. Proteinuria, which is an important potentially modifiable measure of kidney function, has been linked to elevations in the risk of stroke and poor outcomes and death after stroke in populations with and without chronic kidney disease.[26,27] However, the relationship of proteinuria and etiologic classification is unclear. Growing evidences have suggested an independent association between the presence of proteinuria and new-onset AF.[28–30] The biologic role of proteinuria as a marker of endothelial dysfunction, sympathetic activation may explain the possible mechanisms of the elevated risk of vascular events and new-onset AF.[31,32] Previous studies reported different effects of the estimated glomerular filtration rate (eGFR) and proteinuria on the risk of AF.[27,28,30] In our study, we analyzed both eGFR and proteinuria and only proteinuria reached statistical significance. We did not see definite interaction between eGFR and proteinuria. This controversy need further study with more data on kidney dysfunction. Current stroke risk scoring systems for patients with AF include a diagnosis of diabetes mellitus.[33,34] The association between glycosylated hemoglobin, a measure of glycemia during the prior 3 months, and ischemic stroke among diabetes mellitus patients has been investigated in general populations[35] and patients with AF.[36] Metabolic changes of diabetes could lead to atrial structural remodeling, atrial electrical remodeling, atrial electromechanical remodeling, and atrial autonomic remodeling.[37] So diabetes was closely related to AF which usually caused embolic stroke. Therefore, the correlation between glycosylated hemoglobin and brain cortical embolism was support by previous studies.
There were several limitations in our study. First, the exact etiologic classification of stroke or TIA could not be obtained due to lack of imaging information on large artery diseases. The cortex-involved lesions were used to represent the etiologic classification of cardioembolism. However, it must be acknowledged that accurate etiologic classification of stroke may not be possible in every case of stroke even with advanced neuroimaging and vascular imaging techniques. Second, the paroxysmal AF or pre-existing AF might be included in patients with newly detected AF on account of the reality of insufficient screening for AF before stroke or TIA. Finally, the small sample size restricted to subdivide the duration of AF into shorter or longer hours which possibly affected on the risk of embolism. Although there are many unknowns, the findings merit attention and need further research.
In conclusion, although newly detected AF persistent >24 h was associated with cortex-involved lesions, which was highly suggestive of cardioembolism. Further studies are needed to question how isolated newly detected AF causes embolic events.
Funding
This study was supported by grants from Beijing Municipal Administration of Hospitals Incubating Program (PX2018022), the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2015BAI12B04 and 2015BAI12B02), and the Beijing Municipal Commission of Health and Family Planning (SML20150502).
Conflicts of interest
This study was sponsored and funded by Bayer HealthCare Pharmaceuticals. Bayer HealthCare Pharmaceuticals took no part in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Footnotes
How to cite this article: Li SY, Yang XM, Zhao XQ, Liu LP, Wang YL, Jiang Y, Wang YJ. Newly detected atrial fibrillation is associated with cortex-involved ischemic stroke. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000390
References
- 1.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 2.Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 2018; 39:1330–1393. doi: 10.1093/eurheartj/ehy136. [DOI] [PubMed] [Google Scholar]
- 3.Sposato LA, Cipriano LE, Saposnik G, Ruiz Vargas E, Riccio PM, Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 2015; 14:377–387. doi: 10.1016/S1474-4422(15)70027-X. [DOI] [PubMed] [Google Scholar]
- 4.Yang X, Li S, Zhao X, Liu L, Jiang Y, Li Z, et al. Atrial fibrillation is not uncommon among patients with ischemic stroke and transient ischemic stroke in China. BMC Neurol 2017; 17:207.doi: 10.1186/s12883-017-0987-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wachter R, Groschel K, Gelbrich G, Hamann GF, Kermer P, Liman J, et al. Holter-electrocardiogram-monitoring in patients with acute ischaemic stroke (Find-AFRANDOMISED): an open-label randomised controlled trial. Lancet Neurol 2017; 16:282–290. doi: 10.1016/S1474-4422(17)30002-9. [DOI] [PubMed] [Google Scholar]
- 6.Brachmann J, Morillo CA, Sanna T, Di Lazzaro V, Diener HC, Bernstein RA, et al. Uncovering atrial fibrillation beyond short-term monitoring in cryptogenic stroke patients: three-year results from the cryptogenic stroke and underlying atrial fibrillation trial. Circ Arrhythm Electrophysiol 2016; 9:e003333.doi: 10.1161/CIRCEP.115.003333. [DOI] [PubMed] [Google Scholar]
- 7.Martin DT, Bersohn MM, Waldo AL, Wathen MS, Choucair WK, Lip GY, et al. Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices. Eur Heart J 2015; 36:1660–1668. doi: 10.1093/eurheartj/ehv115. [DOI] [PubMed] [Google Scholar]
- 8.Sposato LA, Riccio PM, Hachinski V. Poststroke atrial fibrillation: cause or consequence? Critical review of current views. Neurology 2014; 82:1180–1186. doi: 10.1212/WNL.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 9.Ustrell X, Tagawa M. Some light in the shadows of atrial fibrillation and stroke: to look or not to look. Neurology 2017; 89:1536–1537. doi: 10.1212/WNL.0000000000004504. [DOI] [PubMed] [Google Scholar]
- 10.Kim BJ, Sohn H, Sun BJ, Song JK, Kang DW, Kim JS, et al. Imaging characteristics of ischemic strokes related to patent foramen ovale. Stroke 2013; 44:3350–3356. doi: 10.1161/STROKEAHA.113.002459. [DOI] [PubMed] [Google Scholar]
- 11.Pepi M, Evangelista A, Nihoyannopoulos P, Flachskampf FA, Athanassopoulos G, Colonna P, et al. Recommendations for echocardiography use in the diagnosis and management of cardiac sources of embolism: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur J Echocardiogr 2010; 11:461–476. doi: 10.1093/ejechocard/jeq045. [DOI] [PubMed] [Google Scholar]
- 12.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Kang DW, Chalela JA, Ezzeddine MA, Warach S. Association of ischemic lesion patterns on early diffusion-weighted imaging with TOAST stroke subtypes. Arch Neurol 2003; 60:1730–1734. doi: 10.1001/archneur.60.12.1730. [DOI] [PubMed] [Google Scholar]
- 14.Healey JS, Oldgren J, Ezekowitz M, Zhu J, Pais P, Wang J, et al. Occurrence of death and stroke in patients in 47 countries 1 year after presenting with atrial fibrillation: a cohort study. Lancet 2016; 388:1161–1169. doi: 10.1016/S0140-6736(16)30968-0. [DOI] [PubMed] [Google Scholar]
- 15.Freedman B, Potpara TS, Lip GYH. Stroke prevention in atrial fibrillation. Lancet 2016; 388:806–817. doi: 10.1016/s0140-6736(16)31257-0. [DOI] [PubMed] [Google Scholar]
- 16.Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014; 370:2467–2477. doi: 10.1056/NEJMoa1311376. [DOI] [PubMed] [Google Scholar]
- 17.Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014; 370:2478–2486. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- 18.Boriani G, Glotzer TV, Santini M, West TM, De Melis M, Sepsi M, et al. Device-detected atrial fibrillation and risk for stroke: an analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J 2014; 35:508–516. doi: 10.1093/eurheartj/eht491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Gelder IC, Healey JS, Crijns H, Wang J, Hohnloser SH, Gold MR, et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J 2017; 38:1339–1344. doi: 10.1093/eurheartj/ehx042. [DOI] [PubMed] [Google Scholar]
- 20.Sposato LA, Cerasuolo JO, Cipriano LE, Fang J, Fridman S, Paquet M, et al. Atrial fibrillation detected after stroke is related to a low risk of ischemic stroke recurrence. Neurology 2018; 90:e924–e931. doi: 10.1212/WNL.0000000000005126. [DOI] [PubMed] [Google Scholar]
- 21.Wessels T, Wessels C, Ellsiepen A, Reuter I, Trittmacher S, Stolz E, et al. Contribution of diffusion-weighted imaging in determination of stroke etiology. AJNR Am J Neuroradiol 2006; 27:35–39. doi: 10.1016/j.neuroimage.2005.07.039. [PMC free article] [PubMed] [Google Scholar]
- 22.Wen HM, Lam WW, Rainer T, Fan YH, Leung TW, Chan YL, et al. Multiple acute cerebral infarcts on diffusion-weighted imaging and risk of recurrent stroke. Neurology 2004; 63:1317–1319. doi: 10.1212/01.wnl.0000140490.22251.b6. [DOI] [PubMed] [Google Scholar]
- 23.Yaghi S, Rostanski SK, Boehme AK, Martin-Schild S, Samai A, Silver B, et al. Imaging parameters and recurrent cerebrovascular events in patients with minor stroke or transient ischemic attack. JAMA Neurol 2016; 73:572–578. doi: 10.1001/jamaneurol.2015.4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jing J, Meng X, Zhao X, Liu L, Wang A, Pan Y, et al. Dual antiplatelet therapy in transient ischemic attack and minor stroke with different infarction patterns: subgroup analysis of the CHANCE randomized clinical trial. JAMA Neurol 2018; 75:711–719. doi: 10.1001/jamaneurol.2018.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim Y, Lee SH. Embolic stroke and after-admission atrial fibrillation. Int J Cardiol 2016; 222:576–580. doi: 10.1016/j.ijcard.2016.07.265. [DOI] [PubMed] [Google Scholar]
- 26.Wang A, Liu X, Su Z, Chen S, Zhang N, Wu S, et al. Two-year changes in proteinuria and the risk of stroke in the Chinese population: a prospective cohort study. J Am Heart Assoc 2017; 6: doi: 10.1161/JAHA.117.006271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandsmark DK, Messe SR, Zhang X, Roy J, Nessel L, Lee Hamm L, et al. Proteinuria, but not eGFR, predicts stroke risk in chronic kidney disease: chronic renal insufficiency cohort study. Stroke 2015; 46:2075–2080. doi: 10.1161/STROKEAHA.115.009861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bansal N, Zelnick LR, Alonso A, Benjamin EJ, de Boer IH, Deo R, et al. eGFR and albuminuria in relation to risk of incident atrial fibrillation: a meta-analysis of the Jackson heart study, the multi-ethnic study of atherosclerosis, and the cardiovascular health study. Clin J Am Soc Nephrol 2017; 12:1386–1398. doi: 10.2215/CJN.01860217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molnar AO, Eddeen AB, Ducharme R, Garg AX, Harel Z, McCallum MK, et al. Association of proteinuria and incident atrial fibrillation in patients with intact and reduced kidney function. J Am Heart Assoc 2017; 6: doi: 10.1161/JAHA.117.005685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alonso A, Lopez FL, Matsushita K, Loehr LR, Agarwal SK, Chen LY, et al. Chronic kidney disease is associated with the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011; 123:2946–2953. doi: 10.1161/CIRCULATIONAHA.111.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation 2003; 107:87–92. [DOI] [PubMed] [Google Scholar]
- 32.Huang SY, Chen YC, Kao YH, Hsieh MH, Chen YA, Chen WP, et al. Renal failure induces atrial arrhythmogenesis from discrepant electrophysiological remodeling and calcium regulation in pulmonary veins, sinoatrial node, and atria. Int J Cardiol 2016; 202:846–857. doi: 10.1016/j.ijcard.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870. [DOI] [PubMed] [Google Scholar]
- 34.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010; 137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 35.Selvin E, Coresh J, Shahar E, Zhang L, Steffes M, Sharrett AR. Glycaemia (haemoglobin A1c) and incident ischaemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Lancet Neurol 2005; 4:821–826. doi: 10.1016/S1474-4422(05)70227-1. [DOI] [PubMed] [Google Scholar]
- 36.Ashburner JM, Go AS, Chang Y, Fang MC, Fredman L, Applebaum KM, et al. Effect of diabetes and glycemic control on ischemic stroke risk in AF patients: ATRIA study. J Am Coll Cardiol 2016; 67:239–247. doi: 10.1016/j.jacc.2015.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tadic M, Cuspidi C. Type 2 diabetes mellitus and atrial fibrillation: from mechanisms to clinical practice. Arch Cardiovasc Dis 2015; 108:269–276. doi: 10.1016/j.acvd.2015.01.009. [DOI] [PubMed] [Google Scholar]


