Abstract
Objective:
Dermoscopy is a useful technique for improving the diagnostic accuracy of various types of skin disorders. In China, dermoscopy has been widely accepted, and domestic researchers have made tremendous progress in the field of dermoscopy. The main purpose of this review is to summarize the current status of dermoscopy in China and identify its future directions.
Data sources:
Articles included in this review were obtained by searching the following databases: Wanfang, China National Knowledge Infrastructure, PubMed, and the Web of Science. We focused on research published before 2019 with keywords including dermoscopy, dermoscopic, dermoscope and trichoscopy.
Study selection:
A total of 50 studies were selected. Of these studies, 20 studies were in Chinese and 30 in English, research samples of all the studies were collected from Chinese populations.
Results:
Since 2000, more than 380 articles about dermoscopy have been published in domestic or foreign journals. Dermoscopy can improve the diagnostic accuracy of neoplastic diseases, evaluating the therapeutic effect of treatment, and determining the treatment endpoint, and it can also assist in the differential diagnosis of inflammatory diseases and in the assessment of the severity of the disease. In addition, researches about the applications of dermoscopy during surgical treatment have been published. Training courses aiming to improve the diagnostic ability of dermatologists, either face-to-face or online, have been offered. The Chinese Skin Image Database, launched in 2017 as a work platform for dermatologists, has promoted the development of dermoscopy in China. Computer-aided diagnostic systems based on the Chinese population are ready for use. In the future, cooperation, resource sharing, talent development, image management, and computer-aided diagnosis will be important directions for the development of dermoscopy in China.
Conclusion:
Dermoscopy has been widely used and developed in China, however, it still needs to address more challenges in the future.
Keywords: Dermoscopy, China, Current status, Future
Introduction
Non-invasive technologies, such as dermoscopy, reflectance confocal microscopy, and optical coherence tomography, can be used to provide further examination of suspicious lesions, which can increase the accuracy of diagnosis and allow unnecessary biopsies to be avoided.[1] Dermoscopy can capture characteristics of lesions and eliminate the influence of surface light, so it can effectively assist clinicians in diagnosis. It is the main tool used for early diagnosis, screening, and computer-aided detection systems. In the past decade, non-invasive imaging tools have been widely used in China, especially dermoscopy. Sub-surface structures such as the epidermis, dermal papilla, and color can be better observed in vivo, by eliminating surface reflection at the skin air interface.[2] Dermoscopy can improve the diagnostic sensitivity for various types of skin disorders without reducing the specificity.[3] In addition, dermoscopy can help dermatologists to better define the tumor margin, measure post-treatment effects and classify diseases.
From the literatures of the China National Knowledge Infrastructure, Wan Fang, PubMed, and Web of Science, we can observe that the volume of articles on this topic in China has an upward trend. Based on preliminary work, the Chinese Skin Image Database (CSID), a work platform for dermatologists, was launched in 2017. CSID has set up an Open Research Funding of CSID (CSID-ORF) and have promoted scientific research in the field of dermoscopy in China.[4] The dermoscopic features of diseases, dermoscopic application value, the evaluation of the therapeutic effect, and the classification of the disease are the main research directions of the field of dermoscopy in China.
Diagnostic value of dermoscopy
Dermoscopy as a non-invasive diagnostic technique can increase the sensitivity of detection of skin cancer[5] and inflammatory dermatoses,[6] and decrease the number of unnecessary biopsies for benign lesions. In China, more than half of studies on dermoscopy involve describing the dermoscopic characteristics of skin disease. The identified dermoscopic features can assist dermatologists in making diagnoses. The sensitivity and specificity of dermoscopy for diagnosis in China are summarized in Table 1.
Table 1.
The sensitivity and specificity of dermoscopy for diagnosis.
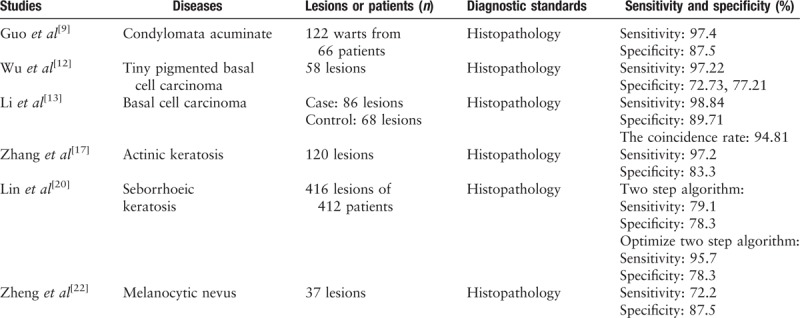
Condylomata acuminata (CA) is a type of infectious disease caused by the human papilloma virus, and it usually occurs in genital areas. Dermoscopic patterns of CA include non-specific pattern, fingerlike pattern, mosaic pattern and knoblike pattern.[7] CA has characteristic vascular features, mainly including dotted, annular, dendritic, and hairpin vessels.[7,8] However domestic dermatologists also express dermoscopic features in regards to the lesion's appearance, such as a papillary pattern or a flat pattern.[8,9] Compared to the sensitivity of naked-eye examination (87.7%), dermoscopy improves the sensitivity of diagnosis CA (to 97.4%).[9] Dermoscopy is a non-invasive, convenient, and efficient diagnostic technique for CA, and it can identify lesions that are invisible to the naked eye.
Basal cell carcinoma (BCC) is one of the most common skin cancers in China. The dermoscopic criteria of BCC are influenced by several factors, including histopathologic sub-type, location, gender, age, and pigmentary traits.[10] BCC-related criteria include arborizing vessels, superficial fine telangiectasias, blue-gray ovoid nests, multiple blue-gray dots and globules, in-focus dots, maple leaf-like areas, spoke wheel areas, concentric structures, ulceration, multiple small erosions, shiny white/red structureless areas, and short white streaks.[10] An overseas meta-analysis showed that the pooled sensitivity and specificity of dermoscopy for BCC was 91.2% and 95.0%, respectively.[11] In China, the sensitivity of dermoscopy for the diagnosis of tiny pigmented BCCs was as high as 97.2%, while the specificity was 70.0% or 87.5% (with two dermatologists).[12] Li et al[13] concluded that the sensitivity and specificity of demoscopy diagnosis of BCC were 98.8% and 89.7%, respectively. The dermoscopic indications used by Li et al to diagnose BCC included: classic diagnostic models, non-classical indications, and other indications,[13] which may be the reason for the improved specificity. The difference in skin pigmentation between the Chinese population and the white population may affect its specificity, and melanocyte lesions may be more difficult to distinguish from BCC in China. Moreover, doctors’ knowledge of dermoscopy can also directly affect the specificity of the diagnosis. Despite all this, dermoscopy has a high correlation with histopathology in the diagnosis of BCC.
Actinic keratosis (AK) is a pre-cancerous lesion related to long-term ultraviolet exposure that has a risk of progressing to squamous cell carcinoma (SCC). The dermoscopic features of non-pigmented AK are keratin/scales, a strawberry’ pattern, a red pseudo network, targetoid-like folliculus, and a rosette sign.[14,15] For pigmented AK, a brownish to gray pseudo network, “annular granular” structures and dark-brown dots and globules around the follicular ostia can be observed.[14,15] When the apparent keratin and the large blood vessels around the hair follicle appear simultaneously, it is highly suspected that the lesion has progressed to SCC.[16] Compared with histopathology, the sensitivity, and specificity of dermoscopy for the diagnosis of AK were 97.2% and 83.3%, respectively.[17] An individual rosette sign has no value in diagnosing AK; however, this sign can help diagnose AK with the addition of other clinical information or dermoscopic features.[18] Dermoscopy can help dermatologists diagnose SCC developing from AK early, which can effectively avoid any damage from treating skin cancer. An expert consensus about squamous cell neoplasms diagnosed by dermoscopy was proposed in 2018[19]. The sensitivity and specificity of glomerular vessels for the diagnosis of intraepidermal carcinoma were 60% and 94%, and white circles had the highest specificity (87%) for the diagnosis of the keratoacanthoma and SCC.[5]
Melanoma, nevus, and seborrheic keratosis (SK) are three types of clinically confusing lesions. Melanoma is a malignant type of tumor; nevus and SK are benign tumors. Melanoma and nevus are both types of tumors derived from melanocytes, and it is difficult for dermatologist to distinguish due to their similar clinical manifestations. Because of the melanin deposition in the hyperplastic epidermal cells, SK with inflammation or irritative damage is very similar to melanoma. A two-step algorithm has been proposed for the diagnosis of SK, including a first step of applying an exclusion algorithm and a second step of applying a diagnostic algorithm. Lin et al[20] added some criteria to the two-step algorithm, such as lack of a blue-gray or blue-white color, a sharp demarcation, mica-like structures and a yellowish color, which increased the sensitivity from 79.1% to 95.7% while maintaining the same specificity. Another study[21] showed better sensitivity and specificity (sensitivity: 91.7%, 95.8%; specificity: 83.6%, 86.0%) by studying the diagnostic accuracy of doctors. SK has a large number of dermoscopic features, but the features lack specificity. For nevus, a study based on 32 lesions achieved a sensitivity of 72.2% and a specificity of 87.5%.[22] This low sensitivity may be related to too harsh dermoscopic diagnostic criteria and a small sample size. An expert consensus about nevus diagnosis by dermoscopy was proposed in 2017.[23] Unfortunately, there are no reliable data on melanoma in China. In overseas studies, the sensitivity of clinical diagnosis of melanoma was increased from around 60% to 90% through adding dermoscopy to clinical examination.[24]
Factors affecting the accuracy and specificity of dermoscopic diagnosis include sample size, diagnostic level, ethnicity,[20] diagnostic criteria, etc. Although dermatologists can usually distinguish most lesions, future studies on the dermoscopic patterns of lesions and regulated diagnostic algorithms are necessary to improve the sensitivity and specificity of diagnosis.
Treatment efficacy
Dermoscopy not only can amplify skin lesions, but also can be used to observe micro-structures that are invisible to the naked eye. In view of the above advantages, a dermoscope can accurately record and judge changes in the lesion's condition. Evaluation of treatment efficacy by dermoscopy in China is summarized in Table 2.
Table 2.
Functions of dermoscopy in evaluating the therapeutic efficacy.
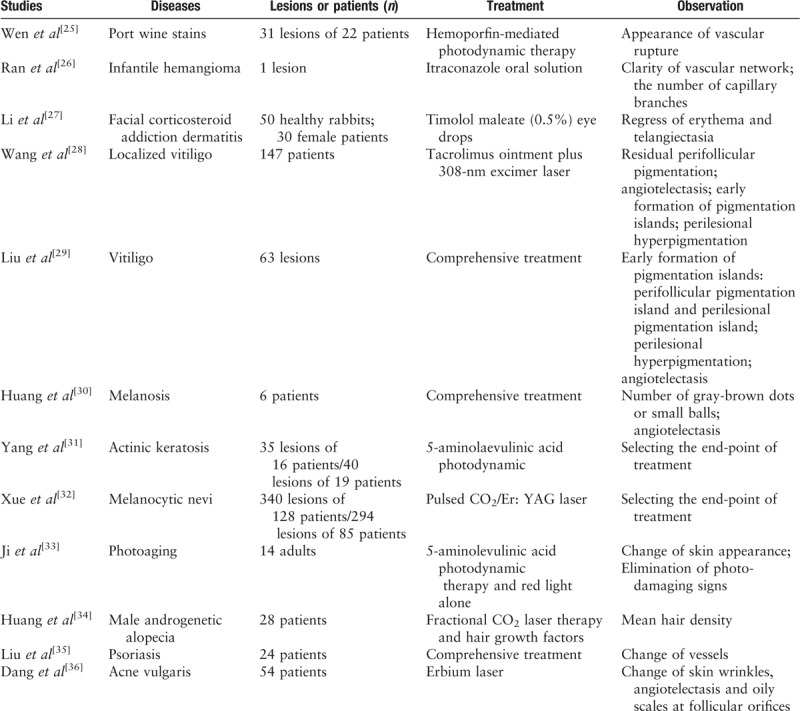
For treatment of port wine stains, signs of vascular rupture and punctate or globular hemorrhagic shadows indicate better efficacy, an appropriate dose and the end-point of treatment, which can be observed through a dermoscope.[25] When observing infantile hemangiomas with a dermoscope, the regression of the vasculature is a major phenomenon at the end of treatment.[26] Facial corticosteroid addiction dermatitis is caused by improper use of topical steroids on the face, mainly characterized by persistent facial erythema and telangiectasia. The regression of erythema and telangiectasia often suggest effective treatment, which can be dynamically and objectively observed through a dermoscope.[27] Dermoscopy can be used to observe the changes of blood vessels in a timely and dynamic manner, and then guides the end-point of treatment.
Pigmented skin diseases refer to increase or decrease of melanin in the skin. The formation of an early pigmentation island is the most important manifestation seen by dermoscopy to judge the therapeutic effect in patients with vitiligo.[28,29] The number of gray-brown dots or small balls is also suitable to assess the treatment efficacy of melanosis.[30] Dermoscopy is a technology for evaluating the therapeutic efficacy in patients with pigmented skin diseases and it can detect changes invisible to the naked eye, which improves patient compliance and thus treatment efficacy.
For neoplastic disease, the aim of dermoscopic techniques is to choose the endpoints of treatment and follow-up.[31,32] Clinicians use dermoscopy to observe whether there is any residual lesion after-treatment of the neoplastic disease, and the patient can be followed up to determine whether the disease has recurred. In addition, dermoscopy can also be used to observe the therapeutic effects of facial rejuvenation[33] and androgenetic alopecia (AGA).[34]
Liu et al[35] performed a continuous dynamic observation of patients with psoriasis by dermoscopy and found that the severity changes of psoriasis were positively correlated with the vascular changes on the dermoscopy. As the treatment works, the diameter of the glomerular vessel begins to decline. After the treatment of acne vulgaris with an erbium laser, Dong et al[36] observed that the skin texture became thinner as the pores shrank and that the oily scales of the hair follicles disappeared with no significant telangiectasia by dermoscopy. In summary, evaluating the therapeutic effects of various diseases by dermoscopy is feasible.
Disease classification
The features seen under a dermoscope have certain prospects in terms of disease staging, and we can more accurately stage the disease and determine its severity with the application of dermoscopy. Therefore, its application in more diseases needs to be explored. The application of dermoscopy in disease staging and typing is summarized in Table 3.
Table 3.
Auxiliary classification by dermoscopy.
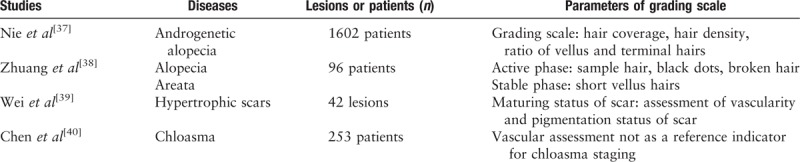
The use of dermoscopy in classification of three diseases (alopecia areata, scar, and AGA) has been reported. AGA is a progressive hair loss disease that can begin shortly after puberty and is the most common type of alopecia in men and women. AGA in men is divided into 12 grades according to the severity and hair coverage based on the Hamilton-Norwood classification. However, AGA in women is divided into three grades. Hair parameters, such as hair coverage, and the hair density, ratio of vellus to terminal hairs, are measured using dermoscopy. By combining relevant dermoscopic parameters and clinical images, we obtained a good consistency and reproducibility with grading scales for AGA in men and women.[37]
Alopecia areata is a sudden, unexplained, inflammatory non-scarring alopecia disease. Alopecia areata can be divided into active periods, quiescent periods, and recovery periods. Previous grading scales were subjective and often lacked reliable clinical information. Dermoscopic signs of the hair loss area of patients with alopecia areata include yellow spots, black dots, broken hair, sample hairs, and short vellus hairs. Black dots and broken hair are characteristic features of the active phase, and the appearance of short vellus hairs is a sign of disease recovery.[38]
There was a positive correlation between the number of melanocytes in the chloasma and the number of vascular counts and morphology scores by dermoscopy in China,[39] but there was no difference in the vascular morphology scores for different chloasma stages.[40] One study showed that the vascular morphology score was statistically significant in assessing the treatment of chloasma.[41] Vascular morphology scores may be related to the color of the lesion but not to the stage of the disease.
Pigmentation, pliability, vascularity, and thickness are important indicators for assessing scars. By comparing the Red-Green-Blue redness and lightness values of the dermoscopic picture with the redness and lightness measured by a spectrocolorimeter, to the Vancouver scar scale vascularity and pigmentation score, Wei et al[42] found that dermoscopy is a potential tool to assess the vascularity and pigmentation of hypertrophic scars.
Determination of the tumor margin
In China, there are few studies on the application of dermoscopy in surgery. Foreign research on dermoscopy in surgery mainly focused on determinating the margin of BCC[43] and SCC,[44] and the combined application of Mohs micrographic surgery.[45] The comparison between using a naked eye and a dermoscope showed that using dermoscopic detection of the surgical excision margin of BCC is superior to observation with the naked eye.[46] For traditional surgery, dermoscopy can more accurately trace the tumor boundary before surgery, so that the tumor tissue can be excised as cleanly as possible to avoid recurrences and secondary surgery. It is feasible to use dermoscopy to determine tumor boundaries and surgical excision margins. However, we need more cases and studies to confirm the best margins for reducing surgical trauma.
In addition, one case showed that a dermoscope can be used to guide suturing when performing a surgery in a small space. Of course, the head of the dermoscope needs to be fixed at a distance of 5 to 10 cm from the skin lesion.[47] In summary, the application of dermoscopy in surgery can be more extensive and flexible.
Training
As a real-time, multipoint, multiple-time, non-invasive diagnostic tool, dermoscopy avoids unnecessary biopsies, minimizes skin trauma, and enables early detection and treatment of diseases. However, the accuracy of dermoscopy diagnosis depends on the experience of the examiner. The doctor who performs dermoscopic examination should not only receive dermoscopic training courses but also receive formal dermatology and skin oncology training.[48] Gender, region, age, dermoscopic training, and owning dermoscopic books have influences on the rate of dermoscopic use, and more convenient programs for dermoscopic training can increase the enthusiasm of dermatologists for the use of dermoscopes. An American survey pointed out the fact that despite the growing number of practitioners incorporating dermoscopy into their daily practices, there remain significant barriers, such as lack of training resources, which are preventing its widespread adoption.[49]
In recent years, online learning has become a widely accepted learning method, with the advantages of a flexible time schedule, cost savings and teacher resources sharing. With the help of UMER platform (a platform including online training courses about dermocopy and more than 20,000 registered dermatologists), the “CSID-Skin Imaging College” was officially launched in 2017 after an extensive preparation time. “CSID Skin Imaging College” is characterized by “distributed version” skin imaging training (the course is short, approximately 5 to 10 min, and focuses on specific issues), a “honeycomb” modular learning mode (the trainer can make full use of downtime to learn and carry out targeted self-learning based on their knowledge state) and a “systematic” knowledge structure. The first batch of 30 “micro-courses” has been launched. So for, 80 “micro-courses” have been published online, and 200 to 300 courses will be published within 2 years. The establishment of the “CSID-Skin Imaging College” has opened up a new model of standardized training of skin imaging technology in China, which not only helps to improve the level of dermoscopic diagnosis of dermatologists but also indirectly improves the level of dermatology in China. Since the establishment of the “CSID-Skin Imaging College,” the number of online scholars has exceeded 6000, and it has become a “maximum class” in the field of skin imaging.[4] In addition to the “CSID-Skin Imaging College,” traditional training courses are still an important channel to acquire skin image related knowledge and improve dermoscopic skills for dermatologists in China.
Computer-aided diagnosis
In addition to general visual screening, early diagnosis of skin diseases based on dermoscopy is the most commonly used method. Compared with other skin tools, the dermoscope can be used to observe deeper characteristics that are invisible to the naked eye, thereby significantly improving the sensitivity of the diagnosis. However, dermoscopic image analysis relying on real-time visual observation is not only time-consuming and laborious, but the diagnosis results can also be susceptible to subjective factors such as doctors’ experience. With the development of computer-aided diagnosis (CAD) systems, the automatic recognition and classification of skin diseases based on dermoscopic images can improve the efficiency and accuracy of diagnosis.
The traditional CAD system based on dermoscopic images mainly includes four steps: artifact and noise reduction, lesion segmentation, feature extraction, and classification. Complex clinical and pathological types; highly similar lesion appearances; dermoscopic image noise such as uneven illumination, black frame, hair, skin texture, etc, bring great difficulties to the classification of skin diseases based on dermoscopic images.
For dermoscopic images, uneven illumination, defocus blur, low contrast, hair, and bubbles also introduce difficulties for CAD system, and influence lesion segmentation. Dermoscopic image quality problems due to defocus blur, uneven illumination[50] or both can be assessed by a non-reference quality assessment algorithm,[51,52] which is highly consistent with visual evaluation. Objective image evaluation ensures high-quality images for physicians and CAD, and improves the accuracy of analysis and diagnosis. Xie et al[53] have investigated dermoscopic images of melanoma that were repaired by a partial differential equation (PDE)-based unsupervised repair algorithm and found this approach was more convenient for dermatologists to observe and improve the accuracy of lesion segmentation. Another novel hair repaired algorithm that aimed to remove hair and restore the occluded texture of tumors was compared with three other algorithms (linear interpolation method, non-linear PDE and exemplar-based repairing techniques),[54] and we have summarized the advantages and disadvantages of the three algorithms in Table 4.
Table 4.
Advantages and disadvantages of three algorithms.

Boundary detection or lesion segmentation is one of the most important steps for a CAD system. Accurate segmentation of skin lesions is the key to CAD systems for tumors. The factors affecting the automated border detection include a great variety of lesion shapes, sizes, colors, and artifact noises. In China, skin dynamic programing (SDP), an automated approach to border detection for skin disease, was applied to lesion border detection. Compared with statistical region merging, dermatologist-like tumor extraction algorithm and region-based active contour, SDP had higher sensitivity and specificity for lesion segmentation in dermoscopic images.[55] Abbas et al[56] proposed improved dynamic programming, which added color and texture weights. This improved method has superiority in skin tumor area extraction with a sensitivity of 96.64%, a specificity of 98.14%, and an error probability of 5.23%.[56] Subsequently, researchers proposed a variety of algorithms for skin lesion extraction, such as: automated saliency-based lesion segmentation,[57,58] adaptive segmentation based on a multi-classification model, two-dimensional image analysis,[59] fuzzy entropy with a level set algorithm,[60] and saliency combined with an Otsu threshold.[61]
In China, the program of dermoscopic image classification is mainly based on deep learning algorithms. Many factors may influence image classification, such as parameter settings, extracted features or feature combinations, and quality of the experimental samples. Xie et al[62] proposed a new network of ensemble models to classify melanomas, especially non-representative ones, and achieved an improved result. Li et al[63] proposed a combined framework (multi-scale fully convolutional residual networks and a lesion index calculation unit to simultaneously carry out segmentation and classification of melanoma lesions). In 2006, Yu et al[56] designed a more than 50 layers of convolutional networks for melanoma classification and took measures to avoid the impact of insufficient data. Yang et al[64] classified dermoscopic melanoma images by convolutional neural networks based on region average pooling, which can directly add the lesion location information into the classification, and they reached an excellent classification result. It has been revealed that embedding diagnosis/classification scenarios in deep neural network algorithms can improve the accuracy of image classification.[65] In a competition between dermatologists and CAD systems in 2018, deep convolutional neural networks for dermoscopic images gave the same results as experienced dermatologists for the diagnosis of nevus and SK.[66]
To improve the research and application levels of CAD in the field of dermatology, a substantial amount of basic training needs to be broadly performed and actively promoted. We should establish a skin image database based on the Chinese population as soon as possible, and we should obtain a unified standard of massive skin image data (including clinical, dermoscope, reflectance confocal microscopy, high-frequency skin ultrasound, histopathology, etc) and accurately label it, which will provide a basis for CAD. At present, CSID has initiated a quality system construction for skin images and has collected more than 200,000 multi-dimensional skin imaging data of various skin diseases (more than 300 types). In addition, CSID has cooperated with a team that has the ability to develop big data to jointly develop annotation software, which can realize fast, accurate and intelligent labeling of skin image data. Using this software, CSID has completed the annotation of 180,000 sets of high-quality “multi-dimensional” skin image data (including multiple skin imaging data and histopathological images), and it has become the core database for the development of CAD systems.
The recently released “Youzhi Skin” (a software installed on a smartphone) also better meets the needs of dermatologists for image collection and management in clinical work and scientific research. Based on the CSID database and through deep learning, Youzhi Skin achieves dermoscope-based disease diagnoses. In an evaluation by an expert group, the recognition rate of benign and malignant skin tumors by Youzhi Skin 2.0 reached 91.2% and the coincidence rate of disease categories reached 81.4%. More than 3000 users in China have started using the Youzhi Skin application.
Future trend of dermoscopy in China
Dermoscopy expands the visual reach of the dermatologist and truly records the manifestations and changes of the skin disease. According to CSID-ORF, 98.6% of the applicants are from tertiary hospitals.[4] Although dermoscopy has achieved certain developments and has been accepted and favored by increasing numbers of dermatologists in China, the use of a dermoscope has mainly been concentrated in tertiary hospitals.
It is necessary to strengthen cooperation and implement large-scale, multi-center clinical research and case-control studies. There are a large number of patients and many kinds of skin diseases in China. However, current research on dermoscopy has the disadvantages of only including a limited number of disease types and using small sample sizes. To some extent, the distribution of dermoscopic traits, especially pigmented skin diseases, are affected by the color of the skin. Dermoscopic diagnostic patterns usually consist of combinations of criteria.[10] Through cooperation and resource sharing, clinical research based on skin image data of the Chinese population is necessary, which can then result in the identification of more accurate dermoscopic features.
It is also necessary to improve the dermatologists’ ability to use dermoscope. The shortage of equipment and lack of training are two obstacles for the development of dermoscopy in China. Professional training is necessary for dermatologists, and dermatologists without training perform no better with a dermoscope than with naked eye examinations.[2] Although regular training courses have been offered, either face-to-face or online, only a small number of dermatologists have received systematic and long-term training. Improving the dermatologist's ability to use a dermoscope is one of the necessary conditions for ensuring the accuracy of disease diagnosis.
It is vital to establish a reasonable and standardized dermoscopic image database. The operation of CAD mainly relies on dermoscopic images, and the availability of high-quality images can increase the sensitivity and specificity of the results. Images can be evaluated by image quality evaluation systems, and the results can be reported to operators quickly.
A future trend is the use of teledermoscope and artificial intelligence. With the development of dermoscopic image analysis technology on network platforms, teledermoscopy and skin health self-inspection have become a trend. The development and application of teledermoscopy can improve the level of diagnosis in primary hospitals. Meanwhile, with the use of smartphones, patients can use mobile phone software for skin self-testing.
Conclusions
In recent years, the application of dermoscopy in China has become more extensive, and research on dermoscopy has been increasing. Clinically, a dermoscope is used not only for the diagnosis of diseases but also during surgery. The ability of CAD systems to classify skin tumors among Chinese has reached the level of experienced dermatologists. In response to the lack of dermoscopic courses affecting the development and use of this technology, CSID opened a free CSID skin imaging college for dermatologists. Although dermoscopy has achieved good results in China, it still needs to address more challenges in the future.
Funding
This work was supported by grants from Milstein Medical Asian American Partnership Foundation Research Project (No. MMAAP2016023), Open Research Funding of China Skin Image Database (No. CSID-ORF-201711 and No. CSID-ORF-201918), and the Fundamental Research Funds for the Central Universities of China (No. 3332018182).
Conflicts of interest
None.
Footnotes
How to cite this article: Shen X, Yu RX, Shen CB, Li CX, Jing Y, Zheng YJ, Wang ZY, Xue K, Xu F, Yu JB, Meng RS, Cui Y. Dermoscopy in China: current status and future prospective. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000396
Xue Shen and Rui-Xing Yu contributed equally to this work.
References
- 1.Bakos RM, Blumetti TP, Roldan-Marin R, Salerni G. Noninvasive imaging tools in the diagnosis and treatment of skin cancers. Am J Clin Dermatol 2018; 19 Suppl 1:3–14. doi: 10.1007/s40257-018-0367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones OT, Jurascheck LC, Utukuri M, Pannebakker MM, Emery J, Walter FM. Dermoscopy use in UK primary care: a survey of GPs with a special interest in dermatology. J Eur Acad Dermatol Venereol 2019; Epub ahead of print. doi: 10.1111/jdv.15614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yelamos O, Braun RP, Liopyris K, Wolner ZJ, Kerl K, Gerami P, et al. Usefulness of dermoscopy to improve the clinical and histopathologic diagnosis of skin cancers. J Am Acad Dermatol 2019; 80:365–377. doi: 10.1016/j.jaad.2018.07.072. [DOI] [PubMed] [Google Scholar]
- 4.Shen CB, Xue K, Yu RX, Meng RS, Liu J, Xu F, et al. A system platform promotes research, education and application of China skin imaging (in Chinese). Dermatol Bull 2018; 35:125–130. [Google Scholar]
- 5.Wolner ZJ, Yelamos O, Liopyris K, Rogers T, Marchetti MA, Marghoob AA. Enhancing skin cancer diagnosis with dermoscopy. Dermatol Clin 2017; 35:417–437. doi: 10.1016/j.det.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook LC, Hanna C, Foulke GT, Seiverling EV. Dermoscopy in the diagnosis of inflammatory dermatoses: systematic review findings reported for psoriasis, lupus, and lichen planus. J Clin Aesthet Dermatol 2018; 11:41–42. [PMC free article] [PubMed] [Google Scholar]
- 7.Dong H, Shu D, Campbell TM, Fruhauf J, Soyer HP, Hofmann-Wellenhof R. Dermatoscopy of genital warts. J Am Acad Dermatol 2011; 64:859–864. doi: 10.1016/j.jaad.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Jiang S, Lin H, Guo X, Zou X. Application of dermoscopy image analysis technique in diagnosing urethral condylomata acuminata. An Bras Dermatol 2018; 93:67–71. doi: 10.1590/abd1806-4841.20186527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang YJ, Zou XB, Guo XJ, Lin H. Application value of dermoscopy in the diagnosis of condyloma acuminatum (in Chinese). Chin J Dermatol 2017; 50:493–496. doi: 10.3760/cma.j.issn.0412-4030.2017.07.006. [Google Scholar]
- 10.Lallas A, Apalla Z, Ioannides D, Argenziano G, Castagnetti F, Moscarella E, et al. Dermoscopy in the diagnosis and management of basal cell carcinoma. Future Oncol (London, England) 2015; 11:2975–2984. doi: 10.2217/fon.15.193. [DOI] [PubMed] [Google Scholar]
- 11.Reiter O, Mimouni I, Gdalevich M, Marghoob AA, Levi A, Hodak E, et al. The diagnostic accuracy of dermoscopy for basal cell carcinoma: a systematic review and meta-analysis. J Am Acad Dermatol 2019; 80:1380–1388. doi: 10.1016/j.jaad.2018.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Wu XY, Chen XD, Gu LX. Dermoscopy in the diagnosis of tiny pigmented basal cell carcinoma: a preliminary evaluation (in Chinese). Chin J Dermatol 2011; 44:847–850. doi: 10.3760/cma.j.issn.0412-4030.2011.12.007. [Google Scholar]
- 13.Li WW, Tu P, Yang SX, Li H, Wang Y. Dermoscopy in the differential diagnosis of basal cell carcinoma: a preliminary study (in Chinese). Chin J Dermatol 2013; 46:480–484. doi: 10.3760/cma.j.issn.0412-4030.2013.07.009. [Google Scholar]
- 14.Li YB, Pan Yi, Xu F, Xu JH, Jiang J, Liu B. Study on the characteristics of 51 cases of actinic keratosis of the skin under the microscope (in Chinese). Hunan Normal Univ (Med Sci) 2016; 13:28–31. doi: 10.3969/j.issn.1673-016X.2016.06.010. [Google Scholar]
- 15.Lee JH, Won CY, Kim GM, Kim SY. Dermoscopic features of actinic keratosis and follow up with dermoscopy: a pilot study. J Dermatol 2014; 41:487–493. doi: 10.1111/1346-8138.12282. [DOI] [PubMed] [Google Scholar]
- 16.Hu C, Yang XQ, Liu XJ, Zhang GL, Cao Z, Wen Z, et al. Dermoscopic features of solar keratosis of different grades and cutaneous squamous cell carcinoma (in Chinese). Geriatr Health Care 2018; 24:511–514. [Google Scholar]
- 17.Zhang Q, Zhang K, Bu WB, Fang F. The value of dermascopy in the diagnosis of actinic keratosis (in Chinese). Chin J Aesthet Med 2018; 27:13–15. [Google Scholar]
- 18.Li WW, Wu WT, Zhang H, Jin QZ, Zhang Q, Zhang CL. Diagnostic value of rosette sign under a polarized dermoseope (in Chinese). Chin J Dermatol 2018; 51:220–223. doi: 10.3760/cma.j.issn.0412-4030.2018.03.015. [Google Scholar]
- 19.Liu J. Experts consensus on the dermoscopic features of squamous squamous cell neoplasms (2017) (in Chinese). Chin J Dermatol 2018; 51:87–91. doi: 10.3760/cma.j.issn.0412-4030.2018.02.001. [Google Scholar]
- 20.Lin J, Han S, Cui L, Song Z, Gao M, Yang G, et al. Evaluation of dermoscopic algorithm for seborrhoeic keratosis: a prospective study in 412 patients. J Eur Acad Dermatol Venereol 2014; 28:957–962. doi: 10.1111/jdv.12241. [DOI] [PubMed] [Google Scholar]
- 21.Yang XR, Sun R, Yuan DF, Deng H. The consistency of dermoscopy and histopathology in the diagnosis of melancytic nevus, seborrheic keratosis and solar keratosis (In Chinese). Chin J Leprosy Skin Dis 2016; 32:456–459. [Google Scholar]
- 22.Zheng YJ, Shen X, Jing Y, Wu YT, Yu RX, Wang L, et al. Diagnostic value of reflectance confocal microscopy combined with dermoscopy for melanocytic nevus (in Chinese). Chin J Dermatol 2017; 50:517–520. doi: 10.3760/cma.j.issn.0412-4030.2017.07.011. [Google Scholar]
- 23.Qiao JJ, Zou XB, Dong HT, Xin LL. Dermoscopic features of melanocytic nevi (in Chinese). Chin J Leprosy Skin Dis 2017; 33:65–69. [Google Scholar]
- 24.Dinnes J, Deeks JJ, Chuchu N, Ferrante di Ruffano L, Matin RN, Thomson DR, et al. Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults. Cochrane Database Syst Rev 2018; 12:CD011902.doi: 10.1002/14651858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen L, Zhang Y, Zhang L, Liu X, Wang P, Shen S, et al. Application of different noninvasive diagnostic techniques used in HMME-PDT in the treatment of port wine stains. Photodiagnosis Photodyn Ther 2019; 25:369–375. doi: 10.1016/j.pdpdt.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Ran RP, Xu XQ, Ran X, Zhuang KW, Chen S, Mu C, et al. A case of infantile hemangioma successfully treated by itraconazole oral solution: dermoscopy monitory the vascular regression (in Chinese). Chin J Derm Venerol 2017; 31:45–47. doi: 10.13735/j.cjdv.1001-7089.201607074. [Google Scholar]
- 27.Li YF, Chen XY, Lei TC. Inhibitory effect of timolol on topical glucocorticoidinduced skin telangiectasia. Mol Med Rep 2018; 18:2823–2831. doi: 10.3892/mmr.2018.9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang LM, Lu WJ, Yuan JT, Zeng BB, Li D, Zhang F, et al. Utility of dermoscopy for evaluating the therapeutic efficacy of tacrolimus ointment plus 308-nm excimer laser combination therapy in localized vitiligo patients. Exp Ther Med 2018; 15:3981–3988. doi: 10.3892/etm.2018.5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, Zhang JL, Liu XJ, Ma XL. Function for monitoring skin pigment recovery of 63 patients with vitiligo by dermoscopy (in Chinese). Chin J Derm Venerol 2015; 29:148–150. doi: 10.13735/j.cjdv.1001-7089.201407109. [Google Scholar]
- 30.Hu J, Xu AE. Application of reflectance confocal microscopy and dermoscopy in the efficacy evaluation of comprehensive treatment of melanosis (in Chinese). Chin J Dermatol 2018; 51:440–442. doi: 10.3760/cma.j.issn.0412-4030.2018.06.010. [Google Scholar]
- 31.Yang X, Hu C, Wen L, Xu Y, Keyal U, Zhang G, et al. Dermoscopic monitoring for treatment and follow-up of actinic keratosis with 5-aminolaevulinic acid photodynamic therapy. Technol Cancer Res Treat 2018; 17:1533033818820091.doi: 10.1177/1533033818820091. [Google Scholar]
- 32.Xue S. Adjunctive use of dermoscopy to reduce recurrence of melanocytic nevi following laser treatment: a study in an Asian population. Int J Clin Exp Med 2017; 10:7117–7122. [Google Scholar]
- 33.Ji J, Zhang LL, Ding HL, Wang HW, Huang Z, Wang XX, et al. Comparison of 5-aminolevulinic acid photodynamic therapy and red light for treatment of photoaging. Photodiagnosis Photodyn Ther 2014; 11:118–121. doi: 10.1016/j.pdpdt.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Huang Y, Zhuo F, Li L. Enhancing hair growth in male androgenetic alopecia by a combination of fractional CO2 laser therapy and hair growth factors. Laser Med Sci 2017; 32:1711–1718. doi: 10.1007/s10103-017-2232-8. [DOI] [PubMed] [Google Scholar]
- 35.Liu XJ, Zhang JL, Kong XJ, Huang YJ. Studies on correlation between the changes of vessels and lesions before and after treatments of psoriasis by dermoscopy dynamic observation (in Chinese). Chin J Derm Venerol 2019; 33:32–37. doi: 10.13735/j.cjdv.1001-7089.201804053. [Google Scholar]
- 36.Dang YL, Liu YL, Zeng WH, Geng SM. Application of dermoscopy in assessing the efficacy of erbium laser for acne vulgaris (in Chinese). Chin J Derm Venereol 2017; 31:508–510. doi: 10.13735/j.cjdv.1001-7089.201607153. [Google Scholar]
- 37.Nie J, Hou W. A semiquantitative grading scale for frontal and vertex of androgenetic alopecia. Int J Dermatol 2019; 58:582–588. doi: 10.1111/ijd.14324. [DOI] [PubMed] [Google Scholar]
- 38.Zhuang LQ, Tang XH, LI JS, Cao SX, Zhang XQ. A retrospective analysis of clinical feature of dermoscopy in 96 cases with alopecia areata (in Chinese). J Diagn Ther Dermato-Venereol 2015; 22:366–369. doi: 10.3969/j.issn.1674-8468.2015.05.004. [Google Scholar]
- 39.Huang J, Xu AE. Application of reflectance confocal microscopy combined with dermoscopy in assessing melanin and blood vessels in chloasma lesions (in Chinese). Chin J Dermatol 2016; 49:591–594. doi: 10.3760/cma.j.issn.0412-4030.2016.08.015. [Google Scholar]
- 40.Chen R, Xu AE. Morphological analysis of melasma lesions at different clinical stags by using three different skin imaging techniques (in Chinese). Chin J Dermatol 2019; 52:103–106. doi: 10.3760/cma.j.issn.0412-4030.2019.02.007. [Google Scholar]
- 41.Huang J, Hu WT, Zhang LL, Xu AE. Efficacy evaluation of compound glycyrrhizin injection in the treatment of melasma (in Chinese). Chin J Dermatol 2018; 51:299–301. doi: 10.3760/cma.j.issn.0412-4030.2018.04.012. [Google Scholar]
- 42.Wei Y, Li-Tsang CWP, Luk DCK, Tan T, Zhang W, Chiu TW. A validation study of scar vascularity and pigmentation assessment using dermoscopy. Burns 2015; 41:1717–1723. doi: 10.1016/j.burns.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 43.Caresana G, Giardini R. Dermoscopy-guided surgery in basal cell carcinoma. J Eur Acad Dermatol Venereol 2010; 24:1395–1399. doi: 10.1111/j.1468-3083.2010.03652.x. [DOI] [PubMed] [Google Scholar]
- 44.Paoli J. Predicting adequate surgical margins for cutaneous squamous cell carcinoma with dermoscopy. Br J Dermatol 2015; 172:1186–1187. doi: 10.1111/bjd.13727. [DOI] [PubMed] [Google Scholar]
- 45.Jayasekera PSA, Dodd J, Oliphant T, Langtry JAA, Lawrence CM. Dermoscopy prior to Mohs micrographic surgery does not improve tumour margin assessment and leads to fewer Mohs stages. Br J Dermatol 2018; 178:565–566. doi: 10.1111/bjd.15903. [DOI] [PubMed] [Google Scholar]
- 46.Zhou Y, Chen W, Liu ZR, Wang DG. Auxiliary detection of the surgical excision margin of basal cell carcinoma using dermoscopy (in Chinese). Chin J Aesthet Med 2019; 28:45–48. [Google Scholar]
- 47.Chuh A. Dermatoscope-guided suturing for an open wound adjacent to the lacrimal sac and the nasolacrimal duct. Australas J Dermatol 2018; 59:153–154. doi: 10.1111/ajd.12710. [DOI] [PubMed] [Google Scholar]
- 48.De Giorgi V, Grazzini M, Rossari S, Gori A, Alfaioli B, Papi F, et al. Adding dermatoscopy to naked eye examination of equivocal melanocytic skin lesions: effect on intention to excise by general dermatologists. Clin Exp Dermatol 2011; 36:255–259. doi: 10.1111/j.1365-2230.2010.03963.x. [DOI] [PubMed] [Google Scholar]
- 49.Terushkin V, Oliveria SA, Marghoob AA, Halpern AC. Use of and beliefs about total body photography and dermatoscopy among US dermatology training programs: an update. J Am Acda Dermatol 2010; 62:794–803. doi: 10.1016/j.jaad.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Lu YA, Xie FY, Wu YF, Jiang ZG, Meng RS. No reference uneven illumination assessment for dermoscopy images. IEEE Signal Process Lett 2015; 22:534–538. doi: 10.1109/LSP.2014.2357015. [Google Scholar]
- 51.Lu YN, Xie FY, Zhou SX, Jiang ZG, Meng RS. Non-reference quality assessment of dermoscopy images with defocus blur and uneven illumination distortion (in Chinese). Acta Automatica Sinica 2014; 40:480–488. doi: 10.3724/sp.j.1004.2014.00480. [Google Scholar]
- 52.Xie F, Lu Y, Bovik AC, Jiang Z, Meng R. Application-driven no-reference quality assessment for dermoscopy images with multiple distortions. IEEE Trans Biomed Eng 2016; 63:1248–1256. doi: 10.1109/tbme.2015.2493580. [DOI] [PubMed] [Google Scholar]
- 53.Xie FX, Qin SY, Jiang ZG, Meng RS. PDE-based unsupervised repair of hair-occluded information in dermoscopy images. Comput Med Imaging Graph 2009; 33:275–282. doi: 10.1016/j.compmedimag.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 54.Abbas Q, Celebi ME, Garcia IF. Hair removal methods: a comparative study for dermoscopy images. Biomed Signal Proces 2011; 6:395–404. doi: 10.1016/j.bspc.2011.01.003. [Google Scholar]
- 55.Abbas Q, Celebi ME, Fondon Garcia I, Rashid M. Lesion border detection in dermoscopy images using dynamic programming. Skin Res Technol 2011; 17:91–100. doi: 10.1111/j.1600-0846.2010.00472.x. [DOI] [PubMed] [Google Scholar]
- 56.Abbas Q, Celebi ME, Fondon Garcia I. Skin tumor area extraction using an improved dynamic programming approach. Skin Res Technol 2012; 18:133–142. doi: 10.1111/j.1600-0846.2011.00544.x. [DOI] [PubMed] [Google Scholar]
- 57.Euijoon A, Lei B, Youn Hyun J, Jinman K, Changyang L, Fulham M, et al. Automated saliency-based lesion segmentation in dermoscopic images. Conf Proc IEEE Eng Med Biol Soc 2015; 2015:3009–3012. doi: 10.1109/embc.2015.7319025. [DOI] [PubMed] [Google Scholar]
- 58.Ahn E, Kim J, Bi L, Kumar A, Li C, Fulham M, et al. Saliency-based lesion segmentation via background detection in dermoscopic images. IEEE J Biomed Health 2017; 21:1685–1693. doi: 10.1109/jbhi.2017.2653179. [DOI] [PubMed] [Google Scholar]
- 59.Abbas Q, Fondon I, Rashid M. Unsupervised skin lesions border detection via two-dimensional image analysis. Comput Meth Prog Bio 2011; 104:e1–e15. doi: 10.1016/j.cmpb.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 60.Maolood IY, Al-Salhi YEA, Lu S. Thresholding for medical image segmentation for cancer using fuzzy entropy with level set algorithm. Open Med-Warsaw 2018; 13:374–383. doi: 10.1515/med-2018-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fan H, Xie F, Li Y, Jiang Z, Liu J. Automatic segmentation of dermoscopy images using saliency combined with Otsu threshold. Comput Biol Med 2017; 85:75–85. doi: 10.1016/j.compbiomed.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 62.Xie F, Fan H, Li Y, Jiang Z, Meng R, Bovik A. Melanoma classification on dermoscopy images using a neural network ensemble model. IEEE Trans Med Image 2017; 36:849–858. doi: 10.1109/tmi.2016.2633551. [DOI] [PubMed] [Google Scholar]
- 63.Li Y, Shen L. Skin lesion analysis towards melanoma detection using deep learning network. Sensors 2018; 18:556.doi: 10.3390/s18020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang J, Xie F, Fan H, Jiang Z, Liu J. Classification for dermoscopy images using convolutional neural networks based on region average pooling. IEEE Access 2018; 6:65130–65138. doi: 10.1109/ACCESS.2018.2877587. [Google Scholar]
- 65.Zhang X, Wang S, Liu J, Tao C. Towards improving diagnosis of skin diseases by combining deep neural network and human knowledge. BMC Med Inform Decis 2018; 18 Suppl 2:59.doi: 10.1186/s12911-018-0631-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang SQ, Liu J, Zhu CY, Shu C, Zhou HN, Xie FY, et al. Comparison of diagnostic performance of dermatologists versus deep convohtional ncurM network for dermoscopic images of pigmented nevus and seborrheic keratosis (in Chinese). Chin J Dermatol 2018; 51:486–489. doi: 10.3760/cma.j.issn.0412-4030.2018.07.002. [Google Scholar]


