Abstract
Cannabinoids act at the CB1 receptor to inhibit adenylate cyclase activity via a pertussis toxin-sensitive G-protein. Within the striatum, CB1 receptors have been shown to be localized on the same neurons as Gi-coupled dopamine D2 receptors. In this study we have examined the interactions of CB1 and D2 receptors on adenylate cyclase. In striatal neurons in primary culture, both the CB1 receptor agonist [3-(1,1-dimethylheptyl)-11-hydroxy-Δ8tetrahydrocannabinol] (HU210) and the D2 receptor agonist quinpirole inhibited forskolin-stimulated cAMP accumulation when applied separately. In contrast, HU210 and quinpirole in combination augmented cAMP accumulation. This augmentation was blocked by the CB1 receptor antagonist SR141716A or the D2 antagonist sulpride. Pertussis toxin treatment of striatal neurons prevented the inhibition of cAMP accumulation by D2 receptors but unmasked a cannabinoid receptor-mediated stimulatory effect on cAMP accumulation. The cannabinoid receptor-stimulated accumulation of cAMP was blocked in a concentration-dependent manner by SR141716A, suggesting that the response was regulated through the CB1 receptor. Similar augmentation of cAMP accumulation after pertussis toxin treatment was observed in Chinese hamster ovary (CHO) cells transfected with, and stably expressing, the CB1 receptor. This stimulation of cAMP was not Ca2+-sensitive and was unaffected by a range of protein kinase inhibitors. Treatment of the pertussis toxin-treated cells with cholera toxin before CB1 receptor activation amplified the stimulatory pathway, suggesting that this response was mediated through a Gs-type G-protein. Stimulation of cAMP accumulation was not observed after pertussis toxin treatment of CHO cells expressing the human CB2 receptor, suggesting that this novel signaling pathway is unique to the cannabinoid CB1 receptor.
Keywords: cannabinoid, G-protein, adenylate cyclase, CB1 receptor, dopamine, D2 receptor, striatum
Cannabinoids exert many of their effects through activation of Gi-protein-coupled receptors. Two subtypes of cannabinoid receptors have been identified. The CB1 receptor (Matsuda et al., 1990) is distributed predominantly in the CNS and testis (Matsuda et al., 1990; Gerard et al., 1991; Westlake et al., 1994), and the CB2 receptor (Munro et al., 1993) is localized in peripheral tissue, including the marginal zone of the spleen (Munro et al., 1993). Within the CNS, the CB1 receptor is localized densely in the basal ganglia (Herkenham et al., 1991a, b; Glass et al., 1997b), and a role for cannabinoids in normal and abnormal physiology of basal ganglia function is emerging (for review, see Glass et al., 1997a). Within the striatum, the CB1 receptor has been localized to the medium spiny neurons (Herkenham et al., 1991a) that act as the major output neurons of the striatum. Medium spiny neurons can be divided in two subclasses based on where their projections terminate and based on their neuropeptide content (Beckstead and Kearsey, 1985; Beckstead and Cruz, 1986; Gimenez-Amaya and Graybiel, 1990). One population of neurons releases substance P and projects to the substantia nigra, whereas the other subset contains enkephalin and projects to the globus pallidus (Graybiel, 1990). The two populations also contain different populations of dopamine receptors. Dopamine D1 receptors are localized predominantly on neurons releasing substance P, and dopamine D2 receptors are localized on enkephalin releasing neurons (Le Moine et al., 1991). CB1 receptors have been identified on both subpopulations of medium spiny neurons (Mailleux and Vanderhaeghen, 1992).
Previous studies have demonstrated the functional coupling of CB1 receptors to the inhibition of adenylate cyclase and voltage-dependent calcium channels via pertussis toxin-sensitive G-proteins (Felder et al., 1992; Mackie and Hille, 1992). Both D1 and D2 dopamine receptors also couple to adenylate cyclase via G-proteins. Dopamine D1 receptors stimulate adenylate cyclase via Gs-proteins (Monsma et al., 1990), whereas dopamine D2 receptors inhibit adenylate cyclase via Gi-proteins (Sibley and Monsma, 1992). Although there is evidence for associations between the actions of cannabinoids and dopamine within the striatum (for review, see Glass et al., 1997a), signaling interactions between these receptors have not been investigated extensively. Previously, CB1 receptors have been shown to inhibit D1 receptor-mediated cAMP accumulation (Bidaut-Russell and Howlett, 1991). However, an interaction between D2 and CB1 receptors has not been established. We now report that concurrent activation of D2 and CB1 receptors results in an increase in cAMP accumulation in contrast to the inhibition of cAMP accumulation normally observed with activation of either receptor alone. We suggest that these data demonstrate a novel pathway in which the CB1 receptor couples to a Gs-protein, indicating that cannabinoid receptor function may be more complex than the simple Gi linkage described previously.
MATERIALS AND METHODS
Cell Culture
Primary striatal culture. Striatum was dissected from embryonic rat pups on embryonic day 18 (E18) and placed in D1 saline (100 U/ml papain, 100 mm CaCl2, 50 mm EDTA, and 1.5 mm NaOH) for 15 min at 37°C, washed for 5 min with 10FC [10% fetal calf serum, 50 U/ml penicillin/streptomycin, 4 mm glutamine in minimum essential media (MEM) containing 2.5 mg/ml trypsin inhibitor and 2.5 mg/ml bovine serum albumin (BSA)]. Cells were then dissociated in 10FC and plated at 3 million/well in six-well plates in neurobasal media (B27 supplement, Life Technologies, Gaithersburg, MD; 0.5 mml-glutamine; and 25 μmglutamate) on a confluent background of astrocytes. Astrocytes were prepared identically but plated in 10FC. Confluent astrocyte cultures were treated with 6.6 μg/ml 5′fluoro-2′deoxyurindine (mitotic inhibitor). Neurons were fed with neurobasal media without glutamate on days 3 and 7, and cAMP assays were performed between days 4 and 8. All cell culture media were obtained from BioWhittaker (Walkersville, MD).
Chinese hamster ovary (CHO)-K1 cells were obtained from American Type Culture Collection (Rockville, MD) and maintained as described previously (Felder et al., 1990). The human cannabinoid CB1 and CB2 receptor cDNA were expressed stably in CHO cells as described previously (Felder et al., 1992). CHO cells stably expressing the D2 receptor were provided generously by Dr. David Sibley (National Institute of Neurological Disorders and Stroke, Bethesda, MD).
Assay of cAMP accumulation. Measurement of cAMP accumulation was performed as described previously with the following modifications (Felder et al., 1990). All ligands were diluted in silonized-glass test tubes with Eagle’s No. 2 media containing 50 mg/ml fatty acid-free BSA. The final BSA concentration was 5 mg/ml. Assays were performed in silonized glass, with ∼4 × 105cells/0.25 ml final assay volume, over a period of 5 min at 37°C. The reaction was stopped with the addition of an equal volume of 0.1N HCl, after which 50 μl was removed for radioimmunoassay of cAMP as described previously (Felder et al., 1990). HU210 and HU211 were provided generously by Dr. Raphael Mechoulam (Hebrew University, Jerusalem, Israel). SR141716A was provided by the National Institute of Drug Abuse (Rockville, MD). Quinpirole and sulpride were obtained from Research Biochemicals International (Natick, MA). The cells were washed twice and suspended in calcium-free Eagle’s No. 2 media with 0.5 mm EGTA to determine the role of extracellular calcium on cAMP accumulation. The role of intracellular calcium was determined by pretreatment in the above buffer with 1 mm ATP for 10 min at 37°C to deplete intracellular calcium stores (Singer-Lahat et al., 1996). The ATP was removed by washing, and the cells were suspended in the calcium-free media. When required, cells were treated overnight with pertussis toxin (5 ng/ml; Calbiochem, La Jolla, CA). Treatment with cholera toxin (2 μg/ml; Calbiochem) was performed in serum-free α-MEM for 1 hr before the start of the experiment. The possible involvement of protein kinases was examined using selected inhibitors (Calbiochem) at the concentrations shown in Table 1. Cells were preincubated with the inhibitors in the assay buffer for 10 min at 37°C before the addition of forskolin and HU210.
Table 1.
Effect of extracellular calcium removal or intracellular calcium depletion on cAMP accumulation
| Untreated | Pertussis toxin-treated | |||||
|---|---|---|---|---|---|---|
| Control | No Ca2+-ex | No Ca2+in/ex | Control | No Ca2+ ex | No Ca2+ in/ex | |
| Basal | 3.3 ± 0.6 | 3.1 ± 0.2 | 2.8 ± 0.5 | 2.8 ± 0.1 | 2.9 ± 0.3 | 3.0 ± 0.1 |
| 50 nm FSK | 46.0 ± 2.7 | 40.5 ± 2.8 | 20.9 ± 1.8 | 20.2 ± 2.0 | 15.8 ± 1.2 | 11.2 ± 1.0 |
| + 1 μm HU210 | 4.7 ± 0.2 | 4.7 ± 0.3 | 4.4 ± 0.3 | 51.2 ± 4.9 | 37.8 ± 2.5 | 26.9 ± 3.0 |
Measurement of intracellular calcium concentration in single fura-2-loaded cells. Cells grown on glass coverslips coated with Vitrogen (300 μg/ml; Collagen Corp., Palo Alto, CA) were loaded with 5 μm fura-2/AM ester (Molecular Probes, Eugene, OR) for 30 min at 37°C. Fura-2 fluorescence was measured using an intensified CCD video camera at an emission wavelength of 510 nm in single cells mounted on a Nikon (Melville, NY) Diaphot microscope illuminated alternately with 340 and 380 nm light (bandpass 4 nm), using a SLM-AMINCO (Urbana, IL) DMX-1000 spectroflourometer and Universal Imaging (West Chester, PA) Metafluor software.
RESULTS
Interaction of dopamine and cannabinoid receptors in primary striatal culture
Primary striatal cultures from E18 rats were tested functionally for the presence of cannabinoid CB1 and dopamine D2 receptors. As shown in Figure 1, the CB1 receptor agonist HU210 and the dopamine D2 receptor agonist quinpirole both inhibited cAMP accumulation in primary striatal culture. The inhibition of cAMP accumulation was blocked by either the CB1 receptor antagonist SR141716A (1 μm) or the D2 receptor antagonist sulpride (1 μm), respectively (Fig. 1). CB1 and D2 receptors were then activated simultaneously in the presence of forskolin to determine whether the inhibitory responses were synergistic. In contrast to the data shown in Figure 1, activation of CB1 receptors in the presence of quinpirole resulted in a dose-dependent augmentation of cAMP accumulation (Fig. 2). The augmentation of cAMP accumulation was blocked completely by either SR141716A (1 μm) or sulpride (1 μm)(data not shown).
Fig. 1.
Effect of CB1 and D2 agonists on forskolin-stimulated cAMP accumulation in primary striatal cultured neurons. Forskolin-stimulated (50 nm) cAMP accumulation was measured in primary culture of the striatum in the presence or absence of quinpirole (100 nm) and HU210 (1 μm) as described in Materials and Methods. The inhibition of cAMP accumulation by these compounds was blocked by the D2 receptor antagonist sulpride (1 μm) or the CB1 antagonist SR141716A (1 μm), respectively. Data are the mean ± SEM of at least three experiments performed in duplicate. One hundred percent accumulation is equivalent to 39 ± 8.5 pmol/mg cAMP.
Fig. 2.
Effect of concurrent activation of D2 and CB1 receptors on cAMP accumulation in primary striatal culture. Forskolin-stimulated (50 nm) cAMP accumulation was measured in primary striatal culture as described in Materials and Methods in combination with 100 nm quinpirole and increasing concentrations of HU210. HU210 reversed the quinpirole-mediated inhibition of forskolin-stimulated cAMP accumulation in a concentration-dependent manner to levels above that demonstrated with forskolin alone (EC50 = 6.1 ± 1.2 nm). The stimulation of cAMP accumulation in the presence of forskolin and quinpirole at 1 μm HU210 was blocked by 1 μm SR141716A. Data are the mean ± SEM of at least three experiments performed in duplicate.
Cells were treated overnight with pertussis toxin to determine the contribution of Gi to the interaction of CB1 and D2 receptors. Pertussis toxin treatment uncouples receptors from Gi-proteins by catalyzing ADP ribosylation of the α-subunit thereby preventing the activation of the G-protein. After overnight treatment of the primary striatal cultures with pertussis toxin (5 mg/ml), quinpirole no longer inhibited forskolin-stimulated cAMP accumulation (data not shown). In contrast, HU210 stimulated a concentration-dependent increase in the forskolin-stimulated accumulation of cAMP that was prevented with SR141716A (1 μm), consistent with a CB1 receptor-mediated mechanism (Fig. 3A).
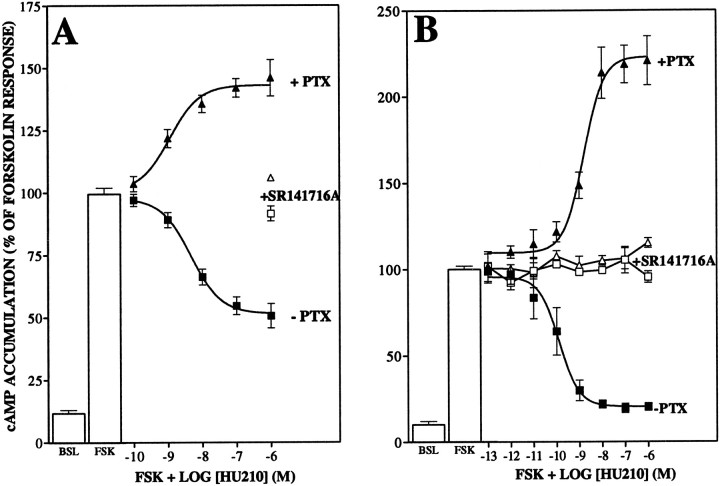
Fig. 3.
Effect of pertussis toxin on HU210-mediated inhibition of forskolin-stimulated cAMP accumulation in primary striatal culture (A) and CHO-hCB1 cells (B). Forskolin-stimulated (50 nm) cAMP accumulation was measured in primary striatal culture and CHO-hCB1 as described in Materials and Methods. Pertussis toxin (+PTX;5 ng/ml) was added ∼18 hr before the measurement of cAMP accumulation. In the absence of pertussis toxin, HU210 inhibited forskolin-stimulated cAMP (A, EC50 = 4.6 ± 1.2 nm; B, EC50 = 0.17 ± 0.01 nm). After pertussis toxin treatment, HU210 resulted in an increase in forskolin-stimulated cAMP (A, EC50 = 1.2 ± 1.1 nm;B, EC50 = 1.6 ± 0.04 nm). Both the inhibition and the stimulation of cAMP were prevented by concurrent exposure to 1 μm SR141716A (open symbols). Data are the mean ± SEM of at least three experiments performed in triplicate. One hundred percent accumulation is equivalent toA, 39 ± 8.5 pmol/ml; B, 39.4 ± 3 pmol/ml.
cAMP accumulation in CHO cells transfected with the human CB1 receptor
CB1 receptor stimulation of cAMP accumulation was investigated further in a clonal CHO cell line stably expressing the human CB1 receptor (CHO-hCB1). In these cells, HU210 demonstrated a high-potency concentration-dependent inhibition of forskolin-stimulated cAMP accumulation (Fig. 3B). As seen in the primary striatal cultures, overnight treatment with pertussis toxin abolished the inhibitory response and unmasked an HU210-dependent increase in cAMP accumulation above forskolin-stimulated levels (Fig. 3B). This stimulation was blocked by SR141716A (1 μm). Pertussis toxin treatment of CHO cells expressing either CB2 or D2 receptors abolished receptor-mediated modulation of cAMP in these cells but did not result in an increase in cAMP accumulation (data not shown), indicating that the stimulatory response is not common to all Gi-linked receptors.
Several controls were used to ensure that the accumulation of cAMP in pertussis toxin-treated CHO-hCB1 cells was mediated by the CB1 receptor. HU211, the inactive enantiomer of HU210, failed to produce any effect on forskolin-stimulated cAMP accumulation in CHO-hCB1 (data not shown). Furthermore, HU210 did not alter cAMP accumulation in nontransfected CHO-K1 cells (data not shown). Cannabinoids have been reported to exert a number of nonreceptor-mediated effects (Razdan, 1986; Felder et al., 1992). Experiments were performed to ensure that the interaction observed between CB1 and D2 receptors in cultured neurons was not attributable to HU210 altering the ability of the D2 receptor to inhibit cAMP. In CHO cells expressing only the dopamine D2 receptor, HU210 (1 μm) did not influence the inhibition of forskolin-stimulated cAMP accumulation by quinpirole (data not shown).
The coupling of the CB1 receptor to stimulation of cAMP accumulation may be mediated through the stimulatory protein Gs-α. Gs-α and forskolin have been shown to be synergistic in their activation of adenylate cyclase (Downs and Aurbach, 1982;Barovsky and Brooker, 1985). Consistent with this activity, in the presence of pertussis toxin, enhanced cAMP accumulation was observed when HU210 and forskolin were added together, compared with the level of stimulation observed when each agent was added alone (Fig.4A). Cholera toxin, which selectively ADP ribosylates and constitutively activates Gs-αsubunits, provides a mechanism to demonstrate turnover of Gs-proteins (Gill and Meren, 1978). The Gs-αsubunits only become available as a substrate for the toxin as they are released from the heterotrimeric G-protein, such as occurs after receptor stimulation. Therefore, after preincubation with cholera toxin, stimulation of receptors coupled to Gs releases α subunits, enabling them to be activated permanently by cholera toxin, and resulting in amplification of agonist-dependent increases in cAMP accumulation. As shown in Figure 4B, cholera toxin treatment increased basal levels of cAMP, and this increase was partially inhibited by HU210. At higher concentrations of HU210 a reversal of the inhibition of cAMP accumulation was observed, suggesting an underlying stimulatory component. Therefore, the same experiment was performed in the presence of pertussis toxin to remove the HU210-mediated inhibition of cholera toxin-stimulated cAMP accumulation (Fig. 4C). In the presence of pertussis toxin, HU210 enhanced the cholera toxin response, indicating mediation through Gs.
Fig. 4.

Evidence suggesting a Gs linkage for the CB1 receptor. A, CHO-hCB1 cells were treated overnight with pertussis toxin. HU210 did not affect basal levels of cAMP accumulation in the absence of forskolin. Increasing concentrations of forskolin resulted in an amplification of HU210-induced cAMP accumulation above forskolin levels. Data are mean ± SEM from a representative experiment. B, cAMP levels after pretreatment with cholera toxin. Cholera toxin results in an increase in basal cAMP accumulation. HU210 stimulation in the absence of forskolin resulted in a U-shaped concentration response curve, with inhibition at lower doses but stimulation at high doses. Data are mean ± SEM from a representative experiment.C, Cells were treated overnight with pertussis toxin before treatment with cholera toxin. An amplified signal was observed after cholera toxin treatment (+PTX/CTX), compared with pertussis treatment (+PTX) alone. All experiments were performed in the absence of forskolin. Data are mean ± SEM from a representative experiment.
Additional mechanisms of regulation of the CB1 receptor-mediated stimulatory response were investigated. Removal of extracellular calcium or depletion of intracellular calcium stores had no effect on the inhibitory or stimulatory responses mediated through the CB1 receptor (Table 1). A spectrum of protein kinase inhibitors also had no effect on the inhibitory or stimulatory responses mediated through the CB1 receptor (Table 2). Furthermore, no calcium mobilization was observed at the concentrations of HU210, which produce stimulation of cAMP accumulation in pertussis toxin-treated cells.
Table 2.
Effect of protein kinase inhibitors on cAMP accumulation
| cAMP accumulation (pmol/mL) | ||
|---|---|---|
| Untreated | PTX-treated | |
| Basal | 2.9 ± 0.1 | 2.8 ± 0.2 |
| 50 nm Fsk | 30.4 ± 1.9 | 16.4 ± 0.6 |
| Fsk + 1 μm HU210 | 3.9 ± 0.1 | 32.0 ± 0.7 |
| + 100 nmbisindolylmaleimide I (PKC) | 4.0 ± 0.2 | 34.5 ± 2.7 |
| + 100 nm H89 (PKA) | 4.0 ± 0.2 | 30.4 ± 2.1 |
| +1 μm KT5823 (PKG) | 5.2 ± 0.1 | 26.5 ± 1.7 |
| + 10 nm KT5926 (MLCK) | 4.0 ± 0.3 | 29.6 ± 1.1 |
| + 1 μm KN93 (CaM kinase II) | 3.8 ± 0.1 | 28.6 ± 0.1 |
| + 100 nm staurosporine (broad spectrum) | 4.0 ± 0.3 | 29.4 ± 0.3 |
Cannabinoid agonists have been shown previously to stimulate a receptor-independent release of calcium from intracellular stores at high concentrations, suggesting a nonspecific pharmacological effect (Felder et al., 1992). Overnight incubation with pertussis toxin (5 ng/ml) did not alter the receptor-independent calcium-mobilizing effects of 50 μm HU210.
DISCUSSION
This study has examined the interactions between cannabinoid CB1 and dopamine D2 receptors within the striatum. Activation of either CB1 or D2 receptors in primary striatal culture resulted in an inhibition of cAMP accumulation. In contrast, simultaneous activation of both receptors resulted in an augmentation of cAMP accumulation. Pertussis toxin treatment of striatal neurons prevented the inhibition of adenylate cyclase by both CB1 and D2 receptors and unmasked a CB1 but not D2 receptor-mediated stimulation of cAMP accumulation. This stimulation of cAMP accumulation after pertussis toxin treatment exhibited a similar EC50 to that observed with concurrent CB1 and D2 receptor stimulation and was blocked with the CB1 receptor antagonist SR141716A. These results suggest the existence of an alternate CB1 receptor-mediated signaling pathway involving the stimulation of adenylate cyclase. Previous studies have described cannabinoid agonist-mediated increases in cAMP accumulation (for review, see Martin et al., 1994). However, in these studies, accumulation of cAMP occurred only at high micromolar concentrations of the cannabinoids and were likely attributable to the membrane-perturbing effects of these hydrophobic compounds (Hillard and Bloom, 1983) and not mediated through the CB1 receptor (Felder et al., 1992). The cannabinoid agonist-mediated accumulation of cAMP described in this study occurred at low, pharmacologically relevant concentrations of the agonist and were blocked by a CB1 receptor antagonist, indicating a receptor-mediated pathway.
Transfected cell lines stably expressing the human CB1 receptor have proven to be useful models in understanding the molecular pharmacology of cannabinoid receptors (Felder et al., 1992, 1995; Vogel et al., 1993). In CB1 receptor-transfected CHO cells, HU210 potently inhibited cAMP accumulation. Pertussis toxin treatment prevented the inhibition of adenylate cyclase and unmasked a concentration-dependent accumulation of cAMP, similar to that seen in striatal neurons. The cannabinoid-mediated accumulation of cAMP in CHO-hCB1 cells was prevented by SR141716A, confirming that it was mediated by the CB1 receptor. Pertussis toxin treatment of CHO cells expressing two other Gi-linked receptors, the human CB2 receptor and the dopamine D2 receptor, blocked the ability of these receptors to inhibit cAMP accumulation but did not result in any receptor-mediated accumulation of cAMP, suggesting that the stimulatory pathway is a novel signaling pathway associated with the CB1 receptor rather than a common feature of Gi-linked receptors.
The ability of cholera toxin and forskolin to amplify the CB1 receptor-mediated accumulation of cAMP supports a role for Gs-proteins in the stimulatory response (Gill and Meren, 1978; Downs and Aurbach, 1982; Barovsky and Brooker, 1985). The requirement for pertussis toxin pretreatment to unmask the stimulation fully suggests that the coupling of the CB1 receptor to Gsis less efficient than its coupling to Gi. It is possible that D2 receptor activation could enhance the coupling of CB1 receptors to Gs, by altering the ratio of Gi- versus Gs-proteins available to the CB1 receptor. However, the relatively high abundance of Gi-proteins compared with Gs-proteins makes this possibility unlikely. Alternatively, D2 receptor stimulation may provide a mechanism for amplification of the CB1 receptor-coupled Gs signal through the release of βγ-subunits. G-protein βγ-subunits can increase Gαs stimulation of type II and type IV adenylate cyclase (Feinstein et al., 1991; Gao and Gilman, 1991). Therefore, stimulation of D2 receptors would result in the release of Giα and βγ-subunits, and the βγ-subunits in concert with CB1 stimulated Gαs would result in stimulation of cAMP as the dominant response.
G-protein coupled receptors have been shown previously to couple to more than one G-protein (Law et al., 1993; Wu et al., 1993; Chabre et al., 1994; Wang et al., 1995; Herrlich et al., 1996). For example, C10, C4, and C2 subclones of the α-2 adrenergic receptor couple to Gi- and Gs-proteins (Eason et al., 1992). D1 receptors couple to Gs- and Gq-proteins in the striatum (Wang et al., 1995) and kidney (Felder et al., 1989). The coupling of CB1 but not CB2 receptors to Gs observed in this study suggests that these receptors differ in their ability to couple to G-proteins and signaling effectors. This is consistent with the finding that CB1 receptors, but not CB2 receptors, couple to Q-type calcium channels and inwardly rectifying potassium channels in transfected AtT-20 cells (Felder et al., 1995). Thus, whereas their pharmacological profiles suggest similar ligand-binding properties, the intracellular responses to activation of either receptor are largely different. CB1 and CB2 receptors demonstrate 68% sequence homology at the active site, but only 44% homology overall (Munro et al., 1993). Previous studies have demonstrated the third intracellular loop to be important for receptor–G-protein coupling (Kobilka et al., 1988;Liggett et al., 1991; Okamoto et al., 1991; Liu and Wess, 1996). This region is substantially larger in the CB1 receptor than in the CB2 receptor (Munro et al., 1993), suggesting that this may be a useful area for future consideration of the molecular determinants for Gs coupling to receptors.
At least eight subtypes of adenylate cyclase have been identified (for review, see Cooper et al., 1995) and although all eight types can be activated by Gs, alternative mechanisms of activation also exist for certain subtypes. For example, protein kinase C (PKC) stimulates type II and VII adenylate cyclase, whereas Ca2+ stimulates type I, III, and VIII but inhibits type V and VI. We investigated whether either of these mechanisms could explain the CB1-mediated accumulation of cAMP after pertussis toxin treatment. Neither inhibition of PKC nor depletion of calcium influenced the ability of HU210 to stimulate adenylate cyclase in pertussis toxin-treated CHO-hCB1 cells. Furthermore, in agreement with previous studies (Felder et al., 1992), HU210 did not seem to induce the mobilization of intracellular Ca2+ at the concentrations used in this study.
The stimulation of cAMP after concurrent activation of CB1 and D2 receptors suggests a possible physiological relevance for the coupling of CB1 to Gs. Increasing evidence indicates that a single receptor subtype may be linked to the formation of multiple, parallel, intracellular signals. This spectrum of heterogenous effectors may be produced through activation of one or several G-proteins. However, it is unlikely that all the signals driven by a single receptor subtype are equally operative under all circumstances, but as evidenced in this study, it seems that the functional weight of one pathway relative to another can be altered by interactions with other receptors. The specificity of these receptor interactions requires further study. For example, does the CB1 receptor interact with just D2 receptors or with all Gi-coupled receptors? Discriminatory interactions between the CB1 receptor and other Gi-linked receptors would provide a mechanism for differential G-protein and effector coupling of the CB1 receptor in different regions of the brain, based on which other receptors are colocalized on the same neurons. Such interactions would provide an additional mechanism for discrete regulation of neurotransmitter signals, beyond the receptor subtype specificity.
Footnotes
M.G. is supported by a postdoctoral fellowship from the Health Research Council of New Zealand. We thank Dr. J. K. Northup for helpful discussions.
Correspondence should be addressed to Dr. Christian C. Felder, Lilly Research Laboratories, Eli Lilly Corporate Center, Indianapolis, IN 46285.
REFERENCES
- 1.Barovsky K, Brooker G. Forskolin potentiation of cholera toxin-stimulated cyclic-AMP accumulation in intact C6–2B cells. Evidence for enhanced Gs-C coupling. Mol Pharmacol. 1985;28:502–507. [PubMed] [Google Scholar]
- 2.Beckstead RM, Cruz CJ. Striatal axons to the globus pallidus entopenduncular nucleus and substantia nigra come mainly from separate cell populations in cat. Neuroscience. 1986;19:147–158. doi: 10.1016/0306-4522(86)90012-6. [DOI] [PubMed] [Google Scholar]
- 3.Beckstead RM, Kearsey KS. Immunohistochemical demonstration of differential substance P-, met-enkephalin- and glutamic acid decarboxylase-containing cell body and axon distributions in the corpus striatum of the cat. J Comp Neurol. 1985;232:481–498. doi: 10.1002/cne.902320406. [DOI] [PubMed] [Google Scholar]
- 4.Bidaut-Russell M, Howlett A. Cannabinoid receptor-regulated cyclic AMP accumulation in the rat striatum. J Neurochem. 1991;57:1769–1773. doi: 10.1111/j.1471-4159.1991.tb06379.x. [DOI] [PubMed] [Google Scholar]
- 5.Chabre O, Conklin BR, Brandon S, Bourne HR, Limbird LE. Coupling of the α2A-adrenergic receptor to multiple G-proteins. A simple approach for estimating receptor-G-protein coupling efficiency in a transient expression system. J Biol Chem. 1994;269:5730–5734. [PubMed] [Google Scholar]
- 6.Cooper DM, Mons N, Karpen JW. Adenylyl cyclases and the interaction between calcium and cAMP signaling. Nature. 1995;374:421–424. doi: 10.1038/374421a0. [DOI] [PubMed] [Google Scholar]
- 7.Downs RW, Jr, Aurbach GD. The effects of forskolin on adenylate cyclase in S49 wild type and cyc-cells. J Cyclic Nucleotide Res. 1982;8:235–242. [PubMed] [Google Scholar]
- 8.Eason MG, Kurose H, Holt BD, Raymond JR, Liggett SB. Simultaneous coupling of alpha-2-adrenergic receptors to two G-proteins with opposing effects. J Biol Chem. 1992;267:15795–15801. [PubMed] [Google Scholar]
- 9.Feinstein PG, Schrader KA, Bakalyar HA, Tang WJ, Krupinksi J, Gilman AG, Reed RR. Molecular cloning and characterization of a Ca2+/calmodulin-insensitive adeylyl cyclase from rat brain. Proc Natl Acad Sci USA. 1991;88:10173–10177. doi: 10.1073/pnas.88.22.10173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felder CC, Blecher M, Jose PA. Dopamine-1 mediated stimulation of phospholipase-C activity in renal cortical membranes. J Biol Chem. 1989;264:8739–8745. [PubMed] [Google Scholar]
- 11.Felder CC, Dieter P, Kinsella J, Katsuiko T, Kanterman RY, Axelrod J. A transfected m5 muscarinic acetylcholine receptor stimulates phospholipase A2 by inducing both calcium influx and activation of protein kinase C. J Pharmacol Exp Ther. 1990;255:1140–1147. [PubMed] [Google Scholar]
- 12.Felder CC, Veluz JS, Williams HL, Briley EM, Matsuda LA. Cannabinoid agonists stimulate both receptor and non-receptor mediated signal transduction pathways in cells transfected and expressing cannabinoid receptor clones. Mol Pharmacol. 1992;42:838–845. [PubMed] [Google Scholar]
- 13.Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, Lia Y, Ma A, Mitchell RL. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- 14.Gao B, Gilman AG. Cloning and expression of a widely distributed (type IV) adenylyl cyclase. Proc Natl Acad Sci USA. 1991;88:10178–10182. doi: 10.1073/pnas.88.22.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerard CM, Mollereau C, Vassart G, Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991;279:129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gill M, Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci USA. 1978;75:3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gimenez-Amaya JM, Graybiel AM. Compartmental origins of the striatopallidal projection in the primate. Neuroscience. 1990;34:111–126. doi: 10.1016/0306-4522(90)90306-o. [DOI] [PubMed] [Google Scholar]
- 18.Glass M, Brodie JM, Maneuf YP. Modulation of neurotransmission by cannabinoids in the basal ganglia. Eur J Neurosci. 1997a;9:199–203. doi: 10.1111/j.1460-9568.1997.tb01390.x. [DOI] [PubMed] [Google Scholar]
- 19.Glass M, Faull RLM, Dragunow M. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study on the fetal, neonatal and adult human brain. Neuroscience. 1997b;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- 20.Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- 21.Herkenham M, Lynn AB, de Costa BR, Richfield EK. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res. 1991a;547:267–274. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- 22.Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in the rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991b;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrlich A, Kühn B, Grosse R, Schmid A, Schultz G, Gudermann T. Involvement of Gs and Gi proteins in dual coupling of the luteinizing hormone receptor to adenylyl cyclase and phospholipase C. J Biol Chem. 1996;271:16764–16772. doi: 10.1074/jbc.271.28.16764. [DOI] [PubMed] [Google Scholar]
- 24.Hillard CJ, Bloom AS. Possible role of prostaglandins in the effects of the cannabinoids on adenylate cyclase activity. Eur J Pharmacol. 1983;91:21–27. doi: 10.1016/0014-2999(83)90357-6. [DOI] [PubMed] [Google Scholar]
- 25.Kobilka BK, Kobilka TS, Regan JW, Caron MG, Leftkowitz RJ. Chimeric alpha 2-,beta 2-adrenergic receptors: delineation of domains involved in effector coupling and ligand binding specificity. Science. 1988;240:1310–1316. doi: 10.1126/science.2836950. [DOI] [PubMed] [Google Scholar]
- 26.Law SF, Yasuda K, Bell GI, Reisine T. Gi alpha 3 and G(o) alpha selectively associate with the cloned somatostatin receptor subtype SSTR2. J Biol Chem. 1993;268:10721–10727. [PubMed] [Google Scholar]
- 27.Le Moine C, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing the D1 dopamine receptor gene. Proc Natl Acad Sci USA. 1991;88:4205–4209. doi: 10.1073/pnas.88.10.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liggett SB, Caron MG, Lefkowitz RJ, Hnatowich M. Coupling of a mutated form of the human beta 2-adrenergic receptor to Gi and Gs. Requirement for multiple cytoplasmic domains in the coupling process. J Biol Chem. 1991;266:4816–4821. [PubMed] [Google Scholar]
- 29.Liu J, Wess J. Different single receptor domains determine the distinct G-protein coupling profiles of members of the vasopressin receptor family. J Biol Chem. 1996;271:8772–8778. doi: 10.1074/jbc.271.15.8772. [DOI] [PubMed] [Google Scholar]
- 30.Mackie K, Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Natl Acad Sci USA. 1992;89:3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mailleux P, Vanderhaeghen JJ. Localization of cannabinoid receptor in the human developing and adult basal ganglia. Higher levels in the striatonigral neurons. Neurosci Lett. 1992;148:173–176. doi: 10.1016/0304-3940(92)90832-r. [DOI] [PubMed] [Google Scholar]
- 32.Martin BR, Welch SP, Abood M. Progress toward understanding the cannabinoid receptor and its second messenger systems. Adv Pharmacol. 1994;25:341–397. doi: 10.1016/s1054-3589(08)60437-8. [DOI] [PubMed] [Google Scholar]
- 33.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 34.Monsma FJ, Mahan LC, McVittie LD, Gerfen CR, Sibley DR. Molecular cloning and expression of a D1 dopamine receptor linked to adenylyl cyclase activation. Proc Natl Acad Sci USA. 1990;87:6723–6727. doi: 10.1073/pnas.87.17.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 36.Okamoto T, Murayama Y, Hayashi Y, Inagaki M, Ogata E, Nishimoto I. Identification of a Gs activator region of the beta 2-adrenergic receptor that is autoregulated via protein kinase A-dependent phosphorylation. Cell. 1991;67:723–730. doi: 10.1016/0092-8674(91)90067-9. [DOI] [PubMed] [Google Scholar]
- 37.Razdan RK. Structure activity relationships in cannabinoids. Pharmacol Rev. 1986;38:75–149. [PubMed] [Google Scholar]
- 38.Sibley DR, Monsma FJ. Molecular biology of dopamine receptors. Trends Pharmacol Sci. 1992;13:61–69. doi: 10.1016/0165-6147(92)90025-2. [DOI] [PubMed] [Google Scholar]
- 39.Singer-Lahat D, Wess J, Liv J, Felder CC. The third intracellular domain of the m3 muscarinic receptor determines coupling to calcium influx in transfected Chinese hamster ovary cells. FEBS Lett. 1996;386:51–54. doi: 10.1016/0014-5793(96)00398-5. [DOI] [PubMed] [Google Scholar]
- 40.Vogel Z, Barg J, Levy R, Saya D, Heldman E, Mechoulam R. Anandamide, a brain endogenous compound interacts specifically with cannabinoid receptors and inhibits adenylate cyclase. J Neurochem. 1993;61:352–355. doi: 10.1111/j.1471-4159.1993.tb03576.x. [DOI] [PubMed] [Google Scholar]
- 41.Wang HY, Undie AS, Friedman E. Evidence for the coupling of Gq protein to D1-like dopamine sites in rat striatum: possible role in dopamine mediated inositol phosphate formation. Mol Pharmacol. 1995;48:988–994. [PubMed] [Google Scholar]
- 42.Westlake TM, Howlett AC, Bonner TI, Matsuda LA, Herkenham M. Cannabinoid receptor binding and messenger RNA expression in human brain: an in vitro receptor autoradiography and in situ hybridization histochemistry study of normal aged and Alzheimer’s brains. Neuroscience. 1994;63:637–652. doi: 10.1016/0306-4522(94)90511-8. [DOI] [PubMed] [Google Scholar]
- 43.Wu D, LaRosa GJ, Simon MI. G protein-coupled signal transduction pathways for interleukin-8. Science. 1993;261:101–103. doi: 10.1126/science.8316840. [DOI] [PubMed] [Google Scholar]