Abstract
Objective:
This review aimed to summarize research progress regarding congenital cytomegalovirus (cCMV) infection-related nervous system diseases and their mechanisms.
Data sources:
All literature quoted in this review was retrieved from PubMed and Web of Science using the keywords “Cytomegalovirus” and “Neurologic disease” in English. To identify more important information, we did not set time limits.
Study selection:
Relevant articles were selected by carefully reading the titles and abstracts. Then, different diagnosis and clinical treatment methods for human CMV infection-related neurologic diseases were compared, and the main mechanism and pathogenesis of neurologic damage caused by CMV were summarized from the selected published articles.
Results:
cCMV infection is a major cause of neonatal malformation. cCMV can infect the fetal encephalon during early gestation and compromise neurodevelopment, resulting in varying degrees of neurologic damage, mainly including hearing impairment, central nervous system (CNS) infection, neurodevelopmental disorders, ophthalmic complications, cerebral neoplasms, infantile autism, epilepsy, and other neurologic abnormalities.
Conclusions:
cCMV infection-induced neurodevelopmental abnormalities, which were directly caused by fetal encephalon infection, thus inducing neuroimmune responses to damage nerve cells. Such abnormalities were also caused by suppression of the proliferation and differentiation of neural progenitor cells by CMV's gene products. cCMV infection in the fetal encephalon can also inhibit neuronal migration and synapse formation and indirectly trigger placental inflammation and thus disrupt the oxygen supply to the fetus.
Keywords: Cytomegalovirus, Neurological disease, Mechanism
Introduction
Human cytomegalovirus (CMV), which belongs to the neurotropic beta-herpesvirus family, is the largest and most complex member of the human herpesvirus family[1] and can infect almost every cell type, including epithelial cells, endothelial cells, smooth muscle cells, neurocytes, and sustentacular cells of the central nervous system (CNS), retinal epithelial cells, dermal fibroblasts, and monocytes/macrophages. However, the production of infectious virions in different cell types varies widely, ranging from very low (macrophages) to very high (fibroblasts). Infection with CMV is highly species specific; humans are the only hosts of human CMV.
The development and maturity of the nervous system are part of a stepwise process involving differentiation of neural tissue and morphologic differentiation of the encephalon, which can be divided into four major stages: neurogenesis, migration, neurite outgrowth, and synapse formation. Histologically, this process is very complicated and includes cell proliferation, differentiation, migration, death, and formation and modification of synapses, while the differentiation of the brain involves its development from the nerve plate into the CNS and the peripheral nervous system with various forms and functions. Two basic factors affect neurodevelopment, including the interaction of gene expression in nerve cells with external factors and the interdependence of nerve cells and glial cells. Therefore, CMV can invade the CNS during any stage of neurodevelopment, resulting in congenital or perinatal infection, and cause neurodevelopmental disorders or other neurologic diseases through acute or persistent viral infection to impede the proliferation and differentiation of neural stem cells.
Congenital CMV (cCMV) infection affects 0.2% to 2.2% (average 1%) of neonates in the United States,[2] but only approximately 10% of newborns have obvious clinical manifestations. However, 10% to 15% of asymptomatic human CMV infections result in long-term neurologic sequelae.[3] The pathogenesis of human CMV infection of the CNS during fetal development is still poorly understood, although studies have indicated that human CMV infection of the CNS caused different structural abnormalities at different fetus ages, including periventricular calcification, ventriculomegaly, and various developmental anomalies. Sensorineural hearing loss (SNHL) is the most common long-term sequelae of cCMV infection.[4] Moreover, cCMV infection is closely related to microcephaly, mental retardation, hydrocephalus, brain tumors, seizures, and autism. This review aimed to summarize neurologic diseases caused by cCMV infection in neonates and the possible pathogenesis of cCMV infection as determined thus far.
Human CMV-related Neurologic Diseases in Neonates
Sensorineural hearing loss
Any structural abnormality or dysfunction in the auditory pathway can manifest as varying degrees of hearing loss. Approximately 700 million people worldwide have a moderate or higher degree of deafness, and more than 20 million people have hearing and speech disabilities in China. Deafness can be classified into organic deafness, sensorineural deafness, and mixed deafness according to different pathologies and lesion areas. Sensorineural deafness is always caused by damaged hair cells in the cochlear helix and the auditory nerve pathways, resulting in sound perception, neurotransmission, and cortical dysfunction. cCMV infection can impair auditory function, and the number of children with hearing loss has gradually increased over the past five decades,[5] with CMV infection as one of the major causes of nongenetic SNHL in developed countries.[6] A related systematic analysis showed that the prevalence of cCMV infection was 0.58%, and 9.8% of cases were symptomatic while 90.2% were asymptomatic. Deafness accounted for 32.8% of symptomatic CMV infections and 9.9% of asymptomatic infections; thus, cCMV infection was considered the leading cause of SNHL.[7] In addition, the study by Smiechura et al[8] found that 17% of children with cCMV infection also had SNHL. In France, cCMV infection is the second most important cause of hearing loss in children except for connexin mutations.[9] The degree of hearing loss caused by cCMV infection can range from unilateral to bilateral hearing loss, and the loss may persist or worsen after the perinatal period. The same hearing impairment may be caused regardless of the affected area of the auditory system or the phases of auditory system development affected by CMV.
The extensive effects of hearing loss in neonates include serious impacts on their learning capacity and ultimately delayed language comprehension,[10] and these negative effects persist into adulthood, implying that early screening, diagnosis and prophylactic treatment are essential for children with cCMV infection. However, because deafness in children is always delayed or progressively intensifies, most children with infections are asymptomatic, with complicating diagnosis and screening during the neonatal period. The most widely used screening methods are as follows. Firstly, the auditory brainstem response is the crucial method for newborn screening without the influence of wakefulness. Secondly, the urinary CMV-DNA viral load has been reported to be associated with neurogenic hearing impairment, and hearing impairment is more likely to occur when the CMV-DNA load reaches 1.415 × 106/mL.[11] Children with thrombocytopenia should also be monitored for hearing strength during CMV infection.[11] However, whether a dried blood spot (DBS) test is effective for predicting SNHL induced by CMV infection remains controversial. A retrospective study suggested that the DBS test was an effective method for retrospective diagnosis of cCMV infection and may serve as a definitive diagnosis method for SNHL.[12] However, Ross et al[13] proposed that polymerase chain reaction (PCR) detection in neonatal DBS samples had very low sensitivity and specificity in predicting SNHL induced by CMV infection at birth and at 4 years old. Moreover, CMV IgM and IgG detected early in serum can be used as prognostic factors for deafness,[14] although the sensitivity and specificity in the diagnosis of cCMV infections are insufficient. Currently, PCR amplification to detect CMV viral DNA is quite sensitive and requires various common clinical samples, such as urine, cerebrospinal fluid, blood, plasma, saliva, and biopsy, although no effective diagnostic imaging methods are available to diagnose cCMV infection-induced deafness, which warrants further exploration.
Regarding the prevention and treatment of hearing loss caused by cCMV infection, ganciclovir treatment of cCMV infection during the perinatal period may improve the long-term persistence and reduce the risk of hearing loss to some extent, whereas this treatment may also cause neutrophilic granulocytopenia.[15] Hearing aids and cochlear implants are effective treatments for hearing-impaired children and can significantly improve language comprehension, especially in younger children,[16] although these devices cannot completely correct the language delays caused by cCMV infection.[17] As long as cochlear implants are placed early in babies, the age at diagnosis and hearing loss progression in infants have little effect on postoperative outcomes.[18] In conclusion, early cochlear implants in children with cCMV-induced hearing loss can contribute to improved language comprehension,[19] although it cannot be fully recovered.
SNHL is the most common disability caused by cCMV infection and has dramatic, long-term impacts on the lives of children. Thus, auditory function must be monitored early in children with cCMV infection to prevent hearing loss through early intervention.[20] Intrauterine growth restriction, petechiae, hepatosplenomegaly, thrombocytopenia, intracerebral calcification, the severity of illness at birth, and the viral load may be valuable predictors of hearing loss in children with symptomatic cCMV infection.[21–25] Identifying an effective imaging method to predict hearing impairment will be significant for the recovery of hearing loss.
Neurodevelopmental disorders
Neurodevelopmental defects are abnormalities in structure, function, metabolism, mind, behavior, and inheritance caused by disorders of embryonic development. Various teratogenic agents can induce different developmental defects by interfering with embryonic development during different stages. The coordination of multiple processes, including neuroinduction, regulation of the cell cycle, expression of neuron-specific genes, and differentiation of neural precursor cells, is important during the development of the CNS, which undergoes a long journey from the 15th day after fertilization until delivery of the fetus (the sensitive period is 15–37 days of oosperm establishment). During this period, many factors, such as hormones, neurotrophins, environmental factors, malnutrition, viral infections, folic acid deficiency, and dementia, may lead to abnormalities in neurodevelopment. Congenital disease and placental damage have long been recognized to be more severe when primary maternal infection with CMV occurs in the first trimester, which may compromise placental development and lead to complications including fetal intrauterine growth restriction, a hallmark of congenital infection, and thus result in structural abnormalities and dysfunction of the nervous system, mainly including microcephaly, calcification around the ventricle, and ventriculomegaly.[24]
Whether cCMV infection can cause mental retardation is still uncertain.[25] Early studies demonstrated that cCMV infection affects the mental development of children, which has a dramatic impact on their long-term behaviors and presentations.[26,27] However, in a later study, Pearl and his colleagues[28] did not find strong evidence of mental retardation caused by cCMV infection. An early 10-year follow-up study showed that children with cCMV infection are unlikely to be at an increased risk of subsequent neurodevelopmental disorders if they did not show any abnormal development at 12 months of age.[29] However, in China, cCMV infection can damage the mental development of children, especially language comprehension, and the mechanism may be related to neurologic damage caused by persistent infection.[30] Microcephaly at birth was the most specific predictor of mental retardation and dyskinesia, but general abnormalities such as abnormal white matter and a single calcification on fetal magnetic resonance imaging (MRI) did not correlate with the state of neurodevelopment.[31,32] Additionally, children with hearing loss caused by cCMV infection were more likely to present neurodevelopmental disorders, which mainly manifested as impaired motor skills, executive functions, perception, language, learning, and social skills and more emotional problems.[33] In conclusion, symptomatic cCMV infection is more likely to compromise neurodevelopment than asymptomatic cCMV infection, which should be brought to the forefront.
CMV infection during early gestation is more likely to generate neurodevelopmental defects,[23,34] possibly because CMV invades the fetus while neurodevelopment is in progress during early gestation. A case report showed that a child was infected with CMV at birth, and B-ultrasonic examination during pregnancy revealed intrauterine growth retardation, ventriculomegaly, and a lack of amniotic fluid. When the child was 5 years old, he had obvious neurologic damage, mainly characterized as brain atrophy, spastic quadriplegia, and cortical blindness,[35] which implied that B-ultrasound examination during gestation shows signs of abnormal vision or brain development that can predict neurologic development-related diseases to some extent.[32] Although fetal MRI has been widely recognized, its role in determining the prognosis of cCMV infection remains inconclusive because the MRI examination is highly sensitive to detecting small lesions in the white matter region and inflammation in the lesion region, which may be reversible without compromising the long-term neurodevelopmental status.[32,36] In terms of the prevention and treatment of neurodevelopmental disorders caused by cCMV infection, studies have shown that intravenous ganciclovir treatment for 6 weeks in children with CNS cCMV infection at ages ranging from 6 to 12 months may improve neurodevelopmental delay.[37] In addition, the state of neurodevelopment should be monitored via B-ultrasound and MRI examinations in children with cCMV infection during the perinatal period, and early treatment may improve the prognosis of neurodevelopmental disorders.
Ophthalmic complications
Organogenesis of the oculus uterque, nose, and encephalon is a coevolutionary process regulated by the same genes, indicating that the oculus uterque is inextricably most closely related to the development of the nervous system. On the 22nd day of embryonic development (at the beginning of the fourth week), the optic sulcus derives from the neural ridge on both sides of the forebrain developed from the neural tube and then initiates the development of the embryo eye. Ocular diseases caused by CMV infection mainly include retinochoroiditis, which manifests as strabismus, optic atrophy, microphthalmia, cataract, retinal necrosis and calcification, blindness, and malformation of the atria and optic disc as detected by pupillography. Infants with symptomatic cCMV infection accounted for approximately 14% of cases of chorioretinitis at birth,[38] which is lower than the rate among patients with congenital toxoplasmosis infection. However, chorioretinitis is difficult to distinguish based on infection sites and clinical manifestations[39] because the choroid is attached to the retina, and choroidal inflammation always involves the retina, which is called choroidal retinitis and is a common fungal disease mainly characterized by blurred vision, central scotoma, visual discoloration, and distortion. The diagnosis of related ophthalmic diseases caused by CMV infection depends on ophthalmologic examination. Currently, no established definitive treatment is available for choroidal retinitis caused by cCMV infection. In 1 case report, a child with cCMV infection presented with multifocal retinal choroiditis approximately 3 months after birth. After 6 weeks of antiviral treatment with ganciclovir, the lesions were less active, although they were not fully resolved,[40] which indicated that antiviral treatment can improve the progression of chorioretinitis caused by cCMV infection.[41]
Cytopathic retinitis and immune reconstitution syndrome, such as chorioretinitis-vitreous, are two different entities of CMV-associated ophthalmologic complications after transplantation,[42] while visual impairment and strabismus are the main ophthalmologic complications associated with symptomatic cCMV infection. Acquired visual impairment and blindness due to CMV infection may be caused by cortical damage, optic atrophy, and retinal abnormalities.[43,44] Although a few experimental studies on cCMV infection-associated ophthalmologic complications have been conducted, no definitive treatment has been established.[45] Without detection, symptomatic cCMV infection can lead to moderate or even severe visual impairment, which will have negative long-term impacts on the lives of children. Therefore, efforts should be increased to monitor visual acuity (VA) and ophthalmologic diseases in children with cCMV infection.
Cerebral neoplasms
Tumors are characterized by abnormal cell proliferation and differentiation resulting from abnormalities of gene expression under the influence of carcinogens. Primary intracranial tumors derived from parenchymal cells of the nervous system are located intracranially, and astrocytoma, the most common primary cerebral neoplasm, accounts for more than 80% of gliomas. The primary cerebral neoplasms derived from nonbrain parenchymal cells are metastatic tumors. Gliomas and medulloblastomas are the most common cerebral neoplasms in children.
CMV gene products, which are only expressed inside tumor cells but not around tumor cells,[46–48] are more frequently observed in cerebral neoplasms, such as glioblastoma (adults) and medulloblastoma (children),[49–51] and are also highly expressed in general tumors, such as breast cancer, colon cancer, and prostatic carcinoma. Cobbs et al[51,52] first observed immediate-early 1 (IE1) gene product IE1-72 immunoreactivity in biopsies of malignant gliomas, which was not found in patients with Alzheimer disease, stroke, or encephalitis. Although they did not establish a causal role for CMV in the glioma pathogenesis, their findings indicated that CMV can facilitate glioma progression to some extent. The CMV infection rates and the expression of IE proteins were high in primary medulloblastoma, medulloblastoma cells, and allograft medulloblastoma, and the high expression of CMV gene US28 resulted in signal transducer and activator of transcription 3 (STAT3) phosphorylation, activation of the Wnt pathway, enhancement of cyclooxygenase-2 (COX-2) expression, production of vascular endothelial growth factor, increased production of prostaglandin E2 and interleukin-6 (IL-6), and enhanced inflammation.[49] In patients with malignant gliomas, lower levels of CMV viral expression were associated with improved survival, implying that the specific treatment of CMV using anti-virus plus COX-2 inhibitors may provide new ideas for the treatment of cerebral neoplasms.[52] Studies have shown no correlation between the CMV viral load and glioblastoma in peripheral blood and tumor tissues.[50] However, CMV may promote tumor formation and progressive deterioration of cerebral neoplasms through reversion of malignant phenotypes,[53] with US28 promoting the proliferation of malignant tumor cells by activating COX-2[54] and inducing tumor supportive monocytes.[55]
In conclusion, CMV and its gene products can exert both oncogenic and oncomodulatory effects, which may control cell cycle progression through interactions with p53, retinoblastoma protein (Rb), and cycling and promote tumor formation by activating oncogenic signaling pathways such as the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rampamycin (mTOR) pathway and mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (Erk), Wnt, and nuclear factor-kappa-light-chain-enhancer of activator B cells (NF-κB) pathways and inducing oncogene expression and telomerase activity.[56] However, the exact mechanism of CMV-encoded proteins in oncogenesis and tumor progression remains unclear.
Infantile autism
Autism, also known as autistic disorder, is a representative disease of pervasive developmental disorders (PDDs). The main characteristics of autism are social dysfunction, communication disorders, language delays, stereotyped behavioral repetitions, and significant limitations of interest. The Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR) divides PDDs into 5 subclinical types, including autistic disorders, Rett syndrome, childhood disintegrative disorder, Asperger syndrome (AS), and unspecified PDDs. Among these types, autism and AS are more common. The common age at the diagnosis of autism is 3 to 6 years old, but some children are diagnosed at 2 years old.[57] The prevalence of autism is approximately 0.7% to 1.0%, and the prevalence in males is approximately four-times higher than that in females.[58] Genetic variability can be identified in up to 25% of patients diagnosed with autism, which provides a valuable clue for exploring the neurodevelopmental mechanisms.[59] The major genes implicated in autism are associated with metabolism, chromosome modification, mRNA regulation, protein synthesis, and synaptic function.[60] Moreover, an abnormal intrauterine environment and genetic susceptibility may be responsible for the increased prevalence of autism, indicating that environmental abnormalities may be one of the risk factors for the development of autism.[61]
cCMV infection is involved in the occurrence of autism,[62–64] especially CMV infection during the third trimester of gestation, which may increase the risk of autism.[65] Engman et al[66] investigated the incidence of cCMV infection in children with autism by detecting the CMV-DNA load in DBSs and showed that cCMV infection may be one of the causes of autism, especially in patients with intellectual disability. Sakamoto et al[67] retrospectively examined the relationship between cCMV infection and autism and showed that the incidence of autism among children with cCMV infection was significantly higher than that among controls. At present, little is known about the role of cCMV infection in the pathogenesis of autism, and most papers are case reports.[68,69] Nevertheless, the incidence of autism is rapidly increasing, and the long-term prognosis is not promising.[70,71] In addition, an early study showed that congenital rubella virus infection was associated with autism[72] and that the rubella vaccine can reduce the incidence of autism, which may provide new perspectives for research on the pathogenesis and early prevention of autism.[73]
Other neurologic abnormalities
cCMV infection compromises neurodevelopment, which not only results in severe neurologic diseases but also causes some microlesions at a low incidence and mild brain injury. Accumulation of these mild injuries may lead to severe dysfunctions. Early studies suggested a possible association of CMV infection with abnormal electroencephalograms and neurologic dysfunction after febrile convulsions and epilepsy.[74,75] Additionally, CMV virus titers were associated with decreased cognitive ability in healthy adults, and CMV seroprevalence and antibody levels may be related to hippocampal volume.[76] Interestingly, high levels of CMV IgG in patients with schizophrenia and bipolar disorder are closely related to reduced hippocampal volume and poorer episodic verbal memory. In addition, CMV infection-related microcephaly, cerebral cortical dysplasia, white matter abnormalities, brain cleft, and intracranial calcification should not be ignored.[77–80]
Furthermore, in patients with acquired immune deficiency syndrome (AIDS), an early study showed that CMV mainly causes five distinct neurologic syndromes: retinitis, myelitis polyradiculopathy, encephalitis with dementia, ventriculoencephalitis, and multiple mononeuritis.[81] Although detection of CMV-DNA through cerebrospinal fluid PCR has been demonstrated to be a useful tool that appears to be a sensitive and specific diagnostic method for CMV-related CNS disease in patients with AIDS, these CMV-related CNS diseases are uncommon and still difficult to identify before death.[82,83] However, the incidence of CMV retinitis has increased, which is a major cause of vision loss in patients with AIDS, especially in the highly active antiretroviral therapy (HAART) era (CMV retinitis in the posterior pole) and retinitis-related retinal detachment remain common causes of vision loss among patients with CMV retinitis despite the widespread use of HAART.[84] Some investigations have shown that the incidence of VA loss in eyes affected by CMV retinitis was high and that the use of HAART, particularly with subsequent immune recovery, can reduce the incidence of VA loss.[84–87] However, studies on vision loss caused by CMV retinitis in pediatric patients with AIDS are rare, possibly because blindness or visual impairment always occur during the terminal life of patients with AIDS.
Pathogenesis of CMV-related nervous system diseases
CNS infections are unique to cCMV infection, and this manifestation is rare in most immunocompromised transplant patients. Although CMV encephalitis has been reported in patients with AIDS, infection of the CNS with CMV and CMV intrauterine infection are different clinical and pathologic conditions. cCMV infection in infants involving the CNS is always associated with persistent CMV infections, which can cause progressive hearing loss during the first year of life despite a lack of significant structural damage to the CNS.[88]
Little is known about the pathogenesis of CMV infection and the associated damage to the development of the fetal CNS, mainly due to the insufficient number of cases in autopsy studies, and species specificity limits the establishment and development of related animal models. The murine CMV infection model is useful to study the pathogenesis of CMV infection in the CNS, but murine CMV does not cause cCMV infection in mice. By directly injecting murine CMV into the brains of neonatal mice to stimulate cCMV infection, intracranial infection with CMV in neonatal mice has been demonstrated to reduce the proliferation of neural progenitor cells, cause the loss of numerous neuronal cells in the early stages of differentiation, and thus interfere with neurodevelopment.[89] Using the same method, Seleme et al[90] found that tumor necrosis factor-alpha and its downstream molecules are involved in CMV-induced cerebellar dysplasia, which implied that suppressing CMV infection-induced inflammation may reduce its damage to neurodevelopment. Additionally, intrauterine injection on the E15 day of gestation is another method to establish cCMV-infected animal models. The results show that early activation of microglia, peripheral leukocyte infiltration, and transient transcriptional upregulation of some chemokines may play important roles in the initiation phase of intracerebral rat CMV infection during neurodevelopment.[91] However, whether such events are beneficial or harmful to the spread of CMV infection requires further investigation.
Human embryonic tissue is also an effective tool to study the mechanism of cCMV infection in neurodevelopment. Gabrielli et al[92] studied 45 embryos at 21 to 22 weeks and found that a higher virus titer in organs corresponded to more severe immune responses and organ damage. They suggested that cCMV infection-induced CNS developmental defects mainly through the following mechanisms: in the encephalon, CMV infection can directly cause inappropriate immune responses, and in the placenta, CMV infection leads to placental dysfunction and fetal hypoxia, which indirectly compromise encephalon development. An in vitro study confirmed that no difference in susceptibility to CMV infection existed regardless of the gestational age of the donor tissue, and they found that initiation differentiation at least partially promoted CMV infection.[93] CMV can infect almost all types of cells but has markedly higher tropism for stem cells/radial cells. The density of CMV-positive cells and the tropism of CMV for stem/progenitor cells were the two crucial factors determining neuropathologic outcomes at the early stages of fetal development in CMV-infected individuals.[94] The IE protein 2 (IE2) encoded by human CMV can negatively regulate the proliferation and self-renewal of neural stem cells by reducing the number of neural stem cells, leading to microcephaly at postnatal stages and suppressing newborn neuron migration, which disrupts the connectivity between neurons.[95]
In conclusion, CMV infection can inhibit the proliferation and differentiation of neural stem cells, and CMV's gene products are involved in neural stem cell apoptosis and autophagy abnormalities, which lead to nervous system infections and neurodevelopmental disorders.[96,97] In addition, IL-10 may protect brain tissue from neurologic injury due to CMV infection by inhibiting chemokine-induced neuroimmune activation and then restricting encephalon damage.[98] In addition, severe deafness was associated with moderate vestibular dysfunction, and widespread cell degeneration, fibrosis, and calcification have been reported in the cochlea and vestibular system of a 14-year-old patient with extensive sequelae due to cCMV infection,[99,100] suggesting that viral cytopathic effects during the development of the hearing system lead to cell damage and vestibular dysfunction, which may constitute the mechanism of hearing loss caused by cCMV infection (Figure 1).
Figure 1.
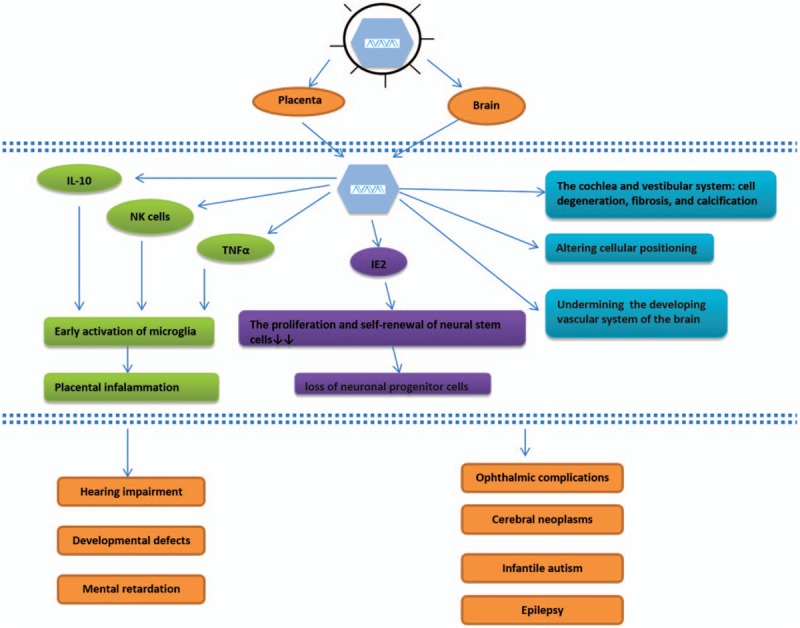
cCMV infection-related neurologic damage and its mechanism. CMV interferes with neurodevelopment by directly infecting the fetal brain to inhibit the proliferation and differentiation of neural progenitor cells or indirectly by triggering placental inflammation to block the oxygen supply to the fetus, which finally causes neurodevelopmental abnormalities. CMV: Cytomegalovirus; cCMV: Congenital cytomegalovirus; IL-10: Interleukin-10; NK cell: Natural killer cell; TNFα: Tumor necrosis factor α; IE2: Intermediate early protein 2.
Conclusions
Human CMV compromises neurodevelopment directly by infecting the fetal encephalon and then inducing neuroimmune responses to damage nerve cells or by its gene products inhibiting the proliferation and differentiation of neural progenitor cells, thus inhibiting neuronal migration and synapse formation, or indirectly by triggering placental inflammation and thus disrupting the oxygen supply to the fetus, ultimately causing neurodevelopmental abnormalities, such as developmental defects, mental retardation, ophthalmic complications, cerebral neoplasms, infantile autism, and epilepsy. Additionally, human CMV infection during the development of the hearing system leads to auditory impairment, which has an extensive influence on the long-term working lives of children (Table 1). Currently, studies on the pathogenesis of neurodevelopmental disorders and hearing loss in infants with cCMV infection are lacking. In addition, neurologic damage in the CNS is mostly irreversible, complicating treatment, and the achievement of breakthroughs in related studies. Therefore, to improve the prognosis of cCMV infection and decrease sequelae, prenatal diagnosis and diagnosis of acquired perinatal infection should be improved, and newborn hearing screening and testing should be increased, which may contribute to the early diagnosis and prevention of CMV infection and a reduction in corresponding neurologic injuries.
Table 1.
Major nervous system diseases caused by human CMV infection.

Funding
This study was supported by a grant of National Natural Science Foundation of China (No. 81271807).
Conflicts of interest
None.
Footnotes
How to cite this article: Zhang XY, Fang F. Congenital human cytomegalovirus infection and neurologic diseases in newborns. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000404
References
- 1.Weller TH. The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. II. N Engl J Med 1971; 285:267–274. doi: 10.1056/NEJM197107292850507. [DOI] [PubMed] [Google Scholar]
- 2.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 2007; 17:253–276. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 3.Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev 2013; 26:86–102. doi: 10.1128/CMR.00062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol 2007; 17:355–363. doi: 10.1002/rmv.544. [DOI] [PubMed] [Google Scholar]
- 5.Stevens G, Flaxman S, Brunskill E, Mascarenhas M, Mathers CD, Finucane M. Global and regional hearing impairment prevalence: an analysis of 42 studies in 29 countries. Eur J Public Health 2013; 23:146–152. doi: 10.1093/eurpub/ckr176. [DOI] [PubMed] [Google Scholar]
- 6.Ciorba A, Bovo R, Trevisi P, Bianchini C, Arboretti R, Martini A. Rehabilitation and outcome of severe profound deafness in a group of 16 infants affected by congenital cytomegalovirus infection. Eur Arch Otorhinolaryngol 2009; 266:1539–1546. doi: 10.1007/s00405-009-0944-5. [DOI] [PubMed] [Google Scholar]
- 7.Goderis J, De Leenheer E, Smets K, Van Hoecke H, Keymeulen A, Dhooge I. Hearing loss and congenital CMV infection: a systematic review. Pediatrics 2014; 134:972–982. doi: 10.1542/peds.2014-1173. [DOI] [PubMed] [Google Scholar]
- 8.Smiechura M, Struzycka M, Konopka W. Congenital and acquired cytomegalovirus infection and hearing evaluation in children. Otolaryngol Pol 2014; 68:303–307. doi: 10.1016/j.otpol.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Avettand-Fenoel V, Marlin S, Vauloup-Fellous C, Loundon N, Francois M, Couloigner V, et al. Congenital cytomegalovirus is the second most frequent cause of bilateral hearing loss in young French children. J Pediatr 2013; 162:593–599. doi: 10.1016/j.jpeds.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Schildroth AN. Congenital cytomegalovirus and deafness. Am J Audiol 1994; 3:27–38. doi: 10.1044/1059-0889.0302.27. [DOI] [PubMed] [Google Scholar]
- 11.Zhang MJ, Yuan TM, Wang LZ. Risk factors for hearing impairment induced by cytomegalovirus infection (in Chinese). Chin J Contemp Pediatr 2016; 18:224–228. doi: 10.7499/j.issn.1008-8830.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer L, Sharon B, Huang TC, Meyer AC, Gravel KE, Schimmenti LA, et al. Analysis of archived newborn dried blood spots (DBS) identifies congenital cytomegalovirus as a major cause of unexplained pediatric sensorineural hearing loss. Am J Otolaryngol 2017; 38:565–570. doi: 10.1016/j.amjoto.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Ross SA, Ahmed A, Palmer AL, Michaels MG, Sanchez PJ, Stewart A, et al. Newborn dried blood spot polymerase chain reaction to identify infants with congenital cytomegalovirus-associated sensorineural hearing loss. J Pediatr 2017; 184:57–61. doi: 10.1016/j.jpeds.2017.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Divya DV, Prasad M, Radhakrishna AN, Reddy SP, Pratyusha K, Kumar K, et al. The serological evidence of cytomegalovirus infection as a potent aetiological factor for cleft lip/palate, mental retardation and deafness. J Clin Diagn Res 2017; 11:C51–C54. doi: 10.7860/JCDR/2017/25118.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimberlin DW, Lin CY, Sanchez PJ, Demmler GJ, Dankner W, Shelton M, et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr 2003; 143:16–25. doi: 10.1016/s0022-3476(03)00192-6. [DOI] [PubMed] [Google Scholar]
- 16.Yamazaki H, Yamamoto R, Moroto S, Yamazaki T, Fujiwara K, Nakai M, et al. Cochlear implantation in children with congenital cytomegalovirus infection accompanied by psycho-neurological disorders. Acta Otolaryngol 2012; 132:420–427. doi: 10.3109/00016489.2011.653442. [DOI] [PubMed] [Google Scholar]
- 17.Viccaro M, Filipo R, Bosco E, Nicastri M, Mancini P. Long-term follow-up of implanted children with cytomegalovirus-related deafness. Audiol Neurootol 2012; 17:395–399. doi: 10.1159/000341160. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida H, Takahashi H, Kanda Y, Kitaoka K, Hara M. Long-term outcomes of cochlear implantation in children with congenital cytomegalovirus infection. Otol Neurotol 2017; 38:e190–e194. doi: 10.1097/MAO.0000000000001483. [DOI] [PubMed] [Google Scholar]
- 19.Dobbie AM. Evaluation and management of cytomegalovirus-associated congenital hearing loss. Curr Opin Otolaryngol Head Neck Surg 2017; 25:390–395. doi: 10.1097/MOO.0000000000000401. [DOI] [PubMed] [Google Scholar]
- 20.Lanzieri TM, Chung W, Flores M, Blum P, Caviness AC, Bialek SR, et al. Hearing loss in children with asymptomatic congenital cytomegalovirus infection. Pediatrics 2017; 139:ii:e20162610.doi: 10.1542/peds.2016-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivera LB, Boppana SB, Fowler KB, Britt WJ, Stagno S, Pass RF. Predictors of hearing loss in children with symptomatic congenital cytomegalovirus infection. Pediatrics 2002; 110:762–767. doi: 10.1542/peds.110.4.762. [DOI] [PubMed] [Google Scholar]
- 22.Walter S, Atkinson C, Sharland M, Rice P, Raglan E, Emery VC, et al. Congenital cytomegalovirus: association between dried blood spot viral load and hearing loss. Arch Dis Child Fetal Neonatal Ed 2008; 93:F280–F285. doi: 10.1136/adc.2007.119230. [DOI] [PubMed] [Google Scholar]
- 23.Pinninti SG, Rodgers MD, Novak Z, Britt WJ, Fowler KB, Boppana SB, et al. Clinical predictors of sensorineural hearing loss and cognitive outcome in infants with symptomatic congenital cytomegalovirus infection. Pediatr Infect Dis J 2016; 35:924–926. doi: 10.1097/INF.0000000000001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Syggelou A, Iacovidou N, Kloudas S, Christoni Z, Papaevangelou V. Congenital cytomegalovirus infection. Ann N Y Acad Sci 2010; 1205:144–147. doi: 10.1111/j.1749-6632.2010.05649.x. [DOI] [PubMed] [Google Scholar]
- 25.Boppana SB, Fowler KB. Insight into long-term neurodevelopmental outcomes in asymptomatic congenital CMV infection. Pediatrics 2017; 140:e20172526.doi: 10.1542/peds.2017-2526. [DOI] [PubMed] [Google Scholar]
- 26.Hanshaw JB, Scheiner AP, Moxley AW, Gaev L, Abel V, Scheiner B. School failure and deafness after “silent” congenital cytomegalovirus infection. N Engl J Med 1976; 295:468–470. doi: 10.1056/NEJM197608262950902. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds DW, Stagno S, Stubbs KG, Dahle AJ, Livingston MM, Saxon SS, et al. In apparent congenital cytomegalovirus infection with elevated cord IgM levels. Casual relation with auditory and mental deficiency. N Engl J Med 1974; 290:291–296. doi: 10.1056/NEJM197402072900601. [DOI] [PubMed] [Google Scholar]
- 28.Pearl KN, Preece PM, Ades A, Peckham CS. Neurodevelopmental assessment after congenital cytomegalovirus infection. Arch Dis Child 1986; 61:323–326. doi: 10.1136/adc.61.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivarsson SA, Lernmark B, Svanberg L. Ten-year clinical, developmental, and intellectual follow-up of children with congenital cytomegalovirus infection without neurologic symptoms at one year of age. Pediatrics 1997; 99:800–803. doi: 10.1542/peds.99.6.800. [DOI] [PubMed] [Google Scholar]
- 30.Zhang XW, Li F, Yu XW, Shi XW, Shi J, Zhang JP. Physical and intellectual development in children with asymptomatic congenital cytomegalovirus infection: a longitudinal cohort study in Qinba mountain area. China J Clin Virol 2007; 40:180–185. doi: 10.1016/j.jcv.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 31.Noyola DE, Demmler GJ, Nelson CT, Griesser C, Williamson WD, Atkins JT, et al. Early predictors of neurodevelopmental outcome in symptomatic congenital cytomegalovirus infection. J Pediatr 2001; 138:325–331. doi: 10.1067/mpd.2001.112061. [DOI] [PubMed] [Google Scholar]
- 32.Farkas N, Hoffmann C, Ben-Sira L, Lev D, Schweiger A, Kidron D, et al. Does normal fetal brain ultrasound predict normal neurodevelopmental outcome in congenital cytomegalovirus infection? Prenat Diagn 2011; 31:360–366. doi: 10.1002/pd.2694. [DOI] [PubMed] [Google Scholar]
- 33.Karltorp E, Lofkvist U, Lewensohn-Fuchs I, Lindstrom K, Westblad ME, Fahnehjelm KT, et al. Impaired balance and neurodevelopmental disabilities among children with congenital cytomegalovirus infection. Acta Paediatr 2014; 103:1165–1173. doi: 10.1111/apa.12745. [DOI] [PubMed] [Google Scholar]
- 34.Oosterom N, Nijman J, Gunkel J, Wolfs TF, Groenendaal F, Verboon-Maciolek MA, et al. Neuro-imaging findings in infants with congenital cytomegalovirus infection: relation to trimester of infection. Neonatology 2015; 107:289–296. doi: 10.1159/000375439. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez TR, Datlow MD, Nidecker AE. Diffuse periventricular calcification and brain atrophy: a case of neonatal central nervous system cytomegalovirus infection. Neuroradiol J 2016; 29:314–316. doi: 10.1177/1971400916665372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doneda C, Parazzini C, Righini A, Rustico M, Tassis B, Fabbri E, et al. Early cerebral lesions in cytomegalovirus infection: prenatal MR imaging. Radiology 2010; 255:613–621. doi: 10.1148/radiol.10090749. [DOI] [PubMed] [Google Scholar]
- 37.Oliver SE, Cloud GA, Sanchez PJ, Demmler GJ, Dankner W, Shelton M, et al. Neurodevelopmental outcomes following ganciclovir therapy in symptomatic congenital cytomegalovirus infections involving the central nervous system. J Clin Virol 2009; 46:S22–S26. doi: 10.1016/j.jcv.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boppana S, Amos C, Britt W, Stagno S, Alford C, Pass R. Late onset and reactivation of chorioretinitis in children with congenital cytomegalovirus infection. Pediatr Infect Dis J 1994; 13:1139–1142. doi: 10.1097/00006454-199412000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Stagno S, Reynolds DW, Amos CS, Dahle AJ, McCollister FP, Mohindra I, et al. Auditory and visual defects resulting from symptomatic and subclinical congenital cytomegaloviral and toxoplasma infections. Pediatrics 1977; 59:669–678. [PubMed] [Google Scholar]
- 40.Brubaker JW, Bale JJ, Ampofo K, Dries DC. Congenital cytomegalovirus infection: progressive postnatal chorioretinitis. J Pediatr Ophthalmol Strabismus 2009; 46:249–251. doi: 10.3928/01913913-20090706-16. [DOI] [PubMed] [Google Scholar]
- 41.Shoji K, Ito N, Ito Y, Inoue N, Adachi S, Fujimaru T, et al. Is a 6-week course of ganciclovir therapy effective for chorioretinitis in infants with congenital cytomegalovirus infection? J Pediatr 2010; 157:331–333. doi: 10.1016/j.jpeds.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 42.Egli A, Bergamin O, Mullhaupt B, Seebach JD, Mueller NJ, Hirsch HH. Cytomegalovirus-associated chorioretinitis after liver transplantation: case report and review of the literature. Transpl Infect Dis 2008; 10:27–43. doi: 10.1111/j.1399-3062.2007.00285.x. [DOI] [PubMed] [Google Scholar]
- 43.Coats DK, Demmler GJ, Paysse EA, Du LT, Libby C. Ophthalmologic findings in children with congenital cytomegalovirus infection. J AAPOS 2000; 4:110–116. doi: 10.1067/mpa.2000.103870. [DOI] [PubMed] [Google Scholar]
- 44.Jin HD, Demmler-Harrison GJ, Coats DK, Paysse EA, Bhatt A, Edmond JC, et al. Long-term visual and ocular sequelae in patients with congenital cytomegalovirus infection. Pediatr Infect Dis J 2017; 36:877–882. doi: 10.1097/INF.0000000000001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Capretti MG, Marsico C, Guidelli GS, Ciardella A, Simonazzi G, Galletti S, et al. Neonatal and long-term ophthalmological findings in infants with symptomatic and asymptomatic congenital cytomegalovirus infection. J Clin Virol 2017; 97:59–63. doi: 10.1016/j.jcv.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Harkins LE, Matlaf LA, Soroceanu L, Klemm K, Britt WJ, Wang W, et al. Detection of human cytomegalovirus in normal and neoplastic breast epithelium. Herpesviridae 2010; 1:8.doi: 10.1186/2042-4280-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soroceanu L, Cobbs CS. Is HCMV a tumor promoter? Virus Res 2011; 157:193–203. doi: 10.1016/j.virusres.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melnick M, Sedghizadeh PP, Allen CM, Jaskoll T. Human cytomegalovirus and mucoepidermoid carcinoma of salivary glands: cell-specific localization of active viral and oncogenic signaling proteins is confirmatory of a causal relationship. Exp Mol Pathol 2012; 92:118–125. doi: 10.1016/j.yexmp.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 49.Baryawno N, Rahbar A, Wolmer-Solberg N, Taher C, Odeberg J, Darabi A, et al. Detection of human cytomegalovirus in medulloblastomas reveals a potential therapeutic target. J Clin Invest 2011; 121:4043–4055. doi: 10.1172/JCI57147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Priel E, Wohl A, Teperberg M, Nass D, Cohen ZR. Human cytomegalovirus viral load in tumor and peripheral blood samples of patients with malignant gliomas. J Clin Neurosci 2015; 22:326–330. doi: 10.1016/j.jocn.2014.06.099. [DOI] [PubMed] [Google Scholar]
- 51.Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res 2002; 62:3347–3350. doi: Published June 2002. [PubMed] [Google Scholar]
- 52.Cobbs CS. Cytomegalovirus and brain tumor: epidemiology, biology and therapeutic aspects. Curr Opin Oncol 2013; 25:682–688. doi: 10.1097/CCO.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 53.Cinatl JJ, Vogel JU, Kotchetkov R, Wilhelm DH. Oncomodulatory signals by regulatory proteins encoded by human cytomegalovirus: a novel role for viral infection in tumor progression. FEMS Microbiol Rev 2004; 28:59–77. doi: 10.1016/j.femsre.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 54.Maussang D, Langemeijer E, Fitzsimons CP, Stigter-van WM, Dijkman R, Borg MK, et al. The human cytomegalovirus-encoded chemokine receptor US28 promotes angiogenesis and tumor formation via cyclooxygenase-2. Cancer Res 2009; 69:2861–2869. doi: 10.1158/0008-5472.CAN-08-2487. [DOI] [PubMed] [Google Scholar]
- 55.Dziurzynski K, Wei J, Qiao W, Hatiboglu MA, Kong LY, Wu A, et al. Glioma-associated cytomegalovirus mediates subversion of the monocyte lineage to a tumor propagating phenotype. Clin Cancer Res 2011; 17:4642–4649. doi: 10.1158/1078-0432.CCR-11-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soderberg-Naucler C, Johnsen JI. Cytomegalovirus infection in brain tumors: a potential new target for therapy? Oncoimmunology 2012; 1:739–740. doi: 10.4161/onci.19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Landa RJ. Diagnosis of autism spectrum disorders in the first 3 years of life. Nat Clin Pract Neurol 2008; 4:138–147. doi: 10.1038/ncpneuro0731. [DOI] [PubMed] [Google Scholar]
- 58.Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcin C, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res 2012; 5:160–179. doi: 10.1002/aur.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huguet G, Ey E, Bourgeron T. The genetic landscapes of autism spectrum disorders. Annu Rev Genomics Hum Genet 2013; 14:191–213. doi: 10.1146/annurev-genom-091212-153431. [DOI] [PubMed] [Google Scholar]
- 60.Gilbert J, Man HY. Fundamental elements in autism: from neurogenesis and neurite growth to synaptic plasticity. Front Cell Neurosci 2017; 11:359.doi: 10.3389/fncel.2017.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Currenti SA. Understanding and determining the etiology of autism. Cell Mol Neurobiol 2010; 30:161–171. doi: 10.1007/s10571-009-9453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ivarsson SA, Bjerre I, Vegfors P, Ahlfors K. Autism as one of several disabilities in two children with congenital cytomegalovirus infection. Neuropediatrics 1990; 21:102–103. doi: 10.1055/s-2008-1071471. [DOI] [PubMed] [Google Scholar]
- 63.Stubbs EG, Ash E, Williams CP. Autism and congenital cytomegalovirus. J Autism Dev Disord 1984; 14:183–189. doi: 0162-3257/04/1000-0583/0. [DOI] [PubMed] [Google Scholar]
- 64.Markowitz PI. Autism in a child with congenital cytomegalovirus infection. J Autism Dev Disord 1983; 13:249–253. doi: 0162-3257/83/0900-0249503.00/0. [DOI] [PubMed] [Google Scholar]
- 65.Yamashita Y, Fujimoto C, Nakajima E, Isagai T, Matsuishi T. Possible association between congenital cytomegalovirus infection and autistic disorder. J Autism Dev Disord 2003; 33:455–459. doi: 0162-3257/03/0800-0455/0. [DOI] [PubMed] [Google Scholar]
- 66.Engman ML, Sundin M, Miniscalco C, Westerlund J, Lewensohn-Fuchs I, Gillberg C, et al. Prenatal acquired cytomegalovirus infection should be considered in children with autism. Acta Paediatr 2015; 104:792–795. doi: 10.1111/apa.13032. [DOI] [PubMed] [Google Scholar]
- 67.Sakamoto A, Moriuchi H, Matsuzaki J, Motoyama K, Moriuchi M. Retrospective diagnosis of congenital cytomegalovirus infection in children with autism spectrum disorder but no other major neurologic deficit. Brain Dev 2015; 37:200–205. doi: 10.1016/j.braindev.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 68.Sweeten TL, Posey DJ, McDougle CJ. Brief report: autistic disorder in three children with cytomegalovirus infection. J Autism Dev Disord 2004; 34:583–586. doi: 0162-3257/04/1000-0583/0. [DOI] [PubMed] [Google Scholar]
- 69.Kawatani M, Nakai A, Okuno T, Kobata R, Moriuchi M, Moriuchi H, et al. Detection of cytomegalovirus in preserved umbilical cord from a boy with autistic disorder. Pediatr Int 2010; 52:304–307. doi: 10.1111/j.1442-200X.2010.03027.x. [DOI] [PubMed] [Google Scholar]
- 70.Maeyama K, Tomioka K, Nagase H, Yoshioka M, Takagi Y, Kato T, et al. Congenital cytomegalovirus infection in children with autism spectrum disorder: systematic review and meta-analysis. J Autism Dev Disord 2018; 48:1483–1491. doi: 10.1007/s10803-017-3412-x. [DOI] [PubMed] [Google Scholar]
- 71.Constantino JN. Measurement of autism symptomatology in children with neurodevelopmental impairment. J Am Acad Child Adolesc Psychiatry 2017; 56:354–355. doi: 10.1016/j.jaac.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 72.Lubinsky M. Behavioral consequences of congenital rubella. J Pediatr 1979; 94:678–679. doi: 10.1016/s0022-3476(79)80054-2. [DOI] [PubMed] [Google Scholar]
- 73.Berger BE, Navar-Boggan AM, Omer SB. Congenital rubella syndrome and autism spectrum disorder prevented by rubella vaccination--United States. BMC Public Health 2011; 11:340.doi: 10.1186/1471-2458-11-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iannetti P, Fiorilli M, Sirianni MC, Pana A, Aiuti F. Nonfebrile seizures after febrile convulsions: possible role of chronic cytomegalovirus infection. J Pediatr 1982; 101:27–31. doi: 10.1016/S0022-3476(82)80174-1. [DOI] [PubMed] [Google Scholar]
- 75.Iannetti P, Del GN, D’Ambrosio E, Balducci L. Neurological and electroencephalographic abnormalities after febrile seizures. Possible association with cytomegalovirus. Ital J Neurol Sci 1981; 2:153–157. doi: 10.1007/bf02335437. [DOI] [PubMed] [Google Scholar]
- 76.Houenou J, D’Albis MA, Daban C, Hamdani N, Delavest M, Lepine JP, et al. Cytomegalovirus seropositivity and serointensity are associated with hippocampal volume and verbal memory in schizophrenia and bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 2014; 48:142–148. doi: 10.1016/j.pnpbp.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 77.Frenkel LD, Gomez F, Sabahi F. The pathogenesis of microcephaly resulting from congenital infections: why is my baby's head so small? Eur J Clin Microbiol Infect Dis 2017; 37:209–226. doi: 10.1007/s10096-017-3111-8. [DOI] [PubMed] [Google Scholar]
- 78.White AL, Hedlund GL, Bale JJ. Congenital cytomegalovirus infection and brain clefting. Pediatr Neurol 2014; 50:218–223. doi: 10.1016/j.pediatrneurol.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 79.Pascual-Castroviejo I, Pascual-Pascual SI, Velazquez-Fragua R, Viano LJ. Congenital cytomegalovirus infection and cortical/subcortical malformations. Neurologia 2012; 27:336–342. doi: 10.1016/j.nrl.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 80.Ahlfors K, Ivarsson SA, Bjerre I. Microcephaly and congenital cytomegalovirus infection: a combined prospective and retrospective study of a Swedish infant population. Pediatrics 1986; 78:1058–1063. [PubMed] [Google Scholar]
- 81.McCutchan JA. Clinical impact of cytomegalovirus infections of the nervous system in patients with AIDS. Clin Infect Dis 1995; 21:S196–S201. doi: 10.1093/clinids/21.Supplement_2.S196. [DOI] [PubMed] [Google Scholar]
- 82.Ginocchio CC. Laboratory diagnosis of human cytomegalovirus (HCMV) central nervous system disease in AIDS patients. Int J Antimicrob Agents 2000; 16:447–453. doi: 10.1016/S0924-8579(00)00274-0. [DOI] [PubMed] [Google Scholar]
- 83.Bestetti A, Pierotti C, Terreni M, Zappa A, Vago L, Lazzarin A, et al. Comparison of three nucleic acid amplification assays of cerebrospinal fluid for diagnosis of cytomegalovirus encephalitis. J Clin Microbiol 2001; 39:1148–1151. doi: 10.1128/JCM.39.3.1148-1151.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thorne JE, Jabs DA, Kempen JH, Holbrook JT, Nichols C, Meinert CL. Causes of visual acuity loss among patients with AIDS and cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Ophthalmology 2006; 113:1441–1445. doi: 10.1016/j.ophtha.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 85.Sittivarakul W, Seepongphun U. Incidence rates and risk factors for vision loss among AIDS-related cytomegalovirus retinitis patients in Southern Thailand. Ocul Immunol Inflamm 2018; 26:82–89. doi: 10.1080/09273948.2017.1283044. [DOI] [PubMed] [Google Scholar]
- 86.Sittivarakul W, Benjhawaleemas T, Aui-Aree N, Jirarattanasopa P, Liabsuetrakul T. Incidence rate and risk factors for contralateral eye involvement among patients with AIDS and cytomegalovirus retinitis treated with local therapy. Ocul Immunol Inflamm 2016; 24:530–536. doi.org/10.3109/09273948.2015.1032307. [DOI] [PubMed] [Google Scholar]
- 87.Kempen JH, Jabs DA, Wilson LA, Dunn JP, West SK, Tonascia JA. Risk of vision loss in patients with cytomegalovirus retinitis and the acquired immunodeficiency syndrome. Arch Ophthalmol 2003; 121:466–476. doi: 10.1001/archopht.121.4.466. [DOI] [PubMed] [Google Scholar]
- 88.Fowler KB, Boppana SB. Congenital cytomegalovirus (CMV) infection and hearing deficit. J Clin Virol 2006; 35:226–231. doi: 10.1016/j.jcv.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 89.Mutnal MB, Cheeran MC, Hu S, Lokensgard JR. Murine cytomegalovirus infection of neural stem cells alters neurogenesis in the developing brain. PLoS One 2011; 6:e16211.doi: 10.1371/journal.pone.0016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seleme MC, Kosmac K, Jonjic S, Britt WJ. Tumor necrosis factor alpha-induced recruitment of inflammatory mononuclear cells leads to inflammation and altered brain development in murine cytomegalovirus-infected newborn mice. J Virol 2017; 91:ii:e01983–ii:e2016. doi: 10.1128/JVI.01983-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cloarec R, Bauer S, Luche H, Buhler E, Pallesi-Pocachard E, Salmi M, et al. Cytomegalovirus infection of the rat developing brain in utero prominently targets immune cells and promotes early microglial activation. PLoS One 2016; 11:e160176.doi: 10.1371/journal.pone.0160176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gabrielli L, Bonasoni MP, Santini D, Piccirilli G, Chiereghin A, Petrisli E, et al. Congenital cytomegalovirus infection: patterns of fetal brain damage. Clin Microbiol Infect 2012; 18:E419–E427. doi: 10.1111/j.1469-0691.2012.03983.x. [DOI] [PubMed] [Google Scholar]
- 93.Pan X, Li XJ, Liu XJ, Yuan H, Li JF, Duan YL, et al. Later passages of neural progenitor cells from neonatal brain are more permissive for human cytomegalovirus infection. J Virol 2013; 87:10968–10979. doi: 10.1128/JVI.01120-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Teissier N, Fallet-Bianco C, Delezoide AL, Laquerriere A, Marcorelles P, Khung-Savatovsky S, et al. Cytomegalovirus-induced brain malformations in fetuses. J Neuropathol Exp Neurol 2014; 73:143–158. doi: 10.1097/NEN.0000000000000038. [DOI] [PubMed] [Google Scholar]
- 95.Han D, Byun SH, Kim J, Kwon M, Pleasure SJ, Ahn JH, et al. Human cytomegalovirus IE2 protein disturbs brain development by the dysregulation of neural stem cell maintenance and the polarization of migrating neurons. J Virol 2017; 91:ii:e00799–ii:e817. doi: 10.1128/JVI.00799-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee KM, Hwang SK, Lee JA. Neuronal autophagy and neurodevelopmental disorders. Exp Neurobiol 2013; 22:133–142. doi: 10.5607/en.2013.22.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Odeberg J, Wolmer N, Falci S, Westgren M, Sundtrom E, Seiger A, et al. Late human cytomegalovirus (HCMV) proteins inhibit differentiation of human neural precursor cells into astrocytes. J Neurosci Res 2007; 85:583–593. doi: 10.1002/jnr.21144. [DOI] [PubMed] [Google Scholar]
- 98.Mutnal MB, Cheeran MC, Hu S, Little MR, Lokensgard JR. Excess neutrophil infiltration during cytomegalovirus brain infection of interleukin-10-deficient mice. J Neuroimmunol 2010; 227:101–110. doi: 10.1016/j.jneuroim.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bernard S, Wiener-Vacher S, Van Den Abbeele T, Teissier N. Vestibular disorders in children with congenital cytomegalovirus infection. Pediatrics 2015; 136:e887–e895. doi: 10.1542/peds.2015-0908. [DOI] [PubMed] [Google Scholar]
- 100.Rarey KE, Davis LE. Temporal bone histopathology 14 years after cytomegalic inclusion disease: a case study. Laryngoscope 1993; 103:904–909. doi: 10.1288/00005537-199308000-00012. [DOI] [PubMed] [Google Scholar]


