Abstract
ATP has been proposed to mediate synaptic transmission in the spinal cord dorsal horn, particularly in the pathway carrying nociceptive information. Using transverse spinal cord slices from postnatal rats, we show that EPSCs mediated by P2Xreceptors, and presumably activated by synaptically released ATP, are evoked in a subpopulation of spinal cord lamina II neurons, a region known to receive strong input from nociceptive primary afferents. The P2X receptors on acutely dissociated dorsal horn neurons are nondesensitizing, insensitive to αβ methylene ATP, and show strong but variable sensitivity to the antagonists suramin and pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS). These characteristics are consistent with a heterogeneous population of P2X receptors, the composition of which includes P2X2, P2X4, and P2X6receptor subtypes. Our results suggest that ATP-activated P2X receptors in lamina II of the rat spinal cord may play a role in transmitting or modulating nociceptive information.
Keywords: ATP, purinergic receptors, synaptic transmission, spinal cord, rat, patch-clamp technique, slice preparation
Holton and Holton (1954) first proposed ATP as a possible neurotransmitter in the dorsal horn over 40 years ago. Since then, its role as a fast neurotransmitter in the peripheral nervous system has been demonstrated (Burnstock et al., 1972; Evans et al., 1992; Silinsky and Gerzanich, 1993; Galligan and Bertrand, 1994). In the CNS only one study, performed on neurons in the medial habenula, has demonstrated that ATP can act as a fast neurotransmitter (Edwards et al., 1992). Within the spinal cord dorsal horn, despite strong evidence implicating ATP as a putative neurotransmitter (Jahr and Jessell, 1983; Fyffe and Perl, 1984; Salter and Henry, 1985; Salter and Hicks, 1994), its role in this regard has not been demonstrated (Li and Perl, 1995).
Several ATP receptor subunits have been cloned from different tissues (Brake et al., 1994; Valera et al., 1994; Chen et al., 1995; Lewis et al., 1995; Buell et al., 1996; Collo et al., 1996; Vulchanova et al., 1996). Of those cloned, the ATP P2X2, P2X4, and P2X6 receptor subunit RNAs have been shown to be expressed in the spinal cord dorsal horn, particularly in the superficial laminae of the dorsal horn (Brake et al., 1994; Buell et al., 1996; Collo et al., 1996; Vulchanova et al., 1996), lending further support to the idea that ATP receptors may participate in synaptic function there. Recently, P2X7 mRNA also was detected in both brain and spinal cord (and elsewhere), but its exact distribution was not described (Surprenant et al., 1996). When P2X2, P2X4, and P2X6 receptor subunits are expressed in heterologous cellular systems in homomeric form, they all mediate a nondesensitizing response to ATP and are insensitive to the agonist αβ methylene ATP (Brake et al., 1994; Collo et al., 1996; Séguéla et al., 1996), making them pharmacologically distinguishable from the other P2X subunits. A further pharmacological distinction among subunits is that, whereas responses mediated by the P2X2subunit are sensitive to the antagonists suramin and pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), P2X4 and P2X6 subunits are relatively insensitive (Buell et al., 1996; Collo et al., 1996) (but seeSéguéla et al., 1996). Using spinal cord slices prepared from rat pups 1–2 weeks after birth, we investigated whether excitatory synaptic transmission in the spinal cord is mediated by the ATP-sensitive P2X receptors. We found that a subset of lamina II neurons receives synaptic inputs mediated by those receptors. However, the subpopulation was <5% of the neurons tested, making detailed pharmacological studies of these synapses difficult. Therefore, we used pharmacological and physiological means to characterize the P2X receptor subunits expressed on acutely dissociated postnatal dorsal horn neurons. Our results provide evidence for the synaptic release of ATP and for postsynaptically localized P2X receptors in the spinal cord dorsal horn, indicating that ATP and P2X receptors have a role in mediating and possibly modulating sensory transmission there.
MATERIALS AND METHODS
Tissue preparation. Acutely prepared transverse cervical spinal cord sections from postnatal (P2–P13) rats were prepared by following the method of Takahashi (1990). Briefly, postnatal rats were anesthetized with isoflurane and decapitated. The cervical spinal column was removed rapidly and placed in ice-cold oxygenated (95% O2/5% CO2) Krebs’ solution. Ventral and dorsal laminectomies were performed and the ventral roots cut as close to the cord as possible. The meninges were removed, except around the roots as they enter the cord, and a 1 cm segment of cervical spinal cord with the dorsal roots attached was isolated. Then the spinal cord was embedded in low-melting-point agarose 2.5% w/v dissolved in Krebs’ at 35–38°C. The agarose block was affixed to a vibratome stage with cyanoacrylate glue and immersed in ice-cold oxygenated Krebs’. Transverse slices of 350–400 μm were cut and incubated in oxygenated Krebs’ at 37°C for 1 hr. Then a slice was transferred to a recording chamber, the agarose removed, and the slice held in the chamber by a platinum grid with nylon wires. The chamber was placed on the stage of an upright microscope (Zeiss Axioskop, Oberkochen, Germany) for recording.
Preparation of acutely dissociated dorsal horn neurons. The preparation of acutely dissociated dorsal horn neurons is described inKyrozis et al. (1996). Postnatal rats (age 7–12 d) were anesthetized with isoflurane and decapitated, and the spinal cord was removed rapidly and submerged in ice-cold Krebs’ solution saturated with 95% O2/5% CO2. The dorsal one-third of the spinal cord slice was excised and digested with 1 mg/ml trypsin (Type III, Sigma, St. Louis, MO) in saturated Krebs’ for 30 min at 37°C, washed three times with Krebs’, and then placed in 1 mg/ml trypsin inhibitor (Type II-O, Sigma) in Krebs’ at room temperature. Several dorsal horn segments were placed in Krebs’ in a 35 mm dish containing a coverslip previously coated with 1 μg/ml poly-d-lysine and washed thoroughly. Then the segments were triturated gently with an ultramicropipette and allowed to adhere to the coverslip for at least 30 min before use.
Infrared imaging. Video-enhanced infrared microscopy was performed by using a modification of a previously described technique (Stuart et al., 1993). Briefly, a spinal cord slice was transferred to a recording chamber and placed on the stage of an upright microscope fit with a 10× [numerical aperture (NA) 0.25] lens, 40× water immersion lens (NA 0.75), and a 775 nm infrared bypass filter. Using the 10× lens, we identified lamina II, although we identified individual neurons in lamina II by using the 40× objective and an infrared filter after offsetting the Köhler illumination by 50% of the visual field under low magnification. The image was enhanced further with a VE-1000 CCD camera (Dage–MTI, Michigan City, IN) and was displayed on a monitor (Bardoni et al., 1995).
Ca2+ imaging. Dissociated neurons were incubated with 5 μm fura-2-AM (Molecular Probes, Eugene, OR) for 20 min at room temperature. The coverslip was transferred to a recording chamber and placed on the stage of an inverted microscope. Neurons were perfused continuously at a gravity-driven flow rate (0.5–1 ml/min) with standard bath containing (in mm): 145 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, 5.5 d-glucose, and 5 × 10−4 TTX, pH 7.3 (325 mOsm/l). A randomly selected neuron was excited at two UV wavelengths, 380 and 340 nm, and emission was collected at 510 nm wavelength. Images were obtained with an intensified CCD camera and fed into a VideoProbe image processor (ETM Systems, Mission Viejo, CA). Background fluorescence was subtracted and [Ca2+]i calculated by using the ratiometric formula of Grynkiewicz et al. (1985). Drugs were applied with a Y-tube for 5 sec. Acutely dissociated dorsal horn neurons were perfused in standard bath solution with 10 μm bicuculline and 5 μm strychnine added.
Electrophysiology. The slices were superfused with 95% O2/5% CO2 saturated Krebs’ flowing at 2–3 ml/min at room temperature (22–24°C). The composition of the Krebs’ solution was (in mm): 125 NaCl, 2.5 KCl, 25 NaHCO3, 1 NaH2PO4, 25d-glucose, 1 MgCl2, and 2 CaCl2; pH of the saturated solution was 7.4, and osmolarity was 320 mOsm. The intracellular solution contained (in mm): 130 K+-gluconate, 10 KCl, 0.1 CaCl2, 1 EGTA, 10 HEPES, and 2 Mg2+-ATP, pH-adjusted to 7.3 with KOH or NaOH and osmolarity adjusted to 305–310 mOsm with glucose (or sucrose). Compounds (from Sigma unless otherwise noted) used were 0.5 μm tetrodotoxin (TTX), 10–20 μm6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, Tocris Cookson, St. Louis, MO), 50 μmd(−)-2-amino-5-phosphonopentanoic acid (d-APV, Tocris Cookson), 5 μmstrychnine, 10 μm bicuculline, 100 μmαβ methylene-ATP, 100–500 μm suramin (Calbiochem, La Jolla, CA), 50–250 μm tetrasodium PPADS (Research Biochemicals International, Natick, MA), 100 μmhexamethonium, and 1 μm 3-tropanyl-indole-3-carboxylate hydrochloride (ICS 205–930, a 5-HT3 receptor antagonist; RBI). Drugs were superfused in the extracellular solution at a gravity-driven flow rate and applied for 5 min before recording.
Recording electrodes were made from thin-walled borosilicate glass and had resistances of 3–5 MΩ when filled with intracellular solution. Whole-cell recordings were made in voltage-clamp configuration at −70 mV. Recorded signals were sampled (10–100 kHz), amplified, and filtered at 1 kHz with an Axopatch 200A patch-clamp amplifier (Axon Instruments, Foster City, CA); the evoked EPSCs were not compensated. Data were stored on a Pentium-based personal computer and analyzed off-line with pClamp 6.0 software (Axon Instruments).
Patch-clamp recordings in whole-cell configuration were obtained from visually identified neurons 50–100 μm below the slice surface. The recording electrode was advanced while positive pressure was applied, and a GΩ seal was obtained. In some cells the ATP-mediated EPSC was evoked by stimulating the ipsilateral white matter, using a concentric bipolar stimulating electrode (Rhodes Medical Instruments, Woodland Hills, CA); in other cases, focal stimulation of the nearby tissue was performed with a glass pipette (∼3 μm tip size) filled with Krebs’ solution. In both conditions the identification of the stimulated presynaptic element (fiber or interneuron) was not possible. Stimuli of 1–15 μA (constant current) and 0.1–0.2 msec usually were required to evoke the ATP-mediated EPSCs in lamina II neurons; stimulus frequencies ranged between 0.1 and 0.5 Hz.
Acutely dissociated neurons were voltage-clamped at −70 mV in the whole-cell configuration, and 100 μm ATP was fast-applied onto the neurons for 4 sec. A 2 sec voltage ramp (from −85 to +20 mV) was started 1 sec after ATP application and terminated 1 sec before the end of ATP application. I–V curves of ATP-evoked whole-cell currents were obtained by subtracting a control voltage ramp from the test ramp. In some experiments recordings were made with cultured dorsal horn neurons in the perforated-patch configuration (Gu et al., 1996).
RESULTS
Fast excitatory synaptic currents mediated by P2X receptors in a subset of lamina II neurons
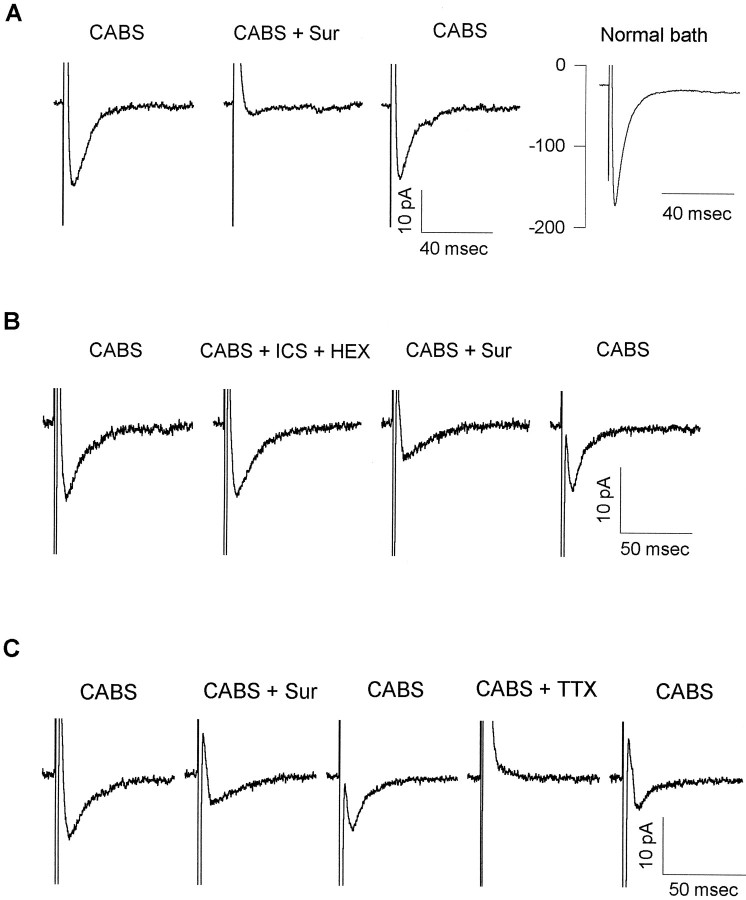
Whole-cell patch-clamp recordings were obtained from lamina II neurons in postnatal rat spinal cord slices. Using video-enhanced infrared microscopy, we chose individual lamina II neurons for recording (Stuart et al., 1993; Bardoni et al., 1995). In most cells focal stimulation of the nearby tissue or stimulation of the ipsilateral white matter or dorsal root evoked postsynaptic responses mediated by glutamate, GABA, and glycine receptors; those responses were blocked by a “cocktail” of 10 μm CNQX, 50 μmd-APV, 10 μm bicuculline, and 5 μm strychnine (CABS). In a subset of lamina II neurons (20 cells of >400 neurons tested) an evoked EPSC remained after application of CABS. Examples of CABS-insensitive EPSCs are shown in Figure 1. In Figure 1A, the EPSC was blocked completely by exposure to the P2 receptor antagonist suramin (500 μm), indicating that it was likely to be mediated by ATP receptors. In Figure 1Athe CABS-insensitive EPSC recovered after washout of suramin. When CABS was washed out, a much larger, presumably glutamatergic, EPSC became apparent.
Fig. 1.
The CABS-insensitive EPSC is mediated by P2 receptors. A, EPSCs not blocked by the “antagonist cocktail” (CABS; 10 μmCNQX, 50 μm APV, 10 μm bicuculline, and 5 μm strychnine to block AMPA, NMDA, GABAA, and glycine receptors, respectively) were blocked by suramin. EPSCs were evoked in a lamina II neuron from a P7 rat in CABS. They were abolished completely by 500 μmsuramin (CABS + Sur). After suramin washout, the CABS-insensitive synaptic current recovered. In normal Krebs’ bath (Normal bath), a larger EPSC was revealed. All traces are averages of five consecutive synaptic responses. B, EPSCs recorded from a lamina II neuron (P11) were resistant to all non-P2X receptor antagonists tested. EPSCs were recorded in CABS as the control solution. Bath application of 100 μmhexamethonium and 1 μm ICS 209–930 (CABS + ICS + HEX) did not affect EPSCs, indicating that the CABS-insensitive EPSCs were not mediated by either nicotinic acetylcholine or 5-HT3 receptors. EPSCs were partially blocked by 500 μm suramin (CABS + Sur). The suramin block was reversible, and almost complete recovery was obtained after a 15 min washout. EPSCs are averages of 10 consecutive synaptic responses. C, TTX blocked the suramin-sensitive EPSC. CABS-insensitive-evoked EPSCs recorded in a lamina II neuron (P10) were blocked by 100 μm suramin (CABS + Sur). The EPSC partially recovered after suramin washout. The suramin-sensitive EPSC was blocked in the presence of 0.5 μm TTX (CABS + TTX), indicating that ATP was released from presynaptic terminals. EPSC partially recovered after washout of TTX. EPSCs are averages of 20 consecutive synaptic responses. CABS-insensitive data for all three cells are shown with holding currents subtracted. In all cases, holding current varied <10 pA throughout the recording period.
The times to peak of the suramin-sensitive EPSCs recorded in CABS were <4 msec, and the decay time constants, estimated between 80–20% of current decay, varied between 4 and 44 msec (n = 19), suggesting that the synaptic currents are mediated by ligand-gated ion channels rather than metabotropic types of receptors. Candidate ionotropic receptors excluded by the blocking cocktail are glutamate, GABAA, and glycine receptors. In addition to the P2X receptors, other fast ligand-gated channels known to be expressed by central neurons include the neuronal nicotinic acetylcholine receptors and the 5-HT3 receptors. To confirm that these channels were not involved in the suramin-sensitive EPSCs recorded in CABS, we added 100 μm hexamethonium (a nicotinic receptor antagonist) and 1 μm ICS 209–930 (a 5-HT3 receptor antagonist) to the antagonist cocktail (Fig.1B; n = 4). Little or no change in the suramin-sensitive EPSCs was observed under these conditions.
In many cases, the focal stimulating electrode evoking the suramin-sensitive EPSCs recorded in CABS was in close proximity to the lamina II neuron under study. This raised the possibility that the synaptic currents were actually artifacts reflecting the electrical stimulus itself. It was also possible that the suramin-sensitive EPSCs recorded in CABS were attributable to nonsynaptically mediated ATP release, rather than a genuine synaptic event. These possibilities were tested readily because evoked synaptic currents were expected to be sensitive to TTX, whereas the above-mentioned stimulation artifacts and the nonsynaptic release of ATP were not. The suramin-sensitive EPSCs recorded in CABS were blocked reversibly by the application of 0.5 μm TTX (Fig. 1C; n = 3), indicating that the EPSCs were genuine synaptic events accompanying stimulation of the presynaptic cell or fiber.
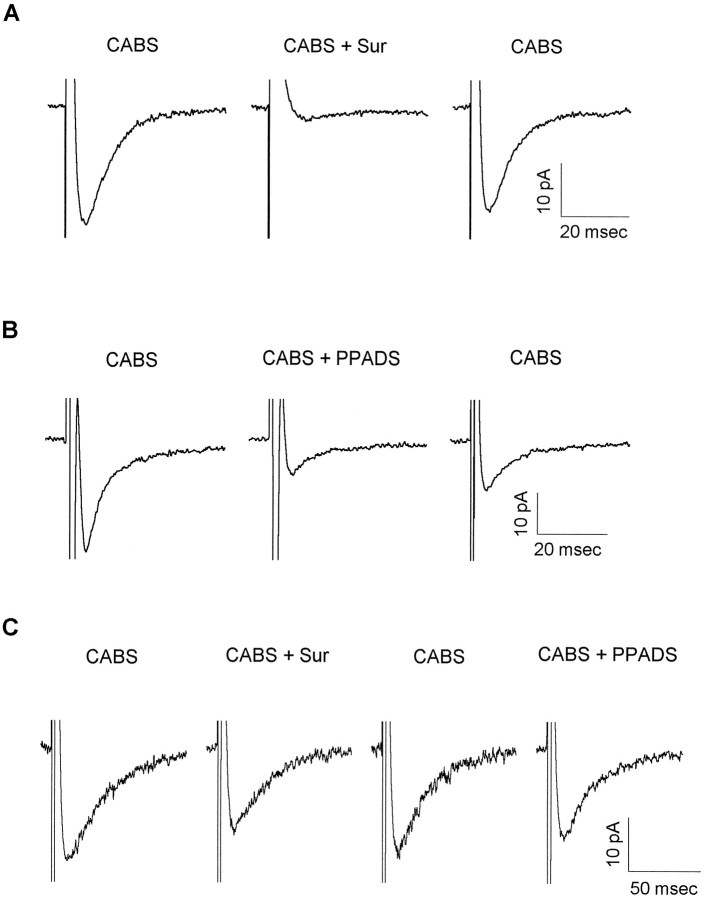
Different subtypes of recombinant P2X receptors show different sensitivity to the antagonists suramin and PPADS. Therefore, we extended our investigation to include the ability of these antagonists to block natively expressed P2X receptors mediating EPSCs recorded in lamina II neurons (Fig. 2). Suramin (100–500 μm) produced a reversible, and in some cells complete, block of the EPSCs (Fig. 2A). The average percentage of inhibition by 500 μm suramin was 64 ± 29 (SD; n = 13). The more specific P2X antagonist PPADS (50–250 μm) blocked the evoked currents less completely (Fig. 2B;n = 6). In two cells a small recovery after PPADS application was observed after 30 min of washout. In a few neurons the antagonists had only a small effect on the CABS-insensitive EPSC, as shown in Figure 2C.
Fig. 2.
P2X receptor antagonists variably block the P2X receptor-mediated EPSCs in lamina II neurons.A, ATP-mediated EPSCs were tested for the degree of suramin block. Fast EPSCs were recorded from a P10 neuron in CABS. Application of 500 μm suramin (CABS + Sur) completely blocked the EPSC. A complete recovery was observed after suramin washout. EPSCs are averages of 10 consecutive traces.B, ATP-mediated EPSCs were tested for the degree of PPADS block. EPSCs were recorded from a lamina II neuron (P6) in CABS. Bath application of 100 μm PPADS (CABS + PPADS) incompletely blocked the EPSC. Partial recovery was obtained after a 10 min wash in CABS. EPSCs are averages of 13–15 consecutive traces. C, P2X receptor-mediated EPSCs were tested for sensitivity to both suramin and PPADS. EPSCs were evoked in a P11 lamina II neuron in the presence of CABS. Suramin (500 μm) (CABS + Sur) partially blocked the EPSC. After a wash in CABS the suramin-blocked component recovered. Application of 50 μm PPADS (CABS + PPADS) slightly depressed the EPSC amplitude. EPSCs are averages of 20 consecutive traces. Data for all three cells are shown with holding currents subtracted. In all cases, holding current varied <10 pA throughout the recording period.
In these experiments high antagonist concentrations were used to help ensure that each cell in our study, located from 50–100 μm deep in the slice, was exposed to saturating concentrations of antagonist. The maximum concentration of suramin tested in our slices, 500 μm, was chosen because it was used in a similar study on spinal cord slices to test for ATP-mediated synaptic transmission in the dorsal horn. In that case, however, an ATP component to the EPSC was not revealed (Li and Perl, 1995). In a study using suramin on hippocampal neurons, high concentrations of suramin were shown to block both the EPSC and directly evoked glutamate currents, indicating that suramin shows poor selectivity for P2 receptors (Motin and Bennett, 1995). Thus, if the CABS-insensitive EPSCs in our studies were residual AMPA receptor-mediated EPSCs, suramin also might block them. However, we believe that the CABS-insensitive synaptic currents were not attributable to a residual AMPA receptor-mediated current, because 10 μm CNQX rapidly and potently blocked the AMPA receptor-mediated synaptic currents in the hundreds of neurons studied; furthermore, in seven of the cells tested showing evidence of P2X receptor-mediated synaptic currents, the CABS included 20 μm CNQX. Additionally, in six cells the more selective P2X receptor antagonist PPADS inhibited the CABS-insensitive EPSCs at concentrations that did not inhibit AMPA receptor-mediated EPSC and mEPSC amplitudes recorded from dorsal horn neurons grown in culture (unpublished observation). Finally, in three of three neurons tested with both suramin and PPADS, both antagonists inhibited the CABS-insensitive EPSCs.
Antagonists were applied for a minimum of 5 min before testing for antagonist block. Nevertheless, the amount of EPSC block because of suramin or PPADS varied widely from cell to cell, with the percentage of suramin block ranging from 8–100% and that for PPADS from 10–73%. No cells were found in which the fast residual component in CABS was completely unaffected by suramin or PPADS. The variability of antagonist action on the EPSCs recorded in the presence of CABS suggests either that P2X subunit expression is heterogeneous within and among cells in lamina II of the dorsal horn or that access of antagonist to dorsal horn neurons in the spinal cord slice preparation is restricted and slowed, or both.
P2X receptor activation measured by calcium imaging and whole-cell recording in dissociated spinal cord neurons
Additional studies on dorsal horn neuron P2X receptor pharmacology were made on acutely dissociated postnatal spinal cord neurons. These neurons were obtained from the dorsal one-third of spinal cord transverse sections, indicating that neurons from several laminae were studied, including at a minimum laminae I, II, and III. Although this preparation does not allow us to identify cells from specific laminae, as in the slice preparation, it has the advantage of allowing rapid and complete drug access to the neurons during pharmacological tests. Ca2+ imaging with the Ca2+ indicator dye fura-2 (Grynkiewicz et al., 1985) was used to perform the pharmacological testing because it provides a way to scan rapidly a field of neurons and to detect the minority of cells sensitive to ATP. Neuronal identity was confirmed by morphology and sensitivity to 100 μm NMDA (Heath et al., 1994).
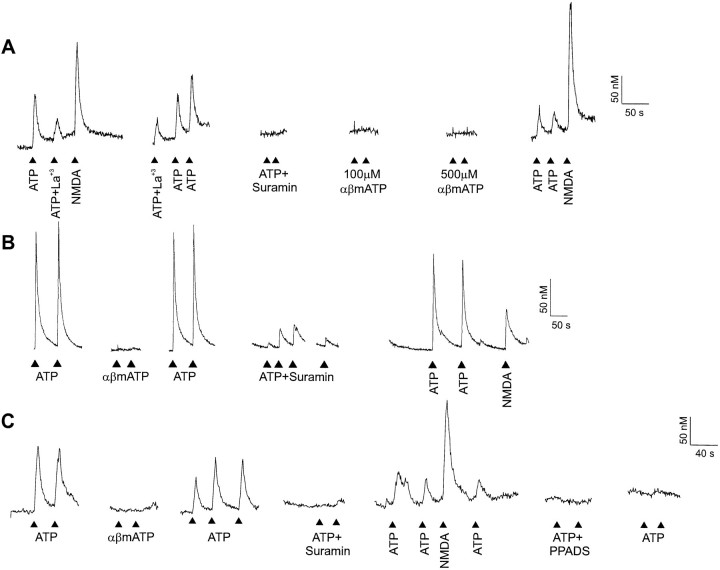
Exogenously applied ATP (100 μm) elevated intracellular Ca2+ concentration ([Ca2+]i) in a small subpopulation (<5%) of acutely dissociated dorsal horn neurons (Fig.3). The increase in [Ca2+]i was blocked only partially by the addition of 30 μm La3+ (Fig.3A; n = 2), a concentration sufficient to block completely the voltage step-activated Ca2+currents (Reichling and MacDermott, 1991) and K+-evoked Ca2+ elevations (Reichling and MacDermott, 1993) in dorsal horn neurons. Thus, the remaining Ca2+ transient in the presence of La3+ is most likely to be attributable to Ca2+ entry through the ATP-activated P2Xreceptor. This is consistent with many observations showing Ca2+ permeability of P2X receptors (Benham and Tsien, 1987; Nakazawa et al., 1990; Rogers and Dani, 1995).
Fig. 3.
ATP-evoked [Ca2+]i transients are mediated by P2X receptors expressed on acutely dissociated dorsal horn neurons. A, ATP caused increases in [Ca2+]i in acutely dissociated dorsal horn neurons. ATP (100 μm) increased [Ca2+]i in a neuron from a P8 animal. This response was blocked completely by 100 μm suramin and partially reduced by 30 μmLa3+, a voltage-gated Ca2+ channel blocker. αβ Methylene (100 and 500 μm) ATP did not evoke an increase in [Ca2+]i. Neuronal response was confirmed by application of 100 μm NMDA.B, The response to ATP in this neuron (P10) exhibited incomplete block by suramin. C, In a dorsal horn neuron from a P7 rat, 50 μm PPADS irreversibly blocked responses to ATP.
In the recordings from the cell shown in Figure 3A, the response to ATP was blocked completely by 100 μm suramin (n = 10), suggesting that the response evoked by ATP in this neuron was mediated by P2X receptors. On the other hand, αβ methylene ATP (n = 10 for 100 μm; n = 6 for 500 μm) failed to elicit an increase in [Ca2+]i. In a different neuron (Fig.3B), however, 100 μm suramin failed to inhibit completely the increase in [Ca2+]i(n = 2; 89.3 ± 3.7% block). This partial block of the Ca2+ response to ATP is similar to the incomplete block by suramin demonstrated for some of the suramin-sensitive EPSCs recorded in CABS from lamina II neurons in the acutely prepared spinal cord slice. This cell (Fig. 3B) was also insensitive to αβ methylene ATP.
In a third neuron (Fig. 3C) an increase in [Ca2+]i again was induced by ATP, but not by αβ methylene ATP. This neuron was tested further with two different antagonists, suramin and PPADS. Both 100 μmsuramin and 50 μm PPADS completely blocked the Ca2+ response to ATP (for PPADS, n = 2); the PPADS block was irreversible. Eleven of the 13 dorsal horn neurons tested with suramin or suramin and PPADS had ATP responses blocked by at least 95%. Only one of the 13 neurons tested responded to αβ methylene ATP. The Ca2+ response to ATP by this αβ methylene ATP-sensitive cell was blocked 95% by suramin.
An important characteristic of P2X receptors that assists functional identification of subunit type is whether or not the response to ATP is desensitizing. Of the nine cells preselected on the basis of their responsiveness to ATP and tested with prolonged 20 sec applications of ATP in our Ca2+ studies (data not shown), all appeared to have nondesensitizing responses. However, because many factors contribute to the kinetics and amplitude of the [Ca2+]i response to ATP, including activation of voltage-gated Ca2+ channels as indicated by the partial block by La3+ (Fig.3A), we directly measured current flow through the P2X receptors by recording ATP-evoked currents (Fig.4). In acutely dissociated neurons voltage-clamped at −70 mV (n = 6 of at least 27 cells tested for sensitivity to ATP), a 2 sec application of 100 μm ATP evoked a nondesensitizing inward current with peak current ranging from −6 to −50 pA (Fig. 4A). The current–voltage curve for the ATP-evoked current was constructed by applying a voltage ramp during the agonist application. An inward rectification of theI–V relationship was evident in four cells tested over the voltage range between −85 and −20 mV. It was difficult to extend the current–voltage curve over the range of −20 to +20 mV in three of the four cells tested because of the small magnitude of the ATP-evoked currents combined with the effect of a ramp subtraction artifact. This subtraction artifact was caused by progressive decrease of voltage-gated Ca2+ currents over the same voltage range over time. Data from the fourth cell are shown in Figure4B.
Fig. 4.
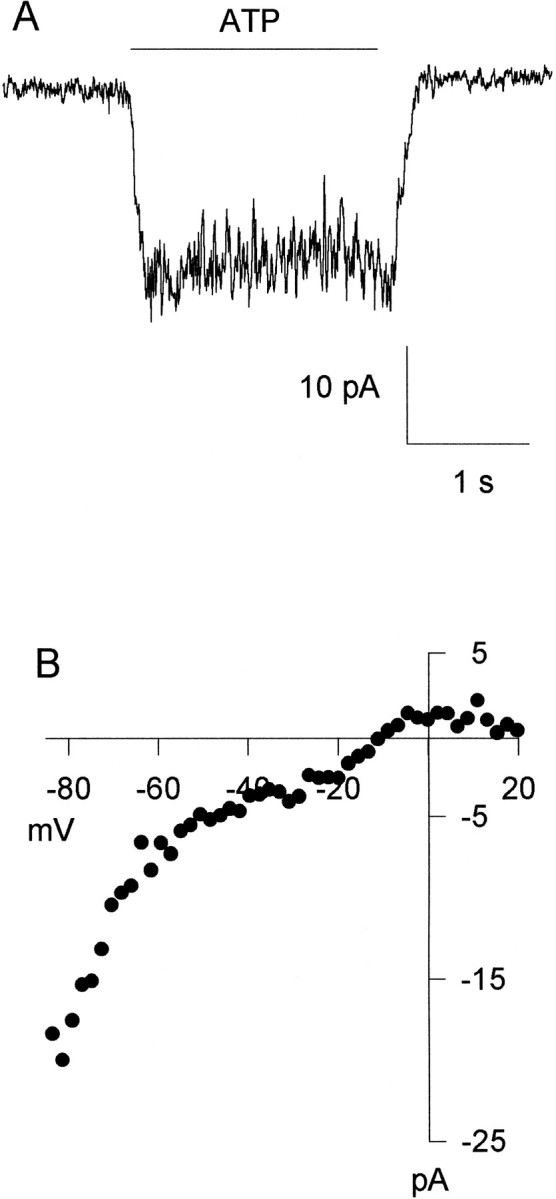
ATP evokes nondesensitizing whole-cell currents in acutely dissociated dorsal horn neurons. A, A 2 sec application of ATP evoked a small, nondesensitizing, inward current in a neuron obtained from a P8 animal. B, The ATP-evoked current shows inward rectification in a different neuron from the same preparation. The current–voltage curve for the ATP-evoked current was constructed by applying a voltage ramp during the agonist application. Inward rectification of theI–V relationship is evident over the voltage range between −85 and −20 mV.
DISCUSSION
We have demonstrated that in <5% of the lamina II neurons tested in the spinal cord dorsal horn, a portion of the evoked EPSCs is mediated by ATP-activated P2X receptors. Although ATP long has been suspected to be a neurotransmitter in the dorsal horn (Jahr and Jessell, 1983; Fyffe and Perl, 1984; Salter and Henry, 1985; Salter and Hicks, 1994), this possibility has proved difficult to demonstrate, leading some investigators to conclude that the primary synaptic role for ATP is as a neuromodulator (Li and Perl, 1995). In the medial habenula, however, Edwards et al. (1992) have shown that some EPSCs are not mediated by glutamate, GABAA, glycine, serotonin, or nicotinic acetylcholine receptors and are inhibited substantially by the desensitizing action of the agonist αβ methylene ATP and the P2 receptor antagonist suramin. These data were the first to establish that ATP mediates a fast synaptic current within the CNS. Since then, no other direct measurements of ATP-mediated synaptic currents in the CNS have been provided. We now have shown that ATP-P2X receptor-mediated synaptic transmission occurs in the spinal cord in a part of the dorsal horn likely to be involved in transmission of nociceptive information.
P2X receptor type and distribution, based on physiological and pharmacological observations
P2X2 receptor subunit RNA is strongly expressed in lamina II of the spinal cord dorsal horn, with little RNA detected in laminae I or III (Collo et al., 1996). In contrast, P2X2subunit protein, as detected by immunoreactivity, is strongly expressed in lamina I of the spinal cord, with a pattern of distribution more consistent with protein expression in primary afferent nerve terminals (Vulchanova et al., 1996) or perhaps in the axons of lamina II neurons projecting to lamina I. Two other P2X receptor subunits, P2X4 and P2X6, have been localized byin situ hybridization studies (Collo et al., 1996) in the external regions of dorsal horn, particularly laminae I and II. The responses to ATP we have obtained from acutely isolated dorsal horn neurons showed mostly a strong response to suramin and PPADS and responses that were insensitive to αβ methylene ATP. This pharmacological profile suggests that, in dorsal horn neurons, P2X receptors include the P2X2 subunit, which is the subunit known to be present in the dorsal horn, strongly blocked by suramin and insensitive to αβ methylene ATP. The CABS-insensitive EPSCs mediated by P2X receptors demonstrated a broadly distributed sensitivity to suramin and PPADS. These observations raise the possibility that the synaptic P2X receptors are heterogeneous in subunit composition. Alternatively, the widely variable sensitivity to the two P2X antagonists in the synaptic experiments simply could be attributable to diffusion-limited access and nonspecific binding of the antagonist before arriving at the synaptic receptors. Although the hypothesis of the presence of a diffusion barrier cannot be excluded, the complete block of glutamatergic, GABAergic, and glycinergic responses obtained by applying their respective antagonists and the high concentrations of P2 receptor antagonists used to block the CABS-insensitive EPSCs make this possibility less likely.
The role of P2X ATP receptors in dorsal horn function
ATP has been shown to be released from the peripheral terminals of dorsal root ganglion neurons after antidromic activation (Holton and Holton, 1954), and these investigators suggested that ATP may be released at the central terminals of the primary afferents as well. Ca2+-dependent release of ATP from dorsal horn synaptosomes supports the idea that ATP may function as a neurotransmitter in the dorsal horn (White et al., 1985). However, the inability of dorsal rhizotomy to depress the Ca2+-dependent release of ATP left it unclear whether the source of the ATP was exclusively primary afferent nerve terminals or whether it also included some intrinsic dorsal horn neurons (White et al., 1985). In our experiments we used a focal stimulating electrode to evoke synaptic responses, increasing the probability of evoking ATP-mediated synaptic currents. This also left us unable to determine the synaptic source of the ATP, i.e., whether it came from intrinsic neurons or primary afferent fibers. However, our experiments do provide strong support for the idea that ATP is a neurotransmitter in the dorsal horn and that P2X receptors are postsynaptically localized there.
An important functional characteristic of P2X receptors is that they are permeable to Ca2+ (Benham and Tsien, 1987; Nakazawa et al., 1990; Rogers and Dani, 1995) and, like Ca2+-permeable AMPA receptors, are opened readily by ligand at negative membrane potentials near the resting membrane potential. This is in contrast to the glutamate-activated NMDA receptors, which, although also highly Ca2+-permeable, have rather limited conductances at negative membrane potentials because of Mg2+ block (Mayer et al., 1984; Nowak et al., 1984). All of the heterologously expressed P2X subunits that have been tested to date, including P2X1, P2X3, and P2X4 receptor subunits, show higher permeability to Ca2+ than to monovalent cations (Valera et al., 1994; Lewis et al., 1995; Buell et al., 1996). In our experiments ATP was able to evoke Ca2+ transients in the presence of 30 μm La3+. This concentration of La3+ has been shown to block voltage step-evoked Ca2+ currents and K+-evoked changes in [Ca2+]i (Reichling and MacDermott, 1991, 1993). Therefore, these data indicate that the P2X receptors on dorsal horn neurons are Ca2+-permeable. Thus, ATP-mediated synaptic transmission is likely to provide a new route for synaptically gated Ca2+ entry into dorsal horn neurons.
Footnotes
This work was supported by Human Frontier Science Program (R.B.), the Whitehall Foundation, and National Institutes of Health (A.B.M.). We thank Steven Roper, Arnold Kriegstein, Pier Cosimo Magherini, Detlev Schild, and Cristóvão de Albuquerque for their thoughtful comments on this manuscript. We also thank Frances Edwards and Megumu Yoshimura for helpful discussions on experimental protocols.
Correspondence should be addressed to Dr. Rita Bardoni at her present address: Dipartimento di Scienze Biomediche, Sezione di Fisiologia, Università di Modena, Via Campi 287, I-41100 Modena, Italy.
REFERENCES
- 1.Bardoni R, Goldstein PA, Lee CJ, MacDermott AB. Patch-clamp recording from visually identified substantia gelatinosa neurons in postnatal rat spinal cord slices. Soc Neurosci Abstr. 1995;21:613. [Google Scholar]
- 2.Benham CD, Tsien RW. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987;328:275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- 3.Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371:519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- 4.Buell G, Lewis C, Collo G, North RA, Surprenant A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO J. 1996;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- 5.Burnstock G, Dumsday B, Smythe A. Atropine-resistant excitation of the urinary bladder: the possibility of transmission via nerves releasing a purine nucleotide. Br J Pharmacol. 1972;44:451–461. doi: 10.1111/j.1476-5381.1972.tb07283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- 7.Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- 9.Evans RJ, Derkach V, Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992;357:503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- 10.Fyffe REW, Perl ER. Is ATP a central synaptic mediator for certain primary afferent fibers from mammalian skin? Proc Natl Acad Sci USA. 1984;81:6890–6893. doi: 10.1073/pnas.81.21.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galligan JJ, Bertrand PP. ATP mediates fast synaptic potentials in enteric neurons. J Neurosci. 1994;14:7563–7571. doi: 10.1523/JNEUROSCI.14-12-07563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grynkiewicz GM, Poenie M, Tsien RWJ. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 13.Gu JG, Albuquerque C, Lee CJ, MacDermott AB. Synaptic strengthening through activation of Ca2+-permeable AMPA receptors. Nature. 1996;381:793–796. doi: 10.1038/381793a0. [DOI] [PubMed] [Google Scholar]
- 14.Heath MJS, Womack MD, MacDermott AB. Substance P elevates calcium in both neurons and glial cells from the dorsal horn of the spinal cord. J Neurophysiol. 1994;72:1192–1198. doi: 10.1152/jn.1994.72.3.1192. [DOI] [PubMed] [Google Scholar]
- 15.Holton FA, Holton PJ. The capillary dilator substances in dry powders of spinal roots; a possible role for adenosine triphosphate in chemical transmission from nerve endings. J Physiol (Lond) 1954;126:124–140. doi: 10.1113/jphysiol.1954.sp005198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jahr CE, Jessell TM. ATP excites a subpopulation of rat dorsal horn neurones. Nature. 1983;304:730–733. doi: 10.1038/304730a0. [DOI] [PubMed] [Google Scholar]
- 17.Kyrozis A, Albuquerque C, Gu J, MacDermott AB. Ca2+-dependent inactivation of NMDA receptors: fast kinetics and high Ca2+ sensitivity in rat dorsal horn neurons. J Physiol (Lond) 1996;495:449–463. doi: 10.1113/jphysiol.1996.sp021606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Perl ER. ATP modulation of synaptic transmission in the spinal substantia gelatinosa. J Neurosci. 1995;15:3357–3365. doi: 10.1523/JNEUROSCI.15-05-03357.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer ML, Westbrook G, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 21.Motin L, Bennett MR. Effect of P2-purinoceptor antagonists on glutamatergic transmission in the rat hippocampus. Br J Pharmacol. 1995;115:1276–1280. doi: 10.1111/j.1476-5381.1995.tb15036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakazawa K, Fujimori K, Takanaka A, Inoue K. An ATP-mediated conductance in pheochromocytoma cells and its suppression by extracellular calcium. J Physiol (Lond) 1990;428:257–272. doi: 10.1113/jphysiol.1990.sp018211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nowak LM, Bregestovksi P, Ascher P, Herbert A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 24.Reichling DB, MacDermott AB. Lanthanum actions on excitatory amino acid-gated currents and voltage-gated calcium currents in rat dorsal horn neurons. J Physiol (Lond) 1991;441:199–218. doi: 10.1113/jphysiol.1991.sp018746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reichling DB, MacDermott AB. Brief calcium transients evoked by glutamate receptor agonists in rat dorsal horn neurons: fast kinetics and mechanisms. J Physiol (Lond) 1993;469:67–88. doi: 10.1113/jphysiol.1993.sp019805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogers M, Dani J. Comparison of quantitative calcium flux through NMDA, ATP, and ACh receptor channels. Biophys J. 1995;68:501–506. doi: 10.1016/S0006-3495(95)80211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salter MW, Henry JL. Effects of adenosine 5′-monophosphate and adenosine 5′-triphosphate on functionally identified units in the cat spinal dorsal horn. Evidence for a differential effect of adenosine 5′-triphosphate on nociceptive vs non-nociceptive units. Neuroscience. 1985;15:815–825. doi: 10.1016/0306-4522(85)90080-6. [DOI] [PubMed] [Google Scholar]
- 28.Salter MW, Hicks JL. ATP-evoked increases in intracellular calcium in neurons and glia from the dorsal spinal cord. J Neurosci. 1994;14:1563–1575. doi: 10.1523/JNEUROSCI.14-03-01563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Séguéla P, Haghighi A, Soghomonian JJ, Cooper E. A novel neuronal P2X ATP receptor ion channel with widespread distribution in the brain. J Neurosci. 1996;16:448–455. doi: 10.1523/JNEUROSCI.16-02-00448.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silinsky EM, Gerzanich V. On the excitatory effects of ATP and its role as a neurotransmitter in coeliac neurons of the guinea-pig. J Physiol (Lond) 1993;464:197–212. doi: 10.1113/jphysiol.1993.sp019630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stuart GJ, Dodt HU, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflügers Arch. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- 32.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi T. Membrane currents in visually identified motoneurones of neonatal rat spinal cord. J Physiol (Lond) 1990;423:27–46. doi: 10.1113/jphysiol.1990.sp018009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valera S, Hussy N, Evans RJ, Adami N, North RA, Surprenant A, Buell G. A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- 35.Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA. Differential distribution of two ATP-gated ion channels (P2X receptors) determined by immunocytochemistry. Proc Natl Acad Sci USA. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White TD, Downie JW, Leslie RA. Characteristics of K+ and veratridine-induced release of ATP from synaptosomes prepared from dorsal and ventral spinal cord. Brain Res. 1985;334:372–374. doi: 10.1016/0006-8993(85)90235-5. [DOI] [PubMed] [Google Scholar]