Abstract
The advancing age of the participants of the European Prospective Investigation into Cancer and Nutrition (EPIC)—Potsdam study was the incentive to investigate frailty as a major parameter of ageing. The aim of this study was to develop a multidimensional tool to measure frailty in an ageing, free-living study population. The “accumulation of deficits approach” was used to develop a frailty index (FI) to characterize a sub-sample (N = 815) of the EPIC-Potsdam (EPIC-P) study population regarding the aging phenomenon. The EPIC-P frailty index (EPIC-P-FI) included 32 variables from the following domains: health, physical ability, psychosocial and physiological aspects. P–values were calculated for the linear trend between sociodemographic and life style variables and the EPIC-P-FI was calculated using regression analysis adjusted for age. The relationship between the EPIC-P-FI and age was investigated using fractional polynomials. Some characteristics such as age, education, time spent watching TV, cycling and a biomarker of inflammation (C-reactive protein) were associated with frailty in men and women. Interestingly, living alone, having no partner and smoking status were only associated with frailty in men, and alcohol use and physical fitness (VO2max) only in women. The generated, multidimensional FI, adapted to the EPIC-P study, showed that this cohort is a valuable source for further exploration of factors that promote healthy ageing.
Introduction
Frailty is a concept of impairment in an elderly population and is used to describe the variability in the ageing process between individuals. Studies showed that frailty is associated with falls, malnutrition, hospitalization, institutionalization, disability and death [1–4]. The existence of frailty in an individual could impair an active and satisfying life in advanced age and is becoming increasingly common in an ageing population.
Although it is widely accepted that frailty exists, searching for an operational common definition or measurement tool of frailty is still an ongoing process [5–7]. Frailty definitions are based on two approaches: the phenotype and the accumulation of deficits approach. The phenotype approach uses the biological syndrome model of frailty, measuring weight loss, fatigue, exhaustion, weakness, low physical activity, slowness, and mobility impairment. Currently, one of the most widely used measures of physical frailty is the frailty phenotype index from Fried et al. [8] which based on a yes/no scoring system of 5 variables and assumes frailty if a person has 3 or more points. In contrast to this approach of measuring frailty, the accumulation of deficit approach uses the burden model of frailty, including symptoms, diseases, conditions and disability [9]. This approach was described by, among others, Rockwood and Mitnitski [10] and includes at least 30 deficits from different domains. These domains should have some relations to health [11]. It is still unclear if disabilities and comorbidities themselves should be a domain to define frailty or if they already constitute the adverse health outcomes.
Although the use of the deficit approach is more challenging due to the higher number of variables needed, it was argued that, compared to Fried’s frailty phenotype scale, a frailty deficit index (FI) is a more sensitive predictor for adverse health outcomes due to its finer graded scale and its multidimensionality [12]. This fine grading of the phenomenon of frailty by the deficit approach could be an advantage in prospective cohort studies due to the need of a profound understanding of the outcome “frailty”. This need is not as urgent in the clinical setting in which the Fried phenotype scale of frailty is often used to characterize patients and their treatment needs.
The European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study could benefit from a tool to define frailty in a comparable manner to other studies for further research regarding the aging phenomenon. With advanced aging of this cohort, studying physiological and lifestyle related aspects of frailty, in connection with ageing itself, seems possible due to the increasing numbers of persons potentially affected by frailty [13, 14].
Therefore, the accumulation of deficits approach of measuring frailty was used in this study to provide a multidimensional tool for the investigation of healthy ageing and to describe frailty in the EPIC-Potsdam cohort.
Materials and methods
Study population
Baseline EPIC study population
EPIC-Potsdam is one out of 23 study centers, which recruited approximately 520,000 participants in 10 European countries in 1992–1998. The EPIC study population represents the general population but was not chosen to provide representative samples. Thus, the recruitment in Potsdam was determined by practical and logistic considerations in order to obtain high participation and long-term follow-up from the study participants. The study procedures and the study population have been described previously [13, 14]. Briefly, the target population was the general population, men aged 40–64 years and women aged 35–64 years living in Potsdam and surrounding municipalities [15]. In the study center, an informed consent form had to be signed and standardized comprehensive interview and physical examinations including the collection of a blood sample had to be completed in order for the person to be accepted as a participant. At the end of the recruitment period from 1994 to 1998, 27,548 subjects had passed successfully through the examination center in Potsdam [15, 16].
Re-examined sub-sample of the original study population
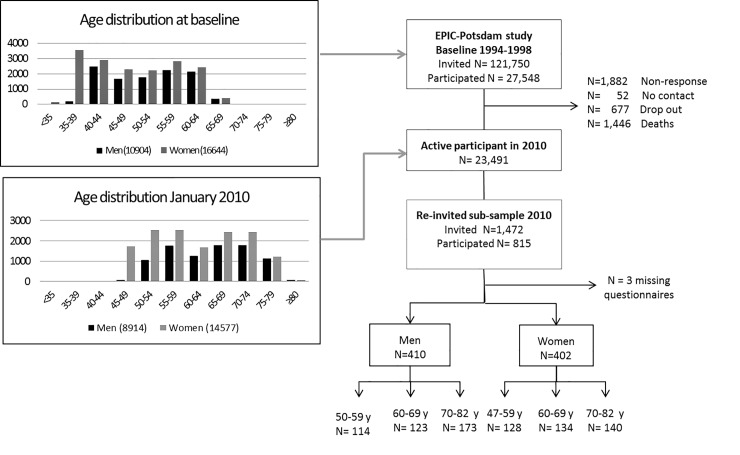
In 2010, a randomly selected sample stratified by gender and age at the time of recruitment was re-invited for a more detailed assessment of exposure variables. Sub-study participants were recruited randomly from the still available study population according to a rectangular sampling scheme, designed to include approximately 300 men and 300 women with equal representation of each of the three 10-year categories for age at baseline (35–44 years, 45–54 years and 55–64 years). 1472 active participants were invited for re-examination, with 815 (55.2%) completing the examination. All participants underwent the proof of exclusion criteria such as severe disease (i.e. heart attack or cancer 6 months prior to the appointment) and gave written informed consent prior to their examination. Participants completed extensive questionnaires, were anthropometrically re-examined and were equipped with a combined heart rate and movement sensor that objectively measured physical activity (PA) continuously over a 7-day period. Three participants did not answer one or more of the questionnaires and were excluded from the analysis. Thus, data from 812 participants (410 men, 402 women) were analyzed (Fig 1). The sub-study was approved by the ethics committee of the State Medical Association of Brandenburg (reference number S 9/2002 ma-ne and 2110/ne).
Fig 1. Flow chart of the EPIC-Potsdam participants at baseline and in the re-invited sub-sample 2010 and age distribution.
Bar graphs show the number of participants in each 5-year category (a) at baseline 1998 and (b) in 2010 for the entire cohort for men (black) and women (grey).
Data collection
Information on socio-demographic factors was available in the database from data collection at recruitment. Data on health, physical ability, psychosocial and physiological aspects were obtained by self-administered questionnaires and physical examinations at the time of the visit of the examination center. Anthropometric variables, including body weight, waist circumference (WC), height and blood pressure (BP) were obtained following standard operating protocols. Body mass index (BMI) was calculated as weight in kilogram (kg) divided by squared height in meters and waist-height-ratio (WHTR) as WC in centimeters divided by height in centimeters. Body fat mass was measured using Bioelectrical Impedance analysis. Hand-grip strength was measured using the Jamar hand dynamometer (Lafayette Instrument Company, USA). The assessment of cardiorespiratory fitness and physical activity has been described in detail previously [17]. In brief, cardiorespiratory fitness was assessed by an 8-min step test (200-mm step; Reebok, Lancaster, UK). Physical activity level (PAL) was calculated from the combined heart rate and movement monitor Actiheart (Cambridge Nurotechnology, Cambridge, UK) which was worn for 8 days by the participants.
Development of the EPIC-P-FI
The EPIC-P-FI was based on the FI framework developed by Rockwood and Mitnitski [10, 18]. According to the framework, the FI should contain at least 30 deficits, which can be: symptoms, signs, limitations and diseases. The selected deficits should be associated with health status, do not saturate too early, have more than 1% prevalence, and should cover a range of health problems and disabilities [11]. To start with, 39 variables of the EPIC-Potsdam data set reflecting deficits from the four domains of health, physical ability, psychosocial and physiological aspects were considered as potential predictors of frailty for the EPIC-P-FI. Variables were dichotomized (1 = presence of deficit; 0 = absence of deficit) or were assigned to three levels (0, 0.5 or 1 point) to reflect differences in severity. The cut-off points were set, whenever possible, according to the literature. Overweight and normal weight were coded as 0; obesity and underweight were given a point [19, 20]. Furthermore, presence of deficit was defined as: WHTR of more than 0.6 [21], the highest quintile of body fat percentage (adjusted for gender), malnutrition (energy intake under 21 kcal per day per kg bodyweight) [22], PAL under 1.4, (very inactive people) [23], losing more than 5% bodyweight in two years without intending it (unwanted weight loss) [8] and using more than five medications (polypharmacy) [24]. The cut-off for hand-grip-strength was set according to BMI and gender as performed by Fried et al. [8].
Thirty-nine potential variables were analyzed for multicollinearity to exclude variables that were not adding information to the FI. Seven variables were excluded by this procedure leaving 32 variables for the final FI. The EPIC-P-FI was calculated by adding all present deficits divided by the total number of deficits available for each participant to produce an EPIC-P-FI between 0 and 1. The individual domains were calculated in the same way: 12 deficits in health, eight deficits in physical ability, seven deficits in psychosocial aspects and five deficits in physiological aspects. Additionally, in a sensitivity analysis, a reduced FI was calculated with 23 deficits (excluding chronic diseases) and compared with the complete EPIC-P-FI. Prevalent diseases (tumor, diabetes, myocardial infarction, stroke, Transient Ischemic Attack, heart failure, angina pectoris, hypertension, osteoporosis) were included. This originates from the fact that it is still unclear if comorbidities are an essential part of frailty or an adverse health outcome of frailty.
Statistical analysis
All analyses were carried out independently in men and women [3]. Sensitivity analyses for participants with missing values were performed to see if findings are sensitive to the inclusion or exclusion of these participants from the analysis. Complete data for all EPIC-P-FI deficits were available for 62% (n = 506) of all participants, 25% (n = 200) had one missing value, 10% (n = 79) had two missing values and the remaining 3% (n = 27) had three to five missing values. Since many participants had missing data for grip strength (male: n = 40, female: n = 51) and PAL (male: n = 62, female: n = 67), a Mann-Whitney U test was conducted to analyze if these participants differ from participants with available data for hand-grip strength and PAL respectively.
Multiple imputation procedures were applied for all variables included in the EPIC-P-FI. The SAS procedure PROC MI was used to create 10 imputed data sets with a maximum number of iterations set at 200 with all variables used to construct the EPIC-P-FI. The correlation between the original EPIC-P-FI and the imputed EPIC-P-FI was very high (R2 = 0.99, P<0.001), and thus we preferred to use the original EPIC-P-FI for analysis.
Descriptive statistics were used to describe demographic characteristics of the study population. Means were age-adjusted to account for different age distributions. The P -values for trend with increasing EPIC-P-FI values were calculated using regression analysis adjusted for age (for all variables except for the variable age). Differences between groups were calculated with chi-square test for categorical data, Kruskal-Wallis test for continuous data, Fisher’s exact test when expected cell counts were smaller than five. Participants were grouped into the categories low, medium and high EPIC-P-FI values based on gender-specific EPIC-P-FI tertiles. The relationship between the EPIC-P-FI values and age was investigated using fractional polynomials (SAS macro mfp8). P<0.05 was used for establishing statistical significance. Statistical analyses were done using SAS Software, version 9.4.
Results
Characteristics of the EPIC-P-FI
Thirty-two variables from four domains (twelve variables in health, seven variables in psychosocial aspects, eight variables in physical ability and five variables in physiological aspects) were included to calculate the EPIC-P-FI. The prevalence of individual EPIC-P-FI variables s presented in the supplemental material (S1 and S2 Tables).
The distribution of the EPIC-P-FI score was skewed to the right, in both men and women from the EPIC-Potsdam study. The EPIC-P-FI had an upper 99% limit of 0.52 for men and women with a range from 0–0.60 (mean = 0.16, median = 0.14) in men and 0–0.70 (mean = 0.18, median = 0.16) in women.
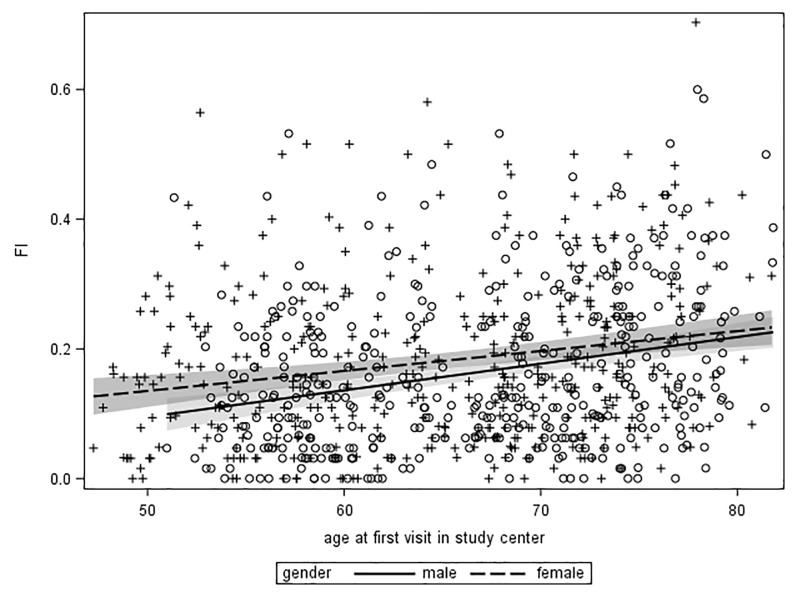
The mean EPIC-P-FI scores were slightly lower in men in all age groups compared to women, and increased across age groups in men (<60 years: FI = 0.13; 60–70 years: FI = 0.15 and >70 years: FI = 0.19, P-trend < .0001) and in women (<60 years: FI = 0.15; 60–70 years: FI = 0.17 and >70 years: FI = 0.22, P-trend < .0001). The EPIC-P-FI correlated linearly with age in men (R2 = 0.07, P<0.001) and women (R2 = 0.04, P<0.001, Fig 2) and increased, on average per year of age, by 0.004 (95% CI: 0.003, 0.006) in men and by 0.003 (95% CI: 0.002, 0.004) in women.
Fig 2. The distribution of the EPIC-P-FI score in relation to age in years in men and women.
Dots for individual data points and prediction lines are shown in the scatter plot, the shaded areas denote the 95% CI for the prediction for men (light) and women (dark).
Correlation of individual domains to the overall EPIC-P-FI
Variables from the four domains of health, physical ability, psychosocial and physiological aspects were combined to calculate the total EPIC-P-FI. In men and women, the domain physical ability showed the highest correlation with the EPIC-P-FI score (ρ = 0.81, P<0.001 and ρ = 0.86, P<0.001 respectively, Table 1).
Table 1. Correlation coefficient rho (ρ) between the total EPIC-P-FI score, the four domains and age.
| Men | |||||
| EPIC-P-FI | Psychosocial aspects | Health | Physical ability | Physiological aspects | |
| Psychosocial aspects | 0.60a | 1 | |||
| Health | 0.76a | 0.23a | 1 | ||
| Physical ability | 0.81a | 0.48a | 0.52a | 1 | |
| Physiological aspects | 0.51a | 0.05 | 0.23a | 0.12a | 1 |
| Age | 0.27a | 0.05 | 0.36a | 0.30a | -0.04 |
| Women | |||||
| EPIC-P-FI | Psychosocial aspects | Health | Physical ability | Physiological aspects | |
| Psychosocial aspects | 0.69a | 1 | |||
| Health | 0.70a | 0.29a | 1 | ||
| Physical ability | 0.86a | 0.54a | 0.54a | 1 | |
| Physiological aspects | 0.52a | 0.08 | 0.19a | 0.23a | 1 |
| Age | 0.21a | -0.02 | 0.30a | 0.18a | 0.12a |
a P-value < 0.05
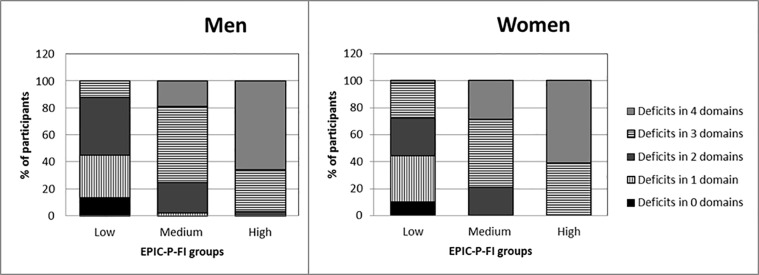
The lowest correlation was between the EPIC-P-FI score and physiological aspects in men and women (ρ = 0.51, P<0.001 and ρ = 0.52, P<0.001 respectively). Of the four domains, the health domain presented the highest correlation with age of men and women (ρ = 0.36, P<0.001 and ρ = 0.30, P<0.001 respectively). The correlation between individual domains ranged from 0.05 (P = 0.2) to 0.54 (P<0.001). Most people in the high EPIC-P-FI group had deficits in three or four domains, with more than 50% of people having deficits in all four domains (Fig 3). In contrast, in the low EPIC-P-FI group most people had no deficits or deficits in only one or two domains.
Fig 3. Number of domains with deficits in the low, medium and high EPIC-P-FI group.
Stacked bar graphs show the percentage of participants with deficits in none (black), 1 (vertical stripes), 2 (dark grey), 3 (horizontal stripes) or 4 (light grey) of the domains for men (left) and women (right) in the low, medium and high EPIC-P-FI group based on EPIC-P-FI score tertiles.
Distribution of characteristics among the EPIC-Potsdam study population across categories of frailty
Characteristics of the EPIC-Potsdam study population are presented in Tables 2 and 3. A total of 410 men and 402 women were included in the analyses. The mean age was 67 years for men and 65 years for women at the time of examination. Participants with missing data for handgrip strength were older (men 70 years vs 67 years P = 0.004; women 67 years vs 65 years P = 0.001) and had higher EPIC-P-FI values (men 0.23 vs 0.15, P<0.001; women 0.22 vs 0.17, P = 0.04) than participants with available hand grip strengths measurements. Participants with missing data for PAL were not different to participants with available PAL measurements. Sensitivity analysis indicated that the reduced FI did not significantly differ from the complete EPIC-P-FI.
Table 2. Characteristics of 410 Men in the EPIC-Potsdam Sub-Study Population by EPIC-P-FI Score in 2010.
| Men | ||||||
|---|---|---|---|---|---|---|
| EPIC-P-FI groups | ||||||
| Variable | Low | Medium | High | P-trend | Total | |
| FI score (mean (min—max)) | 0.04 (0.00–0.08) | 0.14 (0.09–0.20) | 0.31 (0.21–0.60) | 0.16 (0.00–0.60) | ||
| Socio- | Age in years (mean (SE)) | 64.3 (0.7) | 66.7 (0.6) | 69.6 (0.7) | <0.001 | 66.9 (0.4) |
| demographic | Living with other people (%)a | 95.1 | 90.6 | 79.9 | <0.001 | 88.5 |
| factors | Having a partner (%)a | 93.3 | 87.9 | 79.4 | 0.001 | 86.8 |
| Profession (%)a b | ||||||
| higher grade professional | 45.1 | 34.3 | 25.0 | 34.6 | ||
| lower grade professional | 37.4 | 45.0 | 54.2 | 45.6 | ||
| skilled/non-manual worker | 17.1 | 16.0 | 15.2 | 16.1 | ||
| simple manual worker | 0.4 | 4.7 | 5.7 | 3.7 | ||
| Education (%)a | ||||||
| no vocational/vocational training | 18.6 | 31.4 | 38.6 | 29.8 | ||
| technical college | 12.5 | 14.9 | 19.4 | 15.6 | ||
| university | 68.9 | 53.7 | 41.9 | 54.6 | ||
| Lifestyle | Alcohol g/day (mean (SE))a | 14.6 (1.6) | 16.5 (1.5) | 16.6 (1.6) | 0.378 | 16.0 (0.9) |
| factors | Smoking status (%)a | |||||
| never smoker | 45.5 | 34.5 | 17.9 | 32.6 | ||
| ex-smoker | 46.3 | 57.4 | 65.6 | 56.5 | ||
| Smoker | 8.3 | 8.0 | 16.5 | 10.9 | ||
| Sport h/week (mean (SE))a | 3.0 (0.3) | 2.2 (0.3) | 2.2 (0.3) | 0.067 | 2.5 (0.2) | |
| Walking h/week (mean (SE))a | 7.0 (0.7) | 7.8 (0.6) | 8.9 (0.7) | 0.058 | 7.9 (0.4) | |
| DIY h/week (mean (SE))a | 5.1 (0.6) | 4.0 (0.5) | 4.7 (0.6) | 0.602 | 4.6 (0.3) | |
| Housework h/week (mean (SE))a | 4.8 (0.6) | 5.2 (0.6) | 6.2 (0.6) | 0.104 | 5.4 (0.3) | |
| Cycling h/week (mean (SE))a | 3.7 (0.3) | 3.1 (0.3) | 2.4 (0.3) | 0.002 | 3.1 (0.2) | |
| Gardening h/week (mean (SE))a | 5.1 (0.5) | 5.2 (0.5) | 5.3 (0.5) | 0.769 | 5.2 (0.3) | |
| Sleep day h/day (mean (SE))a | 0.4 (0.08) | 0.6 (0.07) | 0.7 (0.08) | 0.018 | 0.6 (0.04) | |
| Sleep night h/night (mean (SE))a | 7.3 (0.08) | 7.4 (0.08) | 7.3 (0.09) | 0.990 | 7.3 (0.05) | |
| Watch TV h/day (mean (SE))a | 2.7 (0.1) | 2.9 (0.1) | 3.3 (0.1) | 0.003 | 3.0 (0.1) | |
| Biological | CRP in μg/l (mean (SE))a | 1.8 (0.6) | 1.8 (0.5) | 4.3 (0.6) | 0.002 | 2.6 (0.3) |
| factors | Blood pressure in mm Hg (mean (SE))a | |||||
| Diastolic | 79.7 (0.8) | 79.8 (0.7) | 78.6 (0.8) | 0.341 | 79.4 (0.5) | |
| Systolic | 137.3 (1.3) | 138.6 (1.2) | 136.4 (1.3) | 0.651 | 137.5 (0.7) | |
| VO2max (mean (SE))a | 29.8 (0.5) | 28.9 (0.5) | 29.3 (0.6) | 0.504 | 29.3 (0.3) | |
a values are age-adjusted
b current profession or if retired, the last carried out profession
Table 3. Characteristics of 402 Women in the EPIC-Potsdam Sub-Study Population by EPIC-P-FI Score in 2010.
| Women | ||||||
|---|---|---|---|---|---|---|
| EPIC-P-FI score | ||||||
| Variable | Low | Medium | High | P-trend | Total | |
| FI score (mean (min—max)) | 0.05 (0.00–0.10) | 0.16 (0.11–0.22) | 0.33 (0.22–0.70) | 0.18 (0–0.7) | ||
| Socio- | Age in years (mean (SE)) | 63.2 (0.7) | 65.0 (0.7) | 66.9 (0.8) | <0.0001 | 65.0 (0.4) |
| demographic | Living with other people (%)a | 75.7 | 77.3 | 71.6 | 0.443 | 74.9 |
| factors | Having a partner (%)a | 73.3 | 75.9 | 70.2 | 0.574 | 73.1 |
| Profession (%)a, b | ||||||
| higher grade professional | 16.8 | 21.2 | 25.0 | 21.0 | ||
| lower grade professional | 70.3 | 68.6 | 60.5 | 66.5 | ||
| skilled/non-manual worker | 12.0 | 8.0 | 11.6 | 10.5 | ||
| simple manual worker | 0.9 | 2.2 | 2.9 | 2.0 | ||
| Education (%)a | ||||||
| no vocational/vocational training | 33.5 | 32.1 | 41.5 | 35.7 | ||
| technical college | 32.2 | 32.9 | 26.9 | 30.7 | ||
| university | 34.3 | 35.0 | 31.6 | 33.7 | ||
| Lifestyle | Alcohol g/day (mean (SE))a | 10.7 (1.0) | 10.6 (1.0) | 7.0 (1.0) | 0.013 | 9.4 (0.06) |
| factors | Smoking status(%)a | |||||
| never smoker | 61.1 | 58.5 | 62.2 | 60.6 | ||
| ex-smoker | 27.0 | 32.0 | 29.1 | 29.4 | ||
| Smoker | 11.8 | 9.4 | 8.7 | 10.0 | ||
| Sport h/week (mean (SE))a | 2.8 (0.3) | 2.2 (0.3) | 2.5 (0.3) | 0.530 | 2.5 (0.2) | |
| Walking h/week (mean (SE))a | 7.4 (0.5) | 6.9 (0.5) | 7.1 (0.5) | 0.694 | 7.2 (0.3) | |
| DIY h/week (mean (SE))a | 1.3 (0.3) | 1.2 (0.3) | 0.6 (0.3) | 0.203 | 1.0 (0.2) | |
| Housework h/week (mean (SE))a | 14.3 (0.7) | 13.5 (0.7) | 14.3 (0.7) | 0.980 | 14.0 (0.5) | |
| Cycling h/week (mean (SE))a | 3.5 (0.3) | 2.6 (0.3) | 2.3 (0.3) | 0.013 | 2.8 (0.2) | |
| Gardening h/week (mean (SE))a | 4.5 (0.4) | 4.2 (0.4) | 4.3 (0.4) | 0.742 | 4.3 (0.3) | |
| Sleep day h/day (mean (SE))a | 0.2 (0.04) | 0.2 (0.04) | 0.4 (0.04) | 0.002 | 0.3 (0.03) | |
| Sleep night h/night (mean (SE))a | 7.4 (0.1) | 7.1 (0.1) | 7.0 (0.1) | 0.011 | 7.2 (0.1) | |
| Watch TV h/day (mean (SE))a | 2.8 (0.1) | 2.8 (0.1) | 3.0 (0.1) | 0.178 | 2.9 (0.07) | |
| Biological | CRP in μg/l (mean (SE)) a | 2.5 (0.6) | 3.2 (0.5) | 4.4 (0.6) | 0.019 | 3.4 (0.3) |
| factors | Blood pressure in mm Hg (mean (SE)) a | |||||
| diastolic | 77.4 (0.8) | 79.7 (0.8) | 78.3 (0.8) | 0.437 | 78.5 (0.5) | |
| systolic | 133.3 (1.4) | 136.3 (1.4) | 133.0 (1.4) | 0.914 | 134.2 (0.9) | |
| VO2max (mean (SE))a | 28.0 (0.4) | 26.8 (0.4) | 26.8 (0.5) | 0.045 | 27.3 (0.3) | |
a values are age-adjusted
b current profession or if retired, the last carried out profession
A small percentage of participants were current smokers (11% of men and 10% of women). Many of the participants stopped smoking (57% of men and 29% of women). The minority of men (12%) and women (25%) were single and lived on their own. Around one third of the participants (37% for men and women) were still working.
Men with high EPIC-P-FI scores were older, more likely to be without a partner and live alone, less educated and less likely to work (Table 2). In women, the same trends could be observed (Table 3). Since the groups of men and women with a high FI were older, the mean values for lifestyle and biological factors were age-adjusted. Men with high EPIC-P-FI values were more likely to smoke, sleep more during the day, cycle less and watch more TV than men with low EPIC-P-FI values (Table 2). Women with high EPIC-P-FI values were more likely to consume less alcohol, watch more TV, sleep more during the day and less during the night and cycle less than women with low EPIC-P-FI scores (Table 3).
Regarding biological factors, both men and women with high EPIC-P-FI values had higher C-reactive protein (CRP) levels than participants with a low EPIC-P-FI score (Tables 2 and 3). In women, VO2max levels were negatively associated with the EPIC-P-FI values. In men, no meaningful associations with the EPIC-P-FI values were observed with blood pressure or cardio-metabolic fitness measured by VO2max. Most of the individuals in the high EPIC-P-FI group had deficits in three or four domains, with more than 50% of people having deficits in all four domains. In contrast, in the low EPIC-P-FI group most people had no deficits or deficits in only one or two domains (Fig 3).
Discussion
The current study provides a multidimensional tool to measure healthy ageing and frailty. This tool was established following an already published guideline and based on health-related questions and examinations conducted in a US cohort of older adults [11]. This newly developed EPIC-P-FI included 32 deficit variables. Men appeared to be slightly less affected by frailty compared to women, with frailty increasing with age in both groups. The physical ability domain showed the highest correlation with the total EPIC-P-FI and the lowest with the physiological domain. Individuals with a high FI value were more likely to sleep more during the day and less during the night, were less physical active i.e. cycling, watched more TV and had higher CRP levels than those with low FI values.
Frailty represents a state of heightened vulnerability in the aging process [25]. In the clinical setting, the Fried’s phenotype scale with its five evaluations is a convenient tool and satisfies the applicants through its quick assessment. However, in epidemiological studies, the measurement of frailty through a FI seems to be more useful [26]. By concept, the accumulation of deficit approach allows the assessment of overall frailty, but also specific deficits and domains and thus, is more suitable to provide a mechanistic understanding of the aging phenomenon [27]. The FI is a robust tool independently of the number of deficits used per domain and also of the number of domains [28–30]. The study by Abete et al showed [31] FI as valid measure of frailty after comprehensive geriatric assessment in an Italian cohort of non-institutionalized patients. Further use of this FI in Italian outpatients older than 65 indicated higher comorbidity and disability in patients with chronic heart failure (CHF), where increased more in presence than in the absence of CHF, with increasing frailty [32]. In the current study, the deficits had been grouped into four domains (health, physical ability, psychosocial and physiological domains). Most of the study participants with highly EPIC-P-FI values had deficits in three or four domains. Deficits were seen in all possible combinations of domains which indicate that frailty presents itself differently in each individual. It is still unclear which domains and which combinations thereof are the most important contributors or indicators of frailty and its health consequences and if it is possible for individuals to compensate deficits in one domain with resources in another domain [33]. Although the multidimensional approach (using nutritional status, physical activity, cognition, mood, social relations and psychological health among others) to measure frailty has been frequently used, only few of the studies reported the impact of individual domains [34–42]. Our study supported the view that several domains should be used for the assessment of frailty and should also be analyzed individually [36, 41]. We should take into account that accuracy and precision can be for selected domains can be differentiated (for example higher for health domain)
The EPIC-P-FI characteristics, such as range, distribution and association with age in the EPIC-Potsdam cohort are similar to those of other published FIs [3, 28, 43–52]. The approach of calibrating the obtained points to the total number of variables is very straightforward, particularly in respect to comparability with other study results. In our experience, neither the number of variables, nor a difference in estimating a deficit will affect the comparability of different FIs if the same principle of constructing the FI had been used. Further, although not a validation in itself, the characteristics indicate that the application of the EPIC-P-FI can generate meaningful results. Sensitivity of the FI was evaluated with a reduced FI calculated with 23 deficits (excluding prevalent chronic diseases). This FI was compared with the complete EPIC-P-FI. This analysis could be considered as a proxy analysis compared to a full analysis of the validity. Especially the increase of the FI with age is considered an indicator of construct validity [3], even though it is just consequential that the total FI score also increases with age if each deficit increases with age [11].
Although frailty differs in men and women, many studies did not analyze frailty separately for gender [42, 46, 53–56]. It is still unknown why women acquire more deficits at a given age but have a lower mortality than men [57]. There may be some factors affecting life expectancy in older people that are not captured by current frailty measures and that these factors are present more often in men than in women. To account for and learn more about gender differences, men and women were analyzed separately in the current study. Having no partner and living alone was associated with frailty in men but not in women while lower education was associated with frailty in men and women, as previously shown [35, 58–63].
Those who were frail were less likely to be active socially. On the other hand, it is possible that active participation in society may retard the onset of frailty [62]. An association between smoking and frailty was observed in men, but not in women in the EPIC-Potsdam cohort. Most studies showed a positive association between smoking and frailty in both genders [56]. Similar to our results, Wang et al. found that smoking and frailty were only associated in men and not in women [55]. As suggested by Woo et al [62] this may be a survivor effect, in that those who were susceptible to smoking-related diseases might have died at an earlier age, and an elderly cohort population is largely consisting of such survivors.
Consuming moderate amounts of alcohol was negatively associated with frailty in women but not in men in the EPIC-Potsdam cohort. Only a few studies have previously analyzed the impact of drinking alcohol on frailty and found that moderate alcohol intake was associated with less frailty in both genders [62, 63]. There was also a trend that frail people slept more during the day and less at night. Mixed results have been published about sleep duration and frailty [64–65]. One reason for this might be the U-shaped association between sleep duration and health. It has been shown that frailty and a long night time sleep duration of 10 or more hours were independently associated with 5-year mortality in older adults [64].
Watching TV was positively associated with frailty in men and women in the EPIC-Potsdam cohort, which is in line with the known link between sedentary behavior and unfavorable health outcome [66–68]. The mechanisms through which television time is associated with metabolic risk, even in this healthy subpopulation, are likely to be of both physiological and behavioral origin. Physiologically, there is emerging evidence that sedentary behavior results in unique metabolic outcomes. Behaviorally, it has been suggested that sedentary time is associated with health outcomes as it displaces time in only a single leisure-time sedentary behavior was measured [66]. On the other hand, no association was observed between self-reported physical activity (except cycling) and frailty. This might be explained by known discrepancies between self-reported and objectively measured physical activity [69]. An association was seen between the physical fitness measured by VO2max and frailty in women, but not in men in the EPIC-Potsdam cohort.
Chronic inflammation has been shown to be associated with frailty and CRP levels have often been used as a marker for inflammation [70, 71]. It was expected that CRP will be more strongly predictive of frailty incidence in women, but accordingly, men and women with high EPIC-P-FI values had higher levels of CRP than people with low EPIC-P-FI values. However, the underlying pathomechanisms are still poorly defined. CRP levels negatively correlated with the rate of skeletal muscle protein synthesis, which may support the idea that low-grade inflammation is implicated in sarcopenia development in frail people [71].
Limitations and strengths
Selection bias might be more pronounced in elderly people, and especially frail people might be underrepresented since they refuse to endure long procedure of examinations. Another problem in generating a FI is the choice of variables and domains and the categorization there, since there is no gold standard yet. So far, the EPIC-P-FI must be considered to be in a developmental stage and therefore, should be validated before applying it to other populations. One of the strengths is the use of the population-based prospective EPIC-Potsdam study whose participants underwent a highly standardized procedure of examinations. The multidimensional EPIC-P-FI developed for this study covers four important domains instead of just the physical domain [7]. The combination of objectively measured and self-reported data is a strong point of this study as is the inclusion of middle-aged people (50–65 years) who are often excluded from frailty research [72] but may give the opportunity to investigate the early changes leading to frailty.
Conclusions
The multidimensional EPIC-P-FI produced a rich assessment of frailty in the EPIC cohort. It is of high importance to investigate frailty separately in both genders since different factors might be involved in the development of frailty in men and women. The EPIC cohort, monitored for more than 20 years, offers the potential to analyze lifestyle factors, health transitions, mortality and frailty prospectively and retrospectively. Exploring frailty in this well-established German population will provide further insights on ageing-associated processes, help to identify factors that predispose to frailty and thereby promote healthy longevity.
Supporting information
(PDF)
(PDF)
Acknowledgments
The preparation of this paper was supported by the MAlNUtrition in the ELderly (MaNuEL) knowledge hub. This work is supported by the Joint Programming Initiative ‘Healthy Diet for a Healthy Life’. The funding agency supporting this work is Germany: Federal Ministry of Food and Agriculture (BMEL) represented by Federal Office for Agriculture and Food (BLE).
We thank the Human Study Centre (HSC) of the German Institute of Human Nutrition Potsdam-Rehbrücke, namely the trustee and the examination unit for the collection of the data and the data hub for the processing of the data.
Data Availability
The EPIC-Potsdam data are ethically and legally restricted because it is an ongoing study with data linked to identifying information. In accordance with German Federal and State data protection regulations, epidemiological data analyses of EPIC-Potsdam may be initiated upon an informal inquiry addressed to the secretariat of the Human Study Center (Office.HSZ@dife.de). Each request will then have to pass a formal process of application and review by the respective PI and a scientific board.
Funding Statement
This work is supported by the Joint Programming Initiative ‘Healthy Diet for a Healthy Life’. The funding agency supporting this work is Germany: Federal Ministry of Food and Agriculture (BMEL) represented by Federal Office for Agriculture and Food (BLE). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Romero-Ortuno R, Kenny RA. The frailty index in Europeans: association with age and mortality. Age Ageing. 2012; 41:684–689. 10.1093/ageing/afs051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Ageing Res Rev. 2013; 12:719–736. 10.1016/j.arr.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 3.Saum KU, Dieffenbach AK, Muller H, Holleczek B, Hauer K, Brenner H. Frailty prevalence and 10-year survival in community-dwelling older adults: results from the ESTHER cohort study. Eur J Epidemiol. 2014; 29:171–179 10.1007/s10654-014-9891-6 [DOI] [PubMed] [Google Scholar]
- 4.Dorner TE, Luger E, Tschinderle J, Stein KV, Haider S, Kapan A, et al. Association between Nutritional Status (MNA(R)-SF) and Frailty (SHARE-FI) in Acute Hospitalised Elderly Patients. J Nutr Health Aging 2014; 18:264–269. 10.1007/s12603-013-0406-z [DOI] [PubMed] [Google Scholar]
- 5.Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S. et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci 2007; 62: 731–737. 10.1093/gerona/62.7.731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez-Manas L, Feart C, Mann G, Viña J, Chatterji S, Chodzko-Zajko W. et al. Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci 2013; 68: 62–67. 10.1093/gerona/gls119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouillon K, Kivimaki M, Hamer M, Sabia S, Fransson EI, Singh-Manoux A. et al. Measures of frailty in population-based studies: an overview. BMC Geriatr 2013;13: 64 10.1186/1471-2318-13-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J. et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001; 56: 146–156. [DOI] [PubMed] [Google Scholar]
- 9.Kane RL, Shamliyan T, Talley K, Pacala J. The association between geriatric syndromes and survival. J Am Geriatr Soc. 2012; 60: 896–904. 10.1111/j.1532-5415.2012.03942.x [DOI] [PubMed] [Google Scholar]
- 10.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I. et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173: 489–495. 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8:24 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci 2007; 62: 738–743. 10.1093/gerona/62.7.738 [DOI] [PubMed] [Google Scholar]
- 13.Boeing H, Korfmann A, Bergmann MM. Recruitment procedures of EPIC-Germany. European Investigation into Cancer and Nutrition. Ann Nutr Metab 1999; 43: 205–215. 10.1159/000012787 [DOI] [PubMed] [Google Scholar]
- 14.Riboli E, Kaaks R. The EPIC Project: rationale and study design. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol 1997; 26 Suppl 1:S6–14. [DOI] [PubMed] [Google Scholar]
- 15.Boeing H, Wahrendorf J, Becker N. EPIC-Germany-A source for studies into diet and risk of chronic diseases. European Investigation into Cancer and Nutrition. Ann Nutr Metab 1999; 43: 195–204. 10.1159/000012786 [DOI] [PubMed] [Google Scholar]
- 16.Kroke A, Bergmann MM, Lotze G, Jeckel A, Klipstein-Grobusch K, Boeing H. Measures of quality control in the German component of the EPIC study. European Prospective Investigation into Cancer and Nutrition. Ann Nutr Metab 1999; 43: 216–224. 10.1159/000012788 [DOI] [PubMed] [Google Scholar]
- 17.Wientzek A, Tormo Diaz MJ, Castano JM, Amiano P, Arriola L, Overvad K. et al. Cross-sectional associations of objectively measured physical activity, cardiorespiratory fitness and anthropometry in European adults. Obesity 2014; 22: 127–134. [DOI] [PubMed] [Google Scholar]
- 18.Mitnitski AB, Song X, Rockwood K. The estimation of relative fitness and frailty in community-dwelling older adults using self-report data. J Gerontol A Biol Sci Med Sci 2004; 59: 627–632. [DOI] [PubMed] [Google Scholar]
- 19.Hoover M, Rotermann M, Sanmartin C, Bernier J. Validation of an index to estimate the prevalence of frailty among community-dwelling seniors. Health Rep 2013; 24: 10–17. [PubMed] [Google Scholar]
- 20.Hubbard RE, Lang IA, Llewellyn DJ, Rockwood K. Frailty, body mass index, and abdominal obesity in older people. J Gerontol A Biol Sci Med Sci 2010; 65: 377–381. 10.1093/gerona/glp186 [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Gonzalez MA, Garcia-Arellano A, Toledo E, Bes-Rastrollo M, Bulló M, Corella D. et al. Obesity indexes and total mortality among elderly subjects at high cardiovascular risk: the PREDIMED study. PloS One 2014; 9: e103246 10.1371/journal.pone.0103246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartali B, Frongillo EA, Bandinelli S, Lauretani F, Semba RD, Fried LP. et al. Low nutrient intake is an essential component of frailty in older persons. J Gerontol A Biol Sci Med Sci 2006; 61: 589–593. 10.1093/gerona/61.6.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.FAO/WHO/UNU. Human energy requirements: Report of a Joint FAO/WHO/UNU Expert Consultation. Rome, 2001.
- 24.Clegg A, Rogers L, Young J. Diagnostic test accuracy of simple instruments for identifying frailty in community-dwelling older people: a systematic review. Age Ageing 2015; 44: 148–152 10.1093/ageing/afu157 [DOI] [PubMed] [Google Scholar]
- 25.Eeles E, Low Choy N. Frailty and Mobility. Interdiscip Top Gerontol Geriatr 2015; 41: 107–120. 10.1159/000381200 [DOI] [PubMed] [Google Scholar]
- 26.Lang PO, Michel JP, Zekry D. Frailty syndrome: a transitional state in a dynamic process. Gerontology 2009; 55: 539–549. 10.1159/000211949 [DOI] [PubMed] [Google Scholar]
- 27.Hubbard RE. Sex Differences in Frailty Interdiscip Top Gerontol Geriatr. 2015; 41: 41–53. [DOI] [PubMed] [Google Scholar]
- 28.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorld Journal 2001; 1: 323–336. 10.1100/tsw.2001.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc 2008; 56: 898–903. 10.1111/j.1532-5415.2008.01656.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr 2002; 2: 1 10.1186/1471-2318-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abete P, Basile C, Bulli G, Curcio F, Liguori I, Della-Morte D, et al. The Italian version of the "frailty index" based on deficits in health: a validation study. Aging Clin Exp Res. 2017;29:913–926. 10.1007/s40520-017-0793-9 [DOI] [PubMed] [Google Scholar]
- 32.Testa G, Liguori I, Curcio F, Russo G, Bulli G, Galizia G, et al. Multidimensional frailty evaluation in elderly outpatients with chronic heart failure: A prospective study. Eur J Prev Cardiol. 2019;26:1115–1117. 10.1177/2047487319827460 [DOI] [PubMed] [Google Scholar]
- 33.Dapp U, Minder CE, Anders J, Golgert S, von Renteln-Kruse W. Long-term prediction of changes in health status, frailty, nursing care and mortality in community-dwelling senior citizens—results from the longitudinal urban cohort ageing study (LUCAS). BMC Geriatr 2014; 14: 141 10.1186/1471-2318-14-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pialoux T, Goyard J, Lesourd B. Screening tools for frailty in primary health care: a systematic review. Geriatr Gerontol Int 2012; 12: 189–197. 10.1111/j.1447-0594.2011.00797.x [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Garcia FJ, Carcaillon L, Fernandez-Tresguerres J, Alfaro A, Larrion JL, Castillo Ce. t al. A new operational definition of frailty: the frailty trait scale. J Am Med Dir Assoc 2014; 15: 371 e7– e13. [DOI] [PubMed] [Google Scholar]
- 36.Sourial N, Wolfson C, Bergman H, Zhu B, Karunananthan S, Quail J. et al. A correspondence analysis revealed frailty deficits aggregate and are multidimensional. J Clin Epidemiol 2010; 63: 647–654. 10.1016/j.jclinepi.2009.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35: 526–529. 10.1093/ageing/afl041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung HW, Kim SW, Ahn S, Lim JY, Han JW, Kim TH. et al. Prevalence and outcomes of frailty in Korean elderly population: comparisons of a multidimensional frailty index with two phenotype models. PloS one 2014; 9: e87958 10.1371/journal.pone.0087958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tocchi C, Dixon J, Naylor M, Jeon S, McCorkle R. Development of a frailty measure for older adults: the frailty index for elders. J Nurs Meas 2014; 22: 223–240. [DOI] [PubMed] [Google Scholar]
- 40.Goggins WB, Woo J, Sham A, Ho SC. Frailty index as a measure of biological age in a Chinese population. J Gerontol A Biol Sci Med Sci 2005; 60: 1046–1051. 10.1093/gerona/60.8.1046 [DOI] [PubMed] [Google Scholar]
- 41.Bielderman A, van der Schans CP, van Lieshout MR, de Greef MH, Boersma F, Krijnen WP. et al. Multidimensional structure of the Groningen Frailty Indicator in community-dwelling older people. BMC Geriatr 2013; 13: 86 10.1186/1471-2318-13-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drubbel I, de Wit NJ, Bleijenberg N, Eijkemans RJ, Schuurmans MJ, Numans ME. Prediction of adverse health outcomes in older people using a frailty index based on routine primary care data. J Gerontol A Biol Sci Med Sci 2013; 68: 301–308. 10.1093/gerona/gls161 [DOI] [PubMed] [Google Scholar]
- 43.Romero-Ortuno R. Frailty Index in Europeans: Association with determinants of health. Geriatr Gerontol Int 2014; 14: 420–429. 10.1111/ggi.12122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones D, Song X, Mitnitski A, Rockwood K. Evaluation of a frailty index based on a comprehensive geriatric assessment in a population based study of elderly Canadians. Aging Clin Exp Res 2005; 17: 465–471. [DOI] [PubMed] [Google Scholar]
- 45.Drubbel I, Bleijenberg N, Kranenburg G, Eijkemans RJ, Schuurmans MJ, de Wit NJ. et al. Identifying frailty: do the Frailty Index and Groningen Frailty Indicator cover different clinical perspectives? a cross-sectional study. BMC Family Practice 2013; 14: 64 10.1186/1471-2296-14-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi J, Yang Z, Song X, Yu P, Fang X, Tang Z et al. Sex Differences in the Limit to Deficit Accumulation in Late Middle-Aged and Older Chinese People: Results From the Beijing Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci 2008; 69: 702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rockwood K, Mitnitski A. Limits to deficit accumulation in elderly people. Mech Ageing Dev. 2006; 127: 494–496. 10.1016/j.mad.2006.01.002 [DOI] [PubMed] [Google Scholar]
- 48.Armstrong JJ, Mitnitski A, Launer LJ, White LR, Rockwood K. Frailty in the Honolulu-Asia Aging Study: Deficit Accumulation in a Male Cohort Followed to 90% Mortality. J Gerontol A Biol Sci Med Sci. 2015; 70: 125–131. 10.1093/gerona/glu089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li G, Ioannidis G, Pickard L, Kennedy C, Papaioannou A, Thabane L. et al. Frailty index of deficit accumulation and falls: data from the Global Longitudinal Study of Osteoporosis in Women (GLOW) Hamilton cohort. BMC Musculoskelet Disord 2014; 15: 185 10.1186/1471-2474-15-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harttgen K, Kowal P, Strulik H, Chatterji S, Vollmer S. Patterns of frailty in older adults: comparing results from higher and lower income countries using the Survey of Health, Ageing and Retirement in Europe (SHARE) and the Study on Global AGEing and Adult Health (SAGE). PloS One 2013; 8: e75847 10.1371/journal.pone.0075847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bennett S, Song X, Mitnitski A, Rockwood K. A limit to frailty in very old, community-dwelling people: a secondary analysis of the Chinese longitudinal health and longevity study. Age Ageing 2013; 42: 372–377. 10.1093/ageing/afs180 [DOI] [PubMed] [Google Scholar]
- 52.Buchman AS, Wilson RS, Bienias JL, Bennett DA. Change in frailty and risk of death in older persons. Exp Aging Res 2009; 35: 61–82. 10.1080/03610730802545051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012; 60: 1487–1492. 10.1111/j.1532-5415.2012.04054.x [DOI] [PubMed] [Google Scholar]
- 54.Ravaglia G, Forti P, Lucicesare A, Pisacane N, Rietti E, Patterson C. Development of an easy prognostic score for frailty outcomes in the aged. Age Ageing 2008; 37: 161–166. 10.1093/ageing/afm195 [DOI] [PubMed] [Google Scholar]
- 55.Wang C, Song X, Mitnitski A, Yu P, Fang X, Tang Z. et al. Gender differences in the relationship between smoking and frailty: results from the Beijing Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2013; 68: 338–346. 10.1093/gerona/gls166 [DOI] [PubMed] [Google Scholar]
- 56.Mello Ade C, Engstrom EM, Alves LC. Health-related and socio-demographic factors associated with frailty in the elderly: a systematic literature review. Cad Saude Publica 2014; 30: 1143–1168. 10.1590/0102-311x00148213 [DOI] [PubMed] [Google Scholar]
- 57.Shi J, Song X, Yu P, Tang Z, Mitnitski A, Fang X, Rockwood K. Analysis of frailty and survival from late middle age in the Beijing Longitudinal Study of Aging. BMC Geriatr 2011; 11: 17 10.1186/1471-2318-11-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guessous I, Luthi JC, Bowling CB, Theler JM, Paccaud F, Gaspoz JM. et al. Prevalence of frailty indicators and association with socioeconomic status in middle-aged and older adults in a swiss region with universal health insurance coverage: a population-based cross-sectional study. J Aging Res 2014:198603 10.1155/2014/198603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pegorari MS, Tavares DM. Factors associated with the frailty syndrome in elderly individuals living in the urban area. Rev Lat Am Enfermagem 2014; 22: 874–882. 10.1590/0104-1169.0213.2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoogendijk EO, van Hout HP, Heymans MW, van der Horst HE, Frijters DH, Broese van Groenou MI. et al. Explaining the association between educational level and frailty in older adults: results from a 13-year longitudinal study in the Netherlands. Ann Epidemiol 2014; 24: 538–544. 10.1016/j.annepidem.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 61.Ng TP, Feng L, Nyunt MS, Larbi A, Yap KB. Frailty in older persons: multisystem risk factors and the Frailty Risk Index (FRI). J Am Med Dir Assoc 2014; 15: 635–642. 10.1016/j.jamda.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 62.Woo J, Goggins W, Sham A, Sham A, Ho SC. Social determinants of frailty. Gerontology 2005; 51: 402–408. 10.1159/000088705 [DOI] [PubMed] [Google Scholar]
- 63.Etman A, Kamphuis CB, van der Cammen TJ, Burdorf A, van Lenthe FJ. Do lifestyle, health and social participation mediate educational inequalities in frailty worsening? Eur J Public Health 2015; 25: 345–350. 10.1093/eurpub/cku093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee JS, Auyeung TW, Leung J, Buyssed DJ, Kaneita Y. Long sleep duration is associated with higher mortality in older people independent of frailty: a 5-year cohort study. J Am Med Dir Assoc 2014; 15: 649–654. 10.1016/j.jamda.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 65.Nobrega PV, Maciel AC, de Almeida Holanda CM, Oliveira Guerra R, Araújo JF. Sleep and frailty syndrome in elderly residents of long-stay institutions: a cross-sectional study. Geriatr Gerontol Int 2014; 14: 605–612. 10.1111/ggi.12144 [DOI] [PubMed] [Google Scholar]
- 66.Healy GN, Dunstan DW, Salmon J, Shaw JE, Zimmet PZ, Owen N. Television time and continuous metabolic risk in physically active adults. Med Sci Sports Exerc 2008; 40: 639–645. 10.1249/MSS.0b013e3181607421 [DOI] [PubMed] [Google Scholar]
- 67.Katzmarzyk PT, Church TS, Craig CL, Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc 2009; 41: 998–1005. 10.1249/MSS.0b013e3181930355 [DOI] [PubMed] [Google Scholar]
- 68.Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. The association between sedentary behaviour, moderate-vigorous physical activity and frailty in NHANES cohorts. Maturitas 2015; 80: 187–191. 10.1016/j.maturitas.2014.11.010 [DOI] [PubMed] [Google Scholar]
- 69.Wientzek A, Vigl M, Steindorf K, Brühmann B, Bergmann MM, Harttig U. et al. The improved physical activity index for measuring physical activity in EPIC Germany. PloS One 2014; 9: e92005 10.1371/journal.pone.0092005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Puts MT, Visser M, Twisk JW, Deeg DJ, Lips P. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol (Oxf) 2005; 63: 403–411. [DOI] [PubMed] [Google Scholar]
- 71.Hubbard RE, O'Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med 2009; 13: 3103–3109. 10.1111/j.1582-4934.2009.00733.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Theou O, O'Connell MD, King-Kallimanis BL, O'Halloran AM, Rockwood K, Kenny RA. Measuring frailty using self-report and test-based health measures. Age Ageing 2015; 44:471–477 10.1093/ageing/afv010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
The EPIC-Potsdam data are ethically and legally restricted because it is an ongoing study with data linked to identifying information. In accordance with German Federal and State data protection regulations, epidemiological data analyses of EPIC-Potsdam may be initiated upon an informal inquiry addressed to the secretariat of the Human Study Center (Office.HSZ@dife.de). Each request will then have to pass a formal process of application and review by the respective PI and a scientific board.