Abstract
Netrin-1 has profound in vitro effects on the growth properties of vertebrate embryonic axons. In addition,netrin-1 mRNA is found in the floor plate of the embryonic nervous system, an intermediate target of many axons, including commissural axons that are affected by netrin-1 in vitro. Moreover, genetic studies of netrin-1 homologs inCaenorhabditis elegans and Drosophilaimplicate these proteins in commissure formation. We raised polyclonal antisera that recognize chick netrin-1 in fixed tissue sections. The antisera were used to immunohistochemically map netrin-1 in the embryonic spinal cord, brain, and retina. The relationship between netrin-1 localization and the growth of pioneering axons suggests roles for netrin-1 in the regulation of circumferential, commissural, and longitudinal axon growth in the spinal cord and brain. The data also suggest that the primary or sole effect of netrin-1 on pioneering spinal cord commissural axons is haptotactic. Furthermore, the pattern of netrin-1 localization raises the possibility that this protein helps mediate neuronal migration in the spinal cord, brain, and retina.
Keywords: axon guidance, brain, circumferential, commissural, floor plate, immunohistochemistry, retina, spinal cord
Nervous system development requires that extending axons precisely navigate their particular, predetermined paths from cell soma through changing environments to their synaptic targets. A rapidly growing literature indicates that the axons are guided through this journey by responding to a variety of substances produced by intermediate and synaptic target tissues (Letourneau et al., 1994;Tessier-Lavigne, 1994; Garrity and Zipursky, 1995; Keynes and Cook, 1995; Goodman, 1996).
Many of the neurons in the dorsolateral embryonic spinal cord extend pioneering axons that can be tracked during their intrasegmental, circumferential growth to the floor plate at the ventral midline, where they decussate and turn to run intersegmentally alongside the floor plate (Holley, 1982; Holley and Silver, 1987; Bovolenta and Dodd, 1990;Yaginuma et al., 1991). This system has served as a fruitful model of vertebrate axon guidance mechanisms primarily because of the relative ease with which these extending axons can be studied in vitro and in vivo (Tessier-Lavigne et al., 1988;Colamarino and Tessier-Lavigne, 1995a).
Several lines of evidence suggest that a recently cloned protein, netrin-1 (Serafini et al., 1994), helps guide these commissural axonsin vivo. First, netrin-1 is homologous to theCaenorhabditis elegans unc-6 gene product (Ishii et al., 1992) and the Drosophila Netrin-A andNetrin-B gene products (Harris et al., 1996; Mitchell et al., 1996) that have been genetically implicated in the guidance of circumferential and commissural axon growth (Hedgecock et al., 1990;Harris et al., 1996; Mitchell et al., 1996). Second, embryonic rat spinal cord axons that normally form the commissural pathway specifically grow through a collagen gel matrix to cells heterologously expressing netrin-1 (Kennedy et al., 1994), indicating that netrin-1 can diffuse through the collagen to act as a chemoattractant for the axons in vitro. Third, embryonic floor plate tissue, the intermediate target of spinal cord commissural axons in vivo, attracts the growth of the axons through collagen in a manner qualitatively identical to that of the netrin-1-expressing cells (Tessier-Lavigne et al., 1988; Kennedy et al., 1994). Finally, in situ hybridization experiments indicate that netrin-1mRNA is expressed by floor plate cells during the period of commissural axon growth to the floor plate (Kennedy et al., 1994). Together these findings suggest that a gradient of soluble netrin-1 protein emanating from the floor plate helps guide commissural axons to the floor platein vivo analogously to the effect of this same protein on these axons in the collagen gel assay. Similar studies of netrin-1 in the brain suggest that a gradient of netrin-1 expressed by the brain floor plate attracts some developing axons and repels others (Kennedy et al., 1994; Colamarino and Tessier-Lavigne, 1995b).
Other data suggest an alternative or additional in vivo role for netrin-1 in nervous system development. All of the detectable netrin-1 activity isolated from tissue and most of the netrin-1 protein heterologously expressed by the tissue culture cells used in the collagen gel assay are present in bound form (Kennedy et al., 1994;Serafini et al., 1994), suggesting that most or all of the netrin-1 expressed in vivo may become bound to plasma membranes or the extracellular matrix and may exert a haptotactic effect on axon growth.
Notably absent from the current literature are studies investigating the in vivo localization of netrin-1 protein. The publishedin situ hybridization studies indicate which cells expressnetrin-1 mRNA and when they express it. However, these studies do not reveal when, where, or in what quantity netrin-1 protein is present. Given the rapid pace at which the commissural axons grow to the floor plate, it is particularly important to determine precisely where netrin-1 is located relative to the extending axons.
We report here the production of anti-netrin-1 antisera and their use in the immunohistochemical mapping of netrin-1 protein in the embryonic chick CNS. This work concentrates on determining the spatial and temporal relationships between netrin-1 localization and the growth of pioneering axons. The resulting data suggest that netrin-1 participates in the regulation of circumferential, commissural, and longitudinal axon growth in the spinal cord and brain. In particular, the data suggest that the primary or exclusive effect of netrin-1 on the in vivo growth of spinal cord commissural axons is haptotactic. The data also raise the possibility that netrin-1 plays a role in neuronal migration mechanisms in the spinal cord, brain, and retina.
MATERIALS AND METHODS
Antibody production. Three peptides (peptide 1, NH2-VVTEKGEEQVRS-COOH; peptide 2, NH2-QIHILKAEKNAD-COOH; and peptide 3, NH2-LGSTEDSPDQSG-COOH) that correspond to potentially antigenic regions of chick netrin-1 (Serafini et al., 1994) and that are not significantly homologous to any other known proteins [in addition, the sequence of peptide 1 is entirely absent from netrin-2, the most closely related known protein (Serafini et al., 1994)] were individually conjugated to keyhole limpet hemocyanin by incubation in 12.5% glutaraldehyde for 1 hr at room temperature. Conjugates were extensively dialyzed against PBS and were emulsified with Freund’s adjuvant before injection into female New Zealand White rabbits (three rabbits per conjugate). Subcutaneous injections of ∼0.5 μmol of conjugated peptide were performed on a biweekly schedule. The first injection contained complete adjuvant, whereas all of the subsequent injections contained incomplete adjuvant. Serum samples were collected 10 d after each injection, and antibody production was evaluated by ELISA. High-titer antisera were affinity-purified with Sepharose 4B columns containing the corresponding antigen peptides conjugated to ovalbumin. Briefly, antisera were diluted in PBS and loaded on the columns that were then extensively washed (15 column vol) with PBS. Purified antisera were eluted in fractions with 0.1 mglycine, pH 2.5, and immediately were neutralized with 1.0m Tris, pH 8.0, dialyzed against PBS and were stored at −80°C after the addition of 2.5 mm sodium azide. ELISA analysis of the purified antisera demonstrated that in each case the antisera recognized the appropriate peptide but did not recognize ovalbumin. The purified antisera were used in immunohistochemistry experiments at concentrations corresponding to dilutions of 1:500–1:2000 of the unpurified antisera.
Immunohistochemistry. White Leghorn chick eggs from the University of Florida Poultry Science Department were incubated at 37°C and 65–75% relative humidity in forced draft, automatic-turning incubators. Embryos were dissected and staged according to the method of Hamburger and Hamilton (1951), rinsed in 4°C PBS, immersion-fixed in Bouin’s solution (71.4% saturated picric acid, 23.8% formaldehyde, and 4.8% glacial acetic acid) for various lengths of time from 2 to 24 hr at 4°C, and finally transferred to 30% sucrose/0.1 m phosphate buffer, pH 7.2, containing 2.5 mm sodium azide for at least 12 hr at 4°C before sectioning.
Thirty micrometer cryostat sections were either collected into PBS to be processed free-floating (primarily used for initial antisera-screening studies) or thaw-mounted and fixed (5 min in Bouin’s solution) on slides (used for all of the adjacent section comparisons). All of the sections were sequentially incubated at room temperature in PBS (7 mmNa2HPO4, 3 mmNaH2PO4, and 130 mm NaCl, pH 7.2) twice for 15 min each, in methanol containing 0.3% H2O2 for 30 min, in PBS for 30 min, in PBS containing 0.4% Triton X-100 for 30 min, in PBS for 15 min, and in blocking solution (PBS containing 0.3% Triton X-100, 0.03% BSA, and 10% normal goat serum) for 1 hr. The sections were subsequently incubated for 3 d at 4°C in PBS containing 0.3% Triton X-100, 0.03% BSA, 2% normal goat serum, and affinity-purified anti-netrin-1 antisera or TuJ1 at 2 μg/ml (an anti-class III β-tubulin mouse monoclonal antibody; Frankfurter et al., 1986). The sections were then sequentially incubated at room temperature in PBS twice for 30 min each, in PBS containing the appropriate biotinylated secondary antibody at 7.5 μg/ml, 0.3% Triton X-100, 0.03% BSA, and 2% normal goat serum for 1 hr, and in PBS twice for 30 min each before staining with the avidin–biotin peroxidase method (ABC elite kit; Vector Laboratories, Burlingame, CA), using 3,3′-diaminobenzidine as a substrate and nickel ammonium sulfate as an enhancer. All observations are based on examination of tissue from at least eight embryos at each stage.
Two modifications of the immunohistochemistry procedure were also used. In some experiments involving the N2D antiserum, slide-mounted sections were autoclaved for 10 min and slowly cooled before the step with PBS containing 0.4% Triton X-100 to improve antigen accessibility (Shin et al., 1991; Bankfalvi et al., 1994). In other experiments, sections were double-labeled by including both anti-netrin-1 and anti-β-tubulin antibodies in the primary antibody incubation step and subsequently using species-specific, fluorescein-labeled (rabbit) and Texas Red-labeled (mouse) secondary antibodies. The fluorescent tags were then visualized by confocal microscopy.
RESULTS
Production and characterization of anti-netrin-1 antisera
Nine rabbits were immunized with one of three synthetic peptides corresponding to nonoverlapping segments of chick netrin-1 (see Materials and Methods). ELISA analyses of resulting antisera indicated that antibody titers progressively increased such that all of the antisera harvested after the fourth injection recognized appropriate antigen peptides at antisera dilutions of >1:10,000.
After affinity purification and fractionation on antigen columns, the antisera were immunohistochemically screened for the presence of antibodies that potentially recognize netrin-1 in fixed tissue sections. These initial experiments were conducted with sections of stage 30 chick heads. Antisera from all six rabbits injected with netrin-1 peptides 1 or 2 labeled the floor plate and retina, although with a wide range of effectiveness (Figs.1A–I,2F–I; data not shown). All of the antisera raised with peptide 3 were clearly ineffective (data not shown). Two antisera raised against peptide 1 (N1D and N1F) and one raised against peptide 2 (N2D) displayed the best signal-to-noise ratios and were chosen for further characterization with stage 15–30 embryos. These studies yielded three critical results indicating that the antisera are able to recognize netrin-1 in tissue sections. First, all three antisera can preferentially label the retina (Fig. 2; data not shown) and the floor plate of the brain and spinal cord at particular stages of development (see Figs. 1, 4, 5, 6, 7). This pattern of labeling is very consistent with the limited expression ofnetrin-1 mRNA (Kennedy et al., 1994). Second, the N1D and N1F antisera produce the same pattern of labeling as the N2D antiserum that was raised against an independent netrin-1 peptide (see Figs.1D,G,H, 2F–H,4I,K; data not shown). Third, the labeling with all three antisera was specifically and completely blocked by incubation of the antisera with the antigen peptide but was unaffected by an identical incubation with an equal concentration of nonantigen peptide (i.e., peptide 1 blocks N1D and N1F labeling but not that of N2D, whereas peptide 2 blocks N2D labeling but not that of N1D or N1F) (see Figs. 1D–I, 2C,D,H,I,6B,C; data not shown). In addition, as expected, no labeling was detected when the primary antibodies were omitted.
Fig. 1.
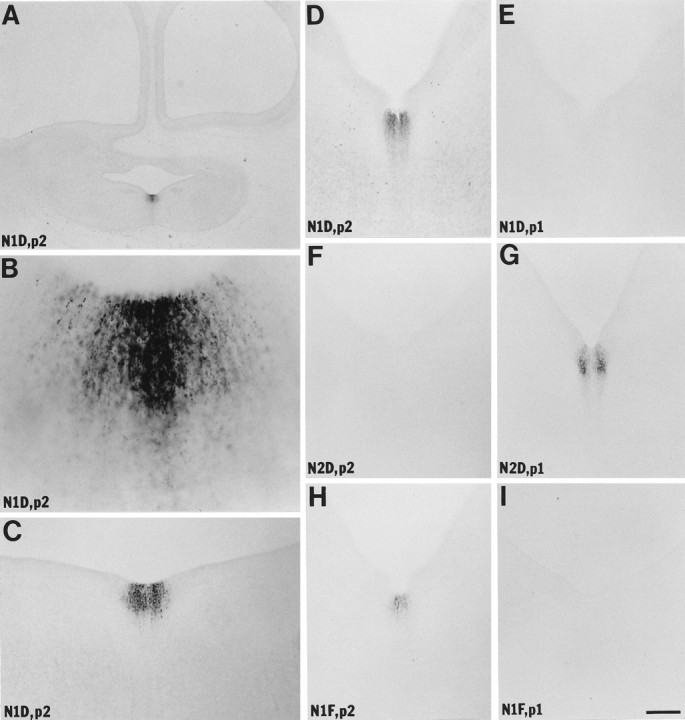
Netrin-1 in stage 30 brain. Netrin-1 IR is found selectively throughout the floor plate with the highest concentrations present at the level of the trochlear nucleus (A,B). Local gradients of netrin-1 IR are detected around and within the floor plate at all levels. C, Level of the fifth nerve. D–I, Approximate levels of the seventh and eighth nerves. All of the sections are approximately transverse.A–E, Processed with anti-netrin-1 antiserum N1D.F, G, Processed with anti-netrin-1 antiserum N2D. H, I, Processed with anti-netrin-1 antiserum N1F. A–D, F,H, Preadsorbed with netrin-1 peptide 2 (p2), the antigen for N2D. E,G, I, Preadsorbed with netrin-1 peptide 1 (p1), the antigen for N1D and N1F. Thus the labeling is specifically blocked by antigen–peptide preadsorption. Scale bar: A, 480 μm; B, 30 μm;C–I, 120 μm.
Fig. 2.
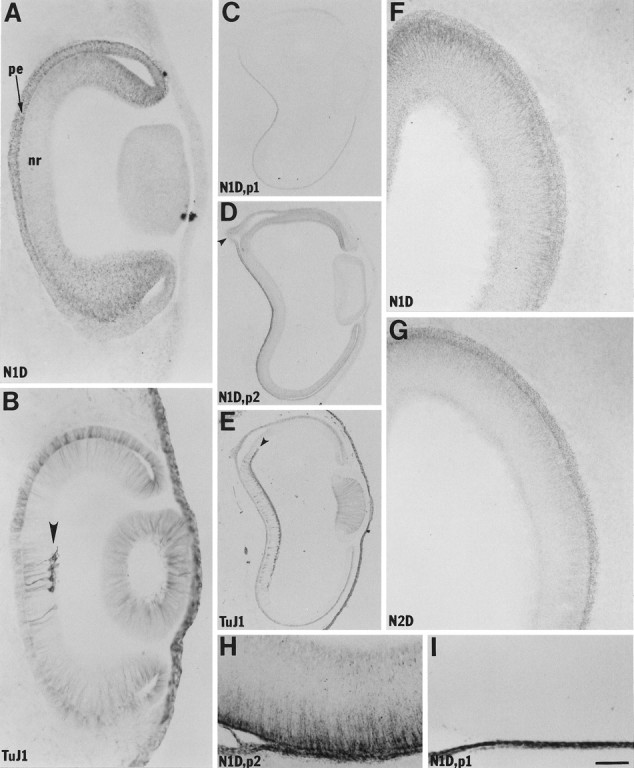
Netrin-1 in retina. A–E, Netrin-1 IR is initially present throughout the inner and outer retina but decreases in the neural retina (nr) and increases in the pigmented epithelium (pe) as retinal ganglion cells (arrowheads in B andE) differentiate. A, B, Adjacent stage 20 sections. C–E, Adjacent stage 24 sections. The optic stalk (arrowhead inD) is also labeled at stages 20–24.F–I, At stage 30, netrin-1 IR is expressed primarily by the ventricular side of the neural retina. A,C, D, F, H,I, Processed with N1D; C,I, Preadsorbed with the antigen peptide netrin-1 peptide 1 (p1); D, H, Preadsorbed with netrin-1 peptide 2 (p2).B, E, Processed with a β-tubulin antibody (TuJ1). G, Processed with N2D. Note that at stage 24 the pigmented epithelium signal is composed of both netrin-1 IR (preadsorbable) and the pigment of the pigmented epithelium (C vs D), whereas at stage 30 there is no detectable netrin-1 IR component to the pigmented epithelium signal (H vs I). Scale bar:A, B, H, I, 60 μm; C–E, 240 μm; F,G, 120 μm.
Fig. 4.
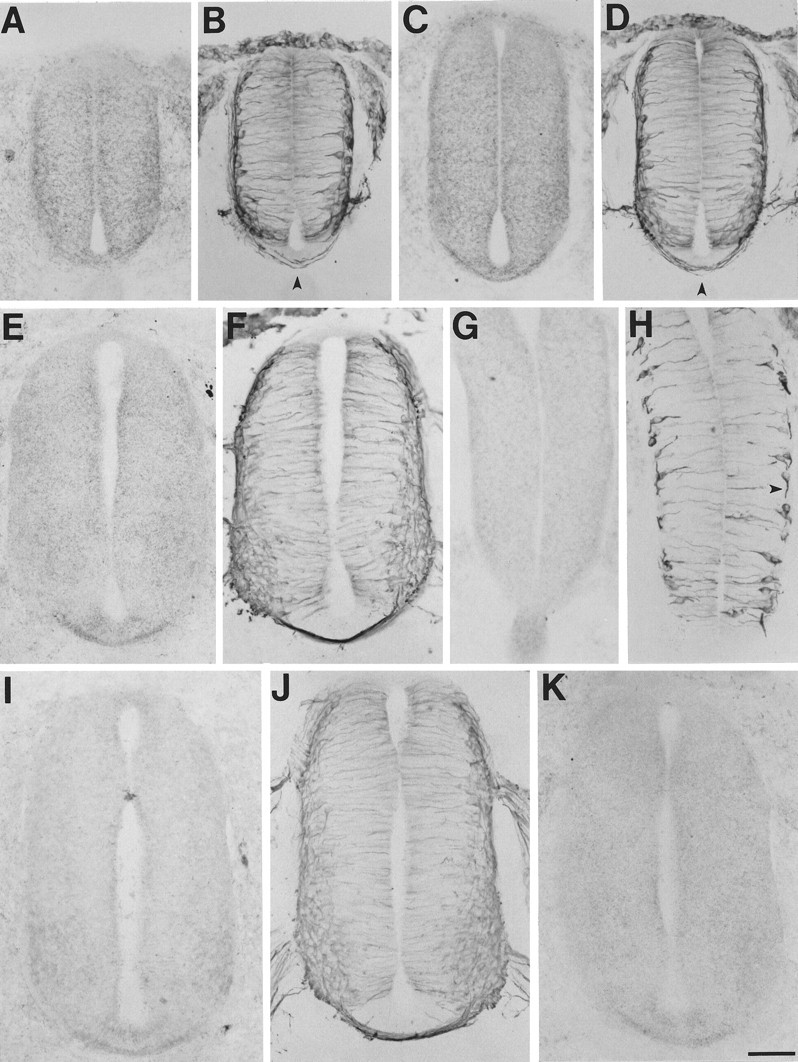
Netrin-1 in stage 20 spinal cord.A–F, Netrin-1 IR concentrates in the floor plate as fibers cross (e.g., arrowheads in B andD). A band of lower netrin-1 IR also develops immediately dorsal to the floor plate (e.g., C).G, H, In very caudal cord where the commissural fibers are just beginning (e.g., arrowheadin H), netrin-1 IR is concentrated along their path to the floor plate (G, H, oblique cuts). I–K, Rostral cord. A,C, E, K, Processed with N1D. G, I, Processed with N2D.B, D, F, H,J, Adjacent sections processed with β-tubulin antibody. Scale bar, 60 μm.
Fig. 5.
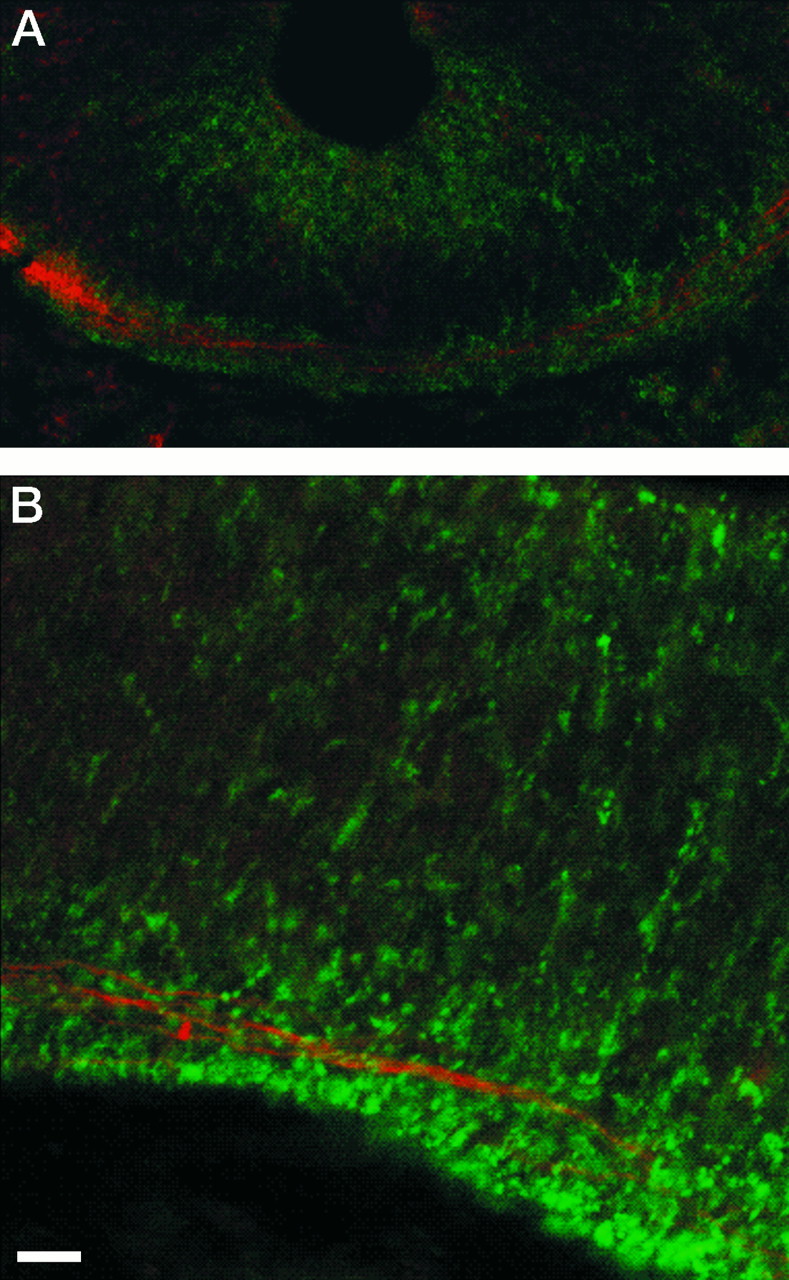
Double-labeling of netrin-1 and commissural fibers. Netrin-1 IR (green) is located around pioneering axons (red) that are either crossing the floor plate in the spinal cord (A) or in the mesencephalon (B, with midline slightly beyond the right edge of the photomicrograph). Little if any netrin-1 IR is present on the axons (areas with both green and redsignals are yellow). Transverse sections of stage 20 embryos were simultaneously processed with N1D and the β-tubulin antibody. Fluorescently tagged secondary antibodies were visualized by confocal microscopy. Scale bar: A, 7 μm;B, 5 μm.
Fig. 6.
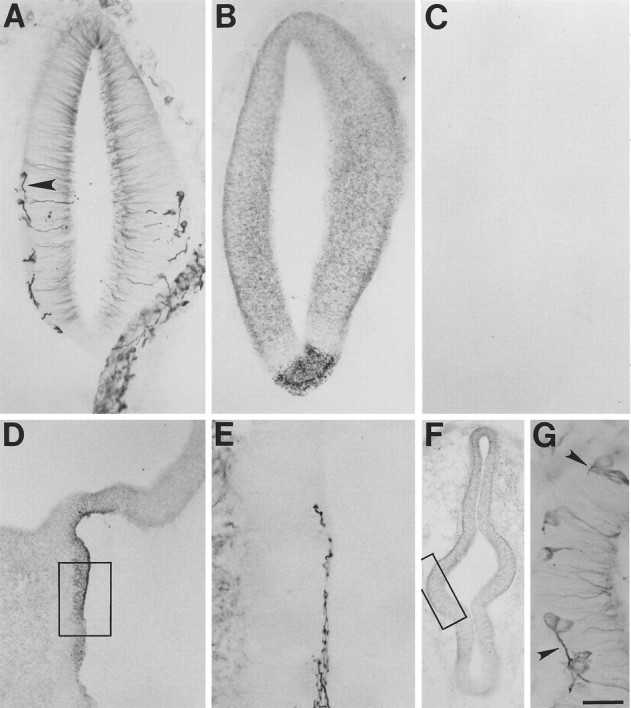
Netrin-1 in stage 15 brain. The highest levels of netrin-1 IR are found in the floor plate (B,D). Lower levels are found more dorsally and rostrally and tend to concentrate along outer borders of the neuroepithelium (B, F). Pioneering axons were detected traveling along paths of elevated netrin-1 IR (A, E, G).A–C, Transverse sections at approximately the junction of the metencephalon and mesencephalon. Note that the section inA is partially folded at the bottom right. D, E, Approximately parasagittal sections through the floor plate (rostral to thetop and ventral to the right).F, G, Approximately transverse sections through the prosencephalon. A, E,G, Processed with β-tubulin antibody.B, C, D, F, Adjacent sections processed with N1D; C, Preadsorbed with antigen peptide. Boxes in D andF designate approximate regions shown at higher power inE and G, respectively. Scale bar:A–C, 60 μm; D, F, 120 μm; E, G, 30 μm.
Fig. 7.
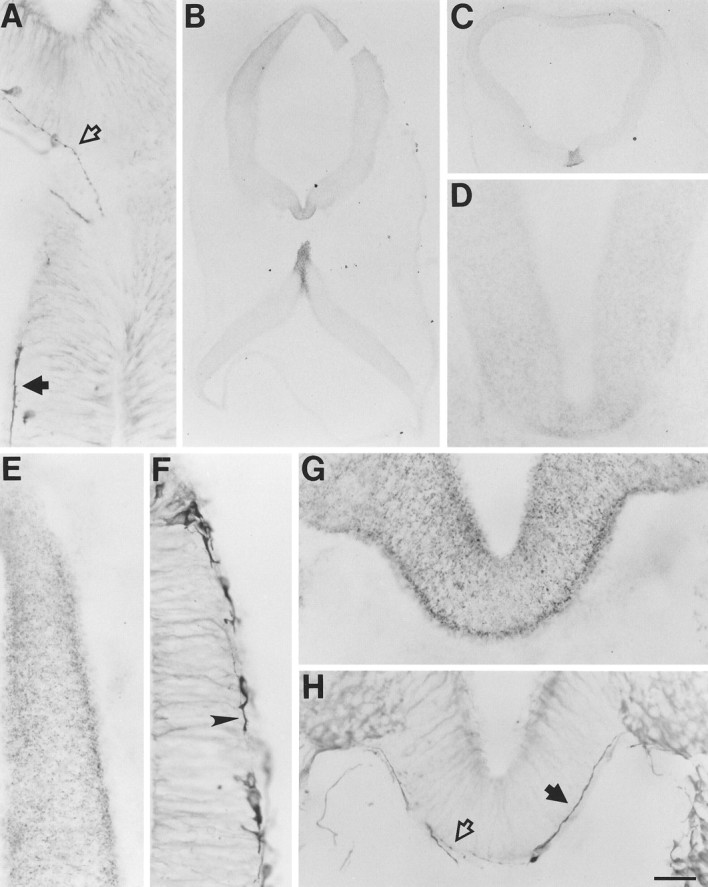
Netrin-1 in stage 20 brain. Netrin-1 IR is concentrated primarily in the floor plate (B,C, D, G). Pioneering longitudinal (A), circumferential (F), and commissural (H) fibers were detected growing through regions of elevated netrin-1 IR. B, An approximately horizontal section through heavily labeled metencephalic floor plate that, because of the neural tube flexure, also contains an approximately transverse cut near the level of the third cranial nerve (top) and more dorsal aspects of more caudal regions (bottom). A, Higher power of adjacent section corresponding to the heavily labeled regions ofB. C, Transverse section through rostral metencephalon. D, Transverse section of caudal myelencephalon. E, F, Dorsal neuroepithelium of the prosencephalon. G,H, Transverse sections at the level of the third cranial nerve (oculomotor cell bodies labeled in top right andleft of H). A,F, H, Processed with β-tubulin antibody. B, Processed with N1F. C,E, G, Processed with N1D.D, Processed with N2D. Thin varicose fibers (open arrows) and thicker fibers with pronounced growth cones (solid arrows) are indicated in A andH. E and G, Sections adjacent to F and H, respectively. Scale bar: A, E–H, 30 μm; B,C, 240 μm; D, 60 μm.
Netrin-1 in the spinal cord
The anti-netrin-1 antisera were used to study the relationship between netrin-1 and developing commissural axons in the spinal cord. Adjacent sections were processed with the netrin-1 antisera and a β-tubulin antibody that labels developing chick neurons including spinal cord commissural axons (Yaginuma et al., 1990). The commissural pathway of the spinal cord develops with a rostral-to-caudal temporal gradient throughout stages 15–24 (Holley, 1982; Yaginuma et al., 1990). Therefore, multiple stages from the period were examined, and sections were taken from throughout the rostrocaudal extent of the cord.
In the caudal stage 15 spinal cord, at the level at which the first commissural neurons are migrating and differentiating but have yet to extend processes ventrally, netrin-1 immunoreactivity (IR) was uniformly highest along the most lateral borders of the cord with relatively low levels in the floor plate (Fig.3A,B). More rostrally, where the neurons have extended short processes that begin to trace the lateral borders of the cord on their way to the floor plate, an increasing dorsal-to-ventral gradient of netrin-1 IR seems to develop along this same path, but little if any netrin-1 IR is present in the floor plate (Fig.3C,D). At the level at which the leading processes approach and enter the floor plate, the floor plate seems to express a relatively small amount of netrin-1 IR in its most basal aspect but as a whole still contains significantly less netrin-1 IR than the ventral–lateral borders of the cord (Fig. 3E,F). More rostrally, where the first few axons have decussated, the floor plate begins to display significant amounts of netrin-1 IR, whereas the ventral–lateral borders continue to contain comparable amounts (Fig.3G,H).
Fig. 3.
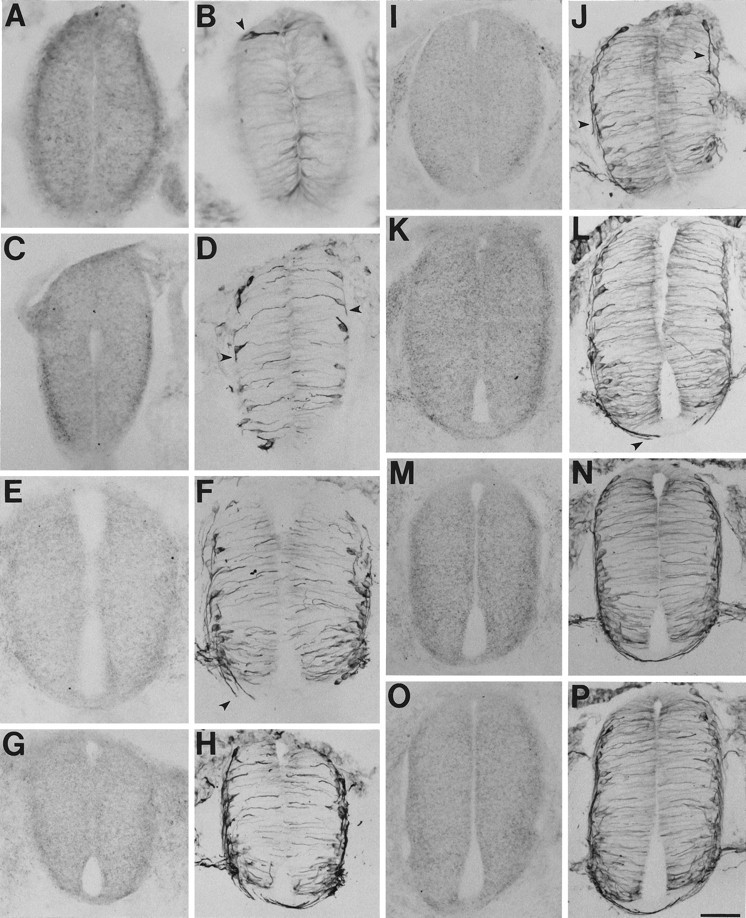
Netrin-1 in stage 15–18 spinal cord. Netrin-1 IR is concentrated along the outer borders of the neuroepithelium before and during the growth of commissural fibers to the floor plate (ventral–midline region at the bottom). The floor plate also begins to display elevated netrin-1 IR after the crossing of the first few fibers. A–H, Transverse sections of stage 15 cord from caudal to rostral. I–P, Transverse sections of stage 18 cord from caudal to rostral. A,C, E, G, I,K, M, O, Processed with N1D. B, D, F,H, J, L, N,P, Adjacent sections processed with the β-tubulin antibody. Arrowheads designate early differentiating neurons before commissural fiber initiation (B) and commissural fibers growing ventrally to the floor plate (D, J) or crossing the floor plate (F, L). Scale bar: A,B, 30 μm; C–P, 60 μm.
Examination of stage 18, 20, and 24 embryos indicated that this relationship between commissural fiber growth and netrin-1 IR observed at stage 15 is maintained throughout the development of the commissural system (Figs. 3I–N,4A,B,G,H; data not shown). In addition, at rostrocaudal levels of these later stages, at which several more fibers have crossed the floor plate, a band of somewhat lower netrin-1 IR is present immediately dorsal to the floor plate (e.g., Figs. 3O,P, 4C,D). Furthermore, as many fibers cross, netrin-1 IR becomes more concentrated in the floor plate and less concentrated along the lateral borders of the cord (Fig.4E,F,I–K).
The data collected with the adjacent-sections approach clearly indicate that, across several stages, the concentration of netrin-1 IR in the floor plate increases as the number of crossing fibers increases. However, this method lacks the resolution required to determine whether a significant proportion of the netrin-1 in the floor plate is located on the crossing fibers. To address this issue, we simultaneously processed stage 20 spinal cord with anti-netrin-1 antibodies and the anti-β-tubulin antibody. Confocal microscopy to detect fluorescently labeled secondary antibodies revealed that little if any of the netrin-1 IR in the floor plate is found on crossing fibers (Fig. 5A). Instead the netrin-1 IR seems to be preferentially located in close proximity to the fibers.
Netrin-1 in the brain
The anti-netrin-1 antisera were also used to study netrin-1 in the developing brain. Once again an adjacent section approach was used to examine the relationship between netrin-1 and pioneering axons.
At stage 15 the floor plate of the brain was labeled by the netrin-1 antisera (Fig. 6B,D). The most intense labeling was found at the level of the metencephalon and the caudal mesencephalon. This netrin-1 IR was significantly more concentrated than that detected elsewhere in the embryo, including the spinal cord. An area of very low netrin-1 IR was found immediately dorsal to the most intensely labeled floor plate (Fig.6B). Dorsal to that area, moderate levels of netrin-1 IR were detected throughout the neuroepithelium with elevated levels present along its outer borders (Fig. 6B). Staining of adjacent sections with the β-tubulin antibody identified a small population of differentiating neurons beginning to extend axons ventrally through this neuroepithelium toward the floor plate (Fig.6A). Parasagittal sectioning revealed pioneering axons coursing longitudinally through the heavily labeled floor plate (Fig. 6E).
The ventral–midline labeling decreased more rostrally until at the level of the rostral diencephalon little if any netrin-1 IR was detected (Fig. 6F), a finding consistent with the reported rostrocaudal extent of netrin-1 mRNA expression in the ventral midline (Kennedy et al., 1994) and with the expression of several floor plate markers (Colamarino and Tessier-Lavigne, 1995a). However, the labeling throughout the more dorsal regions of the prosencephalon is similar to that seen more caudally, in that the neuroepithelium is moderately labeled, with highest levels detected along outer borders (Fig. 6F). As is the case more caudally, this prosencephalic netrin-1 IR coincides with the ventral growth of axons from early differentiating neurons (Fig.6F,G).
At stage 20, netrin-1 IR continues to be present throughout the floor plate, with the most intense labeling still found in the metencephalon and caudal mesencephalon (Fig. 7B,C). The β-tubulin antibody identified several pioneering, longitudinal, and commissural axons traveling through these regions of high netrin-1 IR (Fig. 7A,B,G,H). The axons could be morphologically distinguished as thin varicose fibers or thicker fibers with pronounced growth cones (Fig. 7A,H). In most cases, the axons clearly follow a path of elevated netrin-1 IR. Double-labeling experiments indicated that little if any of the netrin-1 IR is located on the fibers (e.g., Fig. 5B). As with stage 15 embryos, netrin-1 IR was also detected in more dorsal structures, predominately along the outer borders of prosencephalic neuroepithelium that contains young neurons, the axons of which extend along the same borders (Fig.7E,F).
By stage 30, netrin-1 IR in the brain is found almost exclusively in the floor plate, and the concentrations found there are relatively high (Fig. 1). The level of the trochlear nucleus is the most heavily labeled (Fig. 1A,B). The netrin-1 IR displays medial–lateral and dorsal–ventral gradients in intensity (Fig. 1). Caudal to the level of the trochlear nucleus, the floor plate midline contains somewhat lower levels than the slightly more lateral regions of the floor plate that contain the highest netrin-1 IR. At the level of the trochlear nucleus, the midline is most intensely labeled. Lower concentrations of netrin-1 IR were observed more laterally and ventrally throughout the rostrocaudal extent of the floor plate.
Netrin-1 in the retina
Initially both the outer (presumptive pigmented epithelium) and inner (presumptive neural retina) layers of the optic cups contain moderate levels of netrin-1 IR (data not shown). As the retinal ganglion cells begin to differentiate and reach their final vitreal position in the then neural retina, netrin-1 IR decreases in the affected regions of the neural retina and increases in the adjacent pigmented epithelium (Fig. 2A–E). Thus, these changes in netrin-1 IR parallel the appearance of the retinal ganglion cells, occurring first in the deepest portion of the optic cups and expanding outward toward the margins of the cups (Fig.2A–E). The optic stalks are moderately labeled during the period in which the first retinal ganglion cell axons exit the retinas through the stalks (Fig. 2D, data not shown).
By stage 30, when differentiated retinal ganglion cells are present throughout the retina and many of their axons contribute to the optic nerve, moderate-to-intense netrin-1 IR is found at the ventricular surface of the neural retina, and a decreasing gradient of netrin-1 IR extends toward the vitreal surface (Fig.2F–H). The vitreal surface also displays a slightly elevated level of netrin-1 IR (Fig.2F–H). At this stage, the pigmented epithe lium and the optic stalk and nerve contain little if any netrin-1 IR (Fig. 2H,I, data not shown).
DISCUSSION
The goal of the present studies was to localize netrin-1 protein relative to pioneering axons during several critical stages of chick nervous system development. Accordingly, anti-netrin-1 polyclonal antisera were raised and immunohistochemically screened for the presence of antibodies capable of recognizing netrin-1 in fixed tissue sections. Three lines of evidence indicate that such antibodies were obtained. First, antisera raised against independent netrin-1 peptides yielded the same pattern of labeling, thereby providing evidence of recognition of netrin-1 as opposed to cross-reactivity with some unidentified protein that happens to share an epitope with one of the peptides. Second, incubation of the antisera with their respective antigen peptides blocked labeling, whereas identical incubation with unrelated netrin-1 peptides had no effect (see double dissociation demonstrated in Fig.1D–I). Third, the antisera preferentially labeled the same limited set of structures shown previously to preferentially express netrin-1 mRNA.
Spinal cord netrin-1 and commissural axon growth
As outlined in the introductory remarks, published results, including the in vitro effects of netrin-1 and the expression of netrin-1 mRNA in the spinal cord floor plate during the development of the commissural system, combine to suggest that the commissural fibers are guided to the floor plate by a gradient of soluble netrin-1 diffusing from the floor plate. In this model, netrin-1 synthesized by the floor plate acts as a chemoattractant (i.e., a soluble molecule acting while soluble to attract). However, if netrin-1 is synthesized by the floor plate and diffuses to the oncoming commissural fibers where it acts as a chemoattractant, the highest concentration of netrin-1 should be found in the floor plate, the source of the diffusing substance (a basic characteristic of diffusion), with a continuous, decreasing gradient of netrin-1 extending out to the fibers. Moreover, the documented tendency for netrin-1 to bind to expressing cells in vitro (Kennedy et al., 1994) and to the floor plate in vivo (Serafini et al., 1994) would be expected to increase the relative amount of netrin-1 found in the floor plate further. In contrast to this expectation, significant levels of netrin-1 IR were never observed in the floor plate before the crossing of several axons. In fact, during the period of ventral growth by the first commissural axons, the floor plate netrin-1 IR was generally as low or lower than anywhere else in the spinal cord. It is very unlikely that all of the antisera are unable to detect the expected netrin-1 in the floor plate during this period, given that all of them recognize netrin-1 in the floor plate soon after the fibers cross. It is also very unlikely that the floor plate contains relatively high quantities of soluble netrin-1 that all of the antibodies (raised against separate regions of netrin-1) somehow fail to detect, given that biochemical analysis indicates that the embryonic rat floor plate contains no detectable soluble netrin-1 in vivo, as assessed with a sensitive bioassay (Serafini et al., 1994). Presumably, the netrin-1 mRNA detected in the floor plate before the arrival of the commissural fibers (Kennedy et al., 1994) leads to the netrin-1 protein synthesized after the crossing of the first fibers. Interestingly, similar results have recently been reported for Drosophila homologs of netrin-1 that have been genetically implicated in commissural fiber growth (Harris et al., 1996; Mitchell et al., 1996). Thus, mRNAs encoding Netrin-A and Netrin-B are expressed by midline cells (the floor plate equivalent) before the arrival of the commissural fibers at the midline (Harris et al., 1996; Mitchell et al., 1996), but Netrin-A and Netrin-B proteins, although found lateral to the midline at earlier stages, are only detected in the midline cells after the crossing of pioneering axons (Klambt et al., 1991; Harris et al., 1996).
It has also been suggested that netrin-1 may, as a floor plate-derived chemoattractant, direct the growth of later developing commissural axons that navigate through the expanding pool of motor neurons in a ventromedial path to the floor plate (Colamarino and Tessier-Lavigne, 1995a). Although netrin-1 IR was detected at relatively high levels in the floor plate during the growth of these axons, the present data do not support such a proposal, because during this period a band of relatively low netrin-1 IR was consistently observed immediately dorsal to the floor plate, directly in the path of the axons and contrary to the expected pattern of a floor plate-derived, soluble substance guiding the axons (e.g., Figs. 3O,P,4C–F).
We found netrin-1 IR concentrated at the lateral borders of the spinal cord before and during commissural fiber growth to the floor plate along the same path. This localization and timing suggest that netrin-1 at the lateral borders of the cord helps guide commissural fibers. In addition, this elevated netrin-1 IR develops during commissural fiber growth to the floor plate into what seems to be a ventrally increasing gradient. Thus, the present data suggest that a gradient of netrin-1 helps guide the commissural axons to the floor plate, as suggested previously (Kennedy et al., 1994; Serafini et al., 1994). The primarily linear pattern of the netrin-1 IR suggests that the axons respond to a gradient of bound netrin-1.
It is unlikely that a significant fraction of the netrin-1 found along the lateral borders of the spinal cord is synthesized by the cells of the intermediate and dorsal cord, because previous work (Kennedy et al., 1994) did not detect any netrin-1 mRNA in these regions either before or during the period of commissural fiber growth to the floor plate, whereas the techniques used were sufficiently sensitive to detect netrin-1 mRNA easily in several other nearby tissues in the same sections. Therefore, it is most likely that the netrin-1 protein diffuses from another source or sources and binds to netrin-1-binding proteins localized along the lateral borders of the cord, where netrin-1 produces a haptotactic effect on commissural fiber growth. It is possible that this netrin-1 is synthesized by the floor plate. However, given the tendency of netrin-1 to bind to expressing cells (Kennedy et al., 1994) and the finding that all of the detectable netrin-1 activity in the embryonic floor plate is bound, not soluble (Serafini et al., 1994), it seems more likely that the netrin-1 originates in the notochord and/or other tissues near the cord, such as the sclerotome and dermamyotome that express netrin-1 mRNA before and during commissural fiber growth to the floor plate (Kennedy et al., 1994) but that, unlike the floor plate, also contain significant amounts of netrin-1 during this growth period (Figs.4G, 8A–D). The relatively high concentration of netrin-1 found along the borders of the cord presumably reflects the accumulation of netrin-1 by netrin-1-binding proteins.
Fig. 8.
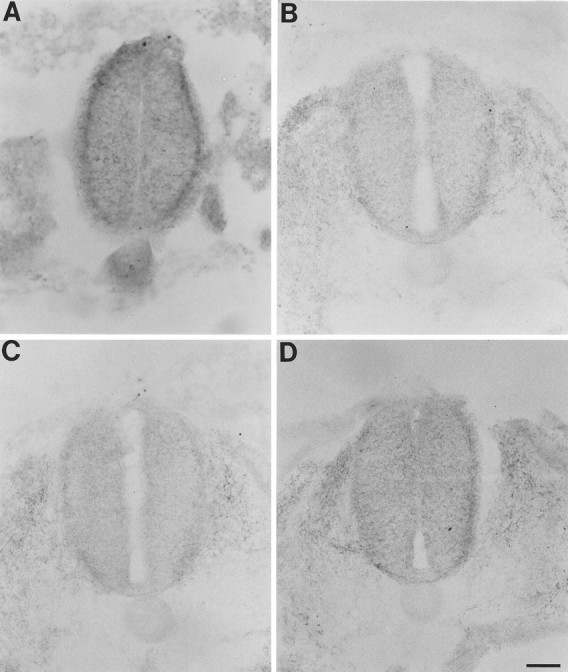
Netrin-1 in tissues adjacent to the spinal cord. Elevated levels of netrin-1 IR were detected in tissues adjacent to the spinal cord before and during the period of pioneering commissural fiber growth to the floor plate. This was true of the notochord (located immediately ventral to the spinal cord) only before and during the early stages of commissural fiber growth, such as that in the very caudal cord at stage 15 (A) and stage 20 (Fig.4G). The early, temporally limited presence of netrin-1 IR in the notochord is consistent with the similarly restricted expression of netrin-1 mRNA in this structure (Kennedy et al., 1994). Elevated netrin-1 IR was also observed during the period of pioneering commissural fiber growth to the floor plate (e.g., stage 15, B and C, and stage 18,D) in the sclerotome located immediately lateral to the cord (with a potential contribution by migrating neural crest cells) and, to a lesser but more variable extent, in the more laterally located dermamyotome. Note that the paraxial tissues frequently became partially dissociated from the cord during harvesting, sectioning, mounting, or processing. A, Same section as in Figure3A. B, Section of Figure3E. D, Section of Figure3K. All of the sections were processed with N1D. Scale bar: A, 30 μm; B–D, 60 μm.
The netrin-1 observed in the floor plate after the arrival of the commissural axons may also play a haptotactic role in helping guide these axons rostrally to form the ventral funiculi. Thus, we find a rostrally increasing gradient of netrin-1 in the floor plate during the period in which almost all of the commissural fibers turn and grow rostrally beside the floor plate (Oppenheim et al., 1988; Bovolenta and Dodd, 1990; Yaginuma et al., 1990).
Surprisingly, the first commissural axons grow down a rather steep netrin-1 gradient as they enter the floor plate from the ventral–lateral cord. It is not clear whether the axons are at that time programmed to accept the decreasing gradient and to decussate guided by the small amounts of netrin-1 detected in the most basal aspect of the floor plate at that stage. Alternatively, or in addition, some factor(s) other than netrin-1 may be primarily responsible for guiding the axons across the floor plate (Stoeckli and Landmesser, 1995).
Netrin-1 and pioneering axons in the brain
The pattern of netrin-1 localization in the brain shares some characteristics with that in the spinal cord but also displays some unique properties. In the stage 15 prosencephalon, rostral to the floor plate, netrin-1 IR is present throughout the dorsal neuroepithelium, with the highest concentrations found at the outer borders where processes are extending ventrally from young neurons. This relationship between netrin-1 IR and extending axons is somewhat similar to that observed during the early stages of spinal cord commissural axon growth. The same relationship is also observed in the mesencephalon and metencephalon. However, at these levels an intensely netrin-1 immunoreactive floor plate is located at the ventral midline. Whereas in the spinal cord significant floor plate netrin-1 IR is observed only after several axons have reached and crossed the floor plate, the mesencephalic and metencephalic floor plate contains relatively high concentrations of netrin-1 IR before and during the arrival and crossing of pioneering commissural axons. In most cases, it is unlikely that the commissural axons are chemotropically responding to a gradient of soluble netrin-1 emanating from the floor plate, particularly in regions where a band of very low netrin-1 IR is present immediately dorsal to the floor plate (e.g., Fig. 6B) or where the edges of the floor plate netrin-1 label are sharply defined (e.g., Fig. 7C). However, at the level of the third nerve (e.g., Fig. 7B,G), it is possible that very local and shallow gradients of netrin-1, originating in the floor plate, chemotropically influence commissural axon growth, although the preferential concentration of netrin-1 along the path of the axons seems more consistent with a haptotactic role.
We also detected pioneering axons projecting longitudinally through the regions of the floor plate that contain high levels of netrin-1 IR in stage 15–20 brain. This correlation suggests that netrin-1 participates in mechanisms responsible for the formation of early longitudinal pathways in the brain. Interestingly, the floor plate has been implicated in the formation of analogous tracts in the zebrafish (Hatta, 1992).
Many of the early longitudinal fibers of the chick brain have already grown ventrally along the outer borders of the neuroepithelium before turning longitudinally (Windle and Austin, 1935). Therefore, as in the spinal cord, the relationship between netrin-1 IR and axon growth suggests that netrin-1 may guide pioneering axons throughout their journey to the floor plate and their subsequent longitudinal growth.
General comments
In vitro heterologous expression studies and genetic data clearly indicate that netrin-1 and its homologs can profoundly affect the pathfinding of extending embryonic axons (Hedgecock et al., 1990; Kennedy et al., 1994; Serafini et al., 1994; Colamarino and Tessier-Lavigne, 1995b; Harris et al., 1996; Mitchell et al., 1996). The data reported here indicate that chick netrin-1 is temporally and spatially regulated in vivo such that it is frequently present in the path of extending axons, including those classes of axons that respond to it in vitro. Therefore, the data support the hypothesis that netrin-1 guides vertebrate axons in vivo. As outlined above, the observed relationship between netrin-1 localization and pioneering axon growth does little to support a chemotactic role for this protein. We cannot, of course, rule out the possibility that patterns of netrin-1 localization consistent with a chemotactic effect are present in small regions or during periods that we did not sample.
Recently, the deleted in colorectal cancer (DCC) protein has been identified as a netrin-1 receptor component necessary for the in vitro effects of netrin-1 on spinal cord commissural axons (Keino-Masu et al., 1996). Studies of DCC homologs inDrosophila and in C. elegans suggest that DCC acts as a netrin-1 receptor component in vivo (Chan et al., 1996; Kolodziej et al., 1996). Interestingly, DCC seems to be expressed by the same circumferential, commissural, and longitudinal fibers of the spinal cord and brain that we find associated with paths of concentrated netrin-1 (Keino-Masu et al., 1996). Thus, the DCC protein may regulate the growth properties of these pioneering axons by responding to this netrin-1.
The C. elegans netrin-1 homolog UNC-6 has been genetically shown to participate in cell migration mechanisms (Hedgecock et al., 1990). Therefore, it is tempting to speculate that the netrin-1 detected throughout several regions of developing neuroepithelium may play a role in guiding the many cells migrating through the neuroepithelium. Thus, the present study demonstrates that immature cells in the spinal cord, brain, and retina migrate up or down gradients of netrin-1 on their way to their final destinations. In particular, the relatively steep gradient that forms in the retina may help determine not only the direction but also the extent of migration by individual cell types, as the different cell types may migrate to characteristic netrin-1 concentrations. Moreover, the cells of the spinal cord and brain that migrate toward higher netrin-1 concentrations may express a different netrin-1 receptor(s) than retinal cells migrating down a netrin-1 gradient, as suggested by studies of UNC-6 and its receptors (Hedgecock et al., 1990;Leung-Hagesteijn et al., 1992; Hamelin et al., 1993; Chan et al., 1996;Wadsworth et al., 1996).
Finally, a report published after the submission of this manuscript (Serafini et al., 1996) describes the effects of a netrin-1gene mutation on mouse brain and spinal cord development. Consistent with previous netrin-1 studies and with the present findings, this report finds that mice homozygous for the mutation, which greatly reduces functional netrin-1 production, display several abnormalities in axon growth. Of particular relevance to the results reported here, most pioneering spinal cord commissural axons do not reach the floor plate but stall en route or grow medially away from the lateral borders and toward the ventricle. In addition, specific inspection early in axon development at the level of the fourth nerve nuclei (the region containing the highest levels of netrin-1 IR in the chick) revealed defects in longitudinal tract formation. Dramatic alterations in commissure formation in the brain were also observed later in development. However, when relating chick and mouse studies of netrin-1, it should be noted that, at least in the early spinal cord, mouse netrin-1 seems to serve the roles performed by netrin-1 and netrin-2 in the chick (Serafini et al., 1996).
Footnotes
This research was supported by Public Health Service Grants DA07244 to A.J.M. and AA09128 to M.B.H. We thank Dr. Anthony Frankfurter for the generous gift of TuJ1 (the β-tubulin antibody); Drs. Kevin Anderson, Alfred Chung, Michael King, Paul Linser, and David Muir for valuable advice; and Michael Paiva for excellent technical assistance.
Correspondence should be addressed to Dr. A. John MacLennan, Department of Neuroscience, Box 100244, University of Florida Health Science Center, Gainesville, FL 32610-0244.
REFERENCES
- 1.Bankfalvi A, Navabi H, Bier B, Bocker W, Jasani B, Schmid KW. Wet autoclave pretreatment for antigen retrieval in diagnostic immunohistochemistry. J Pathol. 1994;174:223–228. doi: 10.1002/path.1711740312. [DOI] [PubMed] [Google Scholar]
- 2.Bovolenta P, Dodd J. Guidance of commissural growth cones at the floor plate in embryonic rat spinal cord. Development (Camb) 1990;109:435–447. doi: 10.1242/dev.109.2.435. [DOI] [PubMed] [Google Scholar]
- 3.Chan SS-Y, Zheng H, Su M-W, Wilk R, Killeen MT, Hedgecock EM, Culotti JG. UNC-40, a C. elegans homolog of DCC (deleted in colorectal cancer), is required in motile cells responding to UNC-6 netrin cues. Cell. 1996;87:187–195. doi: 10.1016/s0092-8674(00)81337-9. [DOI] [PubMed] [Google Scholar]
- 4.Colamarino SA, Tessier-Lavigne M. The role of the floor plate in axon guidance. Annu Rev Neurosci. 1995a;18:497–529. doi: 10.1146/annurev.ne.18.030195.002433. [DOI] [PubMed] [Google Scholar]
- 5.Colamarino SA, Tessier-Lavigne M. The axonal chemoattractant netrin-1 is also a chemorepellent for trochlear motor axons. Cell. 1995b;81:621–629. doi: 10.1016/0092-8674(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 6.Frankfurter A, Binder LI, Rebhun LR. Limited tissue distribution of a novel β-tubulin isoform. J Cell Biol. 1986;103:273A. [Google Scholar]
- 7.Garrity PA, Zipursky SL. Neuronal target recognition. Cell. 1995;83:177–185. doi: 10.1016/0092-8674(95)90159-0. [DOI] [PubMed] [Google Scholar]
- 8.Goodman CS. Mechanisms and molecules that control growth cone guidance. Annu Rev Neurosci. 1996;19:341–377. doi: 10.1146/annurev.ne.19.030196.002013. [DOI] [PubMed] [Google Scholar]
- 9.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 10.Hamelin M, Zhou Y, Su M-W, Scott IM, Culotti JG. Expression of the UNC-5 guidance receptor in the touch neurons of C. elegans steers their axons dorsally. Nature. 1993;364:327–330. doi: 10.1038/364327a0. [DOI] [PubMed] [Google Scholar]
- 11.Harris R, Sabatelli LM, Seeger MA. Guidance cues at the Drosophila CNS midline: identification and characterization of two Drosophila netrin/UNC-6 homologs. Neuron. 1996;17:217–228. doi: 10.1016/s0896-6273(00)80154-3. [DOI] [PubMed] [Google Scholar]
- 12.Hatta K. Role of the floor plate in axonal patterning in the zebrafish CNS. Neuron. 1992;9:629–642. doi: 10.1016/0896-6273(92)90027-b. [DOI] [PubMed] [Google Scholar]
- 13.Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 1990;2:61–85. doi: 10.1016/0896-6273(90)90444-k. [DOI] [PubMed] [Google Scholar]
- 14.Holley JA. Early development of the circumferential axonal pathway in mouse and chick spinal cord. J Comp Neurol. 1982;205:371–382. doi: 10.1002/cne.902050406. [DOI] [PubMed] [Google Scholar]
- 15.Holley JA, Silver J. Growth pattern of pioneering chick spinal cord axons. Dev Biol. 1987;123:375–388. doi: 10.1016/0012-1606(87)90396-4. [DOI] [PubMed] [Google Scholar]
- 16.Ishii N, Wadsworth WG, Stern BD, Culotti JG, Hedgecock EM. UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron. 1992;9:873–881. doi: 10.1016/0896-6273(92)90240-e. [DOI] [PubMed] [Google Scholar]
- 17.Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS-Y, Culotti JG, Tessier-Lavigne M. Deleted in colorectal cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 19.Keynes R, Cook GMW. Axon guidance molecules. Cell. 1995;83:161–169. doi: 10.1016/0092-8674(95)90157-4. [DOI] [PubMed] [Google Scholar]
- 20.Klambt C, Jacobs JR, Goodman CS. The midline of the Drosophila central nervous system: a model for the genetic analysis of cell fate, cell migration, and growth cone guidance. Cell. 1991;64:801–815. doi: 10.1016/0092-8674(91)90509-w. [DOI] [PubMed] [Google Scholar]
- 21.Kolodziej PA, Timpe LC, Mitchell KJ, Fried SR, Goodman CS, Jan LY, Jan YN. Frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell. 1996;87:197–204. doi: 10.1016/s0092-8674(00)81338-0. [DOI] [PubMed] [Google Scholar]
- 22.Letourneau PC, Condic ML, Snow DM. Interactions of developing neurons with the extracellular matrix. J Neurosci. 1994;14:915–928. doi: 10.1523/JNEUROSCI.14-03-00915.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung-Hagesteijn C, Spence AM, Stern BD, Zhou Y, Su M-W, Hedgecock EM, Culotti JG. UNC-5, a transmembrane protein with immunoglobulin and thrombospondin type 1 domains, guides cell and pioneer axon migrations in C. elegans. Cell. 1992;71:289–299. doi: 10.1016/0092-8674(92)90357-i. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell KJ, Doyle JL, Serafini T, Kennedy TE, Tessier-Lavigne M, Goodman CS, Dickson BJ. Genetic analysis of netrin genes in Drosophila: netrins guide CNS commissural axons and peripheral motor axons. Neuron. 1996;17:203–215. doi: 10.1016/s0896-6273(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 25.Oppenheim RW, Shneiderman A, Shimizu I, Yaginuma H. Onset and development of intersegmental projections in the chick embryo spinal cord. J Comp Neurol. 1988;275:159–180. doi: 10.1002/cne.902750202. [DOI] [PubMed] [Google Scholar]
- 26.Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 27.Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 28.Shin R-W, Iwaki T, Kitamoto T, Tateishi J. Hydrated autoclave pretreatment enhances TAU immunoreactivity in formalin-fixed normal and Alzheimer’s disease brain tissues. Laboratory Investigation. 1991;64:693–702. [PubMed] [Google Scholar]
- 29.Stoeckli ET, Landmesser LT. Axonin-1, Nr-CAM, and Ng-CAM play different roles in the in vivo guidance of chick commissural neurons. Neuron. 1995;14:1165–1179. doi: 10.1016/0896-6273(95)90264-3. [DOI] [PubMed] [Google Scholar]
- 30.Tessier-Lavigne M. Axon guidance by diffusible repellants and attractants. Curr Opin Genet Dev. 1994;4:596–601. doi: 10.1016/0959-437x(94)90078-h. [DOI] [PubMed] [Google Scholar]
- 31.Tessier-Lavigne M, Placzek M, Lumsden AGS, Dodd J, Jessell TM. Chemotropic guidance of developing axons in the mammalian central nervous system. Nature. 1988;336:775–778. doi: 10.1038/336775a0. [DOI] [PubMed] [Google Scholar]
- 32.Wadsworth WG, Bhatt H, Hedgecock EM. Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron. 1996;16:35–46. doi: 10.1016/s0896-6273(00)80021-5. [DOI] [PubMed] [Google Scholar]
- 33.Windle WF, Austin MF. Neurofibrillar development in the central nervous system of chick embryos up to 5 days’ incubation. J Comp Neurol. 1935;63:431–463. [Google Scholar]
- 34.Yaginuma H, Shiga T, Homma S, Ishihara R, Oppenheim RW. Identification of early developing axon projections from spinal interneurons in the chick embryo with a neuron specific β-tubulin antibody: evidence for a new ’pioneer’ pathway in the spinal cord. Development (Camb) 1990;108:705–716. doi: 10.1242/dev.108.4.705. [DOI] [PubMed] [Google Scholar]
- 35.Yaginuma H, Homma S, Kunzi R, Oppenheim RW. Pathfinding by growth cones of commissural interneurons in the chick embryo spinal cord: a light and electron microscopic study. J Comp Neurol. 1991;304:78–102. doi: 10.1002/cne.903040107. [DOI] [PubMed] [Google Scholar]


