Abstract
Background:
Serum human chorionic gonadotrophin (hCG) is higher in twin than that in singleton pregnancies. As hCG stimulates the thyroid to produce more free thyroxine (FT4), which may lead to decreased thyroid-stimulating hormone (TSH) levels, the reference ranges of thyroid-related indicators may differ between singleton and twin pregnancies in the first trimester. This study aimed to establish reference ranges for thyroid-related indicators in early twin pregnancies and to compare them with singleton pregnancies.
Methods:
Data of 820 twin-pregnant women were extracted from the established database of all pregnant women who delivered at Peking University First Hospital from October 2013 to May 2018; 160 who met National Academy of Clinical Biochemistry criteria were included to establish TSH and FT4 reference ranges. We screened 480 (3:1 paired) women with singleton pregnancies from the same database as controls. The Mann-Whitney test for TSH and FT4 levels was applied for comparisons between singleton and twin pregnancies.
Results:
First-trimester reference ranges (4–12 gestational weeks) for twin pregnancies were: TSH 0.69 (0.01–3.35) mIU/L and FT4 16.38 (12.45–23.34) pmol/L. Median TSH was significantly lower at 7 to 12 gestational weeks than that at 4 to 6 gestational weeks (0.62 vs. 0.96 mIU/L, Z = −1.964, P = 0.049); FT4 was not significantly different between the two groups. Compared to singleton pregnancies, median TSH was significantly lower (0.69 vs. 1.27 mIU/L, Z = −6.538, P = 0.000), and FT4 was significantly higher (16.38 vs. 14.85 pmol/L, Z = −7.399, P = 0.000) in twin pregnancies in the first trimester.
Conclusions:
Specific reference ranges for thyroid-related indicators for twin pregnancies are needed to avoid a misdiagnosis of thyroid dysfunction. Moreover, establishment of separate reference ranges for 4 to 6 and 7 to 12 gestational weeks in twin pregnancies may be considered.
Keywords: Twin pregnancy, Reference ranges, Thyroid-stimulating hormone, Free thyroxine
Introduction
Thyroid dysfunction is common in pregnant women. The latest meta-analysis showed the prevalence of overt hypothyroidism, sub-clinical hypothyroidism, isolated hypothyroidism, overt hyperthyroidism, and sub-clinical hyperthyroidism to be 0.5%, 3.47%, 2.05%, 0.91%, and 2.18%.[1] In China, the prevalence of clinical hypothyroidism, sub-clinical hypothyroidism, and thyroid peroxidase antibody (TPOAb) positivity during the first half of pregnancy is reportedly 0.6%, 5.27%, and 8.6%, respectively.[2] Numerous studies have shown that abnormal thyroid function or thyroid autoimmunity during pregnancy can increase the risk of adverse pregnancy outcomes (such as premature delivery and miscarriage) and obstetric complications (such as gestational diabetes, gestational hypertension, and preeclampsia) and may even influence the development of the offspring's neurocognitive function.[3–5]
To accurately diagnose thyroid dysfunction during pregnancy, the 2012 Chinese Guidelines for the Diagnosis and Management of Thyroid Disease during Pregnancy and Postpartum clearly indicate that different regions should establish their own specific reference ranges for thyroid-related indicators according to National Academy of Clinical Biochemistry (NACB) recommendations.[2] However, most of the established thyroid-specific reference ranges for pregnancy were established based only on singleton pregnancies. Indeed, we retrieved only three studies[6–8] on the establishment of reference ranges for thyroid indicators in twin pregnancies in the literature; moreover, studies involving the Chinese population are relatively scarce. Because different ethnicities and iodine status may influence the establishment of reference ranges, data for Chinese twin-pregnant women are urgently needed. In addition, the reference range for early pregnancy in the published literature was mainly derived from cohorts at 11 to 14 weeks of gestation. However, following guidelines, most pregnant women in China seek prenatal care before 8 weeks of gestation, and some may even visit clinics before 6 weeks of gestation. Therefore, the clinical significance of establishing reference ranges for thyroid-related indicators for much earlier gestational periods should also be given more attention.
Thyroid indicators may differ between singleton and twin pregnancies. In the first trimester, free thyroxine (FT4) levels increase slightly due to stimulation by human chorionic gonadotrophin (hCG), leading to a modest reduction in secretion of thyroid-stimulating hormone (TSH).[9] Compared to singleton pregnancies, twin pregnancies produce higher levels of hCG and for longer periods, which may result in a more pronounced physiological suppression of TSH.[10] Thus, using the reference range for singleton-pregnant women to assess thyroid function may lead to misdiagnosis in twin-pregnant women.
After issuance of the 2012 Guidelines for the Diagnosis and Management of Thyroid Disease during Pregnancy and Postpartum for Chinese People,[2] we began in January 2013 to screen the serum TSH, FT4, and TPOAb levels of all pregnant women in their first trimester at Peking University First Hospital. This study aimed to establish reference ranges for thyroid-related indicators for twin-pregnant women in the first trimester at our hospital and to further compare the reference ranges for thyroid-related indicators between singleton and twin pregnancies in early pregnancy.
Methods
Ethical approval
This study was approved by the Ethics Committee of Peking University First Hospital (No. E20130716) and conducted in accordance with the Declaration of Helsinki. Given the retrospective study design and the fact that data analysis was performed anonymously, this study was exempt from obtaining informed consent from patients.
Subjects
Data of 820 twin-pregnant women were extracted from our established database of all the pregnant women who delivered at Peking University First Hospital from October 2013 to May 2018. As shown in Figure 1, because the use of ovulation-induction agents (such as gonadotrophins, gonadotrophin-releasing hormone analogs, or antagonists combined with gonadotrophins) can alter thyroid hormone levels,[11] we first excluded 486 women who conceived via in vitro fertilization and embryo transfer. In addition, 121 women without thyroid testing results in early pregnancy at our hospital were excluded, including 14 women for whom thyroid function was not detected in the first trimester, 35 for whom thyroid function was detected after 13 weeks of gestation, 24 transferred from other hospitals for prenatal care in the second or third trimester, and 48 for whom thyroid function was detected in the first trimester at other hospitals where the detection method and reference ranges were different from ours. Furthermore, according to NACB recommendations,[12] 30 women were excluded due to TPOAb positivity, 12 women with known thyroid disease history, and 11 women receiving thyroid-related intervention medications were also excluded. The screening process strictly followed the inclusion and exclusion criteria step by step. Ultimately, 160 twin-pregnant women were enrolled in the study to establish reference range.
Figure 1.
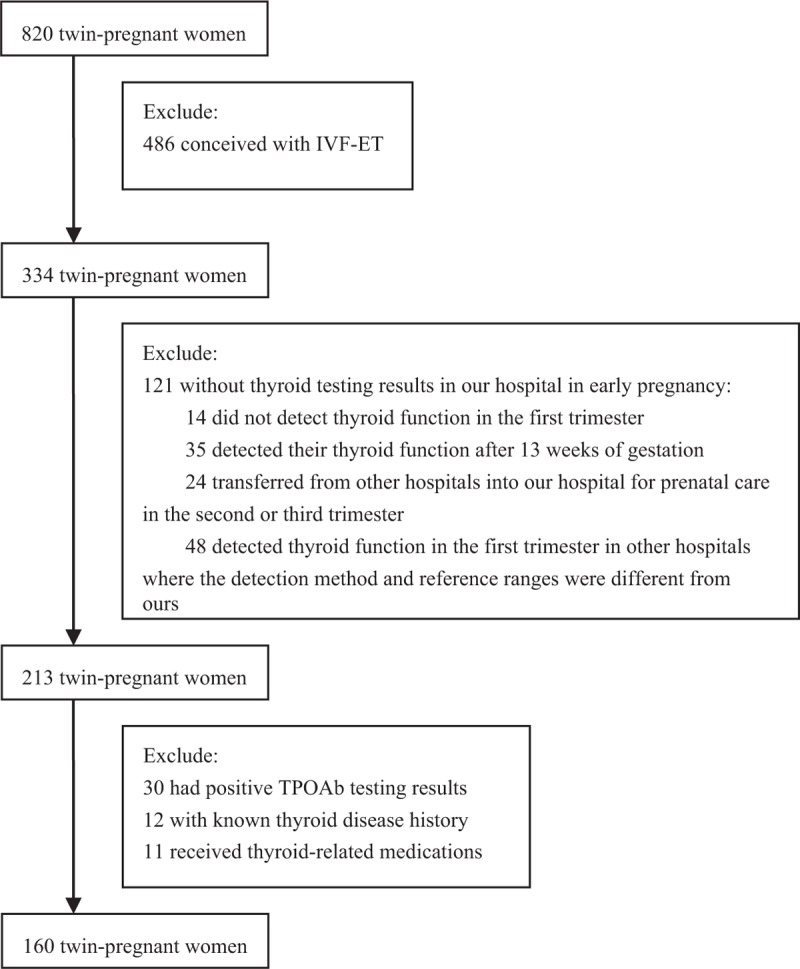
Screening flow chart of this study. IVF-ET: in vitro fertilization and embryo transfer; TPOAb: Thyroid peroxidase antibody.
Using the same enrollment criteria, we screened 480 (3:1 paired) women with singleton pregnancies from our established database as controls. The singleton-pregnant women had similar ages, were at the same gestational age and underwent thyroid function detection at the same gestational weeks as the twin-pregnant women. In addition, the time interval of thyroid function detection between the paired single- and twin-pregnant women was within 2 months.
Laboratory methods
Serum TSH and FT4 were measured using electrochemiluminescence immunoassays (ADVIA Centaur, Siemens Healthcare Diagnostics, Tarrytown, NY, USA), and TPOAb was measured using electrochemiluminescence immunoassays with a Cobas 601 immunoanalyzer (Roche Diagnostics, Mannheim, Germany). The normal reference ranges of the kit for the non-pregnant population were as follows: TSH 0.55 to 4.78 mIU/L and FT4 11.48 to 22.70 pmol/L; TPOAb over 34 IU/mL was considered positive. The inter-assay coefficients of TSH, FT4, and TPOAb were as follows: 2.05% to 5.31%, 0.8% to 2.7%, and 2.8% to 4.8%.
Statistical analysis
All data were entered into the database by two trained workers, and the accuracy of the data was ensured. The process of data extraction and the quality control process were completed under the guidance and supervision of the statisticians.
All statistical analyses were performed using the statistical software package SPSS 22.0 (SPSS, Inc., Chicago, IL, USA). The mean ± standard deviation was calculated for measurement data with a normal distribution. Skewed-distribution data are shown as the median with interquartile range. The reference ranges for TSH and FT4 were calculated based on 2.5th and 97.5th percentiles. The Mann-Whitney test for independent samples was applied for comparisons between two groups of non-normally distributed data. Differences were considered statistically significant at P < 0.05. We plotted kernel density plots for TSH using Stata 15.1 (StataCorp, College Station, TX, USA).
Results
Demographic characteristics of 160 twin-pregnant women for reference ranges establishment
A total of 160 twin-pregnant women were enrolled to establish reference ranges for TSH and FT4 in the current study. The median age was 31 (28–33) years, and the median gestational age at delivery was 37.00 (35.71–37.57) weeks. The median time of thyroid function detection was 9.00 (7.57–10.86) weeks.
Reference ranges for TSH and FT4 in early pregnancy for twin-pregnant women
The medians and reference ranges for TSH and FT4 levels in twin pregnancies in the first trimester were as follows: TSH 0.69 (0.01–3.35) mIU/L and FT4 16.38 (12.45–23.34) pmol/L. Furthermore, we compared TSH and FT4 levels between the groups at 4 to 6 (n = 21) and 7 to 12 (n = 139) gestational weeks and found that the median level of TSH during weeks 7 to 12 of gestation was significantly lower than that during weeks 4 to 6 (0.62 vs. 0.96 mIU/L, Z = −1.964, P = 0.049), though no significant difference in FT4 levels between the 7 to 12-weeks group and 4 to 6-weeks group was observed (16.46 vs. 16.08 pmol/L, Z = −0.889, P = 0.374) [Table 1].
Table 1.
Reference ranges for serum TSH and FT4 levels in twin- and singleton-pregnant women at different gestational weeks in the first trimester.
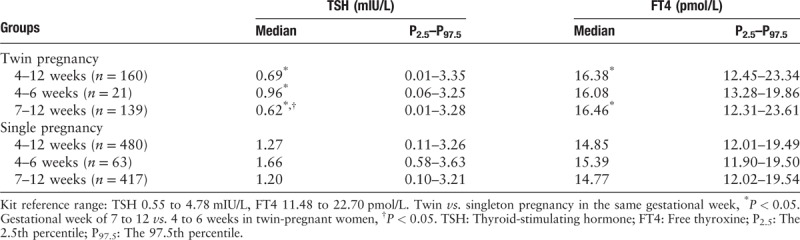
Comparisons of serum TSH and FT4 levels between singleton- and twin-pregnant women
For this comparison, using the same enrollment criteria, we screened 480 (3:1 paired) singleton-pregnant women of similar age as the twin-pregnant women as controls and who underwent thyroid testing at same gestational weeks as the twin-pregnant women. For singleton-pregnant women, the median age was 30 (28–33) years, and the median gestational age at delivery was 39.43 (38.41–40.29) weeks. The median time of thyroid function detection was 9.00 (7.43–10.86) weeks.
As shown in Table 1, compared with singleton-pregnant women, the median level of TSH was significantly lower (0.69 vs. 1.27 mIU/L, Z = −6.538, P < 0.001) and the FT4 level was significantly higher (16.38 vs. 14.85 pmol/L, Z = −7.399, P < 0.001) in twin-pregnant women in the first trimester (4–12 gestational weeks). At 4 to 6 gestational weeks, TSH in the twin-pregnant women was significantly lower (0.96 vs. 1.66 mIU/L, Z = −2.691, P = 0.007), and an increasing trend in FT4 levels was observed in twin-pregnant women (16.08 vs. 15.39 pmol/L, Z = −1.823, P = 0.068). However, there was no statistically significant difference between the two groups. At 7 to 12 gestational weeks, TSH was lower (0.62 vs. 1.20 mIU/L, Z = −6.165, P < 0.001) and FT4 was higher (16.46 vs. 14.77 pmol/L, Z = −7.163, P < 0.001) in twin- than that in singleton-pregnant women.
Figure 2 illustrates the distribution of TSH in single- and twin-pregnant women at different gestational weeks. Compared with single-pregnant women, the distribution of TSH in twin-pregnant women was obviously shifted to the left in all three groups (4–12, 4–6, and 7–12 gestational weeks), whereas only a few twin-pregnant women had a TSH level exceeding 3 mIU/L. Therefore, the median TSH level was lower in twin-pregnant women than in single-pregnant women.
Figure 2.
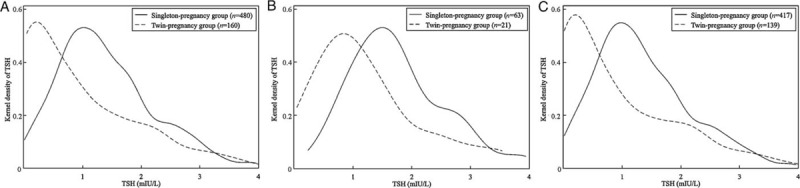
The distribution of TSH at 4 to 12 (A), 4 to 6 (B), and 7 to 12 (C) gestational weeks between single- and twin-pregnant women. Compared with single-pregnant women, the distribution of TSH in twin-pregnant women is shifted obviously to the left in all three groups. TSH: Thyroid-stimulating hormone.
Discussion
To our knowledge, relatively few studies have aimed to establish a reference range for thyroid-related indicators in twin-pregnant women. We strictly complied with NACB guidelines[12] to establish reference ranges for thyroid indicators in twin pregnancies. Moreover, it is universally accepted that the establishment of reference ranges is influenced by iodine status.[13] Most European countries in the published literature are mildly iodine deficient,[14–16] but Shan et al's study[17] and our previous study[18] showed that Beijing is an iodine-adequate region.
According to our results, the distribution of TSH levels in twin-pregnant women is shifted to the left compared to single-pregnant women in the first trimester. The median TSH level in twin-pregnant women was significantly lower than that in singleton-pregnant women in an iodine-adequate region. Because the α-hCG sub-unit is structurally similar to TSH, hCG might exhibit thyrotropic properties, especially in the first trimester of pregnancy.[19,20] Jean-Paul et al[10] first discovered that compared to singleton pregnancy, the peak of both intact and free β-hCG was strikingly higher for a significantly prolonged duration at 8 to 16 weeks of gestation in twin-pregnant women. As a sharp and long-lasting rise in hCG is likely to cause a decrease in TSH levels, a marked decrease in TSH levels was found to be three times more common in twin-pregnant women than in singleton-pregnant women.[10] Ashoor et al[7] also reported that free β-hCG levels were twice as high in women with twin pregnancies than in those with singleton pregnancies at 11 to 13 gestational weeks. These findings explain why the median and lower limits of TSH for twin-pregnant women are lower than those for singleton-pregnant women. In clinical practice, use of reference ranges for singleton pregnancies to assess the thyroid function of twin-pregnant women may lead to misdiagnosis of hyperthyroidism or sub-clinical hyperthyroidism. Moreover, if inappropriate anti-thyroid treatment is applied, it may result in maternal leukopenia and liver dysfunction or offspring deformation. Therefore, it is reasonable to establish a specific reference range for twin pregnancies to evaluate thyroid function.
In our study, the upper limit of reference ranges for TSH in the twin-pregnant women was slightly higher than that in the singleton-pregnant women (3.35 vs. 3.28 mIU/L). However, because the sample size of the former was quite small, the upper limit of the reference ranges for TSH would be elevated even if only a few cases of twin-pregnant women showed high TSH levels. Additionally, women with serum TSH levels above 2.5 mIU/L who received levothyroxine (L-T4) treatment were excluded from our study, which may to some extent suppress the upper limit of the reference range for TSH. As a result, the upper limit for the remaining subjects who did not receive L-T4 intervention was quite similar between the single- and twin-pregnant women.
We also found that serum FT4 was significantly higher in twin pregnancies than that in singleton pregnancies in the first trimester. This result is consistent with other studies,[10,21] both of which demonstrated that fetal number correlated positively with serum FT4 level. This may explain why FT4 is higher in twin-pregnant women.
Among available studies, the reference range for early pregnancy has mainly been derived from cohorts of women at 11 to 14 gestational weeks.[6–8] Conversely, data for much earlier time periods (4–11 weeks) are relatively few. Li's SHEP research[22] first revealed that the median TSH level at 4 to 6 weeks gestation was significantly higher than that at 7 to 12 weeks in singleton pregnancy, while there was no significant difference between non-pregnant women and those at 4 to 6 weeks gestation. Our previous study[18] confirmed this result. In accordance with these two studies, the current research demonstrated that the median serum TSH level in the 7 to 12-week group was significantly lower than that in the 4 to 6-week group in twin pregnancies, which might be attributed to the greater impact of hCG on TSH in twin pregnancies. Following the Chinese guideline recommendation, some pregnant women may seek prenatal care at 6 weeks of gestation or even earlier. Hence, establishing reference ranges for thyroid indicators for much earlier gestational periods (4–6 weeks) has clinical significance for early diagnosis of thyroid dysfunction and intervention. Accordingly, it might be reasonable to establish separate thyroid-related indicator reference ranges for 4 to 6 weeks and 7 to 12 weeks of gestation in twin pregnancy.
The upper limit of TSH in the reference range for twin pregnancies was 3.35 mIU/L in our study, which was notably higher than those for Caucasian and African populations.[7] Ghalia et al[7] also reported serum TSH and FT4 concentrations in twin pregnancies in women of African to be lower than those of Caucasians. This verifies that ethnic differences may influence the establishment of reference ranges for TSH.[23]
Our study also had some limitations. First, because it was a retrospective study, we could not obtain the results of serum thyroglobulin antibodies (TgAb) for all the women enrolled; thus, women with positive TgAb may have been included in the study. Second, in screening of standard populations, we enrolled twin-pregnant women who delivered at our hospital from October 2013 to May 2018, and the reference ranges for thyroid-related indicators in normal pregnant women at our hospital were established in 2015. Hence, some women with serum TSH levels above 2.5 mIU/L have received L-T4 treatment according to the 2011 American Thyroid Association guidelines,[24] as opposed to basing on the reference ranges for pregnant women. Excluding these women from our study might have caused a slight decrease in the upper limit of the TSH reference values. Third, the incidence of twin pregnancies was quite low, which led to the limited sample size of our study. Finally, a prospective study is needed to establish reference ranges for the second and third trimesters of twin pregnancies.
In conclusion, serum TSH and FT4 levels in early pregnancy differed among women with singleton and twin pregnancies. Therefore, it is reasonable to establish specific reference ranges for thyroid-related indicators for twin pregnancies to avoid misdiagnosis of thyroid function in the first trimester. Moreover, it might be important to establish separate reference ranges for 4 to 6 weeks and 7 to 12 weeks of gestation for assessment of thyroid function in twin pregnancy.
Funding
This work was supported by grants from the Interdisciplinary Clinical Research Project of Peking University First Hospital (No. 2017CR27), the Capital Foundation of Medical Developments (No. 2018-4-4077), and the Clinical Medicine Plus X-Young Scholars Project of Peking University (supported by the Fundamental Research Funds for the Central Universities).
Conflicts of interest
None.
Footnotes
How to cite this article: Jiang YX, Sun WJ, Zhang Y, Huang Y, Huang YY, Lu GZ, Zhang JQ, Gao Y, Yang HX, Guo XH. Thyroid function of twin-pregnant women in early pregnancy. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000381
Yan-Xin Jiang and Wei-Jie Sun contributed equally to this study.
References
- 1.Dong AC, Stagnaro-Green A. Differences in diagnostic criteria mask the true prevalence of thyroid disease in pregnancy: a systematic review and meta-analysis. Thyroid 2019; 29:278–289. doi: 10.1089/thy.2018.0475. [DOI] [PubMed] [Google Scholar]
- 2.Chinese Society of Endocrinology CSoP. Guidelines for the diagnosis and management of thyroid disease during pregnancy and postpartum (in Chinese). Chin J Endocrinol Metab 2012; 28:354–371. doi: 10.3760/cma.j.issn.1000-6699.2012.05.002. [Google Scholar]
- 3.Korevaar TIM, Medici M, Visser TJ, Peeters RP. Thyroid disease in pregnancy: new insights in diagnosis and clinical management. Nat Rev Endocrinol 2017; 13:610–622. doi: 10.1038/nrendo.2017.93. [DOI] [PubMed] [Google Scholar]
- 4.De Leo S, Pearce EN. Autoimmune thyroid disease during pregnancy. Lancet Diabetes Endocrinol 2018; 6:575–586. doi: 10.1016/S2213-8587(17)30402-3. [DOI] [PubMed] [Google Scholar]
- 5.Korevaar TIM, Tiemeier H, Peeters RP. Clinical associations of maternal thyroid function with foetal brain development: epidemiological interpretation and overview of available evidence. Clin Endocrinol (Oxf) 2018; 89:129–138. doi: 10.1111/cen.13724. [DOI] [PubMed] [Google Scholar]
- 6.Dashe JS, Casey BM, Wells CE, McIntire DD, Byrd EW, Leveno KJ, et al. Thyroid-stimulating hormone in singleton and twin pregnancy: importance of gestational age-specific reference ranges. Obstet Gynecol 2005; 106:753–757. doi: 10.1097/01.AOG.0000175836.41390.73. [DOI] [PubMed] [Google Scholar]
- 7.Ashoor G, Muto O, Poon LC, Muhaisen M, Nicolaides KH. Maternal thyroid function at gestational weeks 11-13 in twin pregnancies. Thyroid 2013; 23:1165–1171. doi: 10.1089/thy.2012.0537. [DOI] [PubMed] [Google Scholar]
- 8.Salek T, Dhaifalah I, Langova D, Havalova J. Maternal thyroid-stimulating hormone reference ranges for first trimester screening from 11 to 14 weeks of gestation. J Clin Lab Anal 2018; 32:e22405.(1–4). doi: 10.1002/jcla.22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harada A, Hershman JM, Reed AW, Braunstein GD, Dignam WJ, Derzko C, et al. Comparison of thyroid stimulators and thyroid hormone concentrations in the sera of pregnant women. J Clin Endocrinol Metab 1979; 48:793–797. doi: 10.1210/jcem-48-5-793. [DOI] [PubMed] [Google Scholar]
- 10.Grun JP, Meuris S, De Nayer P, Glinoer D. The thyrotrophic role of human chorionic gonadotrophin (hCG) in the early stages of twin (versus single) pregnancies. Clin Endocrinol (Oxf) 1997; 46:719–725. doi: 10.1046/j.1365-2265.1997.2011011.x. [DOI] [PubMed] [Google Scholar]
- 11.Poppe K, Unuane D, D’Haeseleer M, Tournaye H, Schiettecatte J, Haentjens P, et al. Thyroid function after controlled ovarian hyperstimulation in women with and without the hyperstimulation syndrome. Fertil Steril 2011; 96:241–245. doi: 10.1016/j.fertnstert.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 12.Demers LM, Spencer CA. Laboratory medicine practice guidelines: laboratory support for the diagnosis and monitoring of thyroid disease. Clin Endocrinol (Oxf) 2003; 58:138–140. doi: 10.1046/j.1365-2265.2003.01681.x. [DOI] [PubMed] [Google Scholar]
- 13.Glinoer D. The regulation of thyroid function during normal pregnancy: importance of the iodine nutrition status. Best Pract Res Clin Endocrinol Metab 2004; 18:133–152. doi: 10.1016/j.beem.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Bath SC, Sleeth ML, McKenna M, Walter A, Taylor A, Rayman MP. Iodine intake and status of UK women of childbearing age recruited at the University of Surrey in the winter. Br J Nutr 2014; 112:1715–1723. doi: 10.1017/S0007114514002797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brander L, Als C, Buess H, Haldimann F, Harder M, Hanggi W, et al. Urinary iodine concentration during pregnancy in an area of unstable dietary iodine intake in Switzerland. J Endocrinol Invest 2003; 26:389–396. doi: 10.1007/BF03345192. [DOI] [PubMed] [Google Scholar]
- 16.Jiskra J, Fait T, Bilek R, Kratky J, Bartakova J, Lukas J, et al. Mild iodine deficiency in women after spontaneous abortions living in an iodine-sufficient area of Czech Republic: prevalence and impact on reproductive health. Clin Endocrinol (Oxf) 2014; 80:452–458. doi: 10.1111/cen.12298. [DOI] [PubMed] [Google Scholar]
- 17.Shan Z, Chen L, Lian X, Liu C, Shi B, Shi L, et al. Iodine status and prevalence of thyroid disorders after introduction of mandatory universal salt iodization for 16 years in China: a cross-sectional study in 10 cities. Thyroid 2016; 26:1125–1130. doi: 10.1089/thy.2015.0613. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Liu F, Sun W, Huang Y, Zhang W, Wang B, et al. Establishment of reference ranges for thyroid-related indicators in normal pregnant women (in Chinese). Natl Med J China 2016; 96:339–343. doi: 10.3760/cma.j.issn.0376·2491.2016.05 005. [DOI] [PubMed] [Google Scholar]
- 19.Glinoer D, de Nayer P, Bourdoux P, Lemone M, Robyn C, van Steirteghem A, et al. Regulation of maternal thyroid during pregnancy. J Clin Endocrinol Metab 1990; 71:276–287. doi: 10.1210/jcem-71-2-276. [DOI] [PubMed] [Google Scholar]
- 20.Mann K, Schneider N, Hoermann R. Thyrotropic activity of acidic isoelectric variants of human chorionic gonadotropin from trophoblastic tumors. Endocrinology 1986; 118:1558–1566. doi: 10.1210/endo-118-4-1558. [DOI] [PubMed] [Google Scholar]
- 21.Ogueh O, Hawkins AP, Abbas A, Carter GD, Nicolaides KH, Johnson MR. Maternal thyroid function in multifetal pregnancies before and after fetal reduction. J Endocrinol 2000; 164:7–11. doi: 10.1677/joe.0.1640007. [DOI] [PubMed] [Google Scholar]
- 22.Li C, Shan Z, Mao J, Wang W, Xie X, Zhou W, et al. Assessment of thyroid function during first-trimester pregnancy: what is the rational upper limit of serum TSH during the first trimester in Chinese pregnant women? J Clin Endocrinol Metab 2014; 99:73–79. doi: 10.1210/jc.2013-1674. [DOI] [PubMed] [Google Scholar]
- 23.Surks MI, Boucai L. Age- and race-based serum thyrotropin reference limits. J Clin Endocrinol Metab 2010; 95:496–502. doi: 10.1210/jc.2009-1845. [DOI] [PubMed] [Google Scholar]
- 24.Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011; 21:1081–1125. doi: 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]


