Abstract
Bacteria have evolved sophisticated uptake machineries in order to obtain the nutrients required for growth. Gram-negative plant pathogens of the genus Pectobacterium obtain iron from the protein ferredoxin, which is produced by their plant hosts. This iron-piracy is mediated by the ferredoxin uptake system (Fus), a gene cluster encoding proteins that transport ferredoxin into the bacterial cell and process it proteolytically. In this work we show that gene clusters related to the Fus are widespread in bacterial species. Through structural and biochemical characterisation of the distantly related Fus homologues YddB and PqqL from Escherichia coli, we show that these proteins are analogous to components of the Fus from Pectobacterium. The membrane protein YddB shares common structural features with the outer membrane ferredoxin transporter FusA, including a large extracellular substrate binding site. PqqL is an active protease with an analogous periplasmic localisation and iron-dependent expression to the ferredoxin processing protease FusC. Structural analysis demonstrates that PqqL and FusC share specific features that distinguish them from other members of the M16 protease family. Taken together, these data provide evidence that protease associated import systems analogous to the Fus are widespread in Gram-negative bacteria.
Author summary
To grow and cause infection bacteria must obtain essential nutrients from their environment or host. The element iron is one such nutrient and is often contained inside proteins, the building blocks of hosts cells. Bacteria that cause disease in plants are able to extract iron from plant proteins, by importing the protein and cutting it up once inside the bacterial cell. While it was known that specific bacteria that infect plants can do this, it was unclear if other bacteria that infect humans and animals are also able to import host proteins. In this work we analysed the genetic sequences of bacteria and found that genes responsible for importing and processing proteins are widespread in bacteria that cause disease in humans, animals and plants. We analysed the structure and chemistry of the protein products of these genes and found that they possess characteristics that are necessary and sufficient for importing and processing proteins. Our conclusion from this work is that the ability to import host proteins to gain nutrients is common in bacteria.
Introduction
Bacteria often experience a scarcity of the resources they require to grow, divide and persist [1]. In many environments this is due to competition with other microorganisms, while during infection of plants or animals, the host employs strategies to deny bacteria essential nutrients to prevent their growth [2]. Iron-limitation is a key host defence strategy and, in order to overcome this, infectious bacteria have evolved sophisticated iron uptake machinery [3].
It was recently shown that the Gram-negative phytopathogens Pectobacterium carotovorum and Pectobacterium atrosepticum are able to obtain the essential nutrient iron from the plant-protein ferredoxin [4, 5]. In Pectobacterium, iron acquisition via plant ferredoxin is mediated by the Ferredoxin uptake system (Fus), a molecular machine consisting of inner and outer membrane transporters and a periplasmically localised protease [6–9]. Intriguingly, the outer membrane transporter from this system, a TonB-dependent transporter (TBDT) designated FusA, imports intact ferredoxin into the periplasm of the bacteria where it is processed by the M16 family protease FusC [6–8]. This is the first example of a bacterium importing an intact protein for nutrient acquisition, with previously described extraction of protein cofactors taking place on the bacterial cell surface [10, 11]. It is also remarkable considering the transported ferredoxin has dimensions barely smaller than the internal pore of FusA [7]. Proteolytic cleavage of ferredoxin by FusC in the periplasm, results in the release of its iron-sulphur cluster [8], which it is hypothesized is transported into the bacterial cytoplasm by the inner membrane transporter FusD [6].
The observation that bacteria import and process ferredoxin for nutrient acquisition is unprecedented [10, 11]. It was unclear, however, whether this ability is specific to Pectobacterium or a more common strategy implemented by Gram-negative bacteria. To address this question, we interrogated available bacterial genomes for sequences related to the outer membrane transporter FusA. This search showed that gene clusters resembling the Fus are widespread across Proteobacteria and are present in bacteria that adopt a variety of different lifestyles, including many bacteria that form an association with plant or animal hosts. The composition of these gene clusters supports a broad role in protein import, with FusA genes commonly associated with putative M16 processing proteases. To confirm the common architecture of these systems, we characterised the gene cluster analogous to the Fus from Escherichia coli: the ydd/pqqL operon. This showed that despite their distant relationship to the Fus from Pectobacterium, Ydd/PqqL shares a common structure, localisation and regulation. In combination with previous studies, these data provide evidence that protein import systems related to the Fus represent a novel family that are widespread in Proteobacteria, where they may function in obtaining iron from host proteins.
Results
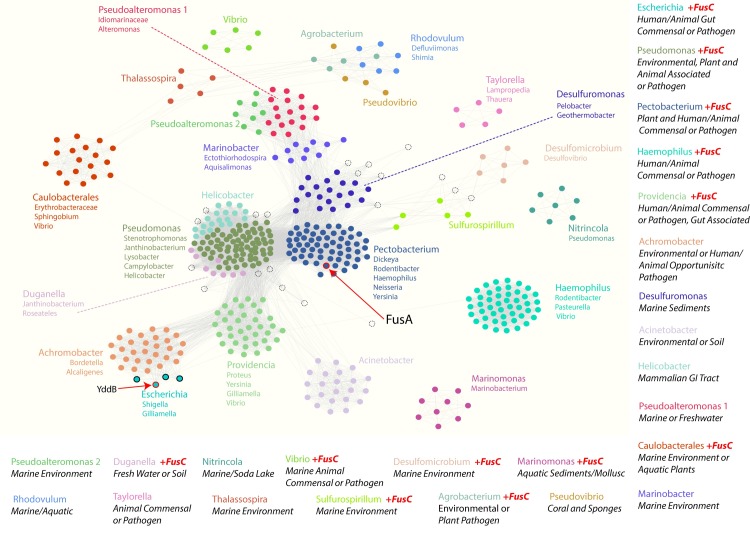
Gene clusters related to the Fus are widespread in Proteobacteria
The sequence of FusA was used to interrogate the UniProtKB database using the HMMER search algorithm [12]. Through this approach, we were able to select the sequences of various FusA-related proteins while excluding other members of the wider TBDT protein superfamily (S1 Fig & S1 Table). Proteins analogous to FusA were identified in a wide variety of species throughout the Proteobacteria. These sequences were clustered via pairwise similarity scores using the program CLANS, forming 24 sequence clusters (Fig 1) [13]. While present in a wide variety of bacteria, FusA homologues were not universally distributed across bacterial clades. For example, Escherichia coli possesses YddB, a distant homologue of FusA (24% amino acid identity), whereas FusA homologues were not detected in closely related genera like Salmonella and Citrobacter. Another striking observation was the diversity of FusA homologues, with the amino acid identity of sequences within CLANS clusters ranging from ~ 30 to 100%. As proteins within each cluster are more closely related to each other than other FusA homologues, the overall diversity of the FusA protein family is very high (S1 Table).
Fig 1. Clustering analysis of FusA homologues.
CLANS similarity network analysis identifies clusters of sequences in the FusA homologues identified by HMMER search of the UniProt reference proteomes dataset. Clusters are named based on the genus of a major species of origin for the cluster, with members of other prevalent genera listed. The presence of FusA sequences associated with the homologues of the protease FusC inside a cluster are noted, as is the most common lifestyle for bacteria in the cluster. Dots represent individual sequences and grey lines represent pairwise similarity relationships. An E-value cut-off of 1e-110 was used for clustering. A full list of sequences and metadata where available is provided in S1 Table.
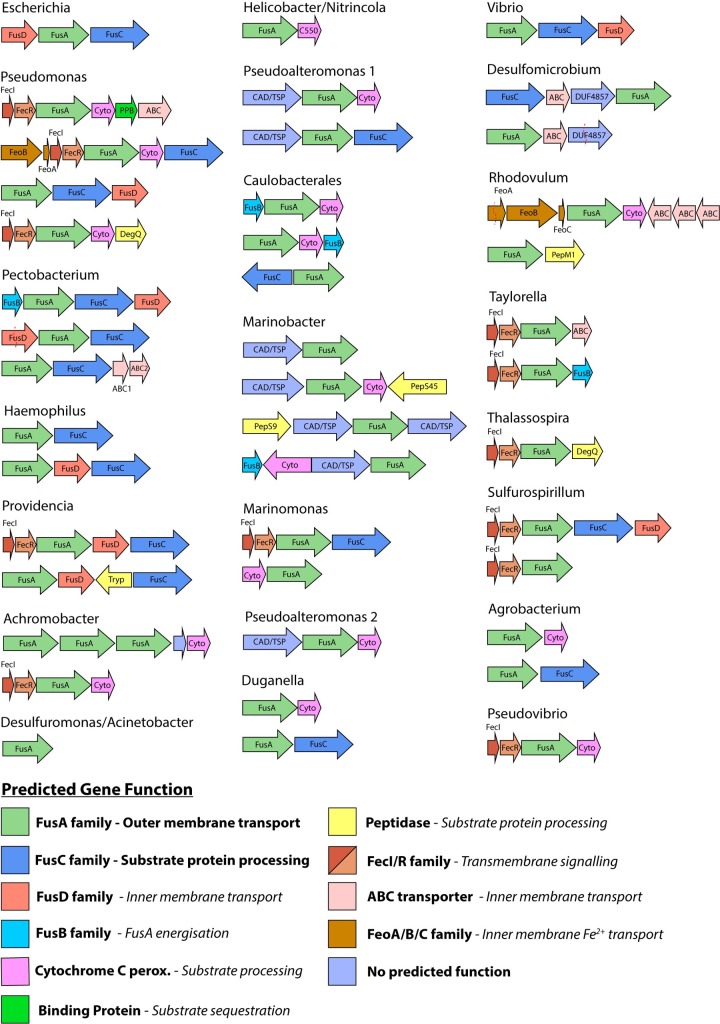
Due to its confirmed role in protein processing upon import [6, 8], the presence of FusC genetically associated with the FusA homologues was examined (Figs 1 and 2). FusC homologues associated with FusA were identified in 12 of the 24 sequence clusters, including the four largest clusters, which we defined by members of the genera predominantly found within the cluster: Pectobacterium, Pseudomonas, Haemophilus and Providencia (Fig 1, S1 Table). An association between FusA and FusC homologues is not restricted to closely related clusters, nor is the presence of a FusC homologue universal to all members of a cluster (Figs 1 and 2 and S1 Table); the stochastic distribution of FusC homologues suggests a related function, rather than evolutionary conservation alone, drives their genetic association with FusA homologues.
Fig 2. Representative genomic context of FusA homologues.
The genetic organisation of FusA homologues identified in HMMER search is shown. Labelled arrows represent genes that were present adjacent to FusA in the sequence clusters outlined in Fig 1. Genes homologous to Fus operon members are labelled accordingly, while other genes are labelled according to the function of their closest characterised homologue. A predicted function for genes is provided below, predictions in bold are based on the function of proteins from the Fus, while predictions in italics are based on function of homologous proteins. A full list of genetic context for FusA homologues, where available, is listed in S1 Table.
The composition of Fus gene clusters inside and between sequence groups was diverse, consisting of different arrangements of FusA and FusC homologues and a variety of other genes with a possible function in iron acquisition and protein import (Fig 2). In some cases, genes encoding other proteases were associated with FusA homologues, suggesting different processing factors may be employed by some systems. In multiple cases where FusC homologues were absent, genes encoding members of the cytochrome c peroxidase family were identified. These dual-haem containing enzymes are redox active [14, 15] and thus may be responsible for reducing an imported substrate protein, inducing it to release an iron containing cofactor without proteolytic processing.
Genome metadata was mined to determine the environment from which the bacteria had been isolated, showing they adopt a variety of lifestyles, which tend to correlate with sequence cluster (S1 Table). For example, members of the clusters defined by Pectobacterium and Hemophillus sequences adopt a commensal or pathogenic relationships with plant or animal hosts, while members of the Marinomonas, Marinobacter and Pseudoalteromonas clusters were isolated from marine or other environmental samples (S1 Table). This suggests that different lineages of these Fus homologues provide an advantage to bacteria adopting specific lifestyles.
Taken together, these data show that homologues of FusA are widespread in Proteobacteria, but their distribution is sporadic, consistent with them providing niche- or lifestyle-specific functions. The presence of associated FusC homologues, or other proteases, in a large number of widely distributed sequence clusters suggests a general role for these Fus gene clusters in protein import and processing.
YddB is structurally analogous to FusA and possesses a large external substrate binding site
In order to determine if FusA homologues possess the structural features required for protein import, we purified and characterised YddB, the FusA homologue from E. coli (Fig 3A). YddB belongs to the outer membrane localised TBDT family and has been detected in the outer membrane and in outer membrane vesicles of E. coli in a number of studies [16–20]. YddB is encoded in an operon with the FusC homologue PqqL, which has been shown to be expressed in response to iron limitation, to be regulated by the ferric uptake regulator (Fur), and to be important in systemic infection of uropathogenic E. coli in a mouse model of infection [21–23]. YddB is distantly related to FusA in the CLANS plot, belonging to a small cluster which is in turn closely associated with a larger cluster containing sequences from pathogenic Bordetella and Achromobacter species (Fig 1, S1 Table). Interestingly, members of this Achromobacter cluster do not contain a FusC homologue but are commonly associated with a cytochrome c peroxidase protein.
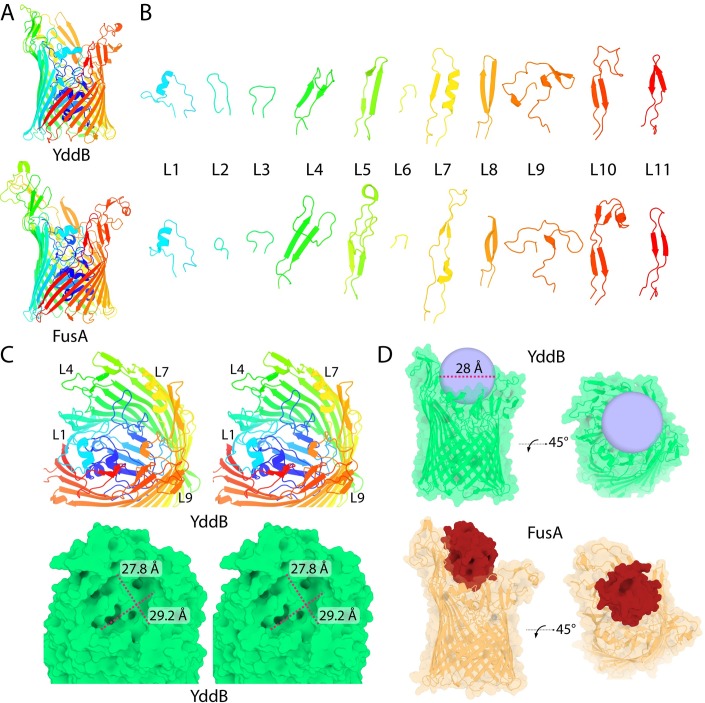
Fig 3. The crystal structure of YddB reveals structural homology to FusA, implying conserved function in protein import.
(A) A cartoon representation of YddB (top) and FusA (bottom) showing structural homology between the two proteins despite limited (24%) sequence identity. (B) Dissection of the eleven extracellular loops of YddB (top) and FusA (bottom), showing that they conform to similar length and structural patterns. (C) A stereo view of the extracellular binding pocket of YddB (top = cartoon, bottom = green surface) showing that it consists of a large cavity capable of binding a small globular protein, in common with FusA. (D) Surface/cartoon representation of YddB (top), showing its extracellular binding pocket can accommodate a globular molecule ~28 Å in diameter. The structure of FusA docked with its ferredoxin substrate (bottom) is shown for reference.
The structure of YddB was solved to a resolution of 2.4 Å by X-ray crystallography by molecular replacement (S2 Table; Fig 3A). Based on identification of structural homologues using the Dali server [24], YddB is most similar to FusA with a RMSD of 2.2 Å and a Z-score of 42.2 (S3 Table). As with FusA, YddB possesses a 22-stranded β-barrel fold, the pore of which is occluded by a globular N-terminal plug-domain. This fold is characteristic of the integral outer membrane TBDT family and like these proteins YddB possesses a hydrophobic transmembrane region (S2 Fig). Comparison of the structure of YddB and FusA by dissection of their extracellular loops confirmed that the two proteins are structurally analogous (Fig 3B); the eleven loops of these proteins share a common structure that is distinct from those of two other TBDTs, FhuE and Fiu (S3 Fig). Substrate capture by TBDTs is mediated with an external binding site composed of these extracellular loops [25]. The capture of the large ferredoxin substrate by FusA was shown to be mediated by a large open extracellular binding site [7]. Analysis of the YddB structure revealed that it possesses an analogous binding site, with dimensions (27.8 × 29.2 Å) capable of accommodating a bulky protein substrate of similar dimensions to ferredoxin (Fig 3C and 3D).
PqqL is a periplasmically localised metalloprotease expressed under iron limiting conditions
We hypothesized that the uncharacterised protein PqqL, encoded in an operon with the yddB gene, is a protease that processes the substrate for this system upon import into the bacterial periplasm. We tested two hypotheses: (i) given the substrate of the ydd/pqqL operon is likely to be an iron containing protein, PqqL production will be increased in response to iron limitation; (ii) in order for PqqL to function as a processing protease, it will be periplasmically localised. The first of these hypotheses is supported by the fact that the ydd/pqqL operon has been shown to be regulated by Fur and is upregulated under iron limiting conditions [22, 23].
To explore the regulation of PqqL expression, we raised an antibody to recognize PqqL and performed cell fractionation and immunoblotting on the model E. coli strain BW25113 and the uropathogenic strain E. coli CFT073. As predicted, low levels of PqqL were detected in cells grown under iron replete conditions in LB medium (Fig 4A). However, the addition of the iron chelator 2’2-bipyridine (BP) to the medium led to increased expression of PqqL in both BW25113 and CFT073 (Fig 4A). No band corresponding to PqqL was detected in a ΔpqqL mutant E. coli BW25113, under any condition, confirming the specificity of PqqL detection (S4 Fig). Expression of PqqL was then tested during growth in human urine, with both BW25113 and CFT073 cells exhibiting an elevated expression relative to growth in LB media (Fig 4A). Expression of PqqL in CFT073 cells grown in urine was generally higher than BW25113, however this was variable between experiments (Fig 4A), possibly attributable to variation in sample composition. As urine is also iron limiting [26], the increase in PqqL expression is consistent to the Fur regulation of the ydd/pqqL operon and the observation that in CFT073, the operon is transcriptionally upregulated during growth in urine and is important for virulence [21].
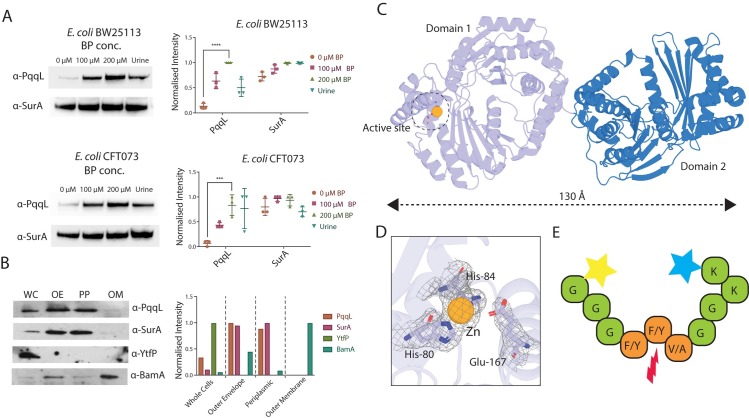
Fig 4. PqqL is an iron regulated, periplasmically localized, metallopeptidase, with an elongated conformation.
A) An anti-PqqL western blot of E. coli BW25113 or CFT073 whole cells grown in the presence or absence of the iron chelator 2’2-bipyridine (BP) or in human urine. Detection of SurA is shown as a loading control. Left shows a representative blot, right shows normalised intensity of blots from 3 biological replicates. This shows more PqqL is produced under iron-limiting conditions. Indicated significant differences between conditions are based on student’s t-test (* p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001; **** p ≤ 0.0001). B) A western blot of cell fractions from E. coli BW25113 grown under iron-limiting conditions (100 μM BP), showing the distribution of PqqL in whole cell (WC), outer envelope (OE) and periplasmic (PP) fractions, but not associated with the outer membrane (OM). Controls using antisera recognizing SurA (periplasmic), BamA (outer membrane) or YtfP (cytoplasmic) are shown. Left shows a representative blot, right shows quantified intensity of this blot C) The crystal structure of PqqL showing that its two ‘clam shell’ domains adopt a highly elongated conformation in the crystal structure, engaging in minimal intra-molecular contacts. D) The crystal structure of PqqL illustrates the presence of a putative Zn ion in the protease active site of the enzyme. E) Peptidase screening assays shows that PqqL is an active peptidase with specificity for peptides containing a F/Y, F/Y, V/A motif (Full data shown in S4 Table).
To determine the cellular location of PqqL, we fractionated E. coli BW25113 cells grown under iron limiting conditions (100 μM BP). Immunoblot analysis of these fractions demonstrated that PqqL is localised to the periplasmic fraction, showing the same fractionation profile as the known periplasmic chaperone SurA (Fig 4B) [27]. PqqL was not detected associated with the outer membrane, fractionating distinctly from the known outer membrane protein BamA [28]. The cytoplasmic protein YtfP was absent from the periplasmic or outer membrane fractions, demonstrating the lack of cytoplasmic contaminants in these fractions (Fig 4B) [29]. Consistent with a periplasmic localisation, immunoprecipition of PqqL from the cell lysate, followed by N-terminal sequencing revealed cleavage of the predicted signal peptide (S5 Fig).
PqqL was expressed recombinantly, purified and crystallised in order to solve its structure (S2 Table). PqqL consists of two clam-shell like halves, each formed from two M16 protease subunits (Fig 4C). This domain structure is analogous to its distant homologue FusC; however, FusC was crystallised in complex with its ferredoxin substrate and adopted a closed clam-shell conformation [6]. In contrast, in the absence of substrate, PqqL adopts an entirely open, highly extended conformation (Fig 4C). This domain arrangement has not previously been observed in structures of M16 proteases [30–32]. In addition to electron density for the PqqL polypeptide chain, density was observed in the predicted peptidase active site of the N-terminal subunit of PqqL, which was attributable to a catalytic metal ion coordinated by histidines 80 and 84 (Fig 4D). In common with other proteases of this family, these residues form part of the catalytic inverzincin H-x-x-E-H motif in which the two histidine residues coordinate a zinc ion, with the glutamate additionally required for catalysis [33]; this suggests the protein binds a putative Zn ion and serves as a metalloprotease.
To test for proteolytic activity and specificity, PqqL was subjected to peptide hydrolysis screening. In this assay, from a pool of 512 tripeptide combinations, PqqL cleaved peptides containing a tripeptide motif composed of F/Y, F/Y and V/A at positions 1, 2 and 3 respectively (Fig 4E, S4 Table). In control experiments, FusC was also shown to be active towards a distinct subset of peptides (S4 Table). PqqL was also tested for proteolytic activity towards a number of potential substrate proteins, including human ferredoxins and globins, as well as plant ferredoxin which is the substrate for FusC (S6 Fig). PqqL did not exhibit activity towards any of these proteins, suggesting they are not the substrates for the YddB/PqqL operon. This narrow specificity of PqqL towards peptide and protein substrates is consistent with that observed for FusC, which was shown to cleave plant ferredoxin but not a number of other small proteins [8]. These data demonstrate that PqqL is an active, periplasmically localised protease with a narrow substrate specificity, supporting the hypothesis that this protein cleaves a discrete imported protein substrate and is functionally analogous to FusC.
PqqL and YddB do not support growth during iron limitation in LB media
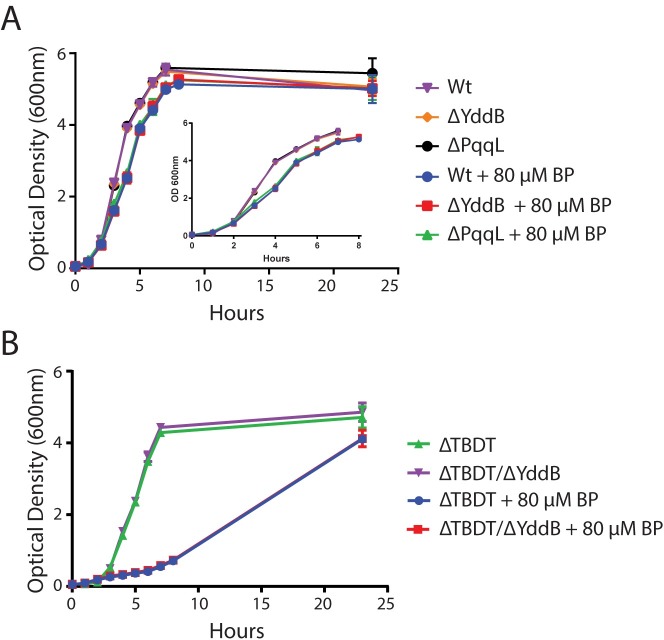
The transcriptional upregulation of the ydd/pqql operon and increased expression of PqqL under iron limitation suggest that this operon may play a general role in iron acquisition [22]. To test this we constructed strains containing a genetic deletion of yddB and pqqL in E. coli BW25113, and a yddB deletion in a previously constructed strain lacking all TBDTs with a role in iron acquisition [25]. We tested these mutants for growth defects in both iron-replete and iron-limited LB media. All strains grew at the same rate as their corresponding wild-type background and reached a comparable final cell density, demonstrating that neither YddB or nor PqqL plays a significant role in iron acquisition under these conditions (Fig 5). This result suggests that the ydd/pqql operon plays a niche specific role in iron acquisition from a discrete protein substrate.
Fig 5. Growth of ΔyddB and ΔpqqL mutant strains is identical to wildtype in iron limited LB media.
(A) E. coli BW25113 wildtype, ΔyddB and ΔpqqL strains were grown in LB media +/- 80 μM 2,2’bipyridine. Growth of ΔyddB and ΔpqqL strains was identical to wildtype under both conditions; all strains exhibited slower growth with 80 μM 2,2’bipyridine due to iron limitation of the media. (B) E. coli BW25113 ΔTBDT and ΔTBDT/ΔyddB were grown as strains in panel A. Growth of ΔTBDT/ΔyddB was identical to the ΔTBDT strain under both conditions; both strains grew slower than wildtype due to poor iron uptake ability, with very slow growth observed with 80 μM 2,2’bipyridine.
Both PqqL and FusC undergo large scale conformational changes in solution
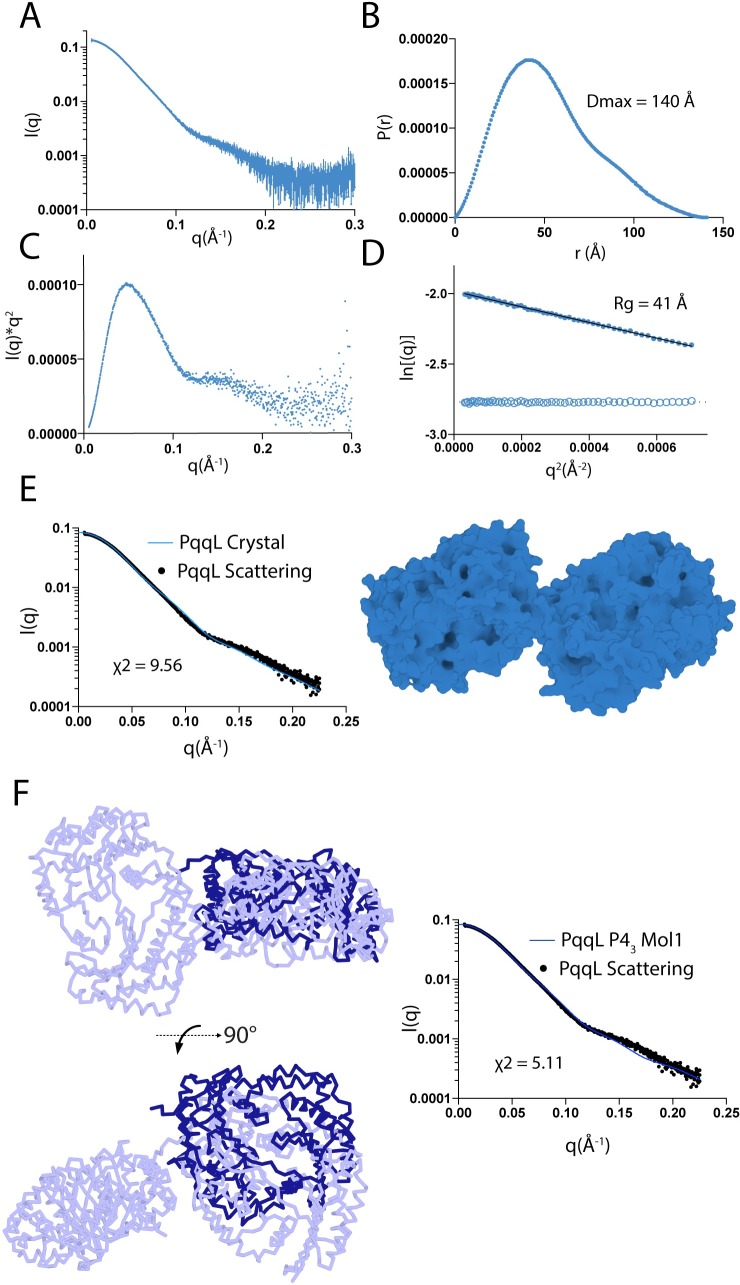
The elongated open conformation of PqqL resolved by X-ray crystallography is intriguing. In all structures previously solved, M16 family proteases adopt a closed or partially closed conformation, including the structure of FusC [6]. In order to determine if the crystal structure of PqqL is consistent with its conformation in solution we utilised small angle X-ray scattering (SAXS) (Fig 6A, S5 Table). In solution, PqqL was found to have maximum dimensions of 140 Å (Fig 6B and 6D), in good agreement with the 130 Å observed in the crystal structure (Fig 4C). In addition, as was previously demonstrated for FusC [6], PqqL was found to exhibit flexibility in solution (Fig 6C), with the simulated scattering of the crystal structure of PqqL representing a poor fit for the SAXS data (Fig 6E). Consistent with this flexibility, during crystallisation of PqqL, an additional poorly diffracting crystal form was obtained. In these crystals, a difference in orientation of the two domains of PqqL is observed, compared to the orientation of the refined crystal structure (Fig 6F, S7 Fig).
Fig 6. PqqL is flexible and adopts an elongated conformation in solution.
Small angle X-ray scattering of PqqL (A) and derived P(r) (B), Kratky (C) and Guinier (D) plots showing that PqqL adopts a highly elongated conformation in solution with a Dmax of 140 Å and has interdomain flexibility. (E) the simulated solution scattering of the PqqL crystal structure is a relatively poor fit for solution scattering data, suggesting that PqqL adopts multiple conformations in solution. (F) PqqL adopts different conformations in crystallo. The light blue ribbon represents PqqL in the refined crystal structure while the dark blue ribbon represents the position of PqqL domain 2 in the low-resolution crystal form shown in S7 Fig, illustrating the difference in domain orientation between to two crystal forms. The fit of this alternative conformation is shown (right).
These data show that, while the crystal structure of PqqL is representative of its maximum dimensions in solution, the protein also possesses flexibility between its two domains. In previous work, solution scattering analysis of FusC revealed that while it adopts a closed conformation in the presence of its substrate in the crystal structure, it adopts an elongated conformation in solution with dimensions of 130 Å [6], very similar to those of PqqL observed in this work.
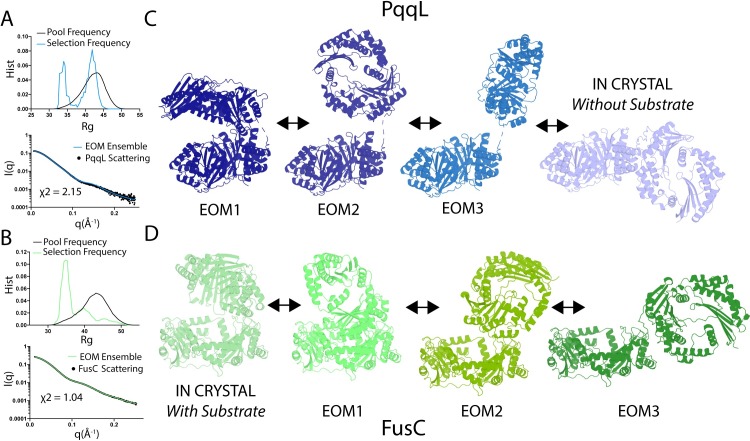
To obtain a clearer picture of the range of conformations PqqL can adopt, we utilised the ensemble optimization method (EOM). Applying this technique, the flexible linker between the two domains of PqqL was specified and molecular dynamics simulations were used to generate an ensemble of 10,000 models, which sampled the physically possible orientations of the two domains. A genetic algorithm was then applied to select a subset of these models that best fit the solution scattering data [34]. EOM selected an ensemble of models that produced a robust fit for the solution scattering data for PqqL (Fig 7A and 7B), providing a far better representation of observed scattering than the single model from the crystal structure (Fig 6E and 6F) [6]. The ensemble of models best representing the scattering data corresponded not only to the open conformation of the crystal structure, but also to partially closed intermediate conformations and a closed conformation analogous to that of the crystal structure of FusC in complex with ferredoxin (Fig 7A and 7C).
Fig 7. PqqL and FusC sample both open and closed conformations in solution.
The radius of gyration (Rg) of the pool of models generated from EOM analysis and those selected the ensemble representative of solution scattering data for PqqL (A top) and FusC (B top). The fit of the ensemble of models selected by EOM for scattering data for experimental scattering for PqqL (A bottom) and FusC (B bottom). A selection of representative models from EOM analysis and the crystal structure of PqqL (C) and Fus (D), showing that both proteins are predicted to sample from open to closed conformations in solution.
We therefore analysed the scattering data for a sample of FusC, and the inverse situation was observed. Along with conformations corresponding to the closed form of FusC evident from the crystal structure [6], a range of intermediate conformations were selected, along with a fully open conformation analogous to that observed in the PqqL crystal structure, and consistent with solution scattering data for FusC [6] (Fig 7B and 7D). These data provide mechanistic insight into how ferredoxin is able to enter the substrate binding cavity of FusC, as folded ferredoxin would be unable to enter the partially closed conformation observed in the crystal structure [6]. The observation that PqqL adopts an analogous range of conformational states to FusC, which are otherwise unprecedented for proteases of this family, further supports a shared, conserved mechanism of function between PqqL and FusC.
Discussion
The discovery of the Fus mediated ability of Pectobacterium species to import plant ferredoxin is a striking example of the creative strategies bacteria engage to satisfy their nutritional requirements [4, 6–8]. In this work we show that gene clusters analogous to the Fus are widely distributed in proteobacterial species. In addition, our analysis of the composition of these gene clusters reveals that they share common features, including the presence of homologues of the ferredoxin processing protease FusC and other genes predicted to be involved in nutrient transport. Furthermore, through structural and biochemical characterisation of the distantly related Fus homologues, YddB and PqqL from E. coli, we show that these proteins share key features with FusA and FusC, suggesting a conserved function. Taken together, these data provide a body of evidence that, rather than being limited to Pectobacterium, protein import systems are widespread in Gram-negative bacteria.
These findings add to a growing body of evidence that TDBTs are highly versatile in terms of both the size and chemical composition of the substrates they import. Initial characterisation of these transporters suggested their substrates were limited to relatively small molecules; predominantly iron binding siderophores [35]. However, their frequent targeting as receptors by antibacterial proteins hinted at the capacity for protein import [9, 36, 37]. Recent work on TDBTs distantly related to those involved in siderophore import has demonstrated their ability to transport unstructured polypeptides and polysaccharides, and even to act as exporters in a protein secretion system [38–40]. Our current work identifies a novel family of putative protein importing TBDTs for which FusA and YddB are archetypical members [7], adding further evidence to the surprising versatility the TBDT family.
In this work we show that PqqL is a protease that shares a common localisation and iron-dependent regulation with FusC [6, 8]. In addition, our structural characterisation provides intriguing clues as to the mechanism of this protease family. Both the crystal structure of FusC and the closed conformation of PqqL identified by our EOM analysis contain an internal cavity capable of accommodating a small globular protein like ferredoxin. However, in this closed conformation, the width of the opening to this cavity would prevent protein entry. By sampling between open and closed conformations in solution, these proteases could capture incoming protein substrates in an open conformation, before adopting a closed conformation for proteolysis. In addition, these conformational changes may account for the apparent ability of FusC to unfold its ferredoxin substrate, and to cleave it at multiple locations consisting of divergent amino acid sequences [6, 8]. While further characterisation is required, the striking similarities between PqqL and FusC demonstrated in this work provide the basis for the definition of a new sub-family of M16 protease.
The distribution of Fus related gene clusters in bacteria adopting such a wide variety of lifestyles, including in both marine and terrestrial environments, raises the question of what protein substrates are targeted by these systems. Given the highly divergent nature of the family, it seems unlikely that ferredoxins are the universal substrate. However, small iron-containing proteins constitute diverse and abundant protein families produced by virtually all organisms [41, 42], so the evolution of Fus-like systems targeting such proteins available in a given niche seems intuitive. For a generalist gut bacterium like E. coli, identification of the substrate for YddB/PqqL may prove difficult, as it could target a substrate produced by its host or a member of the complex gastrointestinal community. However, a number of species shown to possess Fus gene clusters in this work are obligate human pathogens or commensals. In these cases, it is likely that the substrate for these systems will be a host protein. By defining the structure and distribution of the Fus gene cluster family, this study paves the way for future work identifying the substrates of these systems and determining the role they play in bacterial nutrient acquisition and virulence.
Materials and methods
Ethics statement
All urine samples used in this study were collected with the full consent of the providing party and within the ethical guidelines of the institution where they were collected.
Reagents and antisera generation
Human hemoglobin, cytoglobin and myoglobin and horse cytochrome C were obtained from a commercial source (Sigma-Aldrich). Polyclonal rabbit antisera for detection of PqqL, SurA, BamA and YtfP were generated at the Monash Animal Research Platform, from proteins purified in-house. Rabbits were serially injected with purified proteins in combination with complete (first injection) or incomplete (subsequent injections) Freund's adjuvant, over a period of 1–3 months, with rabbit serum periodically tested for reactivity to the target protein. Once acceptable levels of reactivity were achieved rabbits were sacrificed and serum was collected and stored in aliquots at -80°C.
FusA homologue identification and analysis
In order to identify FusA homologues in available bacterial genomes, a HMMER search was performed against the UniProtKB Database using FusA as the search sequence [12, 43]. No E-value cut-off was applied to hits. This search yielded 1063 sequences, which were allocated to groups with >95% sequence identity using CD-HIT [44]. One representative sequence from each group was utilised for further analysis, giving a total of 508 FusA homologue sequences. These sequences were classified by an all-against-all BLAST clustering algorithm, based on pairwise similarities. The resulting data set was visualized with CLANS with an E-value cut off of 1×10−110. Sequence clusters were identified in CLANS using a network-based algorithm, with a minimum group size of 4 [13]. The sequences from each group were ordered via a sequence identity matrix. Species of origin, host of isolation and FusA genetic context were determined from genome metadata where available from the Ensemble and Uniprot databases [45, 46].
Protein expression and purification
The open reading frames encoding YddB and PqqL were amplified by PCR from E. coli BW25113 using primers containing 5’ NcoI and 3’ XhoI restriction sites (S6 Table). They were cloned into a modified pET20b vector with a PelB signal sequence, followed by an N-terminal 10 × his tag and TEV cleavage site, via restriction digestion and ligation. The resulting vector was transformed into E. coli BL21 (DE3) C41 cells (S6 Table) [47]. Protein expression was performed in terrific broth (12 g tryptone, 24 g yeast extract, 61.3 g K2HPO4, 11.55 g KH2PO4, 10 g glycerol) with 100 mg.ml-1 ampicillin for selection. Cells were grown at 37°C until OD600 of 1.0, induced with 0.3 mM IPTG, and grown for a further 14 hours at 25°C. Cells were harvested by centrifugation and lysed using a cell disruptor (Emulseflex) in Ni-binding buffer (50 mM Tris, 500 mM NaCl, 20 mM imidazole [pH 7.9]) for PqqL or Lysis Buffer (50 mM Tris, 200 mM NaCl [pH 7.9]) for YddB, in the presence of 0.1 mg.ml-1 Lysozyme, 0.05 mg.ml-1 DNAse1 and complete protease cocktail inhibitor tablets (Roche).
For PqqL, the resulting lysate was clarified by centrifugation at 30,000 g for 20 minutes and applied to Ni-agarose resin, followed by washing with 10 × column volumes of Ni-binding buffer, and elution of bound proteins with a step gradient of Ni-gradient buffer (50 mM Tris, 500 mM NaCl, 500 mM Imidazole [pH7.9]) of 5, 10, 25 and 50%. Eluted fractions containing recombinant protein were pooled based on their absorbance at 280 nm and incubated with 2 mg.ml-1 TEV protease overnight at 4°C to cleave this his-tag. Protein was then applied to a 26/600 S200 Superdex size exclusion column equilibrated in SEC buffer (50 mM Tris, 200 mM NaCl [pH 7.9]). Eluted fractions containing PqqL, assessed by absorbance at 280 nm, were then pooled, concentrated to 10 mg/ml-1, snap frozen and stored at -80°C. Protein size and purity waswere confirmed by SDS-PAGE.
For YddB, the resulting lysate was clarified by centrifugation at 10,000 g for 10 min and the supernatant was then centrifuged for 1 h at 160,000 g to isolate a membrane fraction. The supernatant was decanted, and the membrane pellet was suspended in lysis buffer using a tight-fitting homogeniser. The resuspended membranes were solubilised by the addition of 10% Elugent (Santa Cruz Biotechnology) and incubated with gentle stirring at room temperature for 20 min. The solubilised membrane protein fraction was clarified by centrifugation at 20,000 g for 10 min. The supernatant containing the solubilized proteins was applied to Ni-agarose resin equilibrated in Ni-binding buffer DDM (50 mM Tris, 500 mM NaCl, 20 mM Imidazole, 0.03% Dodecylmaltoside (DDM) [pH7.9]). The resin was washed with 10–20 column volumes of Ni binding buffer DDM before elution of the protein with a step gradient of, 10, 25 and 50, 100% Ni gradient buffer DDM (50 mM Tris, 500 mM NaCl, 1 M Imidazole, 0.03% DDM [pH7.9]). YddB eluted at the 50 and 100% gradient steps, as assessed by absorbance at 280 nm. Eluted fractions containing YddB were pooled and applied to a 26/600 S200 Superdex size exclusion column equilibrated in SEC buffer DDM (50 mM Tris, 200 mM NaCl, 0.03% DDM [pH 7.9]). To exchange YddB into the detergent Octyl β-D-glucopyranoside (βOG) for crystallographic and biochemical analysis, SEC fractions containing YddB determined by absorbance at 280 nm were pooled and applied to Ni-agarose resin, equilibrated in βOG buffer (50 mM Tris, 200 mM NaCl, 0.8% βOG [pH 7.9]). The resin was washed with 10 column volumes of βOG buffer before elution with βOG buffer + 250 mM imidazole. Fractions containing YddB were pooled, and 6 × histidine tagged TEV protease (final concentration 2 mg.ml-1) and DTT (final concentration 1 mM) were added. This solution was then dialysed against of βOG buffer at 4–6 h at 20°C to allow TEV cleavage of the 10 × histidine tag from YddB and removal of excess imidazole. The sample was then applied to Ni-agarose resin, to remove TEV protease and the cleaved histidine-containing peptide. The flow through containing YddB from this step was collected, concentrated to 10 mg.ml-1 in a 30 kDa cut-off centrifugal concentrator, and snap frozen and stored at -80°C. Protein size and purify were assessed by SDS-PAGE.
The open reading frames (ORFs) for human ferredoxin 1 and 2 (minus the stop codon) were synthesised and cloned into pET21a and expressed in E. coli C41 (DE3). Cells were grown at 37°C and protein expression was induced by the addition of 0.3 mM isopropyl-β-D-thiogalactoside (IPTG) at an OD600 of ∼0.6. Cultures were grown for a further 6 h at 28 °C. Cells were collected and resuspended in 50 mM Tris-HCl, pH 7.9, 500 mM NaCl, 20 mM imidazole, 5% glycerol, 100 μg lysozyme, and complete EDTA-free protease inhibitor cocktail tablets were added. After disruption by sonication, the supernatant was clarified by centrifugation and applied to a HisTrap-nickel agarose column equilibrated in a buffer containing 50 mM Tris-HCl, pH 7.9, 500 mM NaCl, 20 mM imidazole and 5% glycerol. Bound protein was eluted with a linear gradient of 20–250 mM imidazole in lysis buffer. Ferredoxin containing fractions were identified based on colour and analysis by SDS–PAGE, pooled and further purified using a Superdex S75 26/60 column equilibrated in 50 mM Tris, 200 mM NaCl [pH 7.9]. FusC and plant ferredoxin for peptide cleavage assays and structural analysis were purified as previously described [6, 7].
YddB crystallisation, data collection and structure solution
Purified YddB in βOG was screened for crystallisation conditions using commercially available screens (approximately 600 conditions). Crystals grew in a number of conditions, with a condition containing 0.1 M Na cacodylate, 0.15 M Ca acetate, 15% PEG 8000 and 20% glycerol chosen for data collection. Crystals from this condition were looped and flash frozen in liquid N2. Diffraction data was collected at 100 K at the Australian synchrotron and processed in the space group P41212 to 2.4 Å. Initial phases were obtained by molecular replacement, using phaser from the Phenix package, with a search model derived from the crystal structure of FusA from Pectobacterium atrosepticum SCRI1043 [4ZGV]. The crystal structure of YddB was then build and refined using Coot, Phenix package and Buster [48–50].
PqqL crystallisation, data collection and structure solution
Purified PqqL was screened for crystallisation conditions using commercially available screens (approximately 800 conditions). Crystals grew in a number of conditions, with a condition containing 0.1 M Bis-tris propane, 0.2 M NaK tartrate, 20% PEG 3350 [pH 8.5] chosen for optimisation. Crystals initially grew with a diamond morphology and diffracted poorly to >3.2 Å in the space group P43. An additive screen was performed and it was determined that the addition of MgCl2 led to a change in morphology from diamond to rectangular and an improvement in diffraction (S4 Fig). These improved crystals were cryoprotected using paratone oil (Parabar 10312) and flash cooled in liquid N2. Diffraction data was collected at 100 K at the Australian synchrotron and processed in the space group P43212 to 2.6 Å. A partial molecular replacement solution was obtained using Phaser with an ensemble of models of the catalytic portion (approximately residues 1–230) of M16 protease structures identified by a BLAST search of the PDB; however, phases were too poor to allow further model building. Experimental phasing was attempted using selenomethionine labelled protein and numerous heavy atom-soaked crystals, but this proved unsuccessful. As an alternative, in situ proteolysis was undertaken by adding a 1:100 molar ratio of trypsin to PqqL prior to crystals screening. Crystals grew from this screen in 0.1 M phosphate-citrate buffer, 0.2 M NaCl, 20% PEG 8000 [pH 4.2]. Crystals were cryoprotected in the crystallisation solution plus 20% glycerol and flash cooled in liquid N2. Data was collected as for full length PqqL crystals and processed in the space group P21 to 2.0 Å. A partial molecular replacement solution was obtained as with the original PqqL crystals, with two copies of the first half (AA 27 to 494) of PqqL present in the crystallographic asymmetric unit (ASU). Using these data, a model of PqqL27-494 was built and refined using Coot, programs from the Phenix package and Buster [48–50]. PqqL27-494 was then used as a molecular replacement model for the full length PqqL dataset, and a model of full length PqqL was built and refined using the Phenix package, Buster and Coot [48–50].
Determination of the substrate specificity of PqqL using the Rapid Endopeptidase Profiling Library (REPLi)
The REPLi library is a combinatorial peptide library that contains 512 pools of peptides with each pool containing up to eight different variable tripeptides with the template layout of MeOC-GGXXXGG-dipicolinic acid-KK, where each X represents a variable alternative amino acid based on similar physiochemical properties, i.e. A/V, F/Y, I/L, D/E, R/K, D/E, S/T, Q/N, and P [51]. There are no Gly, His, Trp, Cys, or Met residues in the variable tripeptide region. The resulting combinations of variable tripeptides give rise to 3375 different peptides in the library in total. Methoxycarbonyl (MeOC) is the fluorophore, and dipicolinic acid is the fluorophore quencher. The soluble peptide library pools, synthesised by Mimotopes (Melbourne, Australia), contained in 512 wells in six 96-well plates were diluted using FAB to a final concentration of 50 μM. A final concentration of 1 μM PqqL was incubated with the substrate pools in FAB at 37°C. Cleavage of the substrates was monitored by measuring the increase in fluorescence intensity from the MeOC fluorophores using 55 second cycles for 30 cycles, with an excitation wavelength of 320 nm and an emission wavelength of 420 nm, using a BMG microplate reader. The initial velocity of the cleavage was indicated by the slope per unit time of the linear region of the curves.
Based on the REPLi results, 8 individual peptides from the substrate pools containing tripeptidyl sequences of Phe/Tyr- Phe/Tyr—Ala/Val, which displayed the highest rate of cleavage by PqqL, and Ala/Val—Ala/Val—Lys/Arg displayed the highest cleavage rate for FusC, were synthesized (Mimotopes, Melbourne, Australia). To determine the values of the steady-state reaction constants, 950 nM PqqL was mixed with substrate at a range of concentrations from 0 to 600 μM and the initial velocity of reaction was plotted against the substrate concentration, allowing the determination of the Km, Vmax, and kcat values. For FusC, 1 μM was mixed with substrate at a range of concentrations from 0 to 300 μM.
Putative substrate protein cleavage assays
Purified PqqL (1 μM) and potential substrate proteins (10 μM) were incubated in 50 mM Tris-HCl and 50 mM NaCl, pH 7.5, at room temperature. Samples were incubated for 120 min, and the reaction was stopped by the addition of SDS loading buffer. Samples were heated to 95°C for 2 min and then analyzed by SDS-PAGE.
PqqL small angle X-ray scattering and modelling
Size Exclusion Chromatography-Small Angle X-ray Scattering (SEC-SAXS) was performed using Coflow apparatus at the Australian Synchrotron [52, 53]. Purified PqqL was analysed at a pre-injection concentration of 100 μM. Chromatography for SEC-SAXS was performed at 22°C, with an 5/150 Superdex S200 Increase column, at a flow rate of 0.4 ml/min in: 50 mM Tris, 100 mM NaCl, 5% glycerol and 0.2% sodium azide [pH7.9]. The inclusion of glycerol and azide was essential to prevent capillary fouling due to photo-oxidation of buffer components. Scattering data were collected for 1 second exposures over a q range of 0.01 to 0.51 Å-1. A buffer blank for each SEC-SAXS run was prepared by averaging 10–20 frames pre or post protein elution. Scattering curves from peaks corresponding to PqqL were then buffer subtracted, scaled across the elution peak, and compared for inter-particle effects. Identical curves (5–10) from each elution were then averaged for analysis. Data were analysed using the ATSAS package, Scatter and SOMO solution modeler [54].
E. coli ΔpqqL and ΔyddB mutant generation and growth analysis
E. coli BW25113 ΔpqqL and ΔyddB mutant strains were created using the λ Red system [55]. Kanamycin-resistance cassettes flanked by 300 bp of genomic DNA either side of the chromosomal location of yddB and pqqL were amplified by PCR using specific mutants from the E. coli Keio collection [56] as templates, generating the yddB-Kan and pqqL-Kan KO cassettes. Oligonucleotide primer sequences are summarized in S6 Table. Wildtype E. coli BW25113 was transformed with the λ Red recombinase plasmid pKD46 [55] and grown at 30°C (LB broth + 100 μg.ml−1 ampicillin) to an OD600 nm of 0.1 before λ Red recombinase was induced by the addition of 0.2% L-arabinose. The cultures were then grown at 30°C until an OD600 nm of 0.6–0.8 was attained and were transformed with the yddB-Kan or pqqL-Kan KO cassettes using the room-temperature electroporation method [57]. Briefly, bacterial cells were isolated by centrifugation at 3000 g for 3 min and washed twice with a volume of sterile 10% glycerol equal to the volume of culture used. The cells were then resuspended in 10% glycerol to a volume of 1/15 of that of the culture. The yddB-Kan or pqqL-Kan KO cassette DNA (100–500 ng) was then added to 100 μl of the resuspended bacteria and the mixture was electroporated. 1 ml of LB broth was added to the cells post-electroporation, and the culture was recovered at 37°C for 1 h before plating onto LB agar + 30 μg ml−1 kanamycin. PCR was used to validate that colonies did indeed have the KanR cassette in place of the gene of interest.
To remove the KanR gene and generate “clean” yddB or pqqL deletions, the mutant strains were transformed with the plasmid pCP20 [58] containing the `flippase cassette'. Cells were grown under either ampicillin (100 μg ml−1) or chloramphenicol (30 μg ml−1) selection to maintain the plasmid. A single colony of the mutant pCP20-containing strain was used to inoculate 1 ml LB broth (no selection). The culture was grown overnight at 43°C to activate expression of the flippase gene. This culture was then subjected to tenfold serial dilution in sterile LB and plated onto LB agar with no selection. The resulting colonies were patched onto LB agar containing kanamycin, chloramphenicol or no selection. PCR was used to validate colonies that, while unable to grow in the presence of kanamycin or chloramphenicol, grew in the absence of selection and had no remnant of the KanR cassette in the deletion of yddB or pqqL. The E. coli ΔTBDT/ΔyddB strain was created as above, using the previously generated multiple TBDT mutant strain E. coli ΔTBDT, as a starting strain [25]. E. coli ΔTBDT is deficient in iron uptake and grows poorly on LB agar, and this was propagated on LB agar + 100 uM Fe(II)SO4.
Above mutant strains and wildtype E. coli BW25113 were grown in LB broth until stationary phase. These cultures were used to inoculate 20 ml of LB media +/- 80 μM 2,2-bipyridine (BP), to an OD600nm of 0.05. Cultures were grown with shaking and rate of growth was quantified by measuring OD600nm at hourly intervals.
Detection and localisation of PqqL in E. coli cell extracts via western blot
The E. coli model strain BW25113 and the uropathogenic strain CFT073 were grown in 10 ml of LB broth with shaking overnight (S6 Table) [56, 59]. These cultures were used to inoculate 10 ml of LB broth supplemented with either 0, 100, or 200 μM 2,2-bipyridine (BP) or human urine, donated provided by the study’s lead author of the study. Cultures were grown till late log phase and cells were harvested by centrifugation at 3000 g for 20 min at 4°C.
For detection of PqqL in whole cell extracts, E. coli were resuspended in 50 mM Tris, 200 mM NaCl pH 8.0, cell density was normalised based on OD600 measured at harvest. Cell numbers were determined by serial dilution and colony counting, and SDS-PAGE loading buffer was added to buffer containing 4.8x107 cells, samples were then heated at 95°C for 5 min.
For cell fractionation experiments, the Tris-Sucrose-EDTA method was performed [60]. All steps were carried out on ice unless otherwise stated. Cells utilised for these experiments were grown in LB + 100 μM BP. The supernatant was carefully discarded from the sedimented cells and the last few drops were removed by pipette. Cells, where gently resuspended in 1 ml (per 100 ml of bacterial culture) of TSE buffer (200 mM Tris, 500 mM sucrose, 1 mM EDTA [pH 8.0]) using a wire loop. The cells suspension was incubated on ice for 30 min, before sedimentation of cells at 16,000 g for 30 min at 4°C. The supernatant from this step represents the outer-envelope fraction, containing both periplasmic and outer membrane components. This fraction was further centrifuged at 100,000 g to sediment the outer membranes and to yield a more homogenous periplasmic fraction as the supernatant fraction. The outer membrane fraction from this step was resuspended in a minimal quantity of TSE buffer. The protein content of the whole cell, outer envelope, periplasmic and outer membrane fractions was estimated by BSA assay, and protein concentrations were normalised by dilution. Fractions were snap frozen in liquid N2 and stored at -80°C.
For PqqL and control protein detection, samples were separated on a 12% SDS-PAGE gel, which was subsequently blotted to 0.2 μm pore size nitrocellulose membrane. The membranes were blocked by incubation with TBST buffer (50 mM Tris, 150 mM NaCl, 0.1% Tween 20, [pH 7.5]) plus 5% skim milk powder (TBST-B) for 1 h at room temperature. Membranes were then incubated in TBST-B, containing a 1:5,000–1:20,000 dilution of rabbit derived anti-PqqL, anti-BamA, anti-YtfP or anti-SurA polyclonal serum for 1 h at room temperature. Membranes were then washed thoroughly with TBST, before incubation with a 1:20,000 dilution of a HRP-conjugated anti-rabbit secondary antibody for 1 h at room temperature. Membranes were with washed thoroughly with TBST, before protein bands were visualised by chemiluminescence and imaged via X-ray film or CCD-camera. Relative band intensity was quantified from scanned X-ray film or directly from CCD-camera images, by 1-D integration of band intensity using the ‘Plot Bands’ tool in ImageJ [61]. For CCD-images, integrated images were captured within the dynamic range of the detector and for film multiple exposure lengths were captured and those within linear intensity range, determined by plotting the intensity of multiple exposures, were utilised for quantitation. For comparison of blots from different experiments, band intensity was normalised by utilising the formula I/Imax, where I = the intensity of the band and Imax = the intensity of the most intense band from that blot. For blots judging expression levels of PqqL, raw PqqL band intensity was adjusted for loading variability using the formula Iraw/ISurA, where Iraw = the raw intensity of the PqqL band and ISurA = the normalised intensity of the SurA band from the corresponding lane.
PqqL immunoprecipitation
PqqL was isolated from whole cell lysate of E. coli CFT073 grown in LB + 100 μM BP until late log phase, by immunoprecipitation. Protein A agarose beads were washed with binding buffer (50 mM Na Phosphate, 200 mM NaCl, 1mM EDTA [pH 8.0]) and incubated with rabbit derived anti-PqqL serum diluted 1:1 with binding buffer, at room temperature for 1 h. Beads were then washed extensively with binding buffer to remove serum contaminants.
E. coli CFT073 cells were sedimented by centrifugation at 4000 g for 10 min. The cell pellet was resuspended in lysis buffer (50 mM Tris, 150 mM NaCl [pH 8.0]) and lysed by sonication. Lysate was clarified by centrifugation at 14,000 g for 10 min. Clarified lysate was incubated with anti-PqqL loaded protein A agarose for 1 h at room temperature. The beads were washed extensively with lysis buffer, resuspended in 1 × SDS-PAGE sample buffer (62.5 mM Tris-HCl, 2.5% SDS, 0.002% Bromophenol Blue, 10 mM dithiothreitol (DTT), 10% glycerol pH [6.95]) and incubated at 95°C. The sample was then separated on a 12% SDS-PAGE gel. A band corresponding to the size of full length PqqL (~100 kDa) was excised and N-terminal sequencing was performed by Edman degradation.
Supporting information
Representative/structurally characterized TonB-dependent transporters were clustered using CLANS, demonstrating that YddB and FusA form a sequence cluster that is similarly distantly related to other TonB-dependent transporters. FusA/YddB are similarly distant to the main group of transporters as the highly divergent SusC family from Bacteroides spp.; further illustrating a distant relationship between FusA and other transporters. Dots represent individual sequences and grey lines represent pairwise similarity relationships. An E-value cut-off of 1e-110 was used for clustering.
(TIF)
The crystals structure of YddB shown as rainbow cartoon (N-terminus = blue, C-terminus = red) (left), and electrostatic surface (right). The electrostatic surface illustrates the presence of a hydrophobic transmembrane region, which embeds YddB in the membrane. Octyl β-D-glucopyranoisde detergent molecules observed shielding the hydrophobic region in the crystal structure are shown as spheres.
(TIF)
The extracellular loops of YddB (A) are distinct in structure and length from those of FhuE (B) and Fiu (C), transporters for coprogen and catecholate siderophores respectively.
(TIF)
(A) A representative western blot of E. coli BW25113 ΔpqqL whole cells with anti-PqqL (top) and anti-SurA (bottom) in the presence and absence of 2,2’bipyridine, showing no band corresponding to PqqL is detected in this strain. Detection of PqqL in wildtype E. coli BW25113 is shown as a reference. (B) Quantitation of 3 biological replicate of blots of panel A.
(TIF)
PqqL immunoprecipitated using anti-PqqL serum was isolated via SDS page (left) and N-terminally sequenced using Edman degradation. The sequence of the corresponding band (AALPQD) is consistent with the N-terminal sequence of PqqL after cleavage of its predicted signal peptide.
(TIF)
Coomassie brilliant blue stained SDS-PAGE gel visualisation of protease cleavage reactions containing various small iron containing proteins in the presence and absence of PqqL. No proteolytic cleavage by PqqL was observed in these substrates.
(TIF)
(A) In the absence of MgCl2 PqqL formed poorly diffracting crystals in the space group P43, the addition of MgCl2 led to an increase in symmetry and change in space group to P43212. (B) PqqL molecules in crystals of the space group P43 exhibited a difference in conformation between their two domains, indicative of inherent flexibility of PqqL.
(TIF)
Sequence identity of different members of each cluster (labels for the X-axis of the matrix follow those for the Y-axis). The genetic context of FusA homologues and environment of isolation of species are included where available. A, B, C, D = corresponding Fus homologues, other proteins are labelled with name of nearest homologous protein or description of conserved domain, X = unknown function.
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
This research was undertaken on the MX1, MX2 and SAXS/WAXS beamlines at the Australian Synchrotron, part of ANSTO (CAP12312, and M12480). We would like to thank the Monash Crystallisation Facility for their assistance with sample characterisation, crystallographic screening and optimisation.
Data Availability
The crystallographic coordinates and associated structure factors produced in this study are available in the Protein Data Bank (PDB) with the accession codes YddB = 6OFR, PqqL Full Length = 6OFS, PqqL N-terminal Domain = 6OFT. Small angle X-ray scattering data for PqqL is available in the SASBDB accession code SASDFB6.
Funding Statement
The work was funded by the Australian Research Council (ARC; FL130100038) https://www.arc.gov.au/. R.G. was funded by a Sir Henry Wellcome Fellowship award (106077/Z/14/Z) https://wellcome.ac.uk/. T.L. is an ARC Australian Laureate Fellow (FL130100038). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pekkonen M, Ketola T, Laakso JT. Resource Availability and Competition Shape the Evolution of Survival and Growth Ability in a Bacterial Community. PLoS ONE. 2013;8(9):e76471 10.1371/journal.pone.0076471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hibbing ME, Fuqua C, Parsek MR, Peterson SB. Bacterial competition: surviving and thriving in the microbial jungle. Nature Reviews Microbiology. 2010;8(1):15 10.1038/nrmicro2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barber MF, Elde NC. Buried treasure: evolutionary perspectives on microbial iron piracy. Trends in Genetics. 2015;31(11):627–36. 10.1016/j.tig.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grinter R, Milner J, Walker D. Ferredoxin containing bacteriocins suggest a novel mechanism of iron uptake in Pectobacterium spp. PLoS ONE. 2012;7(3):e33033 10.1371/journal.pone.0033033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grinter R, Milner J, Walker D. Beware of proteins bearing gifts: protein antibiotics that use iron as a Trojan horse. FEMS Microbiol Lett. 2013;338(1):1–9. 10.1111/1574-6968.12011 [DOI] [PubMed] [Google Scholar]
- 6.Grinter R, Hay ID, Song J, Wang J, Teng D, Dhanesakaran V, et al. FusC, a member of the M16 protease family acquired by bacteria for iron piracy against plants. PLoS Biol. 2018;16(8):e2006026 10.1371/journal.pbio.2006026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grinter R, Josts I, Mosbahi K, Roszak AW, Cogdell RJ, Bonvin AM, et al. Structure of the bacterial plant-ferredoxin receptor FusA. Nat Commun. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosbahi K, Wojnowska M, Albalat A, Walker D. Bacterial iron acquisition mediated by outer membrane translocation and cleavage of a host protein. Proceedings of the National Academy of Sciences. 2018:201800672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grinter R, Josts I, Zeth K, Roszak AW, McCaughey LC, Cogdell RJ, et al. Structure of the atypical bacteriocin pectocin M2 implies a novel mechanism of protein uptake. Mol Microbiol. 2014;93(2):234–46. 10.1111/mmi.12655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noinaj N, Easley NC, Oke M, Mizuno N, Gumbart J, Boura E, et al. Structural basis for iron piracy by pathogenic Neisseria. Nature. 2012;483(7387):53–8. http://www.nature.com/nature/journal/v483/n7387/abs/nature10823.html-supplementary-information. 10.1038/nature10823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang W, Wilks A. Extracellular Heme Uptake and the Challenge of Bacterial Cell Membranes. Annu Rev Biochem. 2017;(0). [DOI] [PubMed] [Google Scholar]
- 12.Finn RD, Clements J, Eddy SR. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 2011;39(Web Server issue):W29–W37. 10.1093/nar/gkr367 PMC3125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frickey T, Lupas A. CLANS: a Java application for visualizing protein families based on pairwise similarity. Bioinformatics. 2004;20(18):3702–4. 10.1093/bioinformatics/bth444 [DOI] [PubMed] [Google Scholar]
- 14.Schütz B, Seidel J, Sturm G, Einsle O, Gescher J. Investigation of the electron transport chain to and the catalytic activity of the diheme cytochrome c peroxidase CcpA of Shewanella oneidensis. Appl Environ Microbiol. 2011;77(17):6172–80. 10.1128/AEM.00606-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fülöp V, Ridout CJ, Greenwood C, Hajdu J. Crystal structure of the di-haem cytochrome c peroxidase from Pseudomonas aeruginosa. Structure. 1995;3(11):1225–33. 10.1016/s0969-2126(01)00258-1 [DOI] [PubMed] [Google Scholar]
- 16.Martorana AM, Motta S, Di Silvestre D, Falchi F, Dehò G, Mauri P, et al. Dissecting Escherichia coli outer membrane biogenesis using differential proteomics. PLoS ONE. 2014;9(6):e100941 10.1371/journal.pone.0100941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vertommen D, Ruiz N, Leverrier P, Silhavy TJ, Collet JF. Characterization of the role of the Escherichia coli periplasmic chaperone SurA using differential proteomics. Proteomics. 2009;9(9):2432–43. 10.1002/pmic.200800794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee EY, Bang JY, Park GW, Choi DS, Kang JS, Kim HJ, et al. Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics. 2007;7(17):3143–53. 10.1002/pmic.200700196 [DOI] [PubMed] [Google Scholar]
- 19.Scorza FB, Doro F, Rodríguez-Ortega MJ, Stella M, Liberatori S, Taddei AR, et al. Proteomics characterization of outer membrane vesicles from the extraintestinal pathogenic Escherichia coli ΔtolR IHE3034 mutant. Molecular & Cellular Proteomics. 2008;7(3):473–85. [DOI] [PubMed] [Google Scholar]
- 20.Wurpel DJ, Moriel DG, Totsika M, Easton DM, Schembri MA. Comparative analysis of the uropathogenic Escherichia coli surface proteome by tandem mass-spectrometry of artificially induced outer membrane vesicles. Journal of proteomics. 2015;115:93–106. 10.1016/j.jprot.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 21.Subashchandrabose S, Smith SN, Spurbeck RR, Kole MM, Mobley HLT. Genome-Wide Detection of Fitness Genes in Uropathogenic Escherichia coli during Systemic Infection. PLoS Pathog. 2013;9(12):e1003788 10.1371/journal.ppat.1003788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seo SW, Kim D, Latif H, O’Brien EJ, Szubin R, Palsson BO. Deciphering Fur transcriptional regulatory network highlights its complex role beyond iron metabolism in Escherichia coli. Nature communications. 2014;5:4910 10.1038/ncomms5910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McHugh JP, Rodríguez-Quiñones F, Abdul-Tehrani H, Svistunenko DA, Poole RK, Cooper CE, et al. Global iron-dependent gene regulation in Escherichia coli A new mechanism for iron homeostasis. J Biol Chem. 2003;278(32):29478–86. 10.1074/jbc.M303381200 [DOI] [PubMed] [Google Scholar]
- 24.Holm L, Laakso LM. Dali server update. Nucleic Acids Res. 2016;44(W1):W351–W5. 10.1093/nar/gkw357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grinter R, Lithgow T. Determination of the Molecular Basis for Coprogen Import by Gram Negative Bacteria. IUCrJ. 2019;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia EC, Brumbaugh AR, Mobley HL. Redundancy and specificity of Escherichia coli iron acquisition systems during urinary tract infection. Infect Immun. 2011;79(3):1225–35. 10.1128/IAI.01222-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sklar JG, Wu T, Kahne D, Silhavy TJ. Defining the roles of the periplasmic chaperones SurA, Skp, and DegP in Escherichia coli. Genes Dev. 2007;21(19):2473–84. 10.1101/gad.1581007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noinaj N, Kuszak AJ, Gumbart JC, Lukacik P, Chang H, Easley NC, et al. Structural insight into the biogenesis of β-barrel membrane proteins. Nature. 2013;501(7467):385–90. 10.1038/nature12521 http://www.nature.com/nature/journal/v501/n7467/abs/nature12521.html-supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selkrig J, Mosbahi K, Webb CT, Belousoff MJ, Perry AJ, Wells TJ, et al. Discovery of an archetypal protein transport system in bacterial outer membranes. Nat Struct Mol Biol. 2012;19(5):506–10. http://www.nature.com/nsmb/journal/v19/n5/abs/nsmb.2261.html-supplementary-information. 10.1038/nsmb.2261 [DOI] [PubMed] [Google Scholar]
- 30.King JV, Liang WG, Scherpelz KP, Schilling AB, Meredith SC, Tang W-J. Molecular basis of substrate recognition and degradation by human presequence protease. Structure. 2014;22(7):996–1007. 10.1016/j.str.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aleshin AE, Gramatikova S, Hura GL, Bobkov A, Strongin AY, Stec B, et al. Crystal and Solution Structures of a Prokaryotic M16B Peptidase: an Open and Shut Case. Structure. 17(11):1465–75. 10.1016/j.str.2009.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson KA, Bhushan S, Ståhl A, Hallberg BM, Frohn A, Glaser E, et al. The closed structure of presequence protease PreP forms a unique 10 000 Å3 chamber for proteolysis. The EMBO Journal. 2006;25(9):1977–86. 10.1038/sj.emboj.7601080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei Q, Ran T, Ma C, He J, Xu D, Wang W. Crystal structure and function of PqqF protein in the pyrroloquinoline quinone biosynthetic pathway. J Biol Chem. 2016;291(30):15575–87. 10.1074/jbc.M115.711226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tria G, Mertens HD, Kachala M, Svergun DI. Advanced ensemble modelling of flexible macromolecules using X-ray solution scattering. IUCrJ. 2015;2(2):207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noinaj N, Guillier M, Barnard, Travis J., Buchanan SK. TonB-Dependent Transporters: Regulation, Structure, and Function. Annu Rev Microbiol. 2010;64(1):43–60. 10.1146/annurev.micro.112408.134247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cascales E, Buchanan SK, Duché D, Kleanthous C, Lloubes R, Postle K, et al. Colicin biology. Microbiol Mol Biol Rev. 2007;71(1):158–229. 10.1128/MMBR.00036-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grinter R, Roszak AW, Cogdell RJ, Milner JJ, Walker D. The Crystal Structure of the Lipid II-degrading Bacteriocin Syringacin M Suggests Unexpected Evolutionary Relationships between Colicin M-like Bacteriocins. J Biol Chem. 2012;287(46):38876–88. 10.1074/jbc.M112.400150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gómez-Santos N, Glatter T, Koebnik R, Świątek-Połatyńska MA, Søgaard-Andersen L. A TonB-dependent transporter is required for secretion of protease PopC across the bacterial outer membrane. Nature Communications. 2019;10(1):1360 10.1038/s41467-019-09366-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolam DN, van den Berg B. TonB-dependent transport by the gut microbiota: novel aspects of an old problem. Curr Opin Struct Biol. 2018;51:35–43. 10.1016/j.sbi.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 40.Glenwright AJ, Pothula KR, Bhamidimarri SP, Chorev DS, Baslé A, Firbank SJ, et al. Structural basis for nutrient acquisition by dominant members of the human gut microbiota. Nature. 2017;541(7637):407 10.1038/nature20828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer J. Iron–sulfur protein folds, iron–sulfur chemistry, and evolution. JBIC Journal of Biological Inorganic Chemistry. 2008;13(2):157–70. 10.1007/s00775-007-0318-7 [DOI] [PubMed] [Google Scholar]
- 42.Munro AW, Girvan HM, McLean KJ, Cheesman MR, Leys D. Heme and Hemoproteins. Tetrapyrroles: Birth, Life and Death. New York, NY: Springer New York; 2009. p. 160–83. [Google Scholar]
- 43.Chen C, Natale DA, Finn RD, Huang H, Zhang J, Wu CH, et al. Representative Proteomes: A Stable, Scalable and Unbiased Proteome Set for Sequence Analysis and Functional Annotation. PLoS ONE. 2011;6(4):e18910 10.1371/journal.pone.0018910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22(13):1658–9. 10.1093/bioinformatics/btl158 [DOI] [PubMed] [Google Scholar]
- 45.Hubbard T, Barker D, Birney E, Cameron G, Chen Y, Clark L, et al. The Ensembl genome database project. Nucleic Acids Res. 2002;30(1):38–41. 10.1093/nar/30.1.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Apweiler R, Bairoch A, Wu CH, Barker WC, Boeckmann B, Ferro S, et al. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 2004;32(suppl_1):D115–D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miroux B, Walker JE. Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. Journal of molecular biology. 1996;260(3):289–98. 10.1006/jmbi.1996.0399 [DOI] [PubMed] [Google Scholar]
- 48.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr Sect D. 2010;66(4):486–501. 10.1107/S0907444910007493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr Sect D. 2010;66(2):213–21. 10.1107/S0907444909052925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smart OS, Womack TO, Flensburg C, Keller P, Paciorek W, Sharff A, et al. Exploiting structure similarity in refinement: automated NCS and target-structure restraints in BUSTER. Acta Crystallographica Section D: Biological Crystallography. 2012;68(4):368–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas DA, Francis P, Smith C, Ratcliffe S, Ede NJ, Kay C, et al. A broad‐spectrum fluorescence‐based peptide library for the rapid identification of protease substrates. Proteomics. 2006;6(7):2112–20. 10.1002/pmic.200500153 [DOI] [PubMed] [Google Scholar]
- 52.Kirby N, Cowieson N, Hawley AM, Mudie ST, McGillivray DJ, Kusel M, et al. Improved radiation dose efficiency in solution SAXS using a sheath flow sample environment. Acta Crystallographica Section D: Structural Biology. 2016;72(12):1254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kirby NM, Mudie ST, Hawley AM, Cookson DJ, Mertens HD, Cowieson N, et al. A low-background-intensity focusing small-angle X-ray scattering undulator beamline. J Appl Crystallogr. 2013;46(6):1670–80. [Google Scholar]
- 54.Konarev PV, Volkov VV, Sokolova AV, Koch MH, Svergun DI. PRIMUS: a Windows PC-based system for small-angle scattering data analysis. J Appl Crystallogr. 2003;36(5):1277–82. [Google Scholar]
- 55.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proceedings of the National Academy of Sciences. 2000;97(12):6640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, et al. Construction of Escherichia coli K‐12 in‐frame, single‐gene knockout mutants: the Keio collection. Molecular systems biology. 2006;2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tu Q, Yin J, Fu J, Herrmann J, Li Y, Yin Y, et al. Room temperature electrocompetent bacterial cells improve DNA transformation and recombineering efficiency. Scientific reports. 2016;6:24648 10.1038/srep24648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doublet B, Douard G, Targant H, Meunier D, Madec J-Y, Cloeckaert A. Antibiotic marker modifications of λ Red and FLP helper plasmids, pKD46 and pCP20, for inactivation of chromosomal genes using PCR products in multidrug-resistant strains. J Microbiol Methods. 2008;75(2):359–61. 10.1016/j.mimet.2008.06.010 [DOI] [PubMed] [Google Scholar]
- 59.Guyer DM, Kao J-S, Mobley HL. Genomic analysis of a pathogenicity island in uropathogenic Escherichia coli CFT073: distribution of homologous sequences among isolates from patients with pyelonephritis, cystitis, and catheterassociated bacteriuria and from fecal samples. Infection and Immunity. 1998;66(9):4411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quan S, Hiniker A, Collet J-F, Bardwell JC. Isolation of bacteria envelope proteins Bacterial Cell Surfaces: Springer; 2013. p. 359–66. [DOI] [PubMed] [Google Scholar]
- 61.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature methods. 2012;9(7):671 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]