Abstract
Recently, with use of rat dorsal root ganglion (DRG) neurons we have been able to dissociate the binding affinities of vanilloids from their potencies to induce 45Ca uptake, which suggests the existence of distinct classes of the vanilloid receptor (Acs et al., 1996). In the present study, we have demonstrated that the ultrapotent capsaicin analog resiniferatoxin (RTX) desensitized rat DRG neurons to the subsequent induction of 45Ca uptake by capsaicin and RTX with affinity and cooperativity similar to that found for [3H]RTX binding, contrasting with a ∼10-fold weaker potency and lack of cooperativity to induce 45Ca uptake. Likewise, the competitive antagonist capsazepine inhibited RTX-induced desensitization with potency similar to that for inhibition of specific [3H]RTX binding, whereas the potency of capsazepine was ∼10-fold higher for inhibiting RTX-induced45Ca uptake. Finally, the noncompetitive antagonist ruthenium red inhibited both the RTX-induced desensitization and45Ca uptake but showed ∼60-fold selectivity for inhibiting RTX-induced desensitization. The RTX-induced desensitization was not associated with loss of specific [3H]RTX binding, suggesting lack of gross cell toxicity. In contrast to RTX, capsaicin caused desensitization with a potency corresponding to that for 45Ca uptake and did so in a noncooperative manner. Unlike the RTX-induced desensitization, the desensitization by capsaicin was blocked by ruthenium red only at doses that blocked45Ca uptake and depended on external calcium. Our findings provide further support for the existence of vanilloid receptor subtypes on DRG neurons with distinct pharmacology and distinct patterns of desensitization.
Keywords: dorsal root ganglion neurons, capsaicin, resiniferatoxin, desensitization, [3H]RTX binding, 45Ca uptake, capsazepine, ruthenium red, pain, rat
A subpopulation of primary afferent neurons, located in the dorsal root and trigeminal ganglia, can be defined by their selective susceptibility to the effects of capsaicin (Buck and Burks, 1986; Holzer, 1991), the major pungent ingredient of hot peppers of the plant genus Capsicum.
Several years ago we found that resiniferatoxin (RTX), a naturally occurring irritant tricyclic diterpene (Hergenhahn et al., 1975) that combines structural features of the phorbol ester tumor promoters and of capsaicin, functions as an ultrapotent capsaicin analog (Szallasi and Blumberg, 1989). Qualitatively, RTX induces a pattern of responses generally similar to those observed for capsaicin (Szallasi and Blumberg, 1989). RTX and capsaicin differ, however, in their relative potencies for different responses (Blumberg et al., 1993).
As observed for capsaicin, after initial excitation, RTX treatment also leads to desensitization to subsequent RTX application; in addition, desensitization by either compound leads to cross-desensitization to the other (Blumberg et al., 1993). It was postulated decades ago that pungency of capsaicin analogs is proportional to the desensitization that follows (Jancso, 1968); however, this does not seem to be the case for RTX. For example, similar concentrations of RTX and capsaicin cause contraction of isolated rat urinary bladder, but RTX shows a 1000-fold higher potency to induce desensitization (Maggi et al., 1990). Moreover, RTX, but not capsaicin, can desensitize the pulmonary J1 receptors of the rat without previous excitation (Szolcsanyi et al., 1990).
[3H]RTX shows specific, saturable binding to membranes of sensory afferent neurons, displaying appropriate tissue, species, and pharmacological specificity to represent the vanilloid receptor (Blumberg et al., 1993; Acs et al., 1994a,b). Specific [3H]RTX binding by the above membrane preparations displays sigmoidal saturation kinetics, indicating apparent positive cooperativity (Acs et al., 1994a,b).
The basis for the differences in the pattern of responses to capsaicin and RTX and the divergence between the stimulatory and desensitizing potencies of vanilloids has remained unresolved. Consistent with the existence of receptor subclasses (Holzer, 1991; Blumberg et al., 1993), different vanilloid analogs show different potencies for receptor binding and for induction of 45Ca uptake in dorsal root ganglion (DRG) neurons when assayed under similar conditions (Acs et al., 1996). Likewise, both RTX and capsaicin bind to DRG neurons in a positive cooperative fashion but induce 45Ca uptake in a noncooperative manner (Acs et al., 1996).
In the present study, we have examined whether we can identify any responses in the DRG neurons linked to the high-affinity RTX receptor as defined by [3H]RTX binding. We conclude that this site mediates desensitization of 45Ca uptake to subsequent vanilloid challenge. We further demonstrate that stimulation of the 45Ca uptake site by capsaicin can alternatively desensitize the cells to subsequent vanilloid challenge. These two pathways of desensitization show different sensitivities to ruthenium red and different dependence on external Ca2+. On the one hand, our findings strengthen the evidence for multiple vanilloid receptors; on the other hand, they help rationalize the extensive evidence for the complexity of “desensitization” in response to vanilloids (Holzer, 1991).
MATERIALS AND METHODS
Female Sprague Dawley rats (6–8 weeks old, 150–160 gm body weight) were purchased from NCI-FCRDC (Frederick, MD). Animals were allowed to access food and water ad libitum throughout the course of the experiments. Animal protocols were approved by the Animal Care and Use Subcommittee, Division of Basic Sciences, National Cancer Institute. [3H]RTX (37 Ci/mmol) was synthesized by the Chemical Synthesis and Analysis Laboratory, NCI-FCRDC.45Ca (CaCl2, 23.55 mCi/mg) was purchased from DuPont NEN (Boston, MA). Nonradioactive RTX and capsazepine were from LC Laboratories (Woburn, MA). Capsaicin was from Sigma (St. Louis, MO). Ruthenium red was purchased from Research Biochemicals International (Natick, MA).
Cell cultures. Rat DRG neuron cultures were prepared as described (Acs et al., 1995, 1996). Animals were decapitated under CO2 anesthesia. The spinal columns were removed aseptically, and DRGs from all levels were dissected out and collected in ice-cold DMEM (Life Technologies, Gaithersburg, MD) containing 0.5% heat-inactivated fetal bovine serum (Life Technologies), 1 mm sodium pyruvate, and 25 mm HEPES. Ganglia were digested with 0.125% collagenase (Sigma) in DMEM for 90 min at 37°C and then for an additional 90 min in fresh collagenase solution. Ganglia were washed twice with DMEM containing 25 mm HEPES and 1 mm sodium pyruvate and were triturated through a flame-polished Pasteur pipette to form a single cell suspension. The cells were pelleted through a cushion of DMEM containing 15% fatty acid-free bovine serum albumin (BSA) (Sigma) to remove myelin debris. Cells were then washed three times with serum-free DMEM and resuspended in the same medium, and the number of viable cells was determined by the Trypan-blue dye exclusion test. Cells were then plated into MultiScreen-DV 96-well filtration plates (Millipore, Marlborough, MA) at a density of ∼5 × 103 cells/well in 100 μl of serum-free medium.
For desensitization experiments, cells were incubated at 37°C in the presence of RTX or capsaicin for the indicated times before measurement of induction of 45Ca uptake. Alternatively, the cells in suspension were treated with RTX and competing ligands (capsazepine or ruthenium red). After incubation at 37°C for 6 hr, cells were washed three times with serum-free DMEM containing 0.25 mg/ml BSA and then challenged with either capsaicin or RTX to induce 45Ca uptake. The efficacy of the washing procedure was quantitated in the following way. Cells were incubated in the presence of 250 pm [3H]RTX (the concentration of RTX used in most experiments for inducing desensitization) for 6 hr. The radioactivity in the cell suspension was determined before washing and after each washing step by scintillation counting. According to these experiments, ∼95% of the added radioactive RTX was removed by this washing procedure from the suspension (see insert in Fig.5). The number of viable cells was then determined. Cells were then plated into Multiscreen-DV 96-well filtration plates at a density of ∼5 × 103 cells/well in 100 μl serum-free medium and used for 45Ca uptake and [3H]RTX binding assays.
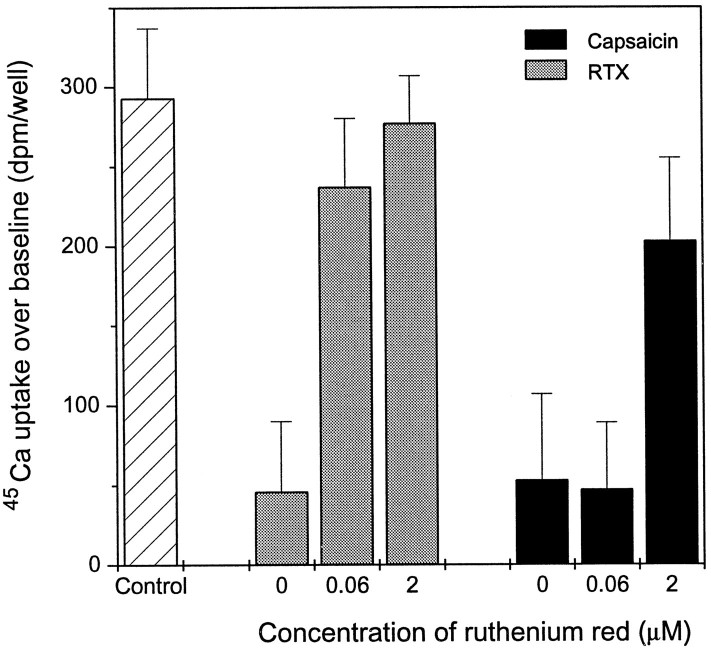
Fig. 5.
Differential inhibition by ruthenium red of capsaicin and RTX-induced desensitization of rat DRG neurons. Cells were incubated with either 250 pm RTX or 1 μmcapsaicin for 6 hr in the presence of 60 nm or 2 μm ruthenium red, washed three times with serum-free DMEM containing 0.25 mg/ml BSA to remove the pretreatment compounds, and then challenged with 3 μm capsaicin to induce45Ca uptake. The return of the 45Ca uptake response in the presence of ruthenium red represents the inhibition of desensitization. Points represent mean values from sets of eight determinations in a single experiment; error bars indicate SEM. Two additional experiments yielded similar results.
Measurement of 45Ca uptake by DRG neurons.Freshly dissociated cells in MultiScreen-DV 96-well filtration plates were incubated in a total volume of 0.25 ml of serum-free DMEM (containing 1.8 mm CaCl2) in the presence of 0.25 mg/ml BSA (included to stabilize the compounds in the aqueous solution), 1 μCi/ml 45Ca, and increasing concentrations of the different compounds for 20 min at 37°C (Acs et al., 1995, 1996). Cells were then washed five times with ice-cold DMEM by filtration using a MultiScreen Vacuum Manifold (Millipore). Filters were dried under a heat lamp and punched out into scintillation vials using MultiScreen disposable punch tips, and the radioactivity was determined by scintillation counting. For each data point in each experiment, eight wells were assayed.
Analysis of 45Ca uptake data. Analysis of the45Ca uptake experiments was performed as described previously (Acs et al., 1996) by computer fit to the Hill equation (Endrenyi et al., 1975). In the case of experiments performed on RTX or capsaicin-pretreated cells, desensitization was defined as the difference (in dpm/well) between the increase in 45Ca uptake in these and in control cells after challenge by capsaicin or RTX. The decrease in the 45Ca uptake induced by vanilloids was plotted against the pretreatment concentration of RTX, and the data were fitted to the Hill equation. Data from competition experiments in which the effect of the desensitizing compound was antagonized by either a competitive (capsazepine) or a noncompetitive (ruthenium red) antagonist were fitted to the modified Hill equation (Davis et al., 1977). Data were fitted to the equations using the computer program MicroCal Origin 3.5 (MicroCal Software, Northampton, MA). For the statistical analysis of the curve fitting to the experimental data, the χ2 test of goodness of fit was used.
Measurement of [3H]RTX binding by DRG neurons. For [3H]RTX binding assays, cells were plated into Multiscreen-DV 96-well filtration plates (Acs et al., 1996). Immediately after plating, 150 μl of DMEM containing 0.25 mg/ml BSA, [3H]RTX, and nonradioactive ligands was added to each well containing the 100 μl cell suspension, and the plates were incubated in triplicate for 60 min at 37°C. Plates were then chilled on ice, and 1 mg of α1-acid glycoprotein (AGP, Sigma) in 50 μl of ice-cold serum-free DMEM was added to each well to reduce nonspecific binding (Szallasi et al., 1992). Cells were then washed four times with DMEM (200 μl/well) containing 0.5 mg/ml AGP by filtration using a MultiScreen Vacuum Manifold (Millipore). Filters were dried under a heat lamp and punched out into scintillation vials using MultiScreen disposable punch tips, and the bound radioactivity was determined by scintillation counting. Binding was expressed as femtomoles/103 cells; nonspecific binding was determined in the presence of 1 μmnonradioactive RTX.
RESULTS
The potencies of RTX and capsaicin to stimulate the uptake of calcium into rat DRG neurons were determined in the presence of 1 μCi/ml 45Ca. As expected, both ligands induced a dose-dependent increase in 45Ca uptake by the cells with ED50 values of 1.24 ± 0.02 nm for RTX and 316 ± 47 nm for capsaicin (mean ± SEM for four experiments each). Hill coefficients for the dose–response curves were close to unity (1.08 ± 0.07 and 1.02 ± 0.06 in the case of RTX and capsaicin, respectively; mean ± SEM for four experiments) (p > 0.05; Student’s t test), suggesting a noncooperative mechanism of action. These values agreed well with those determined previously (Acs et al., 1996).
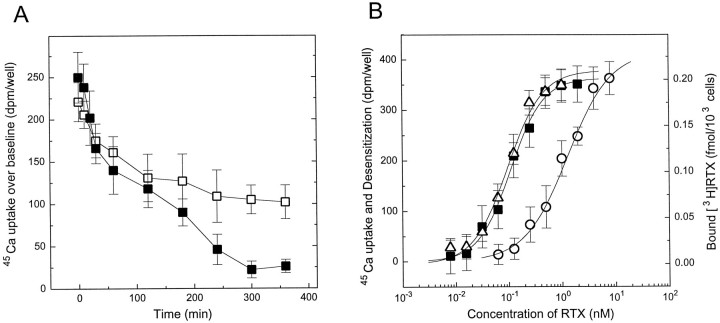
We had reported previously (Acs et al., 1996) that RTX displayed 24-fold greater potency for specific binding to DRG neurons than for induction of 45Ca uptake. We were interested in how the low concentrations of RTX, at which binding was measured, affected the induction of 45Ca uptake on subsequent capsaicin treatment. We first determined the effect of RTX pretreatment for different time intervals on 45Ca uptake after challenge with capsaicin. Preincubation of the neurons with 100 pm (a concentration close to the Kd value of RTX for receptor binding) and 250 pm RTX (a concentration that almost saturates RTX binding sites) (Acs et al., 1996) for 10 min had no effect on the level of 45Ca uptake when the cells were stimulated with 3 μm capsaicin (a dose that by itself induces a maximal stimulation of 45Ca uptake by the cells) (Acs et al., 1995, 1996) (Fig. 1A). In contrast, a significant decrease of 45Ca uptake (20.8 ± 1.3 and 33.6 ± 2.1% decrease compared with control uptake, respectively; mean ± SEM for three experiments each) (p < 0.05; Student’s t test) was observed in both cases after 30 min of preincubation with RTX. A maximal effect of RTX pretreatment (55.7 ± 3.3 and 91.2 ± 1.8% decrease in 45Ca uptake in the case of 100 and 250 pm RTX pretreatment, respectively, compared with control cells; mean ± SEM for three experiments each) was reached in both cases by 5 hr of preincubation. On the basis of the above results, a 6 hr preincubation time was used in the subsequent experiments to induce maximal desensitization to RTX.
Fig. 1.
Inhibition of 45Ca uptake in rat DRG neurons after RTX pretreatment. A, Time course of the effect of RTX pretreatment on 45Ca uptake. Cells were pretreated with 100 pm (□) and 250 pm (▪) resiniferatoxin and then challenged with 3 μm capsaicin. Two additional experiments yielded similar results. B, Comparison of dose–response curves for induction of 45Ca uptake, [3H]RTX binding, and desensitization by RTX in rat DRG neurons. Data are expressed as uptake values above baseline for RTX-induced 45Ca uptake (○) and as specifically bound [3H]RTX (▵) when binding was determined. Desensitization was defined as the difference (in dpm/well) in 45Ca uptake between pretreated and control cells challenged with capsaicin. In the case of desensitization (▪), cells were pretreated with different concentrations of RTX for 6 hr and then challenged with 3 μm capsaicin. Points represent mean values from sets of eight determinations in a single experiment; error bars indicate SEM. The theoretical curves were calculated by fitting the measured values to the Hill equation. In the case of45Ca uptake and desensitization, three additional experiments in each case yielded similar results. The dose–response curve for [3H]RTX binding is a single experiment, yielding binding parameters similar to those we found previously (Acs et al., 1996).
To determine the concentration dependence of RTX-induced desensitization, neurons were incubated with different concentrations of RTX for 6 hr. This procedure resulted in a dose-dependent reduction in the level of 45Ca uptake after challenge with 3 μm capsaicin (Fig. 1B). Fitting the desensitization curves (see Materials and Methods) to the Hill equation yielded an apparent Kd value for desensitization of 81 ± 5 pm and a Hill coefficient of 1.51 ± 0.11 (mean ± SEM for four experiments), suggesting positive cooperativity of RTX action. The potency of RTX for desensitization thus was ∼10-fold higher than that for induction of 45Ca uptake and was similar to its affinity for receptor binding (47 ± 4 pm) (Acs et al., 1996). Moreover, RTX desensitized the cells in a positive cooperative fashion—with a Hill coefficient similar to that determined in receptor binding assays (1.78 ± 0.12) (Acs et al., 1996)—as opposed to its noncooperative action in45Ca uptake induction assays.
In the above experiments, we desensitized with RTX and challenged with capsaicin. In other experiments we challenged the 250 pmRTX-pretreated cells with as high a dose of RTX as 200 nm. The Kd and Hill coefficient values for desensitization were similar to those after capsaicin challenge (Kd and Hill coefficient values were 91 ± 6 nm and 1.62 ± 0.24, respectively; mean ± SEM for five experiments).
The desensitization of 45Ca uptake was independent of the challenging dose of capsaicin or RTX. Pretreatment of the neurons with 250 pm RTX (a concentration that by itself induced only 11.7 ± 1.5% of maximal 45Ca uptake response; mean ± SEM for four experiments) almost completely (92.1 ± 2.8%; mean ± SEM for four experiments, for challenge by 3 μm capsaicin) abolished the induction of 45Ca uptake by capsaicin up to a concentration of 6.4 μm (data not shown). Likewise, pretreatment of the cells with 250 pmRTX for 6 hr also abolished the 45Ca uptake induced by 200 nm RTX by 90.3 ± 3.1% (mean ± SEM for five experiments) (data not shown). These results suggest that the decrease in 45Ca uptake stimulated by a subsequent challenge could not be attributed to competition at the site coupled to the45Ca uptake. Further support for this conclusion comes from experiments in which the cells were washed three times after RTX preincubation immediately before the capsaicin challenge (this procedure removed >95% of the 250 pm RTX used for pretreatment) (for details, see Materials and Methods). The washing had no effect on the level of desensitization caused by the pretreatment (data not shown). We conclude that the RTX-induced desensitization does not require the continuous presence of RTX on the receptors.
As shown in Figure 1B, [3H]RTX displayed specific binding to rat DRG neurons. At 4 nm[3H]RTX, a concentration sufficient to saturate the receptors, the receptor density in control cells was 0.140 ± 0.002 fmol/103 cells. This value agreed well with those determined previously (Acs et al., 1996). Under similar conditions, in cells pretreated with 250 pm RTX for 6 hr and in cells to which 250 pm RTX was added immediately before the binding assay, we determined similar values of specifically bound [3H]RTX of 0.129 ± 0.002 and 0.125 ± 0.001 fmol/103 cells, respectively. When we determined the density of RTX binding sites after washing the cells three times after RTX pretreatment, we observed values of specifically bound [3H]RTX of 0.139 ± 0.002 and 0.141 ± 0.008 fmol/103 cells, respectively. In neither case did the pretreated cells significantly differ from the corresponding control (p > 0.05; Student’s t test). We conclude that the observed decrease in 45Ca uptake after RTX pretreatment could not be attributed to the downregulation of the vanilloid receptor as detected by the [3H]RTX binding assay. This observation further demonstrates the lack of toxicity of the 6 hr incubation with 250 pm RTX. Likewise, the number of viable neurons was not changed after RTX treatment, and the baseline uptake of45Ca was the same in pretreated and control cells (data not shown).
As observed previously (Acs et al., 1996), the competitive antagonist capsazepine (Bevan et al., 1992; Szallasi et al., 1993) inhibited stimulation of 45Ca uptake by 500 nm capsaicin in a noncooperative fashion. Ki and Hill coefficient values were 291 ± 31 nm and 0.98 ± 0.03, respectively (mean ± SEM for three experiments). Capsazepine likewise inhibited 45Ca uptake into rat DRG neurons induced by 200 nm RTX with similarKi and Hill coefficient values of 351 ± 39 nm and 1.07 ± 0.08 (mean ± SEM for four experiments) (data not shown).
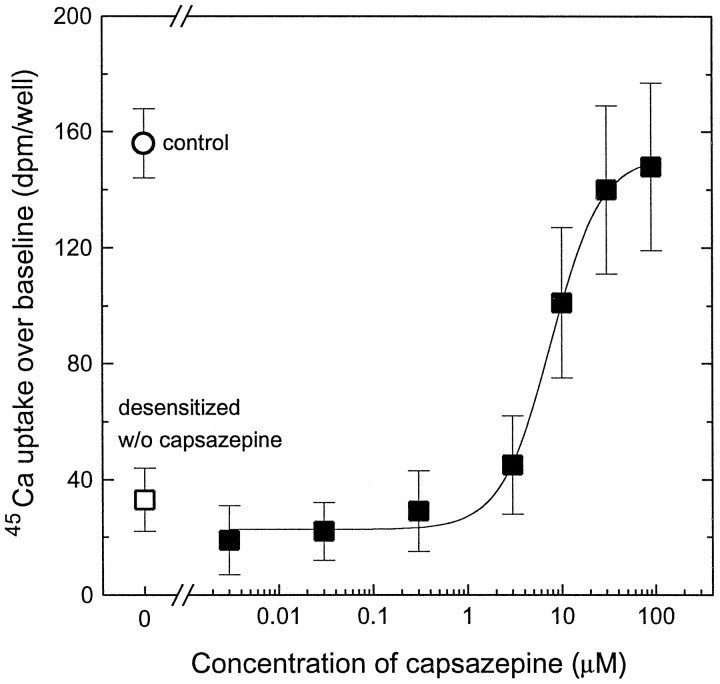
To determine whether capsazepine also acted as an antagonist of RTX-induced desensitization, we pretreated the cells with 250 pm RTX and different concentrations of capsazepine for 6 hr, washed them three times, and then challenged them with 200 nm RTX. This high challenging concentration of RTX (∼200-fold its Kd for induction of45Ca uptake) was used to assure complete displacement of capsazepine from both the 45Ca uptake site and the specific [3H]RTX binding site. Using the washing protocol (see Materials and Methods), we were able to show that capsazepine inhibited the RTX-induced desensitization in a dose-dependent fashion (Fig. 2), yielding a Ki of 3.66 ± 0.71 μm and a Hill coefficient value of 1.83 ± 0.11 (mean ± SEM for four experiments), suggesting apparent positive cooperativity. These data are in good accord with theKi and Hill coefficient values of capsazepine for inhibiting specific [3H]RTX binding to DRG neurons (3.16 ± 0.21 μm and 1.72 ± 0.11, respectively) (Acs et al., 1996) and contrast with the respective values of capsazepine for blocking RTX-induced 45Ca uptake (see above). By itself, capsazepine did not cause desensitization.
Fig. 2.
Inhibition of RTX-induced desensitization of rat DRG neurons by capsazepine. Cells were incubated in the presence of 250 pm RTX and different concentrations of capsazepine for 6 hr. Cells were then washed three times with serum-free DMEM containing 0.25 mg/ml BSA to remove the above compounds and were then challenged with 200 nm RTX to induce 45Ca uptake (▪). Cells not treated with either RTX or capsazepine (○, control) or treated with only 250 pmRTX for 6 hr (□) are shown as control values. Points represent mean values from sets of eight determinations in a single experiment; error bars indicate SEM. The theoretical curve was calculated by fitting the measured values to the Hill equation. Two additional experiments yielded similar results.
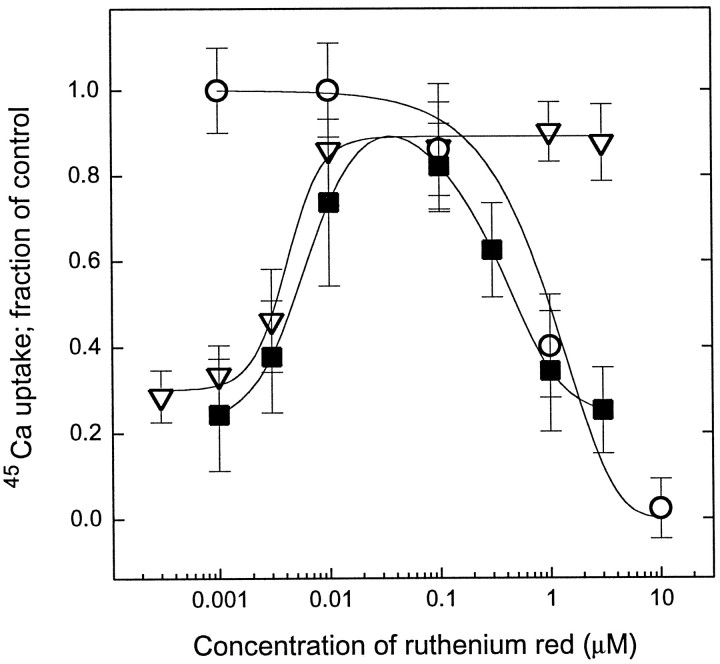
Similar experiments were performed with the noncompetitive vanilloid receptor antagonist ruthenium red (Amann and Maggi, 1991). When applied together with RTX, ruthenium red blocked the 45Ca uptake into the cells induced by 200 nm RTX in a dose-dependent fashion, with an ED50 of 790 ± 40 nm(mean ± SEM for three determinations) (Fig. 3); this value agreed well with those reported previously (Maggi et al., 1988). Furthermore, when administered together with 250 pmRTX during the 6 hr pretreatment, ruthenium red had a biphasic effect on 45Ca uptake induced by a subsequent challenge with 200 nm RTX. At lower concentrations ruthenium red inhibited RTX-induced desensitization, which was reflected by the increased45Ca uptake into the cells. At higher concentrations, however, ruthenium red inhibited the 45Ca uptake induced by the challenging dose of RTX. When the cells were washed three times after pretreatment with RTX and ruthenium red, the second phase of the above curve was eliminated (no ruthenium red was present in the assay to block 45Ca uptake) (Fig. 3). In these latter experiments ruthenium red inhibited 250 pm RTX-induced desensitization with an ED50 of 14 ± 2 nm (mean ± SEM for three determinations), a value markedly different (∼60-fold difference in potencies) from that determined for the inhibition of induction of the 45Ca uptake. The ability of ruthenium red to inhibit selectively the RTX-induced desensitization is of great importance. It argues strongly that the desensitization observed in response to RTX does not reflect simply a long-term consequence of limited Ca2+ influx occurring at a concentration of ligand below the ED50 for stimulation of 45Ca uptake.
Fig. 3.
Effect of ruthenium red on RTX-induced45Ca uptake and on RTX-induced desensitization in rat DRG neurons. Cells were incubated in the presence of 250 pm RTX and different concentrations of ruthenium red for 6 hr and then challenged with 200 nm RTX (▪). To evaluate the effect of ruthenium red just on desensitization, after the above incubation cells were washed three times with serum-free DMEM containing 0.25 mg/ml BSA to remove the pretreatment compounds before the cells were challenged to induce 45Ca uptake (▿). To measure the effect of ruthenium red just on 45Ca uptake, varying concentrations of ruthenium red and 200 nm RTX were applied together, and45Ca uptake was determined (○). Points represent mean values from sets of eight determinations in a single experiment; error bars indicate SEM. The theoretical curve for blocking the RTX-induced45Ca uptake was calculated by fitting the measured values to the modified Hill equation, whereas data for blocking the RTX-induced desensitization was fitted to the Hill equation. Two additional experiments yielded similar results.
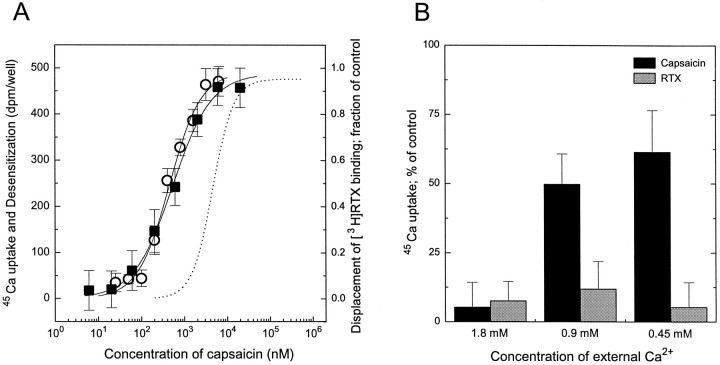
The motivation for the above studies was to determine whether we could identify any responses that were coupled to the vanilloid receptor subtype detected by [3H]RTX binding. We conclude that this receptor subclass is associated with desensitization of subsequent 45Ca uptake in response to vanilloid challenge. These studies do not address the converse issue, whether the vanilloid receptor subtype detected by 45Ca uptake can also induce desensitization. We therefore treated the cultured DRG neurons with capsaicin, which is selective for inducing 45Ca uptake and has weaker affinity for the receptor defined by specific [3H]RTX binding, and we determined the effect of capsaicin pretreatment on the subsequent 45Ca uptake induced by vanilloid challenge. The stimulation of 45Ca uptake was inhibited by capsaicin pretreatment with an ED50of 445 ± 46 nm (Fig.4A). This value compares closely with the ED50 for induction of 45Ca uptake by capsaicin, 316 ± 47 nm, and is thus markedly lower than that for inhibition of [3H]RTX binding by capsaicin (Ki of 4.9 μm) (Acs et al., 1996). Furthermore, capsaicin induced desensitization in a noncooperative manner (with a Hill coefficient of 0.89 ± 0.15; mean ± SEM for three experiments), similar to its action on inducing 45Ca uptake (see above) and in contrast to the displacement of [3H]RTX binding (Hill coefficient of 1.81) (Acs et al., 1996).
Fig. 4.
A, Comparison of dose–response curves for induction of 45Ca uptake, desensitization, and displacement of [3H]RTX binding by capsaicin in rat DRG neurons. Data are expressed as dpm/well values over baseline for capsaicin-induced 45Ca uptake (○). In the case of desensitization (▪), cells were pretreated with different concentrations of capsaicin for 6 hr and then challenged with 3 μm capsaicin. Desensitization was defined as the difference (in dpm/well) in 45Ca uptake between pretreated and control cells challenged with capsaicin. For comparison, the capsaicin dose–response curve for displacement of [3H]RTX binding (dotted line), determined previously by us (Acs et al., 1996), was plotted. Points represent mean values from sets of eight determinations in a single experiment; error bars indicate SEM. The theoretical curves were calculated by fitting the measured values to the Hill equation. Two additional experiments in each case yielded similar results.B, Extracellular Ca2+ dependence of the capsaicin-induced desensitization of rat DRG neurons. Cells were incubated with either 250 pm RTX or 1 μmcapsaicin for 4 hr in medium that contained 1.8 mm, 0.9 mm, or 0.45 mm Ca2+. After incubation, cells were washed with serum-free DMEM containing 1.8 mm Ca2+ and challenged with 3 μm capsaicin to induce 45Ca uptake. Data were expressed as percentage values of control 45Ca uptake determined on untreated cells in the same medium. The external Ca2+ dependence of desensitization is indicated by the return of the 45Ca uptake response. Points represent mean values from sets of eight determinations in a single experiment; error bars indicate SEM. Two additional experiments yielded similar results.
We next compared the dependence of the desensitization induced by either RTX or capsaicin on the concentration of Ca2+in the medium. Because the DRG neurons show toxicity to prolonged incubation in the absence of extracellular Ca2+, we determined that desensitization in media containing decreased Ca2+ concentrations after 4 hr preincubation (Fig.4B). Desensitization by 250 pm RTX was not affected by the change in the concentration of Ca2+ in the medium, whereas desensitization by 1 μm capsaicin was reduced in media containing a decreased concentration of Ca2+.
Finally, consistent with the desensitization in response to capsaicin reflecting the induction of 45Ca uptake, we compared the ability of ruthenium red to block the capsaicin and RTX-induced desensitization. We examined concentrations of ruthenium red that were three- to fourfold the ED50 for blocking RTX-induced desensitization and 45Ca uptake (60 nm and 2 μm, respectively). Only the latter concentration inhibited the desensitization induced by 1 μm capsaicin; in contrast, the desensitization induced by 250 pm RTX was already inhibited by 60 nm ruthenium red, as expected (Fig.5). We conclude that capsaicin induces desensitization through a mechanism distinct from that of RTX. The capsaicin-induced desensitization is linked to the enhanced Ca2+influx; the RTX-induced desensitization is mediated by the receptor detected by specific [3H]RTX binding.
DISCUSSION
Our findings cogently argue for the existence of two pharmacologically defined classes of vanilloid receptors, for which we suggest the designation R(TX)-type and C(apsaicin)-type. The distinct pharmacology of these two receptor subclasses had already been demonstrated in our comparison of [3H]RTX binding and 45Ca uptake in intact DRG neurons (Acs et al., 1996). In the present study, we have extended the evidence by showing that a biological response, desensitization in response to RTX, quantitatively agrees with the pharmacology for the R-type receptor. We further show that ruthenium red, a noncompetitive vanilloid antagonist, shows markedly different affinity for blocking responses through the R- and C-type receptors.
A critical issue was whether the desensitization induced by RTX could simply have been a consequence of a low level of 45Ca uptake induced by RTX occupying a small fraction of the C-type receptors. Three findings argue against this explanation. First, ruthenium red was much more potent for blocking the RTX-induced desensitization compared with 45Ca uptake. Second, the RTX-induced desensitization showed no dependence on external Ca2+, in contrast to the desensitization by capsaicin. Finally, desensitization in response to capsaicin was observed only with an ED50 corresponding to that for induction of 45Ca uptake, not at a concentration an order of magnitude lower.
Elegant previous studies have established that capsaicin-induced desensitization is largely dependent on external Ca2+ (Santicioli et al., 1987; Cholewinski et al., 1993; Docherty et al., 1996). Our results with capsaicin are consistent with these observations and provide further support for distinct mechanisms of desensitization mediated by the R- and C-type vanilloid receptors. Obviously, the ability to separate responses through these two pathways in DRG neurons will depend on ligands of appropriate selectivity and on their use at appropriate concentrations. At higher concentrations, RTX will also act on the C-type receptors and capsaicin on the R-type. An alternative approach for analysis would be to find systems in which only one receptor subtype is expressed. Elsewhere, we will describe characterization of vanilloid responses in a series of non-neuronal cell lines. In these cells, we observe only C-type receptors and not R-type receptors, and the C-type receptors display characteristics quantitatively similar to those described here (T. Biro, M. Maurer, S. Modarres, N. E. Lewin, C. Brodie, G. Acs, P. Acs, R. Paus, and P. M. Blumberg, unpublished observations).
Our findings help structure and interpret various observations by multiple groups in the vanilloid field. Szallasi and colleagues (1993) (Goso et al., 1993) have reported previously that specific RTX binding in some preparations, e.g., airways and colon, is of low affinity (Kd values for RTX are 250 pm and 3 nm, respectively) and lacks cooperativity; these characteristics might fit with these measurements reflecting interaction at the lower affinity, 45Ca uptake-coupled receptor site. Interpretation had been clouded somewhat because of the difficulties in accurately defining this low-affinity binding and the known variability in the cooperativity depending on the conditions of membrane preparation (Szallasi and Blumberg, 1993). Like us, Walpole et al. (1996) found differences between the structure–activity relations for 45Ca uptake and RTX binding. In their case, however, binding was performed on membrane preparations, and because the general trend was similar for both assays, they concluded that “the potencies in the 45Ca uptake assay are generally about 10-fold lower than binding potencies, presumably due to other processes, e.g., uptake into mitochondria being necessary for detection in this assay.” In fact, the differences in structure–activity relations are impressive. If one compares the relative potency for binding versus45Ca uptake, the ratio (Ki/ED50) is 0.04 for RTX compared with 15 for capsaicin; RTX thus permits a 375-fold better selectivity than does capsaicin for the high-affinity RTX binding site compared with the vanilloid receptor mediating 45Ca uptake.
In biological systems, abundant evidence supports distinct structure–activity relations for different biological responses for vanilloids (Holzer, 1991). Such differences are evident not only in comparisons between vanilloids of the RTX and capsaicin classes, but also within each class. Thus, the vanilloid analog olvanil differentially induces desensitization compared with capsaicin (Dickenson et al., 1990; Dray et al., 1990). The dissection of patterns of biological response can be explained most readily by receptor subtypes, although it is also clear that differences in pharmacokinetics can complicate interpretation (Maggi et al., 1990).
Cellular studies of others likewise argue for vanilloid receptor heterogeneity. Liu and Simon (1994), for example, characterized currents induced in single sensory neurons in response to capsaicin and RTX. At least one subset of currents was inducible by capsaicin but not RTX (at the concentration examined), and furthermore, significant differences were found in the desensitization patterns of the different vanilloid-sensitive currents (Liu and Simon, 1996).
The findings reported here have important implications. First, the evidence that vanilloids induce the activation of a nonvoltage-dependent, relatively nonselective cation channel in intact cells (Wood et al., 1988; Winter et al., 1990) and in single-channel patch-clamp preparations (Oh et al., 1996) has strongly supported the argument that the vanilloid receptor either is, or is closely associated with, this ion channel. Our findings suggest that a subset of vanilloid receptors, namely those corresponding to the RTX selective subclass, have a different mechanism. Although further studies will be required to define the mechanism for the R-type vanilloid receptor, the potent inhibition of desensitization by ruthenium red argues for the involvement of calcium, consistent with activation of, e.g., the phosphoinositide pathway. In fact, the stimulation of the phosphoinositide pathway in DRG neurons after vanilloid treatment has already been described (Harvey et al., 1995).
Elegant studies by Walpole and coworkers (1996) have helped define the structural constraints for activity of vanilloids of the capsaicin class and, to a lesser degree, those of the RTX class. Because these analyses have used 45Ca uptake as the measure of activity, it is clear that separate evaluation of structure–activity relations at the R-type vanilloid receptor will be required. Because this latter receptor is coupled to desensitization without 45Ca uptake, derivatives optimized for selectivity to this site may be of particular potential therapeutic interest. Conversely, vanilloid antagonists selective for the 45Ca uptake site should permit enhanced selectivity when used in combination with a vanilloid agonist selective for the R-type receptor. Indeed, on the basis of our limited knowledge of structure–activity relations for the RTX-selective vanilloid receptor, we should be able to enhance the selectivity of RTX by a factor of 10 by coapplication with capsazepine.
Footnotes
G. Acs and T. Biro contributed equally to this work.
Correspondence should be addressed to Dr. Peter M. Blumberg, MMTP/LCCTP/NCI, Building 37, Room 3A01, 37 Convent Drive MSC 4255, Bethesda, MD 20892-4255.
REFERENCES
- 1.Acs G, Palkovits M, Blumberg PM. [3H]resiniferatoxin binding by the human vanilloid (capsaicin) receptor. Mol Brain Res. 1994a;23:185–190. doi: 10.1016/0169-328x(94)90225-9. [DOI] [PubMed] [Google Scholar]
- 2.Acs G, Palkovits M, Blumberg PM. Comparison of [3H]resiniferatoxin binding by the vanilloid (capsaicin) receptor in dorsal root ganglia, spinal cord, dorsal vagal complex, sciatic and vagal nerve and urinary bladder of the rat. Life Sci. 1994b;55:1017–1026. doi: 10.1016/0024-3205(94)00636-9. [DOI] [PubMed] [Google Scholar]
- 3.Acs G, Palkovits M, Blumberg PM. Trifluoperazine modulates [3H]resiniferatoxin binding by human and rat vanilloid (capsaicin) receptors and affects 45Ca uptake by adult rat dorsal root ganglion neurones. J Pharmacol Exp Ther. 1995;274:1090–1098. [PubMed] [Google Scholar]
- 4.Acs G, Lee J, Marquez VE, Blumberg PM. Distinct structure-activity relations for stimulation of 45Ca uptake and for high affinity binding in cultured adult rat dorsal root ganglion neurones and dorsal root ganglion membranes. Mol Brain Res. 1996;35:173–182. doi: 10.1016/0169-328x(95)00204-6. [DOI] [PubMed] [Google Scholar]
- 5.Amann R, Maggi CA. Ruthenium red as a capsaicin antagonist. Life Sci. 1991;49:849–856. doi: 10.1016/0024-3205(91)90169-c. [DOI] [PubMed] [Google Scholar]
- 6.Bevan SJ, Hothi S, Hughes IF, Rang HP, Shah K, Walpole CSJ, Yeats JC. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br J Pharmacol. 1992;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blumberg PM, Szallasi A, Acs G. Resiniferatoxin, an ultrapotent capsaicin analog. In: Wood JN, editor. Capsaicin in the study of pain. Academic; London: 1993. pp. 45–62. [Google Scholar]
- 8.Buck SH, Burks TF. The neuropharmacology of capsaicin: review of some recent observations. Pharmacol Rev. 1986;38:179–226. [PubMed] [Google Scholar]
- 9.Cholewinski A, Burgess GM, Bevan S. The role of calcium in capsaicin-induced desensitization in rat cultured dorsal root ganglion neurons. Neuroscience. 1993;55:1015–1023. doi: 10.1016/0306-4522(93)90315-7. [DOI] [PubMed] [Google Scholar]
- 10.Davis ME, Akera T, Brody TM, Watson L. Opiate receptor: cooperativity of binding observed in brain slices. Proc Natl Acad Sci USA. 1977;74:5764–5766. doi: 10.1073/pnas.74.12.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickenson A, Hughes C, Rueff A, Dray A. A spinal mechanism of action is involved in the antinociception produced by the capsaicin analogue NE 19550 (olvanil). Pain. 1990;43:353–362. doi: 10.1016/0304-3959(90)90032-9. [DOI] [PubMed] [Google Scholar]
- 12.Docherty RJ, Yeats JC, Bevan S, Boddeke HWGM. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurons from adult rats. Pflügers Arch. 1996;431:828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- 13.Dray A, Bettaney J, Rueff A, Walpole CSJ, Wrigglesworth R. NE-19550 and NE-21610, antinociceptive capsaicin analogues: studies on nociceptive fibres of the neonatal tail in vitro. Eur J Pharmacol. 1990;181:289–293. doi: 10.1016/0014-2999(90)90091-j. [DOI] [PubMed] [Google Scholar]
- 14.Endrenyi L, Fajszi C, Kwong FH. Evaluation of Hill slopes and Hill coefficients when the saturation binding or velocity is not known. Eur J Biochem. 1975;51:317–328. doi: 10.1111/j.1432-1033.1975.tb03931.x. [DOI] [PubMed] [Google Scholar]
- 15.Goso C, Evangelista S, Tramontana M, Manzini S, Blumberg PM, Szallasi A. Topical capsaicin administration protects against trinitrobenzene sulfuric acid (TNB)-induced colitis in the rat: characterization of the vanilloid (capsaicin) receptor involved. Eur J Pharmacol. 1993;249:185–190. doi: 10.1016/0014-2999(93)90431-g. [DOI] [PubMed] [Google Scholar]
- 16.Harvey JS, Davis C, James IF, Burgess GM. Activation of protein kinase C by the capsaicin analogue resiniferatoxin in sensory neurones. J Neurochem. 1995;65:1309–1317. doi: 10.1046/j.1471-4159.1995.65031309.x. [DOI] [PubMed] [Google Scholar]
- 17.Hergenhahn M, Adolph W, Hecker E. Resiniferatoxin and other esters of novel polyfunctional diterpenes from Euphorbia resinifera and unispina. Tetrahedron Lett. 1975;19:1595–1598. [Google Scholar]
- 18.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–200. [PubMed] [Google Scholar]
- 19.Jancso N. Desensitization with capsaicin and related acylamides as a tool for studying the function of pain receptors. In: Linn RKS, editor. Pharmacology of pain, Vol 9. Pergamon; Oxford, UK: 1968. pp. 33–55. [Google Scholar]
- 20.Liu L, Simon SA. A rapid capsaicin-activated current in rat trigeminal ganglion neurons. Proc Natl Acad Sci USA. 1994;91:738–741. doi: 10.1073/pnas.91.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L, Simon SA. Capsaicin-induced currents with distinct desensitization and Ca2+ dependence in rat trigeminal ganglion cells. J Neurophysiol. 1996;75:1503–1514. doi: 10.1152/jn.1996.75.4.1503. [DOI] [PubMed] [Google Scholar]
- 22.Maggi CA, Patacchini R, Santicioli P, Giuliani S, Geppetti P, Meli A. Protective action of ruthenium red toward capsaicin desensitization of sensory fibers. Neurosci Lett. 1988;88:201–205. doi: 10.1016/0304-3940(88)90126-7. [DOI] [PubMed] [Google Scholar]
- 23.Maggi CA, Patacchini R, Tramontana M, Amann R, Giuliani S, Santicioli P. Similarities and differences in the action of resiniferatoxin and capsaicin on central and peripheral endings of primary sensory neurons. Neuroscience. 1990;37:531–539. doi: 10.1016/0306-4522(90)90421-y. [DOI] [PubMed] [Google Scholar]
- 24.Oh U, Hwang SW, Kim D. Capsaicin activates a nonselective cation channel in cultured neonatal rat dorsal root ganglion neurons. J Neurosci. 1996;16:1659–1667. doi: 10.1523/JNEUROSCI.16-05-01659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santicioli P, Patacchini R, Maggi CA, Meli A. Exposure to calcium-free medium protects sensory fibers by capsaicin desensitization. Neurosci Lett. 1987;80:167–172. doi: 10.1016/0304-3940(87)90648-3. [DOI] [PubMed] [Google Scholar]
- 26.Szallasi A, Blumberg PM. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience. 1989;30:515–520. doi: 10.1016/0306-4522(89)90269-8. [DOI] [PubMed] [Google Scholar]
- 27.Szallasi A, Blumberg PM. [3H]resiniferatoxin binding by the vanilloid receptor: species-related differences, effects of temperature and sulfhydryl reagents. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:84–91. doi: 10.1007/BF00168777. [DOI] [PubMed] [Google Scholar]
- 28.Szallasi A, Lewin NA, Blumberg PM. Identification of alpha-1-acid glycoprotein (orosomucoid) as a major vanilloid binding protein in serum. J Pharmacol Exp Ther. 1992;262:883–888. [PubMed] [Google Scholar]
- 29.Szallasi A, Goso C, Blumberg PM, Manzini S. Competitive inhibition by capsazepine of [3H]resiniferatoxin binding to central (spinal cord and dorsal root ganglia) and peripheral (urinary bladder and airways) vanilloid (capsaicin) receptors in the rat. J Pharmacol Exp Ther. 1993;267:728–733. [PubMed] [Google Scholar]
- 30.Szolcsanyi J, Szallasi A, Szallasi Z, Joo F, Blumberg PM. Resiniferatoxin: an ultrapotent selective modulator of capsaicin-sensitive primary afferent neurons. J Pharmacol Exp Ther. 1990;255:923–928. [PubMed] [Google Scholar]
- 31.Walpole CSJ, Bevan S, Bloomfield G, Breckenridge R, James IF, Ritchie T, Szallasi A, Winter J, Wrigglesworth R. Similarities and differences in the structure-activity relationships of capsaicin and resiniferatoxin analogues. J Med Chem. 1996;39:2939–2952. doi: 10.1021/jm960139d. [DOI] [PubMed] [Google Scholar]
- 32.Winter J, Dray A, Wood JN, Yeats JC, Bevan S. Cellular mechanism of action of resiniferatoxin: a potent sensory neuron excitotoxin. Brain Res. 1990;520:131–140. doi: 10.1016/0006-8993(90)91698-g. [DOI] [PubMed] [Google Scholar]
- 33.Wood JN, Winter J, James IF, Rang HP, Yeats J, Bevan S. Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. J Neurosci. 1988;8:3208–3220. doi: 10.1523/JNEUROSCI.08-09-03208.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]