Abstract
Background
Bartonellae are intracellular bacteria, which can cause persistent bacteraemia in humans and a variety of animals. Several rodent-associated Bartonella species are human pathogens but data on their global distribution and epidemiology are limited. The aims of the study were to: 1) determine the prevalence of Bartonella infection in rodents and fleas; 2) identify risk factors for Bartonella infection in rodents; and 3) characterize the Bartonella genotypes present in these rodent and flea populations.
Methods and results
Spleen samples collected from 381 rodents representing six different species were tested for the presence of Bartonella DNA, which was detected in 57 individuals (15.0%; 95% CI 11.3–18.5), of three rodent species (Rattus rattus n = 54, Mastomys natalensis n = 2 and Paraxerus flavovottis n = 1) using a qPCR targeting the ssrA gene. Considering R. rattus individuals only, risk factor analysis indicated that Bartonella infection was more likely in reproductively mature as compared to immature individuals (OR = 3.42, p <0.001). Bartonella DNA was also detected in 53 of 193 Xenopsylla cheopis fleas (27.5%: 95% CI 21.3–34.3) collected from R.rattus individuals. Analysis of ssrA and gltA sequences from rodent spleens and ssrA sequences from fleas identified multiple genotypes closely related (≥ 97% similar) to several known or suspected zoonotic Bartonella species, including B. tribocorum, B. rochalimae, B. elizabethae and B. quintana.
Conclusions
The ssrA and gltA sequences obtained from rodent spleens and ssrA sequences obtained from fleas reveal the presence of a diverse set of Bartonella genotypes and increase our understanding of the bartonellae present in Tanzanian. Further studies are needed to fully characterise the prevalence, genotypes and diversity of Bartonella in different host populations and their potential impacts on human health.
Introduction
Bartonella are fastidious, Gram-negative, vector-borne bacteria with worldwide distribution. Bartonella species are known to infect mainly erythrocytes and endothelial cells of various mammals, such as humans, cats, dogs, ruminants, wild rabbits and rodents [1]. Epidemiological studies have demonstrated that rodents and other small mammals are important hosts of Bartonella species and that ectoparasites such as fleas, ticks, sand flies, and lice are key vectors of Bartonella infection [2,3].
In recent years, an increasing number of Bartonella species have been identified as zoonotic pathogens. To date there are roughly 45 Bartonella species and subspecies that have been designated [4], of which at least 20 are rodent-associated [1]. Several studies indicate that rodent-associated Bartonella are the cause of human infections in various regions of the world, particularly in areas where humans are in close contact with rodents [5–10]. However, knowledge of the distribution and epidemiology of Bartonella in rodents and of the role of Bartonella species in human disease in Tanzania is limited.
Clinical manifestations of Bartonella infection in humans can range from mild [7] to life threatening disease and can present as acute or chronic [5,11]. Known sequelae attributed to Bartonella species include endocarditis [8], myocarditis [12], fever and neurologic disorders [13], intraocular neuroretinitis [14], meningitis, splenomegaly and lymphadenopathy [15]. This range of non-specific and variable symptoms makes Bartonella infections hard to diagnose clinically. This contributes to a poor understanding of the current distribution and relative importance of infections caused by this pathogen. The challenges of identifying the causes of non-specific febrile illness are demonstrated by previous research conducted in Moshi, northern Tanzania, where a study of patients admitted to hospital with febrile illness revealed that a range of zoonotic pathogens were responsible for roughly a quarter of the hospital admissions [16]. However, no zoonotic infections were included in the admission differential diagnosis for any patient in that study, indicating lack of awareness and diagnostic capacity for many zoonotic pathogens. Over the past few decades, numerous reports of bartonellosis in febrile humans have been made globally [6,8–10,17,18]. However, in developing countries bartonellosis is often not considered as a potential diagnosis.
Molecular detection and typing methods for Bartonella are widely used due to their greater sensitivity and ease of use in comparison to culture and serology based approaches. Real-time PCR assays are recommended for primary screening of Bartonella species followed by confirmatory assays, using either conventional or real-time PCR and sequencing [19,20]. In Africa, studies conducted in Ethiopia [21], Kenya [22], South Africa [23], the Democratic Republic of Congo [24,25] and Uganda [26] have previously confirmed detection of Bartonella species in rodents using conventional PCR detection methods for multiple gene targets. In northern Tanzania, a study performed in Mbulu, a rural district in northern Tanzania, detected Bartonella in 41% of indigenous rodents using gltA and rpoB PCR targets [25]. Analyses of gltA sequences from these samples revealed the presence of multiple genotypes similar to known Bartonella species, including B. elizabethae, B. tribocorum, B. birtlesii and B. queenslandensis [25].
Bartonella species are present in Tanzania and may contribute to the burden of human febrile illness in northern Tanzania. However, the distribution and epidemiology of Bartonella in Tanzania is largely unknown. The aims of this study were to use molecular diagnostic tools to estimate the prevalence of Bartonella infection in rodents and their fleas sampled in northern Tanzania. Specifically, we aimed to: 1) determine the prevalence of Bartonella infection in rodents and fleas using quantitative real-time polymerase chain reaction (qPCR); 2) identify risk factors for Bartonella infection in rodents; and 3) use sequencing of the gltA and ssrA genes to characterize the Bartonella genotypes present in these rodent and flea populations.
Methods
Ethics statement
Ethical approval for the study was granted by the Tanzania Commission for Science and Technology (COSTECH 2012-471-ER-2005-141 & 2015-71-NA-2011-199); Kilimanjaro Christian Medical Centre (KCMC) Ethics Committee (535 & 537); National Institute of Medical Research (NIMR), Tanzania (NIMR/HQ/R.8a/Vol.IX/1499 & NIMR/HQ/R.8a/Vol.IX/1522); Tanzania Wildlife Research Institute (TAWIRI); University of Glasgow College of Medical, Veterinary and Life Sciences Ethics Committee (200120020), and University of Glasgow Faculty of Veterinary Medicine Ethics and Welfare Committee (01a/13 & 02a/13). Written consent for study participation was obtained for each participating household, using forms translated into Swahili. Rodent sampling was performed in accordance with the UK Guidance on the Operation of the Animals (Scientific Procedures) Act 1986 and American Veterinary Medical Association Guidelines for the Euthanasia of Animals [27,28].
Rodent trapping and sampling
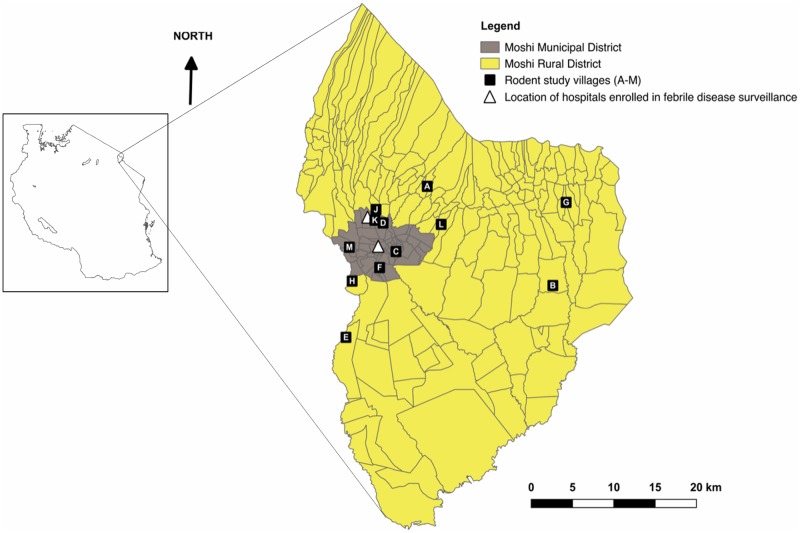
Rodent spleen samples and ectoparasites were obtained from a cross-sectional study conducted to explore the role of rodents in the epidemiology of leptospirosis and other zoonoses in the Kilimanjaro region of northern Tanzania [29]. Rodents were trapped in five villages within Moshi Municipal District and seven villages within Moshi Rural District, as previously described [29] (Fig 1). The target sample size was 50 rodents per village to give sufficient power (α = 0.95, β = 0.8) to detect a minimum infection prevalence of 10% [29]. Villages for sampling were randomly selected from a list, home to people that had sought care, and had been enrolled in previous febrile illness surveillance studies at local hospitals [16]. Rodent trapping was performed in three sessions: 1) May-June 2013 (wet season); 2) May-June 2014 (wet season); and 3) August-September 2014 (dry season). Rodents were trapped in households in a total of 12 villages through cross-sectional visits, with one additional round of repeat sampling conducted in one village (based on high trap success in the initial visit) [29]. Trapped rodents were euthanized by terminal halothane anaesthesia and cervical dislocation. Data gathered for every trapped rodent included: species (determined by observation of phenotypic characteristics and measurement of morphometric features), sex and reproductive maturity status (mature or immature determined based on external sexual characteristics [30]). A full necropsy and tissue sampling were performed for each rodent sampled. A fresh sterile scalpel blade was used for each rodent and all other necropsy equipment was washed using 5% Virkon and dried between usages to avoid cross-contamination. Spleen tissue samples were collected into sterile Eppendorf tubes and stored at -80°C prior to DNA extraction. Ectoparasites observed on trapped rodents were collected and stored in 70–96% ethanol; all ectoparasites from the same rodent were stored together. Collected fleas were identified to species level using a dissecting microscope and a pictorial flea identification guide [31]. Xenopsylla cheopis fleas were selected for DNA extraction and Bartonella testing based on their known contribution to Bartonella transmission [32]. For each rodent with at least one X. cheopis collected, DNA was extracted from one (if only one X. cheopis present on that host) or two (if more than one X. cheopis present on that host). Where multiple X. cheopis were collected from the same rodent, selection of individual fleas for DNA extraction was opportunistic.
Fig 1. Map of Moshi Municipal and Moshi Rural Districts, showing representative locations of rodent study villages in relation to the two hospitals (Kilimanjaro Christian Medical Centre and Mawenzi Regional Referral Hospital) at which febrile illness surveillance has been conducted in previous studies.
Letters indicate the different villages in which rodent trapping was conducted. Polygons in the main image show local administrative boundaries. Insert map on left shows outline of Tanzania and location of study districts within the country. This figure is adapted from a version published previously [29].
DNA extraction
DNA was extracted from approximately 10 milligrams (mg) of spleen tissue using the DNeasy Blood and Tissue Kit spin-column protocol for DNA purification from tissues (Qiagen, Hilden, Germany). DNA from spleen tissues was eluted in 100μl of AE buffer and quantified using a Nano-Drop spectrophotometer (Thermo Scientific, Waltham, MA, USA). DNA from individual whole fleas was also extracted using the DNeasy Blood and Tissue Kit, following the protocol for purification of total DNA from ticks and eluted in a final volume of 65μl AE buffer. For all extractions, a no-template extraction control (PCR-grade water) was included for every 20 samples and DNA extracts were stored at -20°C prior to testing. DNA extracts from spleens were diluted in 20μl of AE buffer to a standard DNA concentration of 10–50 ng/μl for PCR testing, to minimize the potential for PCR inhibition due to the high concentration of host DNA in the rodent spleen extracts. Due to the lower concentration of DNA in extracts from fleas, these were tested directly from extraction concentrations.
Quantitative real-time PCR for detection of Bartonella species ssrA gene
DNA extracts from rodent spleens and fleas were screened using a Bartonella genus-specific real-time PCR assay (qPCR) targeting the transfer-mRNA ssrA gene, using a previously published protocol [20]. The original paper describing this assay reports a lower limit of detection of < 5 fg of Bartonella DNA, equivalent to < 3 genomic copies per reaction when tested against four Bartonella species (B. quintana, B. henselae, B. bovis, and B. elizabethae) [20]. The primers ssrA-F (5ˈ-GCTATGGTAATAAATGGACAATGAAATAA-3ˈ), ssrA-R (5ˈ GCTTCTGTTGCCAGGTG-3ˈ) and 6-carboxyfluorescein FAM-labelled probe (5ˈ-ACCCCGCTTAAACCTGCGACG-3ˈ-BHQ1) were used to amplify an ssrA gene fragment of approximately 300bp. qPCR reactions were carried out in 20 μl volumes comprised of 10 μl QuantiNova Probe PCR mix (Qiagen), 0.8 μl of each primer (10 μM) and probe (5 μM), 2.6 μl nuclease-free water and 5 μl DNA template. Positive control (rodent tissue DNA extract obtained from a previous study [24] positive for Bartonella with closest similarity to B. tribocorum), extraction controls and no-template controls were included in each qPCR run. Assays were performed on a Rotor-Gene Q/6000 (Qiagen) with manufacturer recommended thermocycling conditions as follows: 95 °C for 2 minutes, followed by 45 cycles of 95 °C for 5 seconds and 60 °C for 5 seconds. A qPCR run was considered valid when all negative controls showed no evidence of amplification and the positive controls amplified with a Ct value of < 40. Extracts were tested in duplicate and considered positive when amplification was recorded in one or more test wells with a Ct value ≤40.
PCR amplification and sequencing of Bartonella ssrA gene products
For sequencing, conventional PCR amplification of the ssrA gene was performed on all DNA extracts from both rodent spleens and fleas that were positive in the ssrA qPCR, based on a previously published protocol [20]. Each PCR reaction (25 μl) comprised 2.5 μl of PCR 10X buffer, 0.1μL Platinum Taq polymerase, 0.75μL MgCl2, 0.5 μl dNTPs (10 μM) (Invitrogen, USA) and 0.5 μl of each primer ssrA-F and ssrA-R at 10 μM [20]. Template DNA volume varied from 5–10 μl depending on ssrA assay Ct value. Nuclease-free water was used to make up the total reaction volumes as needed. Amplifications were performed with the following conditions: 94 °C for 2 minutes, followed by 40 cycles of 94 °C for 15 seconds, 60 °C for 60 seconds, and 72 °C for 30 seconds, and then a final extension step of 72 °C for 3 minutes. Positive and negative controls were included in each PCR run. PCR products were visualized by electrophoresis in a 1.5% agarose gel stained with GelRed (Cambridge Bioscience, Cambridge, UK). A sample was considered positive if a clearly defined DNA band of approximately 300 bp was visible in the gel and confirmed as Bartonella by sequencing of the product. To confirm and characterize the genotypes of Bartonella detected, amplicons with the expected size were purified using either a QIAquick PCR or Gel Extraction Purification Kit (Qiagen). Sequencing was performed at Source Biosciences (Nottingham, UK) using the same primers as for the detection PCR [33]. Sequence identity was confirmed using BLASTn, as implemented in the National Centre for Biotechnology Information (NCBI) web portal.
PCR amplification and sequencing of Bartonella gltA gene products
Conventional PCR amplification of the gltA gene was performed on all rodent spleen DNA extracts, based on a previously published protocol [34]. Each PCR reaction (25 μl) comprised 12.5 μl of PCR 2X master mix (Promega, Madison, WI, USA), 1.25 μl 5% dimethyl sulphoxide (DMSO) (Sigma-Aldrich, St. Louis, USA), 1.25 μl molecular grade water (Qiagen), 2.5 μl of each oligonucleotide primer (10 μM), BhCS781.p (5ˈ-GGGGACCAGCTCATGGTGG-3ˈ) and BhCS1137.n (5ˈ- AATGCAAAAAGAACAGTAAACA-3ˈ) [34], and 5 μl DNA template. Amplifications were performed on a PTC-240 DNA-Engine (MJ Research/BioRad Technologies, USA) with the following conditions: 94 °C for 2 minutes, followed by 40 cycles of 94 °C for 30 seconds, 54.3 °C for 30 seconds, and 72 °C for 2 minutes, then a final step of 72 °C for 7 minutes. Positive and negative controls were included in each PCR run. PCR products were visualized by electrophoresis in a 1.5% agarose gel stained with ethidium bromide (Invitrogen, USA). A sample was considered positive if a clearly defined DNA band of approximately 379 bp was visible in the gel and confirmed as Bartonella by sequencing of the product. Purification, sequencing and BLAST analyses were conducted as for the ssrA gene.
Phylogenetic analyses for ssrA and gltA gene products
Incomplete and poor quality sequences (e.g. with ambiguous peaks) were excluded from phylogenetic analysis for both ssrA and gltA gene fragments. For each gene, sequences were aligned using the ClustalW algorithm, implemented in MEGA 7.0 [35]. The model test function in MEGA 7.0 was used to select the best-fitting nucleotide substitution models, which were then incorporated into a phylogenetic analysis based on a maximum likelihood optimality criterion for tree reconstruction, with 1000 bootstrap pseudoreplicates. For the ssrA analysis, rodent spleen and flea sequences from this study were aligned with reference ssrA sequences from cultured Bartonella species downloaded from GenBank (see GenBank accession numbers in Fig 2). A Brucella melitensis sequence was used as the outgroup [36]. For analysis of the gltA data, sequences from study rodent spleens were aligned with those from Bartonella reference strains obtained from GenBank and also with representative sequences from previous studies conducted in East Africa (see GenBank accession numbers in Fig 3). Reference sequences in the alignment included gltA sequences from previous studies of Bartonella in rodents from Tanzania [25], Kenya [22], the Democratic Republic of Congo [24,25] and Uganda [26] that included either similar rodent species or Bartonella gltA sequences similar to those obtained in this study. A B. tamiae gltA sequence obtained from an African bat was used as the outgroup [37].
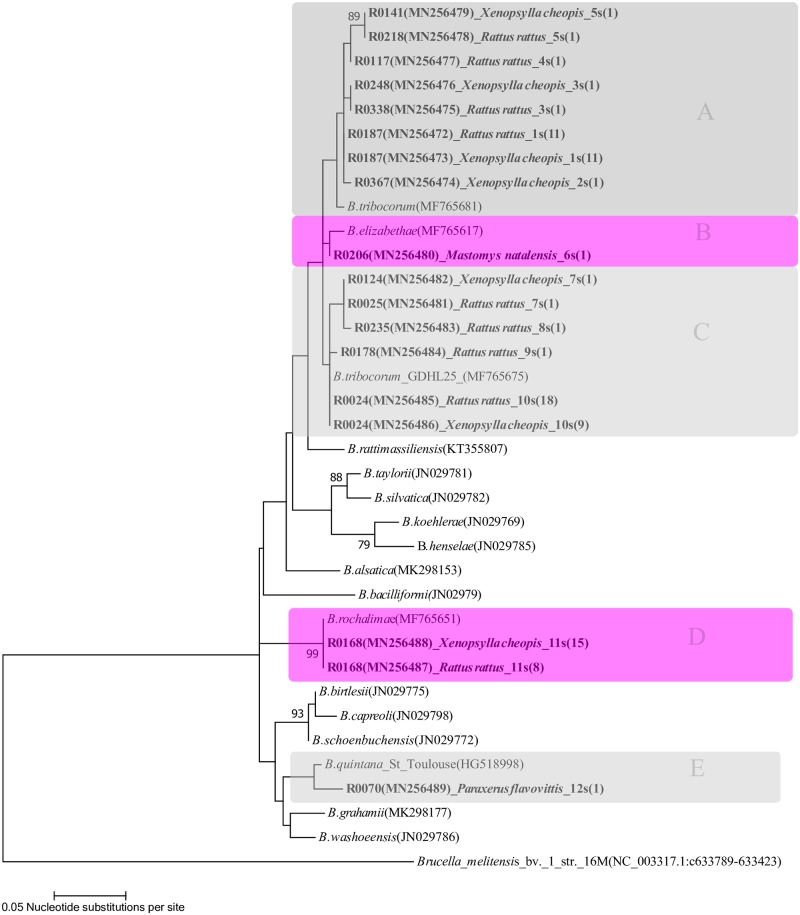
Fig 2. Phylogenetic tree showing the relatedness of the Bartonella ssrA gene sequences (237bp gene fragments) derived from 45 rodent spleen tissue samples (43 R. rattus, 1 M. natalensis and 1 P. flavovottis) and 39 X. cheopis fleas collected in northern Tanzania.
A single representative sample sequence is included for each combination of Bartonella genotype identified in this study and host of origin. Genotypes (1s–12s) and groups (As–Es) are indicated by lettering. Groups A, C and E are shaded grey, with groups B and D in pink. The phylogenetic tree was constructed using the maximum likelihood method based on a Kimura 2-parameter substitution model [41], as determined by Model test as implemented in MEGA 7.0 [35]. The tree with the highest log likelihood is shown and drawn to scale, with branch lengths shown in terms of the number of substitutions per site. Vertical branches indicate identical sequences. The numbers at the nodes correspond to bootstrap values higher than 70% after 1000 replicates. Sequences from this study are labelled with unique identifiers, with prefix “R” followed by sample identifier numbers, Genbank accession number, the rodent or flea host species, the genotype code and the number of samples yielding each genotype (in parentheses). Sequences from reference strains of Bartonella are included with the Bartonella species name and GenBank accession numbers given in parentheses. Brucella melitensis was included as an outgroup.
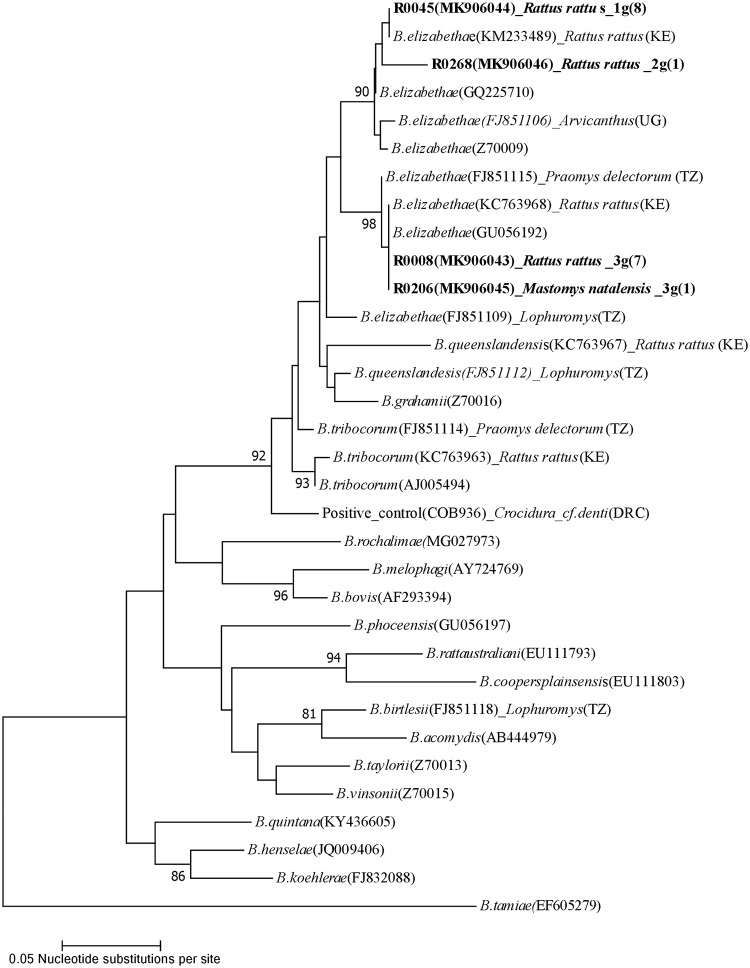
Fig 3. Phylogenetic tree showing the relatedness of the Bartonella gltA gene sequences (283bp fragments) derived from 17 spleen tissue samples from rodents (16 R. rattus and 1 Mastomys natalensis) trapped in northern Tanzania.
A single representative sample sequence is included for each genotype identified in this study, with the exception of genotype 3g to illustrate the identical sequences obtained from R. rattus and M. natalensis. Sequences from this study are labelled with unique identifiers, with prefix “R” followed by sample identifier numbers, Genbank accession number, the rodent or flea host species, the genotype code and the number of samples yielding each genotype (in parentheses). Reference Bartonella sequences from rodents trapped elsewhere in East Africa obtained from GenBank are indicated by GenBank accession numbers in parentheses, rodent species and country code (Kenya (KE) [22], Uganda (UG) [26] Tanzania (TZ) [25], Democratic Republic of Congo (DRC) [25]). The sequence obtained for the known Bartonella positive control sample provided by a colleague from a previous study is included and indicated with a unique identification number (COB936) [24].
Statistical analyses
Statistical analyses were performed in R [38]. Exact binomial proportions and confidence intervals for prevalence estimates were calculated using the package ‘binom’ [39]. Generalized linear mixed models (GLMM), with binomial family and logit link function, were used to examine variables associated with rodent and flea ssrA qPCR test status (qPCR positive vs negative) and implemented using the package ‘lme4’ [40]. For rodents, the dataset for these analyses was limited to R. rattus only, given the dominance of this species. Explanatory variables considered in the GLMM for rodent ssrA qPCR status included host sex and reproductive maturity (mature or immature), which were determined based on external sexual characteristics [30]. Trapping season (wet or dry), trapping district (Moshi Municipal or Moshi Rural) and rodent abundance were also included as explanatory variables for analysis. Adjusted trap success data were calculated by dividing the total number (n) of rodents caught per village by the corrected number of trap nights, which is calculated as: total number of trap nights (number of traps x number of nights) minus lost trap nights (sum of number of closed, damaged or lost traps / 2) and expressed as a percentage [29]. The village identification variable was included as a random effect to account for the clustered sampling strategy. For fleas, rodent ssrA qPCR test status was the only explanatory variable evaluated in the GLMM and the village identification variable was included as a random effect. Initial maximal multivariable models were created including all candidate explanatory variables and likelihood ratio tests were used to compare candidate models and guide model selection.
Results
Sample characteristics
Spleen samples from a total of 381 rodents were available for testing. The majority (n = 317, 83.2%), were from black rats (R. rattus) (Table 1). Other rodent species tested included: house mice (Mus musculus, n = 44, 11.5%); African pygmy mice (Mus minutoides, n = 3, 0.8%); multimammate mice (Mastomys natalensis, n = 8; 2.1%), spiny mice (Acomys wilsonii, n = 6, 1.6%) and striped bush squirrels (Paraxerus flavovittis, n = 3, 0.8%). Of the rodents tested, 219 individuals (57.5%) were female. The majority of all sampled rodents (n = 224, 58.8%) were classified as sexually mature based on external sexual characteristics. A total of 265 of the 381 rodents (69.6%) were trapped during wet season sampling. A total of 513 fleas were collected from 153 of the 381 (40.2%) rodents included in the study. Flea species identified were Xenopsylla cheopis (n = 306), Echidnophaga gallinacea (n = 204) and Ctenocephalides felis (n = 3). DNA extracts from a total of 193 Xenopsylla cheopis collected from 124 rodents (Rattus rattus n = 118, Mus musculus n = 4 and Mastomys natalensis n = 2) were available for Bartonella testing using ssrA.
Table 1. Summary of rodent species and their Bartonella detection status defined by ssrA qPCR and sequence confirmed by gltA PCR testing of spleen samples.
| Rodent species | Number of spleen samples tested | Bartonella ssrA qPCR Positive n (%) | Bartonella ssrA qPCR Ct values of Positives | Bartonella gltA Product Sequence Positive n (%) |
|---|---|---|---|---|
| Acomys wilsonii | 6 | 0 (0) | NA | 0 (0) |
| Mastomys natalensis | 8 | 2 (25.0) | 33.23 & 33.54 | 1 (12.5) |
| Mus minutoides | 3 | 0 (0) | NA | 0 (0) |
| Mus musculus | 44 | 0 (0) | NA | 0 (0) |
| Paraxerus flavovottis | 3 | 1 (33.3) | 36.49 | 0 (0) |
| Rattus rattus | 317 | 54 (17.0) | median value of 33.07, range 24.25–39.56 | 16 (5.0) |
| Total | 381 | 57 (15.0) | 17 (4.4) |
Bartonella detection in rodent spleens and risk factors for rodent infection
Bartonella DNA was detected by ssrA qPCR in a total of 57 of 381 (15.0%, 95% CI 11.5–18.9%) rodents screened (Table 1). Samples derived from Mastomys natalensis, Paraxerus flavovittis and R. rattus (Table 1) were all classified as Bartonella positive by this test. The positive control used in ssrA qPCR runs to test rodent spleen extracts had a mean Ct value of 32. The assay showed a 100% lower limit of detection of 1.8 fg of Bartonella quintana DNA control. For the logistic regression analysis considering data from R. rattus only, rodent reproductive maturity status was the only significant risk factor (LRT: χ2 = 13.30, df = 1, p < 0.0003), with reproductively mature individuals more likely to be ssrA qPCR positive (OR 3.42, 95% CI 1.69–6.89, p < 0.001). None of the other candidate variables evaluated (rodent sex, trapping season, trapping district or rodent abundance at trapping village) were significantly associated with R. rattus ssrA qPCR test status. The breakdown of rodents trapped by village is given in Table 2.
Table 2. Summary of rodent trapping data and the Bartonella ssrA and gltA genotypes detected in trapped rodents by village around Moshi, Tanzania.
| Village code | District | Total number of rodents tested for Bartonella | Adjusted trap success [29] | Bartonella genotypes identified, with data on the number of individuals and rodent species each genotype was detected in | |
|---|---|---|---|---|---|
| ssrA genotypes | gltA genotypes | ||||
| A | Rural | 12 | 9.79 | 1s–2 * Rattus rattus | 3g–2 * Rattus rattus |
| B | Rural | 13 | 4.28 | 7s–1 * Rattus rattus 10s–1 * Rattus rattus |
- |
| C | Municipal | 31 | 4.77 | 10s–1 * Rattus rattus | 1g–1 * Rattus rattus |
| D | Municipal | 25 | 2.68 | 10s–1 * Rattus rattus 2s–1 * Paraxerus flavovittis |
1g–1 * Rattus rattus |
| E | Rural | 39 | 5.28 | 4s–1 * Rattus rattus 10s–2 * Rattus rattus |
1g–1 * Rattus rattus 3g–1 * Rattus rattus |
| F | Municipal | 76 | 10.8 | 1s–5 * Rattus rattus 9s–1 * Rattus rattus 10s–1 * Rattus rattus 11s–5 * Rattus rattus |
1g–1 * Rattus rattus 3g–3 * Rattus rattus |
| F (visit 2) | Municipal | 33 | 4.42 | 1s–1 * Rattus rattus 11s–3 * Rattus rattus |
|
| G | Rural | 15 | 1.94 | 6s–1 * M. natalensis | 3g–1 * M. natalensis |
| H | Rural | 35 | 4.69 | 1s–1 * Rattus rattus 5s–5 * Rattus rattus 8s–1 * Rattus rattus 10s–3 * Rattus rattus |
1g–2 * Rattus rattus 3g–1 * Rattus rattus |
| J | Rural | 19 | 2.70 | 10s–4 * Rattus rattus | 2g–1 * Rattus rattus |
| K | Municipal | 23 | 3.19 | 10s–1 * Rattus rattus | - |
| L | Rural | 22 | 2.93 | 1s–2 * Rattus rattus 10s–3 * Rattus rattus |
1g–2 * Rattus rattus |
| M | Municipal | 38 | 5.06 | 3s–1 * Rattus rattus | |
Bartonella detection in fleas
Bartonella DNA was detected by ssrA qPCR in 53 of 193 (27.5%, 95% CI 21.3–34.3%) X. cheopis flea extracts. All ssrA qPCR positive flea extracts were collected from R. rattus individuals. The positive control used in ssrA qPCR runs to test flea extracts had a mean Ct value of 32. Logistic regression analysis identified a relationship between flea ssrA qPCR test status and the ssrA qPCR test status of the rodent that each flea was collected from (LRT: χ2 = 20.73, df = 1, p < 0.001). X. cheopis fleas collected from ssrA qPCR positive rodents were more likely to themselves be ssrA qPCR positive (OR 7.23, 95% CI 2.90–17.97, p < 0.001).
Characterisation of Bartonella in Tanzanian rodents and fleas by ssrA sequencing
Full length sequences of the ssrA gene target were obtained from 45 of 57 rodents spleen samples (Rattus rattus n = 43, Mastomys natalensis n = 1 and Paraxerus flavovottis n = 1) and 39 of 53 ssrA positive X. cheopis fleas. From the 237bp ssrA sequences amplified, 12 unique genotypes (1s to 12s) were identified in the sequences from rodent and flea populations combined (Table 3; Fig 2). The 12 genotypes were grouped into monophyletic groups with ≥97% similarity within the group. The following groupings were identified: 1) group As: Genotypes 1s to 5s clustering with a B. tribocorum reference sequence from strain GDHL73 (GenBank accession number MF765681); 2) group Bs: genotype 6s was unique but clustered with a B. elizabethae reference sequence (GenBank accession number MF765617); 3) group Cs: genotypes 7s to 10s clustering with a sequence from B. tribocorum strain GDHL25 (GenBank accession number MF765675); 4) group Ds: genotype 11s clustering with a B. rochalimae reference sequence (GenBank accession number MF7651); and 5) group Es: genotype 12s from P. flavovittis, which was mostly closely related to a B. quintana reference sequence (GenBank accession number HG518998). The distribution of ssrA genotypes between trapping villages (Table 2) show that more frequently detected ssrA genotypes (1s, 10s and 11s) were present in rodents trapped at multiple villages and provides no evidence of spatial segregation of the genotypes in this rodent population. Data on ssrA genotypes were available for fourteen pairs of X. cheopis fleas and R. rattus hosts (n = 11 R. rattus including three from which two X. cheopis were collected and tested). For seven pairs the ssrA genotype detected in fleas and rodent hosts were identical, but in the other seven pairs the genotypes differed. At the group level, 11 flea and host pairs had sequences from the same ssrA group and three pairs differed.
Table 3. Summary of Bartonella ssrA genotypes identified in rodent spleens and fleas from Moshi, Tanzania.
| ssrA genotypes | ssrA Group | Rodent species and number of positive samples | Flea species and number of positive samples | Closest Bartonella species (GenBank ID)a | % similarity to closest Bartonella spp. (number of base pair identities/ total base pair length) |
|---|---|---|---|---|---|
| 1s | As | 11 * R. rattus (MN25672) |
11 * X. cheopis (MN25673) |
B.tribocorum (MF765681) |
99 (240/244) |
| 2s | As | - | 1 * X. cheopis (MN25674) |
B.tribocorum (MF765681) | 99 (217/222) |
| 3s | As | 1 * R. rattus (MN25675) |
1 * X. cheopis (MN25676) |
B.tribocorum (MF765681) | 99 (228/233) |
| 4s | As | 1 * R. rattus (MN25677) |
- | B.tribocorum (MF765681) | 99 (239/244) |
| 5s | As | 1 * R. rattus (MN25678) |
1 * X. cheopis (MN25679) |
B.tribocorum (MF765681) | 98 (237/244) |
| 6s | Bs | 1* M. natalensis (MN25680) |
- |
B.elizabethae (MF765617) |
99 (222/224) |
| 7s | Cs | 1 * R. rattus (MN25681) |
1 * X. cheopis (MN25682) |
B.tribocorum (MF765675) |
99 (242/244) |
| 8s | Cs | 1 * R. rattus (MN25683) |
- | B.tribocorum (MF765675) | 99 (239/244) |
| 9s | Cs | 1 * R. rattus (MN25684) |
- | B.tribocorum (MF765675) | 99 (236/237) |
| 10s | Cs | 18 * R. rattus (MN25685) |
9 * X. cheopis (MN25686) |
B.tribocorum (MF765675) | 100 (244/244) |
| 11s | Ds | 8 * R. rattus (MN25687) |
15 * X. cheopis (MN25688) |
B.rochalimae (MF765651) |
100 (246/246) |
| 12s | Es | 1* P. flavovottis (MN25689) |
- |
B.quintana (HG518998) |
98 (233/239) |
The number of individuals of each rodents (n = 45) and fleas (n = 39) species from which each genotype was obtained are shown, as well as data on % similarity to reference Bartonella species sequences, with the number of base pair identities indicated in parentheses. The Genbank accession numbers for each genotype are also indicated in parentheses in columns 2 and 3.
a The closest reference sequences to the study sequences were selected from fully characterized sequences in Genbank obtained from cultures.
Characterisation of Bartonella in Tanzanian rodents by gltA sequencing
Full length sequences of the gltA gene were obtained from 17 rodent spleen DNA extracts (R. rattus n = 16 and M. natalensis n = 1). To be conservative, the alignment was pruned to the length of the shortest sequence (283 bp) and three unique genotypes were identified in this fragment (Fig 3). The correspondence between gltA and ssrA genotypes and groups is shown in Table 4. Only one R.rattus individual had a gltA sequence without a corresponding ssrA sequence. For the 16 remaining gltA genotypes, the same individuals all yielded ssrA sequences falling into groups As to Cs (Table 4). Eight sequences (genotype 1g, GenBank accession number MK906044) collected from R. rattus were identical to a B. elizabethae sequence obtained from a R. rattus sampled previously at a rural site in Kenya (strain B29297 [22], GenBank accession number KM233489). A second genotype (2g, GenBank accession number MK906046) was identified in one R. rattus and showed and 97% similarity (278 of 284 base pair matches) to a B.elizabethae sequence (GQ225710). Eight sequences (genotype 3g) collected from M. natalensis (GenBank accession number MK906045) and from R. rattus (GenBank accession number MK906043) were identical to a sample identified in a R. rattus from an urban site in Kenya (strain B28391 [22], GenBank accession number: KC763968) and to a cultured reference strain of B. elizabethae (strain BR02, GenBank accession number: GU056192).
Table 4. Summary of gltA genotypes identified in rodent spleens (n = 17) from Moshi, Tanzania and the correspondence with ssrA genotypes identified in the same species and individuals.
Data on the % similarity to reference Bartonella species sequences, and the number of base pair identities are indicated. The Genbank accession numbers for each genotype identified in the study are indicated.* The closest reference sequences to the study sequences were selected from fully characterized sequences in Genbank obtained from cultures.
| gltA Genotype | GenBank accession number | Rodent species and number of positive samples | Closest Bartonella species (GenBank ID)*. | % similarity to closest Bartonella spp. (number of base pair identities/ total base pair length) | ssrA group and genotype |
|---|---|---|---|---|---|
| 1g | MK906044 | 8 * R. rattus | B.elizabethae(GQ225710) | 99.65 (282/283) | Group C:10s |
| 2g | MK906046 | 1 * R. rattus | B.elizabethae (GQ225710) | 97 (278/284) | Group C:10s |
| 3g | MK906043 | 5 * R. rattus | B.elizabethae(GU056192) | 100 (283/283) | Group A: 1s |
| 3g | MK906043 | 1 * R. rattus | B.elizabethae(GU056192) | 100 (283/283) | Group A: 4s |
| 3g | MK906045 | 1 * M. natalensis | B.elizabethae(GU056192) | 100 (283/283) | Group B: 6s |
| 3g | MK906043 | 1 * R. rattus | B.elizabethae(GU056192) | 100 (283/283) | No ssrA typing obtained |
Discussion
This study reveals substantial variation in Bartonella genotypes among rodents and their fleas in a previously uncharacterised region of northern Tanzania (the Moshi Municipal and Moshi Rural Districts). Rattus rattus was the most common rodent species trapped and showed a high Bartonella prevalence defined by ssrA qPCR, which is consistent with the global distribution of Bartonella species in Rattus [42]. Within R. rattus, the probability of qPCR positivity was higher in reproductively mature individuals as compared to immature individuals, consistent with other studies (performed in the USA) that have found an association with age [43,44]. Sequencing of ssrA and gltA gene fragments revealed a variety of genotypes and the majority of sequences obtained showed greatest similarity to B. tribocorum and B. elizabethae reference sequences, both of which have been isolated in humans with febrile illness [6]. Sequences similar to B. rochalimae and B. quintana were also identified based on ssrA sequencing. These species were not detected by sequencing of the gltA, indicating reduced sensitivity of the gltA conventional PCR for detection of Bartonella species as compared to the ssrA qPCR. This is consistent with the findings of a previous study [20].
The ssrA qPCR was used to estimate prevalence in rodents and fleas, and the sequencing of ssrA and gltA PCR products to assess genetic variation and characterise the Bartonella detected. The overall prevalence of Bartonella (15%) detected in rodents using the ssrA qPCR was lower than has been detected in many comparable studies of global rodent populations [21,45], including studies that have used a less sensitive gltA assay for prevalence determination [46,47]. In a previous Tanzanian study of indigenous rodent species an overall gltA prevalence of 41% was detected [25]. A Ugandan survey using gltA to test invasive and indigenous rodent populations found variable prevalence across species, with higher prevalence in indigenous species (60% in Arvicanthus niloticus and 61% in Cricetomys gambianus), but low prevalence (1.4%) was recorded in invasive R. rattus [26]. Similarly, in Kenya the Bartonella prevalence determined by culture varied by rodent species [22]. Considering the data from R. rattus only, the prevalence seen in this study and previous African studies reveals consistently lower prevalence in comparison to R. rattus sampled in Asia and tested using ssrA qPCR methods (e.g. 32.5% [48]). The prevalence of Bartonella detection in X. cheopis fleas in this study using the ssrA qPCR was also lower than has been recorded in this species in the USA, where 190 of 200 (95%) X. cheopis tested were positive for Bartonella DNA [32]. The low prevalence of Bartonella in R. rattus and X. cheopis observed in this study are consistent with several other studies conducted in Africa. It has been argued that this pattern of lower Bartonella prevalence in African R. rattus populations could be attributed to host escape during colonization [49]. Further investigation of native and invasive rodent populations across Africa would be needed to investigate this further, and also evaluate the possible implications for human disease risk on the continent.
Phylogenetic analysis of sequences from rodents and their fleas revealed high concordance of sequences between hosts and ectoparasites. Overall, 10 distinct ssrA genotypes were identified that were most similar to reference sequences of B. tribocorum and B. elizabethae (Groups As to Cs), with only one genotype (10s) showing an identical match to a published B. tribocorum sequence in GenBank. However, since all of the reference sequences that were most similar were from a single study in China [50] it is important to recognise the limited reference data available currently and need for future comparison to datasets from other geographic areas to further evaluate these data on the diversity and types of Bartonella found in Tanzania, Moreover, B. elizabethae and B. tribocorum share identical published ssrA sequences in Genbank and our results show clustering in the phylogenetic tree (Fig 2), so the two species cannot be distinguished by this ssrA fragment. The other two Bartonella species identified were: 1) a single sequence (11s) with greatest similarity (98%) to B. quintana obtained from a sample from a P. flavovittis host; and 2) a sequence obtained from multiple samples of R. rattus and X. cheopis that showed an identical match to B. rochalimae, emphasising the diversity of Bartonella present in rodents in Tanzania. To the best of our knowledge, this is the first report of molecular detection and characterization of Bartonella species in rodents and their associated ectoparasites in Africa using the ssrA gene target. The scope for comparison with other sequences is thus limited, as there is currently little reference material on ssrA sequences from Bartonella sampled elsewhere, particularly in Africa.
In contrast, the gltA gene has been widely used to study Bartonella globally. Phylogenetic analysis of sequences from 17 gltA PCR products from this Tanzanian rodent population (16 R. rattus and 1 M. natalensis) showed the highest similarity to reference sequences of B. elizabethae, which has multiple published sequences in Genbank, including many from east Africa. The association of sequences similar to B. elizabethae with Rattus spp. in this study is consistent with similar findings from Malaysia [51] and Thailand [48]. B. elizabethae has also been identified in different rodent species in Africa [25,26]. Identical gltA sequences were amplified from R. rattus and M. natalensis in our study, suggesting possible transmission between different rodent species in the Tanzania site or a shared common source of infection. Identical sequences were also identified previously in R. rattus sampled at both rural (strain B28297, accession number KM233489, from Asembo, Kenya) and urban (strain B28391, accession number KC763968, from Kibera, Nairobi Kenya) sites in Kenya [22]. This suggests that similar Bartonella could be found in rodents in Kenya and Tanzania. However, these comparisons are based on short sequences of a single gene target only. Longer sequences from multiple genes and greater sampling effort across the region would be required to robustly confirm sharing of genotypes to trace source populations or determine patterns of host connectivity
Several studies of zoonotic disease have shown that a variety of pathogens account for high proportions of febrile illness in northern Tanzania but that considerable proportions remain unexplained [16,52]. Bartonella species have been identified as important causes of human febrile illness in several global settings but there has been little investigation of the impact of bartonellosis upon human health, in Africa particularly. The finding of Bartonella genotypes that are most similar to B. elizabethae, B. rochalimae and B. quintana reference sequences in rodents trapped in and around households in Moshi, Tanzania, and the fleas collected from these rodents, indicates the possibility that Bartonella infection may be responsible for an as yet unknown proportion of febrile illnesses in this region. Efforts are needed to determine the clinical impact of bartonellosis in this region and increase awareness about Bartonella and other zoonotic pathogens among physicians and health care workers, especially where the cause of large proportions of febrile illness remains unknown. Our results also demonstrate that molecular detection tools can be effectively used for surveillance and diagnostic of zoonotic pathogens in resource limited settings.
Acknowledgments
The authors would like to thank Professor Gibson Kibiki and Professor Blandina Mmbaga, the former and sitting directors of Kilimanjaro Clinical Research Institute (KCRI) for their support in establishing zoonotic research programmes at KCRI including this study. Special thanks go to laboratory staff at the KCRI biotechnology laboratory, especially Dr. Ireen Kiwelu, Happiness Kumburu, Davis Kuchaka and the late Bora Almasy for assisting in laboratory work. We are grateful to Dassa Nkini and Elizabeth Kussaga for administrative support and to Anne Laudisoit (formerly at University of Antwerp and FRIA, Belgium) for providing positive control materials obtained during a previous study [24].
Data Availability
Datasets supporting this manuscript are available through: http://dx.doi.org/10.5525/gla.researchdata.859. Unique sequences generated through this study are available through GenBank (accession numbers MK906043 to MK906046 and MN256472 to MN256489).
Funding Statement
This study was supported by a Leverhulme - Royal Society Africa Award (grant number AA130131; https://www.leverhulme.ac.uk, https://royalsociety.org). KJA was supported by the Wellcome Trust (grant number 096400/Z/11/Z; https://wellcome.ac.uk/). JEBH, GMS and DTH received support from the Research Councils UK, UK Department for International Development, and UK Biotechnology and Biological Sciences Research Council (BBSRC) (grant numbers BB/ J010367/1, BB/ L018845; http://www.bbsrc.ac.uk/). KMT received support from the Research Councils UK, UK Department for International Development, and UK Biotechnology and Biological Sciences Research Council (BBSRC) (grant number BB/L017679; http://www.bbsrc.ac.uk/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dehio C (2004) Molecular and cellular basis of Bartonella pathogenesis. Annu Rev Microbiol 58: 365–390. 10.1146/annurev.micro.58.030603.123700 [DOI] [PubMed] [Google Scholar]
- 2.Castle KT, Kosoy M, Lerdthusnee K, Phelan L, Bai Y, Gage KL, et al. (2004) Prevalence and diversity of Bartonella in rodents of northern Thailand: a comparison with Bartonella in rodents from southern China. Am J Trop Med Hyg 70: 429–433. [PubMed] [Google Scholar]
- 3.Angelakis E, Khamphoukeo K, Grice D, Newton PN, Roux V, Aplin K, et al. (2009) Molecular detection of Bartonella species in rodents from the Lao PDR. Clin Microbiol Infect 15 Suppl 2: 95–97. 10.1111/j.1469-0691.2008.02177.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okaro U, Addisu A, Casanas B, Anderson B (2017) Bartonella Species, an Emerging Cause of Blood-Culture-Negative Endocarditis. Clin Microbiol Rev 30: 709–746. 10.1128/CMR.00013-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saisongkorh W, Rolain JM, Suputtamongkol Y, Raoult D (2009) Emerging Bartonella in humans and animals in Asia and Australia. J Med Assoc Thai 92: 707–731. [PubMed] [Google Scholar]
- 6.Kosoy M, Bai Y, Sheff K, Morway C, Baggett H, Maloney SA, et al. (2010) Identification of Bartonella infections in febrile human patients from Thailand and their potential animal reservoirs. Am J Trop Med Hyg 82: 1140–1145. 10.4269/ajtmh.2010.09-0778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson BE, Neuman MA (1997) Bartonella spp. as emerging human pathogens. Clin Microbiol Rev 10: 203–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iralu J, Bai Y, Crook L, Tempest B, Simpson G, McKenzie T, et al. (2006) Rodent-associated Bartonella febrile illness, Southwestern United States. Emerg Infect Dis 12: 1081–1086. 10.3201/eid1207.040397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonilla DL, Cole-Porse C, Kjemtrup A, Osikowicz L, Kosoy M (2014) Risk factors for human lice and bartonellosis among the homeless, San Francisco, California, USA. Emerg Infect Dis 20: 1645–1651. 10.3201/eid2010.131655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breitschwerdt EB, Maggi RG, Chomel BB, Lappin MR (2010) Bartonellosis: an emerging infectious disease of zoonotic importance to animals and human beings. J Vet Emerg Crit Care (San Antonio) 20: 8–30. 10.1111/j.1476-4431.2009.00496.x [DOI] [PubMed] [Google Scholar]
- 11.Regier Y, O’Rourke F, Kempf VAJ (2016) Bartonella spp.—a chance to establish One Health concepts in veterinary and human medicine. Parasite Vector 9 ARTN 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosoy M, Murray M, Gilmore RD, Bai Y, Gage KL (2003) Bartonella strains from ground squirrels are identical to Bartonella washoensis isolated from a human patient. J Clin Microbiol 41: 645–650. 10.1128/JCM.41.2.645-650.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welch DF, Carroll KC, Hofmeister EK, Persing DH, Robison DA, Steigerwalt AG, et al. (1999) Isolation of a new subspecies, Bartonella vinsonii subsp. arupensis, from a cattle rancher: identity with isolates found in conjunction with Borrelia burgdorferi and Babesia microti among naturally infected mice. J Clin Microbiol 37: 2598–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knap N, Duh D, Birtles R, Trilar T, Petrovec M, Avsic-Zupanc T (2007) Molecular detection of Bartonella species infecting rodents in Slovenia. Fems Immunol Med Mic 50: 45–50. 10.1111/j.1574-695X.2007.00226.x [DOI] [PubMed] [Google Scholar]
- 15.Probert W, Louie JK, Tucker JR, Longoria R, Hogue R, Moler S, et al. (2009) Meningitis Due to a "Bartonella washoensis"-Like Human Pathogen. J Clin Microbiol 47: 2332–2335. 10.1128/JCM.00511-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crump JA, Morrissey AB, Nicholson WL, Massung RF, Stoddard RA, Galloway RL, et al. (2013) Etiology of Severe Non-malaria Febrile Illness in Northern Tanzania: A Prospective Cohort Study. PLoS Negl Trop Dis 7: e2324 10.1371/journal.pntd.0002324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chinga-Alayo E, Huarcaya E, Nasarre C, del Aguila R, Llanos-Cuentas A (2004) The influence of climate on the epidemiology of bartonellosis in Ancash, Peru. Trans R Soc Trop Med Hyg 98: 116–124. 10.1016/s0035-9203(03)00017-8 [DOI] [PubMed] [Google Scholar]
- 18.Breitschwerdt EB (2014) Bartonellosis: One Health Perspectives for an Emerging Infectious Disease. Ilar J 55: 46–58. 10.1093/ilar/ilu015 [DOI] [PubMed] [Google Scholar]
- 19.Gutierrez R, Vayssier-Taussat M, Buffet JP, Harrus S (2017) Guidelines for the Isolation, Molecular Detection, and Characterization of Bartonella Species. Vector-Borne Zoonot 17: 42–50. 10.1089/vbz.2016.1956 [DOI] [PubMed] [Google Scholar]
- 20.Diaz MH, Bai Y, Malania L, Winchell JM, Kosoy MY (2012) Development of a Novel Genus-Specific Real-Time PCR Assay for Detection and Differentiation of Bartonella Species and Genotypes. J Clin Microbiol 50: 1645–1649. 10.1128/JCM.06621-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meheretu Y, Leirs H, Welegerima K, Breno M, Tomas Z, Kidane D, G et al. (2013) Bartonella prevalence and genetic diversity in small mammals from Ethiopia. Vector Borne Zoonotic Diseases 13: 164–175. 10.1089/vbz.2012.1004 [DOI] [PubMed] [Google Scholar]
- 22.Halliday JE, Knobel DL, Agwanda B, Bai Y, Breiman RF, Cleaveland S, et al. (2015) Prevalence and diversity of small mammal-associated Bartonella species in rural and urban Kenya. PLoS Negl Trop Dis 9: e0003608 10.1371/journal.pntd.0003608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pretorius AM, Beati L, Birtles RJ (2004) Diversity of bartonellae associated with small mammals inhabiting Free State province, South Africa. Int J Syst Evol Microbiol 54: 1959–1967. 10.1099/ijs.0.03033-0 [DOI] [PubMed] [Google Scholar]
- 24.Laudisoit A, Falay D, Amundala N, Akaibe D, de Bellocq JG, Van Houtte N, et al. (2014) High prevalence of Rickettsia typhi and Bartonella species in rats and fleas, Kisangani, Democratic Republic of the Congo. Am J Trop Med Hyg 90: 463–468. 10.4269/ajtmh.13-0216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gundi VA, Kosoy MY, Makundi RH, Laudisoit A (2012) Identification of diverse Bartonella genotypes among small mammals from Democratic Republic of Congo and Tanzania. Am J Trop Med Hyg 87: 319–326. 10.4269/ajtmh.2012.11-0555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Billeter SA, Borchert JN, Atiku LA, Mpanga JT, Gage KL, Kosoy MY (2014) Bartonella species in invasive rats and indigenous rodents from Uganda. Vector Borne Zoonotic Diseases 14: 182–188. 10.1089/vbz.2013.1375 [DOI] [PubMed] [Google Scholar]
- 27.Home Office (2014) Guidance on the Operation of the Animals (Scientific Procedures) Act 1986.
- 28.Leary S, Underwood W, Anthony R, Cartner S, Corey D, Grandin T, et al. (2014) The AVMA Guidelines for the Euthanasia of Animals. Shaumburg, IL: American Veterinary Medical Association. [Google Scholar]
- 29.Allan KJ, Halliday JEB, Moseley M, Carter RW, Ahmed A, Goris MGA, et al. (2018) Assessment of animal hosts of pathogenic Leptospira in northern Tanzania. PLoS Negl Trop Dis 12 ARTN e0006444 10.1371/journal.pntd.0006444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cunningham D, Moors P. (1996) A guide to the identification and collection of New Zealand Rodents. Wellington: New Zealand: Wildlife Service Department. [Google Scholar]
- 31.Pratt HD, Wiseman JS (1962) Fleas of public health importance and their control. Communicable Disease Center, Atlanta, Georgia. [Google Scholar]
- 32.Billeter SA, Gundi VA, Rood MP, Kosoy MY (2011) Molecular detection and identification of Bartonella species in Xenopsylla cheopis fleas (Siphonaptera: Pulicidae) collected from Rattus norvegicus rats in Los Angeles, California. Appl Environ Microbiol 77: 7850–7852. 10.1128/AEM.06012-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanger F, Nicklen S, Coulson AR (1977) DNA Sequencing with Chain-Terminating Inhibitors. P Natl Acad Sci USA 74: 5463–5467. 10.1073/pnas.74.12.5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norman AF, Regnery R, Jameson P, Greene C, Krause DC (1995) Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J Clin Microbiol 33: 1797–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol 33: 187904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frank HK, Boyd SD, Hadly EA (2018) Global fingerprint of humans on the distribution of Bartonella bacteria in mammals. PLoS Negl Trop Dis 12: e0006865 10.1371/journal.pntd.0006865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bai Y, Hayman DTS, Mckee CD, Kosoy MY (2015) Classification of Bartonella Strains Associated with Straw-Colored Fruit Bats (Eidolon helvum) across Africa Using a Multi-locus Sequence Typing Platform. PLoS Negl Trop Dis 9 ARTN e0003478 10.1371/journal.pntd.0003478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R Core Team (2018) R: A Language and Environment for Statistical Computing. Vienna, Austria. [Google Scholar]
- 39.Dorai-Raj S (2009) binom: Binomial Confidence Intervals For Several Parameterizations. R package version 1.0–5.
- 40.Bates D, Maechler M, Bolker B, Walker S (2015) Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw 67: 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 41.Tamura K (1992) Estimation of the Number of Nucleotide Substitutions When There Are Strong Transition-Transversion and G+C-Content Biases. Mol Biol Evol 9: 678–687. 10.1093/oxfordjournals.molbev.a040752 [DOI] [PubMed] [Google Scholar]
- 42.Buffet JP, Kosoy M, Vayssier-Taussat M (2013) Natural history of Bartonella-infecting rodents in light of new knowledge on genomics, diversity and evolution. Future Microbiol 8: 1117–1128. 10.2217/fmb.13.77 [DOI] [PubMed] [Google Scholar]
- 43.Firth C, Bhat M, Firth MA, Williams SH, Frye MJ, Simmonds P, et al. (2014) Detection of zoonotic pathogens and characterization of novel viruses carried by commensal Rattus norvegicus in New York City. MBio 5: e01933–01914. 10.1128/mBio.01933-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peterson AC, Ghersi BM, Alda F, Firth C, Frye MJ, Bai Y, et al. (2017) Rodent-Borne Bartonella Infection Varies According to Host Species Within and Among Cities. Ecohealth 14: 771–782. 10.1007/s10393-017-1291-4 [DOI] [PubMed] [Google Scholar]
- 45.Gundi VA, Kosoy MY, Myint KS, Shrestha SK, Shrestha MP, Pavlin JA, et al. (2010) Prevalence and genetic diversity of Bartonella species detected in different tissues of small mammals in Nepal. Appl Environ Microbiol 76: 8247–8254. 10.1128/AEM.01180-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lipatova I, Paulauskas A, Puraite I, Radzijevskaja J, Balciauskas L, Gedminas V (2015) Bartonella infection in small mammals and their ectoparasites in Lithuania. Microbes Infect 17: 884–888. 10.1016/j.micinf.2015.08.013 [DOI] [PubMed] [Google Scholar]
- 47.Kamani J, Morick D, Mumcuoglu KY, Harrus S (2013) Prevalence and diversity of Bartonella species in commensal rodents and ectoparasites from Nigeria, West Africa. PLoS Negl Trop Dis 7: e2246 10.1371/journal.pntd.0002246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klangthong K, Promsthaporn S, Leepitakrat S, Schuster AL, McCardle PW, Kosoy M, et al. (2015) The Distribution and Diversity of Bartonella Species in Rodents and their ectoparasites across Thailand. Plos One 10 ARTN e0140856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM (2003) Introduced species and their missing parasites. Nature 421: 628–630. 10.1038/nature01346 [DOI] [PubMed] [Google Scholar]
- 50.Li X-Y, Chen J-D, Li C-R (2018) Genotype characteristics of Bartonella and the infection in rodents in Guangdong Province, China. Zhongguo Ren Shou Gong Huan Bing Za Zhi 34: 482–486. [Google Scholar]
- 51.Tay ST, Mokhtar AS, Zain SNM, Low KC (2014) Short Report: Isolation and Molecular Identification of Bartonellae from Wild Rats (Rattus Species) in Malaysia. Am J Trop Med Hyg 90: 1039–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chipwaza B, Mhamphi GG, Ngatunga SD, Selemani M, Amuri M, Mugasa JP, et al. (2015) Prevalence of bacterial febrile illnesses in children in Kilosa district, Tanzania. PLoS Negl Trop Dis 9: e0003750 10.1371/journal.pntd.0003750 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets supporting this manuscript are available through: http://dx.doi.org/10.5525/gla.researchdata.859. Unique sequences generated through this study are available through GenBank (accession numbers MK906043 to MK906046 and MN256472 to MN256489).