Abstract
One important question concerning the homeostatic regulation of many physiological processes is whether the control mechanisms are purely reflexogenic or whether they may involve neural adaptation in the form of learning and memory in the brainstem. Using a brainstem slice preparation in the rat, we studied the modifiability of neural transmission in the first-order synapses of the medial and commissural nucleus tractus solitarius of the medulla. Sustained low-frequency stimulation (5 Hz) of primary afferent fibers in the tractus solitarius resulted in a phasic depression (accommodation) of synaptic strength as reflected by a concomitant decrease in the evoked excitatory postsynaptic potentials. In one group of neurons (type I), synaptic strength recovered rapidly after low-frequency stimulation, whereas in another group of neurons (type II), synaptic strength remained depressed for >30 min, i.e., manifesting long-term depression (LTD). The latter was switched into a short-term depression lasting 15–25 min after pharmacological blockade of NMDA receptor channels withd-APV or chelation of intracellular calcium ions with EGTA, whereas the accommodation phase was unaffected. Application of an AMPA receptor anti-desensitization agent cyclothiazide abolished the LTD, but not the accommodation response. These results suggest the presence of separate postsynaptic sites for the induction of LTD and accommodation, one being sensitive to cyclothiazide, whereas the other is not. Moreover, the maintenance of LTD is dependent on the level of intracellular Ca2+. These phasic and long-term synaptic plasticity in the nucleus tractus solitarius may play a role in the homeostatic regulation of cardiorespiratory functions.
Keywords: nucleus tractus solitarius, cardiovascular control, baroreflex, respiratory control, chemoreflex, vagal reflex, autonomic regulation, homeostasis, brainstem, long-term depression, long-term potentiation, synaptic accommodation, phasic synaptic depression, synaptic plasticity, learning and memory, AMPA receptor desensitization, NMDA receptor, intracellular calcium
The nucleus tractus solitarius (NTS) in the dorsal medulla of the mammalian brainstem is a gateway for many primary afferents from cardiovascular, respiratory, gastrointestinal, and other visceral sensory receptors important for homeostatic regulation (Andresen and Kunze, 1994; Barraco, 1994). Although the physiological significance of the NTS is well recognized, the neural mechanisms underlying the central processing and integration of these afferent signals are not well understood. It has been widely assumed that unlike the complex behavioral functions in the higher brain, involuntary physiological functions in the lower brain, such as cardiorespiratory regulations, are probably largely reflexogenic in nature without much adaptation. However, studies in intact animals in vivo have revealed the possible existence of various memory systems in certain physiological processes involving the NTS, such as respiratory control (for review, see Eldridge and Millhorn, 1986; Poon, 1996a,b) and taste aversion (Houpt et al., 1996). Nevertheless, until now there has been little evidence that NTS neurons are capable of learning and memory in the form of long-term potentiation (LTP) or long-term depression (LTD) as with neurons found in the hippocampus, neocortex, and cerebellum (for review, see Bliss and Collingridge, 1993; Bear and Malenka, 1994;Linden and Connor, 1995).
A key issue regarding synaptic plasticity such as LTP and LTD is the cellular mechanisms underlying their induction and maintenance. Another unresolved question is whether the expression of LTP and LTD is presynaptic or postsynaptic. These important questions must be addressed in understanding synaptic plasticity in the NTS and higher brain centers in general.
Besides long-term synaptic plasticity, many cortical neurons exhibit phasic synaptic depression at repetitive afferent stimulation (Abbott et al., 1997). Early evidence for phasic synaptic plasticity in the NTS was derived from the reported frequency-dependent synaptic depression of these neurons in guinea pig (Miles, 1986). It is not certain, however, whether such phasic response resulted from a depression of excitatory synaptic transmission or a potentiation of inhibitory transmission. Also, little is known about the cellular mechanisms underlying such phasic plasticity and whether it might be related to long-term plasticity, e.g., as a precursor to LTD.
In this investigation, we present direct experimental evidence of both LTD and a highly robust, phasic depression of excitatory synaptic transmission in specific areas of the rat NTS that are involved in cardiorespiratory regulation. More important, our results reveal that the induction and maintenance of LTD are contingent on the desensitization of AMPA receptors and the postsynaptic influx of calcium ions, respectively, whereas the induction of the phasic component is independent of both events and was distinct from LTD. These findings support the notion that differing postsynaptic mechanisms underlie the induction of phasic and LTD. We propose that such phasic and long-term plasticity in the NTS may contribute to the adaptive regulation of autonomic and respiratory functions for the maintenance of homeostatic stability.
MATERIALS AND METHODS
Brainstem slice preparation. Transverse brainstem slices (400 μm) around the level of the obex were prepared from 3- to 21-d-old Sprague Dawley rats, as described previously by Champagnat et al. (1983). Briefly, the animal was craniotomized under metofane anesthesia, and the brainstem and upper cervical spinal cord were removed rapidly and glued to the cutting stage of a vibratome (TPI, Series 1000). Throughout the surgical and sectioning procedure, the brainstem was immersed in chilled artificial cerebrospinal fluid (ACSF) saturated with carbogen (95% O2 and 5% CO2) and containing (in mm): NaCl 125, KCl 3, CaCl2 2.5, NaHCO3 26.5, MgSO4 1.5, NaH2PO4 1, glucose 10, pH 7.4. After stabilization at room temperature in carbogenated ACSF for at least 1 hr, the brainstem slice was transferred to a recording chamber on a microscope stage (Nikon, Diaphot 200), stabilized under a nylon mesh, and submerged continuously and superfused with carbogenated ACSF at 32°C.
Stimulation and recording. Under visual control with a micromanipulator (Newport, MX100R), a monopolar tungsten electrode with an ultrafine tip (FHC; 5 MΩ, 0.005" diameter) was positioned at the tractus solitarius (TS) for electrical stimulation of the primary afferents. Whole-cell patch recordings in NTS cells were obtained using a low-noise amplifier (Axopatch 200A, Axon Instruments, Foster City, CA) with a fluid-filled micropipette (resistance, 4–10 MΩ) containing (in mm): KCl 130, CaCl2 0.4, EGTA 1.1, MgCl2 1, NaCl 5, potassium HEPES 10, Mg2+-adenosine triphosphate 2, and Na2+-guanosine triphosphate 0.1, pH 7.2–7.3. The micropipette was positioned at the commissural or medial NTS using a motorized drift-free micromanipulator. Neurons were approached blind and a gigaohm seal was formed by a gentle suction of the micropipette. Once in the whole-cell mode, a stabilization period of ∼10 min was allowed before recordings began. Membrane characteristics were monitored periodically by injection of hyperpolarizing current pulses (−0.01 nA) through the patch electrode.
EPSPs or EPSCs were induced by single electrical impulses (pulse width, 0.1 msec; intensity, 3–10 V) delivered by a stimulator (S48 with isolation unit, model STU5B, Grass Instruments, Quincy, MA) at 20 sec intervals. The responses were judged to be mediated by first-order synapses of the TS–NTS pathway if their latency was between 3 and 5 msec; stimulus artifacts that preceded the postsynaptic response were eliminated. Amplitude of evoked EPSPs (range, 5–15 mV) or EPSCs was used as a measure of synaptic strength. Low-frequency stimulation (LFS) of TS was applied with similar impulses in a repetitive train (pulse frequency, 5 Hz; train duration, 9 sec; train period, 10 sec) lasting 5 min. During LFS, EPSPs corresponding to the first pulse of each train were recorded. In some cells, a brief period of high-frequency stimulation (HFS; 100 Hz, 2 sec) of TS was applied.
Pharmacology. In all experiments, bicuculline (10–20 μm) was added to the bath solution to suppress inhibitory synaptic transmission via GABAA receptors. Excitatory postsynaptic responses were verified by bath application of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μm) and 2-amino-5-phosphonovalerate acid (d-APV, 50 μm), which are selective antagonists for non-NMDA and NMDA receptors, respectively. Where necessary, desensitization of AMPA receptors was precluded by the addition of cyclothiazide (CYZ, 100 μm) to the perfusate. In some experiments, the patch electrode solution was enriched with EGTA (10 mm) to chelate intracellular Ca2+ in the postsynaptic cell. All neurochemicals were obtained from Research Biochemicals International (Natick, MA).
Data analysis. The raw signals were displayed continuously on a strip chart recorder (Nihon Kohden) and stored on a digital audiotape recorder (DTR-1200, Bio Logic). Episodic data were displayed on a storage oscilloscope and stored on a computer hard disk via an analog-to-digital converter with a commercial data analysis package (pCLAMP6, Axon Instruments). Episodic EPSPs and EPSCs were averaged before, during, and after LFS. Differences between means were tested by means of the Student’s t test at the 5% significance level, unless otherwise stated.
RESULTS
Post-tetanic potentiation
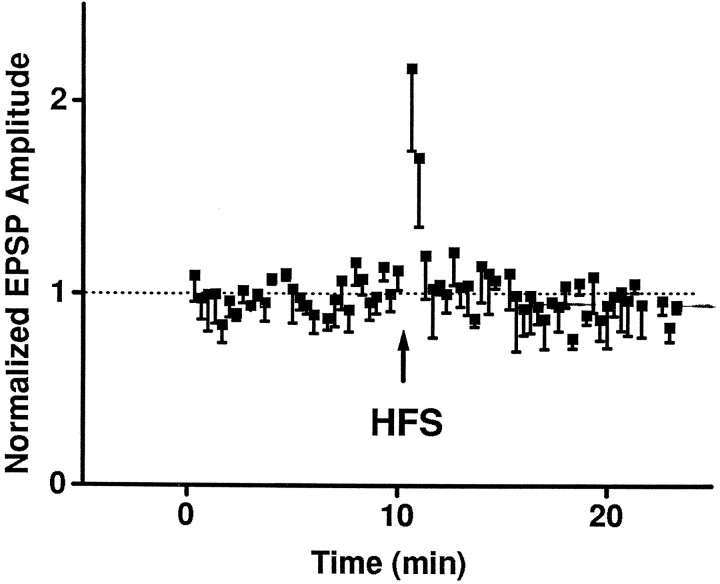
A schematic of the transverse brainstem section and the arrangement of the stimulating electrode and whole-cell patch-recording micropipette is illustrated in Figure 1. First, we verified the stability of the experimental preparation using a high-frequency (tetanic) afferent stimulation (HFS) protocol that has been used previously to study synaptic transmission in the NTS using intracellular recording with sharp electrodes (Fortin et al., 1992). In agreement with the previous study, synaptic strength in the TS–NTS pathway increased transiently during HFS. Thereafter, the evoked EPSPs returned to control level and remained stable (Fig. 2). In some neurons, a short-term (∼1 min) post-tetanic potentiation ensued after HFS. No long-term changes in EPSPs were seen. Thus, the whole-cell recording was stable and similar to sharp electrode recording.
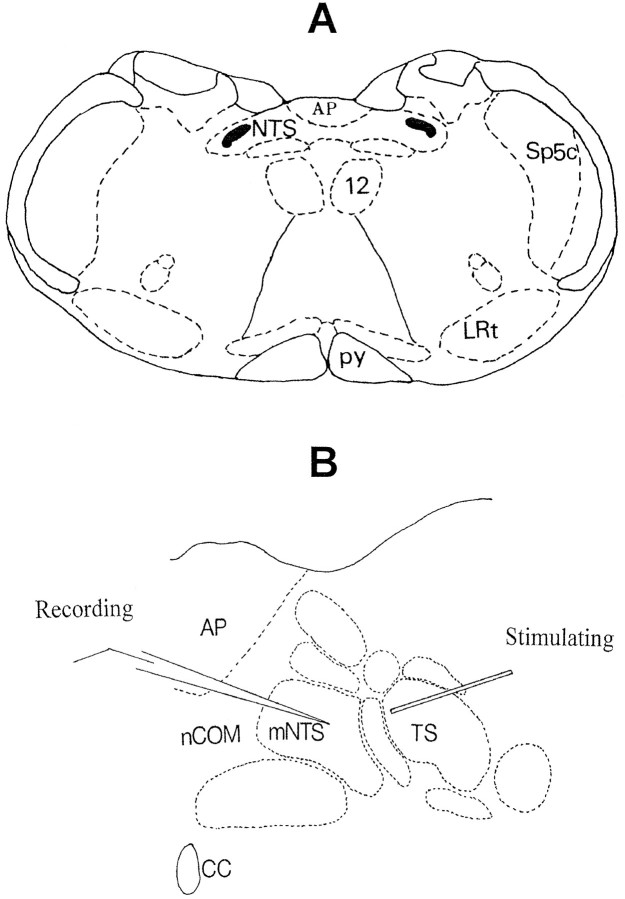
Fig. 1.
A, Schematic of a transverse section of medulla oblongata at the level of the area postrema (AP) slightly rostral to the obex level showing the two dorsomedial columns of the NTS and their fusion along the midline.Solid sections in the lateral NTS are the TS, which are composed mainly of peripheral afferent fibers from vagal, glossopharyngeal, facial, and trigeminal nerves. 12, Hypoglossal nucleus; Sp5c, caudal spinal trigeminal nucleus; LRt, lateral reticular nucleus.B, Enlarged section showing the commissural (nCOM) and medial NTS (mNTS) and the arrangement of stimulating and recording electrodes.
Fig. 2.
HFS (100 Hz for 2 sec) of TS elicited a transient increase in synaptic strength during stimulation (data not shown) and subsequent return to the baseline levels (dotted line). Data are mean ± SE from four NTS neurons. In two neurons, there was a short-term post-tetanic potentiation that decayed in ∼1 min. EPSP amplitudes were normalized by the average value during the control period (10 min) before HFS.
LTD and short-term depression (STD)
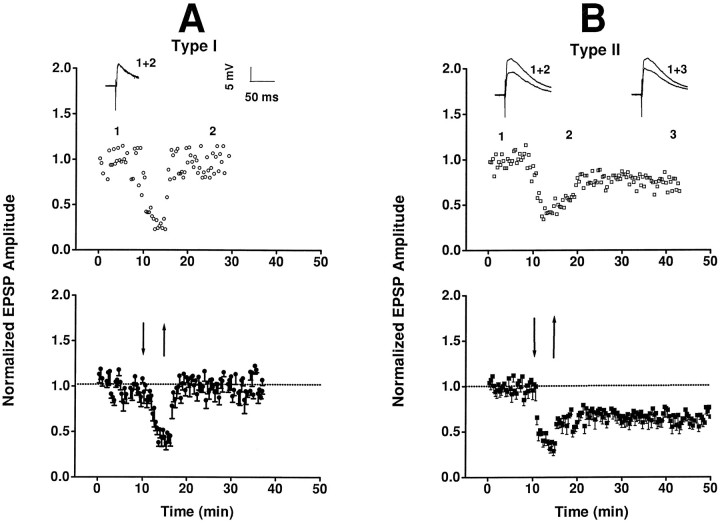
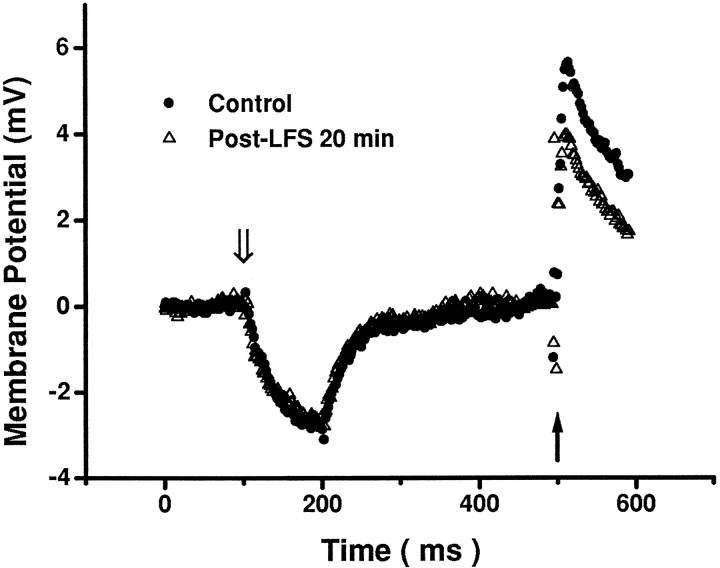
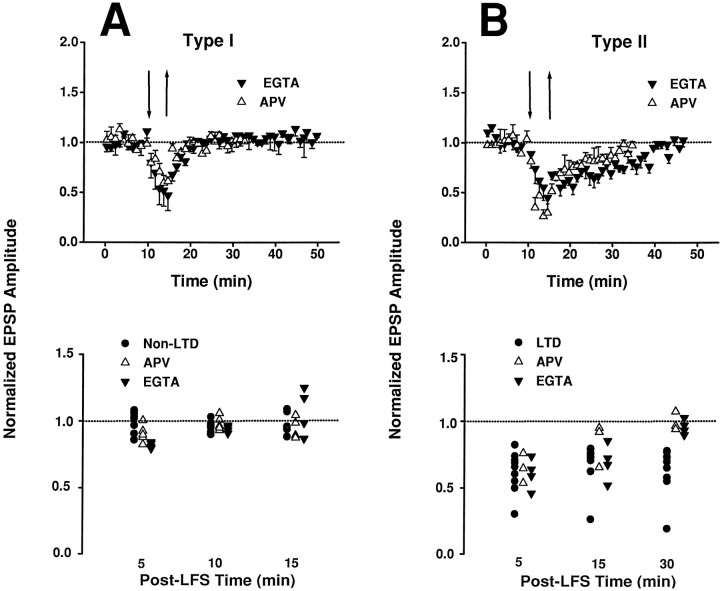
In contrast to HFS, sustained LFS of TS induced various synaptic responses in NTS neurons (Fig. 3). According to their responses after LFS, neurons were divided into two groups. In one group of neurons (type I), synaptic strength recovered rapidly after LFS (Fig. 3A). In another group of neurons (type II), the post-LFS response was biphasic (Fig. 3B): a partial recovery phase within the first 5 min, followed by a prolonged period of sustained depression, or LTD phase. There were no appreciable changes in resting membrane potentials (range, −50 to −78 mV) or membrane resistance and capacitance before and after LFS (Fig.4).
Fig. 3.
Synaptic plasticity in NTS. A, In type I neurons, synaptic strength decreased (to 46.8 ± 6.2% control; mean ± SE) during LFS (5 Hz/5 min, interval demarcated by arrows), showing an accommodation response, and recovered rapidly afterward. Top panel shows episode-by-episode response of one neuron; insets show averaged EPSPs (15 episodes) during control (1) and post-LFS (2) periods. Bottom panel shows the average responses (mean ± SE) in a group of neurons (n = 8). B, In type II neurons, LFS also elicited an accommodation response (43.7 ± 2.6%), but synaptic strength recovered only partially during the initial post-LFS period (∼5 min) and remained depressed for >30 min, resulting in LTD. Insets in top panel show the average EPSPs of one neuron in the control period (1) and 5 and 30 min after LFS (2 and 3, respectively).Bottom panel shows the average responses in a group of neurons (n = 9).
Fig. 4.
Changes in membrane potential from baseline level in an NTS cell. The membrane potential response to a hyperpolarizing current pulse of −10 pA (open arrow) >100 msec was the same before and after LFS. Note that EPSP evoked by a single electrical pulse to the TS (solid arrow) was depressed after LFS. Data represent the average of 20 episodes in each condition. Input resistances estimated in this manner in three cells were similar in control and post-LFS periods (491 ± 28 vs 507 ± 29 MΩ).
The postsynaptic influx of Ca2+ via NMDA receptors is a key step in the induction of LTD in many types of neurons in the higher brain (Bliss and Collingridge, 1992; Debanne and Thompson, 1994; Linden and Connor, 1995). To investigate whether postsynaptic Ca2+and/or NMDA receptors were responsible for the differing post-LFS responses of type I and type II neurons, we repeated the above experiment using (1) bath application ofd-APV (50 mm) to block the NMDA receptors, or (2) an electrode solution filled with a high concentration of EGTA (10 mm) to chelate intracellular Ca2+ in the postsynaptic cell. In one group of neurons, the post-LFS response recovered rapidly in a manner similar to that of type I neurons not treated with d-APV or EGTA (Fig. 5A). In another group of neurons, however, the post-LFS response exhibited a slow-recovery STD lasting 15–25 min (Fig. 5B). Thus, the maintenance of LTD in type II neurons was impaired after d-APV or EGTA treatments.
Fig. 5.
Application of d-APV (50 mm) in bath solution or EGTA (10 μm) in electrode solution did not abolish the accommodation response to LFS in all neurons. Top panels show responses of each group averaged over each minute. Bottom panels show the responses of individual neurons averaged over a 5 min interval preceding the time indicated. A, In one group of neurons, synaptic strength recovered within 5 min after LFS and remained stable for >30 min (top panel) in both the APV group (n = 5) and the EGTA group (n = 4). The time course of recovery in these neurons was similar to that of the type I (untreated) neurons (bottom panel). B, In another group of neurons, synaptic strength recovered after 15–25 min after LFS in the APV group (n = 3) and the EGTA group (n = 4), indicating a slow-recovery short-term depression effect that is distinct from the LTD in the LTD group (top and bottom panels). The LTD and STD in type II neurons are clearly distinguishable from the rapid recovery in type I neurons (compare bottom panels inA and B). The EPSPs of the APV group at 30 min time mark (bottom panel) were derived from corresponding values at 20 min after LFS when the responses had already returned to the baseline level.
The present finding of a critical role of intracellular Ca2+ and NMDA receptor in the maintenance (but not induction) of LTD in NTS neurons is in contrast to previous findings in the hippocampus (Dudek and Bear, 1992;Mulkey and Malenka, 1992; Bolshakov and Siegelbaum, 1994) and neocortex (Brocher et al., 1992; Kirkwood et al., 1993; Castro-Alamancos et al., 1995), in which the application of Ca2+ chelators (such as EGTA or BAPTA) or APV often blocked the induction of LTD. However, a switching of LTD to STD by APV or EGTA/BAPTA was also observed in some neocortical neurons (Brocher et al., 1992; Kirkwood et al., 1993; Castro-Alamancos et al., 1995). Although blockade of LTD byd-APV could result in fast recovery during the post-LFS period as in some hippocampal neurons (Dudek and Bear, 1992; Mulkey and Malenka, 1992), it is highly unlikely that STD could be induced in type I cells in this manner. By comparison of the post-LFS responses in neurons with and without such pharmacological treatments (compare Figs.3 and 5), therefore, it appears most likely that the neuronal groups showing rapid recovery or STD under d-APV or EGTA were associated with type I and type II behaviors, respectively.
Phasic synaptic depression
In addition to LTD, LFS also elicited a phasic synaptic depression as evidenced by a transient decrease in the synaptic strength to <50% of control level after 5 min of LFS (Fig. 3). This phasic response, hereinafter referred to as synaptic accommodation, was consistently observed in all type I and type II neurons. As shown in Figure 5, the accommodation response during LFS was not abolished by application of d-APV or EGTA, suggesting that this phasic component was Ca2+-independent and distinct from the LTD component. Thus, accommodation was highly prevalent and robust in NTS cells.
Such phasic, activity-dependent depression of excitatory synaptic transmission in the rat NTS is in agreement with the frequency-dependent depression of these neurons observed previously in guinea pig (Miles, 1986). In that study, a continuous 5 Hz stimulus resulted in a concomitant decrease of ∼35% in EPSP amplitude within 30 sec, followed by a rapid recovery in the poststimulation period. In comparison, the present results showed a 55–65% reduction in all cells over a 5 min stimulation interval that led to a sustained poststimulation LTD in type II cells. Thus, it appears that accommodation is a phasic phenomenon and is inducible by a brief stimulus, whereas a sufficiently long stimulation duration (several minutes) is required for the induction of STD (and subsequently, LTD) in NTS cells. Such low-frequency, long-duration stimulation protocol is compatible with the 1–5 Hz, 900-pulse protocol suggested for the induction of LTD in hippocampus and visual cortex (Dudek and Bear, 1992; Kirkwood et al., 1993).
CYZ abolishes STD and LTD, but not accommodation
One possible explanation for the accommodation response is that non-NMDA (AMPA/kainate) receptors, which account for much of the glutamatergic EPSPs in NTS neurons (Andresen and Yang, 1990), may be desensitized by glutamate itself and become unresponsive to continued glutamatergic activation. Although the short-term kinetics of AMPA receptor desensitization is quite rapid (with a time constant of ∼10 msec) (Trussell et al., 1993), it remains possible that other slow components of desensitization might be elicited by sustained LFS.
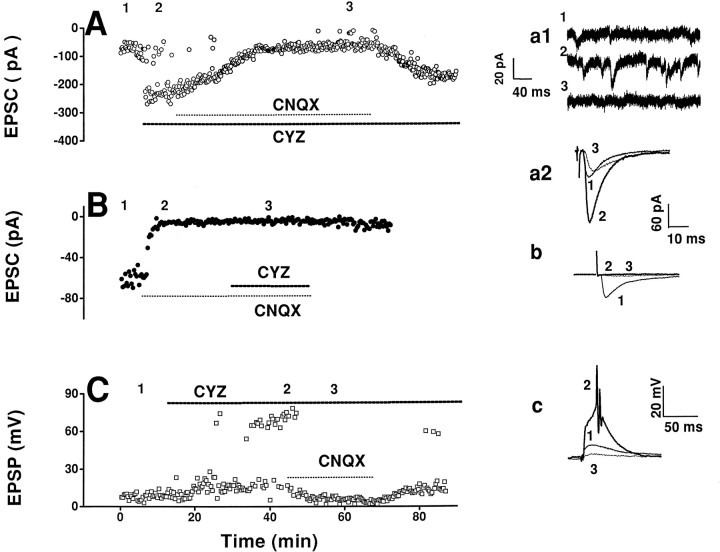
Accordingly, we measured the changes in excitatory synaptic strengths before and after bath application of CYZ (100 mm), a selective AMPA receptor anti-desensitization agent (Partin et al., 1993). CYZ markedly enhanced the amplitudes of both evoked EPSCs and spontaneous EPSCs in voltage-clamp mode (Fig.6A,B) and EPSPs in current-clamp mode (Fig. 6C) of NTS neurons, with the resultant induction of action potentials in the latter. The effect of CYZ on spontaneous EPSCs was almost completed blocked by CNQX (Fig.6a1), suggesting a mediation of the postsynaptic responses by non-NMDA (AMPA/kainate) receptors in these neurons. Furthermore, the evoked postsynaptic responses were almost totally blocked by application of CNQX before CYZ (Fig. 6B), but not after CYZ (Fig.6A,C). The difference in the effects of CNQX before and after CYZ may be attributable to the competitive combination of CNQX and CYZ with AMPA receptors. In Figure7A, LFS not only abolished the facilitation effects of CYZ but also decreased the EPSP amplitudes to <50% of control level (before CYZ). This result suggests that the mechanism of accommodation is distinct from the glutamate-induced, CYZ-blocked, desensitization of AMPA receptors.
Fig. 6.
Effects of CYZ on excitatory synaptic transmission in NTS. A, In one neuron in voltage-clamp mode (holding potential, −80 mV), the amplitude of EPSC increased after bath application of CYZ (100 μm). Co-application of CNQX (10 μm) suppressed the effect of CYZ but did not abolish the EPSC response. a1 and a2(Insets) show, respectively, spontaneous EPSCs and averages of 15 consecutive evoked EPSCs during application of normal perfusion solution (1), CYZ (2), and CYZ with CNQX (3). CYZ significantly increased the amplitudes of spontaneous and evoked EPSCs. B, In another neuron in voltage-clamp mode (holding potential, −70 mV), the EPSCs were almost completely blocked by CNQX; bath application of CYZ after CNQX did not restore the EPSCs. b(Inset) shows averaged EPSCs during application of normal perfusion solution (1), CNQX (2), and CNQX with CYZ (3). C, In another neuron in current-clamp mode (membrane potential, −55 mV), CYZ enhanced the amplitude of evoked EPSPs and induced firing of action potentials (EPSP >60 mV); both effects being reversed by CNQX.c, (Inset) shows averaged EPSPs during bath application of normal perfusion solution (1), CYZ (2), and CYZ with CNQX (3).
Fig. 7.
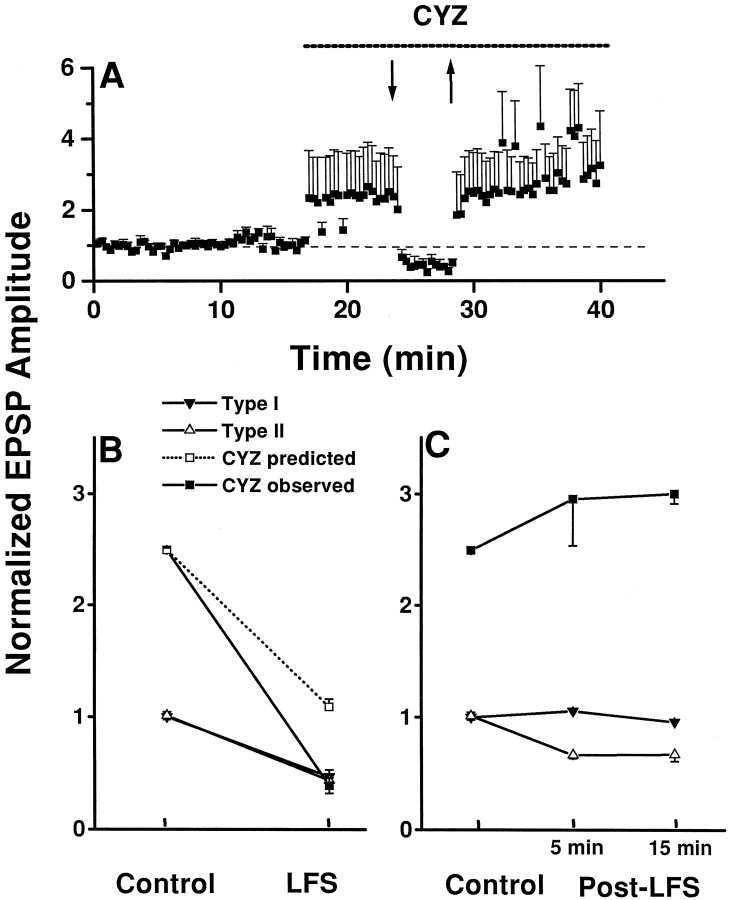
Summary of the effects of CYZ on accommodation and LTD. A, Group data (n = 5) showing the abolition of LFS-induced LTD, but not accommodation, after application of CYZ. Responses under CYZ represent averages of both evoked EPSPs and action potentials from all neurons. Note that neuronal excitability continued to increase under CYZ after termination of LFS.B, Synaptic strength at the end of LFS period was not statistically different under CYZ (38.7.8 ± 6.3% control) than in untreated groups (see Fig. 3A;p > 0.1; two-tailed t test).Dashed line indicates predicted response under CYZ if the site of accommodation was presynaptic, assuming that the effect of LFS was independent of CYZ. Data are replotted from Figures 3 and7A with all data points corresponding to neuronal firing being removed. C, Synaptic strength under CYZ was not depressed after LFS. Data represent averages in the control period and 5 and 15 min after LFS (Post-LFS). None of the cells under CYZ exhibited type II behavior despite variable effects of CYZ on EPSPs.
One important question is whether the mechanism of accommodation is presynaptic or postsynaptic. Evidence both for and against possible presynaptic and postsynaptic mechanisms has been proposed on considerations of the changes in membrane potential and spontaneous synaptic events as well as on quantal analysis of neurotransmitter release (Miles, 1986). To address this question, we compared the magnitudes of the accommodation response to LFS, with and without CYZ. As shown in Figure 7B, the evoked EPSPs had similar amplitudes at the end of the LFS period, whether or not CYZ was present. As a consequence, the degree of accommodation (percent decrease from pre-LFS levels) of the EPSP response was greater under CYZ than that in normal slices (without CYZ). We interpret this result as suggesting that the locus of accommodation was postsynaptic, because a given presynaptic decrease in transmitter release would have led to proportionate decreases in evoked EPSPs from pre-LFS levels in both conditions (Fig. 7B).
Furthermore, although CYZ greatly enhanced control EPSPs, it was ineffective for the accommodated EPSPs during LFS. Thus, the LFS-induced accommodation superseded CYZ action in regulating the conductance of AMPA receptor gated channels.
In contrast to the persistence of the accommodation response under CYZ, the post-LFS evoked EPSP response returned rapidly toward the pre-LFS level under CYZ (Fig. 6C). The time course of the recovery was reminiscent of that for type I cells. None of the cells treated with CYZ exhibited LTD or STD (n = 5) (Fig.7C). The effect of CYZ is statistically significant (p < 0.05) assuming a binomial distribution with a 9:8 population distribution for type I and type II cells (Fig.3). Thus, type II behavior was abolished by CYZ.
DISCUSSION
Phasic and LTD in NTS cells
The present study provides the first experimental demonstration, at a cellular level, of an activity-dependent LTD of excitatory synaptic transmission in a brainstem area important for homeostatic regulation. Such activity-dependent, homosynaptic LTD in NTS is akin to the LTD found in the hippocampus (Dudek and Bear, 1992; Mulkey and Malenka, 1992), visual cortex (Kirkwood et al., 1993), somatosensory and motor cortexes (Castro-Alamancos et al., 1995), as well as the dorsal (Randic et al., 1993) and ventral (Pockett and Figurov, 1993) horns of the spinal cord. Thus, LTD is probably generally expressed in many types of neurons and may subserve a wide range of functions throughout the CNS.
Many questions remain regarding the characteristics of the newly discovered LTD in the NTS. For example, it is not clear whether the LTD is saturable on repeated induction by LFS or is reversible by means of other inputs. Also, it is not certain whether the LTD is specific to the type of input and/or input–output pairing. Nevertheless, it must be emphasized that the observed LTD was not the result of changes in passive membrane characteristics or deterioration of cell function (attributable to cytosolic washout or otherwise) in the whole-cell mode. Because a long stabilization period was allowed before LFS, any washout effect would have been apparent during the control period. Such washout effect was also not apparent in cells subjected to HFS in which the primary response was a potentiation instead of depression, in agreement with previous findings (Fortin et al., 1992). Finally, the absence of LTD in type I cells and the sensitivity of the LTD to various pharmacological agents in type II cells strongly indicate that the observed LTD after LFS was not merely a rundown effect of cellular function.
In contrast to LTD, demonstration of LTP in NTS cells has proved difficult (Fortin et al., 1992). Interestingly, a similar tetanus stimulation protocol led to a sustained potentiation of inhibitory synaptic transmission in the dorsomedial NTS (Glaum and Brooks, 1996). The expression of LTD, and not LTP, of excitatory TS–NTS synapses is analogous to the preponderance of heterosynaptic LTD and absence of LTP in the parallel fibers–Purkinje cell synapses in cerebellum (Ito, 1989; Linden and Connor, 1995). Presumably, the induction of LTP in the NTS may call for experimental protocols different from the present approach, such as the use of θ bursts, which was reportedly more effective in inducing LTP in the hippocampus than tetanic stimulation (Larson et al., 1986).
Another major finding of the present investigation was that LFS elicited not only LTD but also a phasic and highly robust accommodation response. By contrast, a transient facilitation is often observed in hippocampal neurons during LFS before any subsequent decrease in synaptic strength (Mulkey and Malenka, 1992).
Distinct mechanisms for phasic and LTD
More important, our results showed that accommodation and LTD are distinct events elicited by LFS with differing induction mechanisms. Thus, accommodation was expressed in type I cells and in all cells after treatment with CYZ, whereas LTD was not. Also, the accommodation response was not influenced by blockade of NMDA receptor channels and chelation of intracellular Ca2+, whereas the LTD in type II cells was switched into STD under these conditions.
The concurrent absence/presence of both STD and LTD in type I/type II cells and Ca2+-dependent switching of the LTD into STD in type II cells reveal that the induction of STD was a critical step that preceded the subsequent maintenance of LTD in type II cells. Thus, LFS elicited LTD in two steps. In phase I (induction phase), a transient STD of non-NMDA receptors was induced; in phase II (maintenance phase), the transient synaptic depression was maintained by processes (e.g., second messenger systems) triggered by an increased intracellular Ca2+ level resulting from the activation of NMDA receptors. Consequently, the lack of STD in type I cells predisposes to the absence of LTD in these cells.
Furthermore, the absence of STD and LTD in CYZ-treated cells suggests that AMPA receptor desensitization is one the first steps in the chain of events that leads to the induction of STD and the subsequent maintenance of LTD. Taken together, these results suggest that LFS concomitantly elicits a phasic, CYZ-independent accommodation and a CYZ-dependent desensitization of AMPA receptors. In type II cells, the desensitization develops into STD in the poststimulation period, which, in turn, leads to LTD when sufficient Ca2+ ions are present in the postsynaptic cell.
Sites of phasic and LTD
In Figure 7B, the antidesensitization action of CYZ on postsynaptic AMPA receptors (Partlin et al., 1993; Trussell et al., 1993) is superseded by the phasic depression induced by LFS, suggesting that the expression of phasic depression is postsynaptic and CYZ-independent.
The question of whether presynaptic or postsynaptic mechanisms contribute to the induction of hippocampal LTP or LTD has been a subject of considerable controversy. The present finding that CYZ abolished LTD suggests that the induction of STD and subsequent LTD is at least partially postsynaptic and contingent on the desensitization of AMPA receptors. Moreover, the mechanism underlying the induction of STD and LTD must be distinct from that of accommodation, because the latter was not affected by CYZ.
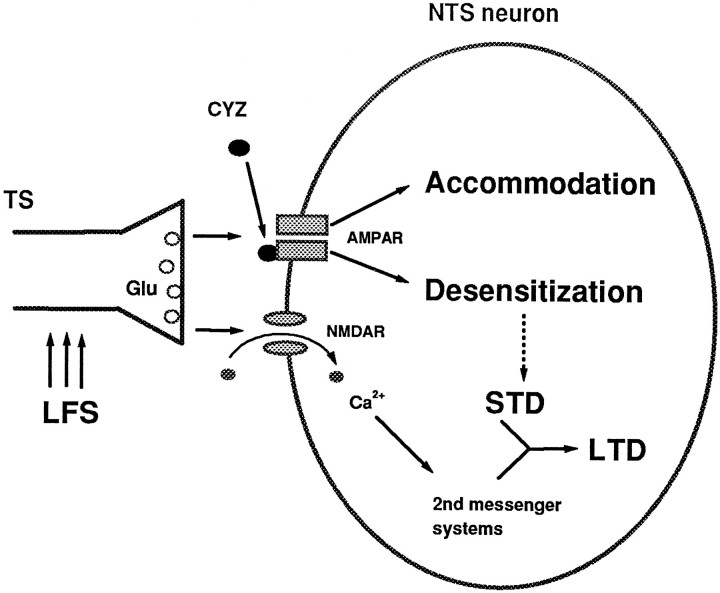
One possible explanation for all of the above findings is that both accommodation and LTD may be brought about by an inactivation of postsynaptic AMPA receptors, but perhaps through different allosteric sites, one being sensitive to CYZ and the other not (Fig.8). A possible initiating mechanism for both the phasic and the LTD components is the activation of postsynaptic metabotropic glutamate receptors (mGluRs), known to trigger some forms of LTD (Linden et al., 1991; Kato, 1993; Shigemoto et al., 1994). However, the AMPA-mediated synaptic currents in NTS neurons were found to be potentiated (rather than depressed) by the mGluR agonist 1S,3R-ACPD (Glaum and Miller, 1993). Nevertheless, it remains possible that other mGluR subtypes insensitive to 1S,3R-ACPD (Westbrook, 1994) might contribute to the accommodation and/or LTD in NTS neurons.
Fig. 8.
Hypothetical model of phasic LTD in NTS neurons based on the results presented in Figures 3, 4, 5, 6, 7. According to this model, LFS of afferent fibers in the TS releases the excitatory neurotransmitter glutamate (Glu). The continued presence of Glu, in turn, inactivates postsynaptic AMPA receptors (AMPAR), but perhaps through different allosteric sites, one being sensitive to CYZ (solid ovals) and the other not. The CYZ-insensitive site accounts for the induction of accommodation, whereas the CYZ-sensitive site contributes to AMPAR desensitization. In type II cells, the AMPAR desensitization develops into a poststimulation STD that is absent (broken arrow) in type I cells. Subsequently, the STD is maintained by second-messenger systems that are triggered by an influx of Ca2+ via NMDA receptors (NMDAR) during LFS, resulting in LTD. Blocking the NMDAR channels and/or chelation of intracellular Ca2+ abolish this maintenance phase, thereby switching the LTD into STD in type II cells.
Physiological significance of phasic and LTD in NTS
In view of the functional diversity of the NTS, it is difficult to extrapolate such phasic and long-term plasticity observed in vitro to corresponding physiological behaviors of the intact animal in vivo. Nevertheless, in light of the prevalence of synaptic accommodation in both type I and type II cells, it is reasonable to surmise that synaptic accommodation is probably a common cellular mechanism underlying many physiological processes involving the NTS regions under study, which are known to receive afferent inputs from baroreceptor, chemoreceptor, and pulmonary receptors important for cardiovascular and respiratory control (Andresen and Kunze, 1994;Barraco, 1994). One well-known phenomenon in which accommodation may be of relevance is the acute resetting of baroreflex control commonly found in experimental animals and humans (Mancia et al., 1986; Chapleau et al., 1995). Specifically, after brief elevation of arterial blood pressure, the baroreflex curve often shifts rightward so that a greater pressure stimulus is needed to elicit a reflex response. It has been shown recently that apart from the passive resetting of baroreceptors, there is a second component of acute baroreflex resetting that is of CNS origin (Heesch and Barron, 1992). Moreover, this central component is inducible within minutes by electrical stimulation of the carotid sinus nerve at an intermediate frequency of ∼10 Hz and recovers rapidly after cessation of the stimulus. Such characteristics of the central component of acute baroreflex resetting determined in ratin vivo (Heesch and Barron, 1992) are strikingly similar to the synaptic accommodation response presently found in the rat NTSin vitro.
Another important effect in which accommodation may play a role is the hypoxic depression of respiration, which has been well documented in experimental animals and humans (Bisgard and Neubauer, 1995). As with acute baroreceptor resetting, hypoxic respiratory depression has both a central and a peripheral (chemoreceptor) component and is manifested as a gradual “roll-off” of the initial response to the hypoxic stimulus. An activity-dependent attenuation of synaptic transmission mediating the hypoxic stimulus has been proposed as a likely mechanism of central hypoxic depression (Poon, 1996a).
Less clear is the possible functional correlate of LTD, which has been observed in only half of the NTS cells. Presumably, LTD is not a generic property of all NTS neurons, but is specific to certain physiological systems. Presently, there has been no evidence of any long-term memory in the neural control of the cardiovascular system, and thus type II cells are probably not associated with circulatory regulation. On the other hand, many studies in both animal models and human subjects in vivo have indicated the possible existence of both long-term and short-term memory in the respiratory system (Eldridge and Millhorn, 1986; Poon, 1996a,b). In particular, the hypoxic respiratory depression in humans is known to persist for some time after withdrawal of the hypoxic stimulus (Bisgard and Neubauer, 1995). This behavior is compatible with a neural memory effect of sustained depression of synaptic transmission and is consistent with the LTD in type II NTS cells (Poon, 1996a).
In conclusion, we have shown that neurons in the NTS exhibit LTD and a phasic, highly robust accommodation on LFS of the afferent inputs in the TS. Both phenomena are expressed postsynaptically, but induction and maintenance of LTD are contingent on AMPA receptor desensitization and Ca2+ influx, respectively, whereas accommodation is independent of both. Such phasic and long-term synaptic plasticity in the NTS may play a role in the homeostatic regulation of cardiorespiratory functions.
Footnotes
This work was supported in part by Office of Naval Research Grant N00014-95-1-0414; National Heart, Lung and Blood Institute Grants HL45261, HL50614, HL52925; and National Science Foundation Grant BCS-9216419. We thank Drs. M. Bear and Y. Frégnac for helpful comments on an early version of this manuscript.
Correspondence should be addressed to Dr. C.-S. Poon, Harvard–MIT Division of Health Sciences and Technology, Room 20A-126, Massachusetts Institute of Technology, Cambridge, MA 02139.
REFERENCES
- 1.Abbott LF, Varela JA, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- 2.Andresen MC, Kunze DL. Nucleus tractus solitarius—gateway to neural circulatory control. Annu Rev Physiol. 1994;56:93–116. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- 3.Andresen MC, Yang M. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol. 1990;259:H1307–H1311. doi: 10.1152/ajpheart.1990.259.4.H1307. [DOI] [PubMed] [Google Scholar]
- 4.Barraco RC. Nucleus of the solitary tract. CRC Press; Boca Raton, FL: 1994. [Google Scholar]
- 5.Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 6.Bisgard GE, Neubauer JA. Peripheral and central effects of hypoxia. In: Dempsey A, Pack AI, editors. Regulation of breathing, Ed 2. II. Lung biology in health and disease, Vol 79. Dekker; New York: 1995. pp. 617–668. [Google Scholar]
- 7.Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 8.Bolshakov Y, Siegelbaum SA. Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science. 1994;246:1148–1152. doi: 10.1126/science.7909958. [DOI] [PubMed] [Google Scholar]
- 9.Brocher S, Artoloa A, Singer W. Intracellular injection of Ca2+ chelators blocks induction of long-term depression in rat visual cortex. Proc Natl Acad Sci USA. 1992;89:123–127. doi: 10.1073/pnas.89.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castro-Alamancos MA, Donoghue JP, Connors BW. Different forms of synaptic plasticity in somatosensory and motor areas of the neocortex. J Neurosci. 1995;15:5324–5333. doi: 10.1523/JNEUROSCI.15-07-05324.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Champagnat J, Denavit-Saubié M, Siggins GR. Rhythmic neuronal activities in the nucleus of the tractus solitarius isolated in vitro. Brain Res. 1983;280:155–159. doi: 10.1016/0006-8993(83)91184-8. [DOI] [PubMed] [Google Scholar]
- 12.Chapleau MW, Cunningham JT, Sullivan MJ, Wachtel RE, Abboud FM. Structural versus functional modulation of the arterial baroreflex. Hypertension. 1995;26:341–347. doi: 10.1161/01.hyp.26.2.341. [DOI] [PubMed] [Google Scholar]
- 13.Debanne D, Thompson SM. Calcium: a trigger for long-term depression and potentiation in the hippocampus. News Physiol Sci. 1994;9:256–260. [Google Scholar]
- 14.Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-d-aspartate receptor blockade. Proc Natl Acad Sci USA. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eldridge FL, Millhorn DE. Oscillation, gating, and memory in the respiratory control system. In: Cherniack NS, Widdicombe JG, editors. Handbook of physiology, Sec 3, The respiratory system, Vol II, Control of breathing. American Physiological Society; Bethesda, MD: 1986. pp. 93–114. [Google Scholar]
- 16.Fortin G, Velluti JC, Denavit-Saubié M, Champagnat J. Responses to repetitive afferent activity of rat solitary complex neurons isolated in brainstem slices. Neurosci Lett. 1992;147:89–92. doi: 10.1016/0304-3940(92)90781-2. [DOI] [PubMed] [Google Scholar]
- 17.Glaum SR, Brooks PA. Tetanus-induced sustained potentiation of monosynaptic inhibitory transmission in the rat medulla: evidence for a presynaptic locus. J Neurophysiol. 1996;76:30–38. doi: 10.1152/jn.1996.76.1.30. [DOI] [PubMed] [Google Scholar]
- 18.Glaum SR, Miller RJ. Activation of metabotropic glutamate receptors produces reciprocal regulation of ionotropic glutamate and GABA responses in the nucleus of the tractus solitarius of the rat. J Neurosci. 1993;13:1636–1641. doi: 10.1523/JNEUROSCI.13-04-01636.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heesch CM, Barron KW. Is there a central nervous system component to acute baroreflex resetting in rats? Am J Physiol. 1992;262:H503–510. doi: 10.1152/ajpheart.1992.262.2.H503. [DOI] [PubMed] [Google Scholar]
- 20.Houpt TA, Philopena JM, Joh TH, Smith GP. c-Fos induction in the rat nucleus of the solitary tract correlates with the retention and forgetting of a conditioned taste aversion. Learn Memory. 1996;3:25–30. doi: 10.1101/lm.3.1.25. [DOI] [PubMed] [Google Scholar]
- 21.Ito M. Long-term depression. Annu Rev Neurosci. 1989;12:85–102. doi: 10.1146/annurev.ne.12.030189.000505. [DOI] [PubMed] [Google Scholar]
- 22.Kato N. Dependence of long-term depression on postsynaptic metabotropic glutamate receptors in visual cortex. Proc Natl Acad Sci USA. 1993;90:3650–3654. doi: 10.1073/pnas.90.8.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirkwood A, Dudek SM, Gold JT, Aizenman CD, Bear MF. Common forms of synaptic plasticity in the hippocampus and neocortex in vitro. Science. 1993;260:1518–1521. doi: 10.1126/science.8502997. [DOI] [PubMed] [Google Scholar]
- 24.Larson J, Wong D, Lynch G. Patterned stimulation at the θ frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368:347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- 25.Linden DJ, Connor JA. Long-term synaptic depression. Annu Rev Neurosci. 1995;18:319–357. doi: 10.1146/annurev.ne.18.030195.001535. [DOI] [PubMed] [Google Scholar]
- 26.Linden DJ, Dickinson MH, Smeyne M, Connor JA. A long-term depression of AMPA currents in cultured cerebella Purkinje neurons. Neuron. 1991;7:81–89. doi: 10.1016/0896-6273(91)90076-c. [DOI] [PubMed] [Google Scholar]
- 27.Mancia G, Ferrari AU, Zanchetti A. Reflex control of the circulation in experimental and human hypertension. In: Zanchetti A, Tarazi RC, editors. Handbook of hypertension, Vol 8, Pathophysiology of hypertension—regulatory mechanisms. Elsevier; New York: 1986. pp. 47–68. [Google Scholar]
- 28.Miles R. Frequency dependence of synaptic transmission in nucleus of the solitary tract in vitro. J Neurophysiol. 1986;55:1076–1090. doi: 10.1152/jn.1986.55.5.1076. [DOI] [PubMed] [Google Scholar]
- 29.Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- 30.Partin KM, Patneau DK, Winters CA, Mayer ML, Buonanno A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron. 1993;11:1069–1082. doi: 10.1016/0896-6273(93)90220-l. [DOI] [PubMed] [Google Scholar]
- 31.Pockett S, Figurov A. Long-term potentiation and depression in the ventral horn of rat spinal cord in vitro. NeuroReport. 1993;4:97–99. doi: 10.1097/00001756-199301000-00025. [DOI] [PubMed] [Google Scholar]
- 32.Poon CS. Synaptic plasticity and respiratory control. In: Khoo MCK, editor. Bioengineering approaches to pulmonary physiology and medicine. Plenum; New York: 1996a. pp. 93–113. [Google Scholar]
- 33.Poon CS. Self-tuning optimal regulation of respiratory motor output by Hebbian covariance learning. Neural Networks. 1996b;9:1367–1383. doi: 10.1016/s0893-6080(96)00022-6. [DOI] [PubMed] [Google Scholar]
- 34.Randic M, Jiang MC, Cerne R. Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. J Neurosci. 1993;13:5228–5241. doi: 10.1523/JNEUROSCI.13-12-05228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shigemoto R, Takaaki A, Nomura S, Nakanish S, Hirano T. Antibodies inactivating mGluR1 metabotropic glutamate receptor block long-term depression in cultured Purkinje cells. Neuron. 1994;12:1245–1255. doi: 10.1016/0896-6273(94)90441-3. [DOI] [PubMed] [Google Scholar]
- 36.Trussell LO, Zhang S, Raman IM. Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron. 1993;10:1185–1196. doi: 10.1016/0896-6273(93)90066-z. [DOI] [PubMed] [Google Scholar]
- 37.Westbrook GL. Glutamate receptor update. Curr Opin Neurobiol. 1994;4:337–346. doi: 10.1016/0959-4388(94)90094-9. [DOI] [PubMed] [Google Scholar]