Abstract
It is increasingly appreciated that the sexes differ in their perception of noxious stimuli and in their responsivity to exogenous and endogenous analgesic manipulations. We previously reported the existence of qualitative sex differences in the neurochemical mediation of nonopioid (i.e., naloxone-insensitive) stress-induced analgesia (SIA) produced by forced swims and suggested that female mice possess a sex-specific SIA mechanism. This female-specific system is now known to be estrogen-dependent, to be ontogenetically organized, and to vary with reproductive status; however, its neurochemical identity remains obscure. In an attempt to identify candidate genes underlying SIA in both sexes, we performed a two-phase quantitative trait locus (QTL) mapping experiment using the BXD/Ty recombinant inbred (RI) set derived from DBA/2J (D2) and C57BL/6J (B6) inbred mouse strains and (B6xD2)F2 hybrid mice derived from these same progenitors. All mice were subjected to 3 min forced swims in 15°C water; nociceptive sensitivity on the 54°C hot-plate assay was assessed immediately before and 2 min after cessation of the swim. We report the localization of a QTL statistically associated with SIA magnitude [p = 0.00000012; logarithm of the odds (LOD) = 6.1] in female mice only. This female-specific QTL, which we name Fsia1, is located on chromosome 8 at 52–84 cM from the centromere and accounts for 17–26% of the overall trait variance in this sex. The present data provide further evidence of the existence of a female-specific SIA mechanism and highlight the important role of both genetic background and gender in the inhibition of pain.
Keywords: sex differences, genetics, antinociception, stress-induced analgesia, gene mapping, quantitative trait locus, nonopioid, pain
It is well known that the CNS contains circuitry that evolved to inhibit ascending nociceptive signals. These endogenous pain inhibition mechanisms can be activated by direct electrical stimulation or pharmacologically, but they evolved to be activated by exposure to environmental stressors, a phenomenon known as stress-induced analgesia (SIA) (Kelly, 1986). Multiple SIA systems are known to exist; in the simplest dissociation they can be divided into opioid or nonopioid forms, on the basis of their sensitivity to antagonism by the prototypic opioid receptor blockers naloxone or naltrexone (Lewis et al., 1980; Watkins and Mayer, 1982;Terman et al., 1984).
It is possible to observe opioid or nonopioid SIA after the application of the same laboratory stressor by altering stress severity parameters. For instance, in Swiss–Webster mice, swims of longer duration and/or colder temperature produce increasingly nonopioid SIA, that is, SIA increasingly refractory to naloxone/naltrexone antagonism (Marek et al., 1992; Mogil et al., 1993, 1996b; but see Tierney et al., 1991).
Much is known regarding the neurochemical and anatomical details of endogenous opioid pain inhibition mechanisms (Basbaum and Fields, 1984). Opioid peptide neurotransmitters are released and act on opioid receptors in the periaqueductal gray and the spinal cord, and serotonin and various peptide neurotransmitters participate as well (Basbaum and Fields, 1984; Mayer and Frenk, 1988). Some details regarding the anatomy of nonopioid pain inhibitory systems have been determined in studies of stimulation-produced analgesia; for example, naloxone-resistant analgesia can be produced by stimulating the dorsal rather than ventral periaqueductal gray (Cannon et al., 1982). In contrast, the neurochemistry of nonopioid mechanisms, as implied by their name, remains largely obscure. There exist a number of reports describing the selective attenuation of nonopioid SIA—variously supporting the involvement of H2 histamine receptors (Gogas et al., 1986; Gogas and Hough, 1989), serotonin 5-HT1A(Rodgers and Shepherd, 1989; Kavaliers and Colwell, 1991), 5-HT2, and 5-HT3 receptors (Rodgers et al., 1990), GABAA receptors (Rodgers and Randall, 1987;Kavaliers, 1988), α2-adrenergic receptors (Bodnar et al., 1983; Coderre and Rollman, 1984; Chance, 1986; Watkins et al., 1992), NMDA receptors (Marek et al., 1991; Marek et al., 1992; Ben-Eliyahu et al., 1993), or the parallel activation of multiple spinal opioid receptor types (Watkins et al., 1992)—but contradictory data abound, and no consensus has emerged.
We further complicated this field several years ago when in an attempt to replicate our study showing that 3 min swims in 15°C water produce nonopioid SIA that is attenuated by low doses (0.075 mg/kg, i.p.) of the NMDA antagonist dizocilpine (MK-801), we observed that female mice were wholly insensitive to such antagonism (Mogil et al., 1993). Nonetheless, females exhibited equipotent SIA from the swims compared with males, implying the existence of a female-specific, nonopioid, non-NMDAergic SIA mechanism. We (Sternberg et al., 1994, 1996) and others (Kavaliers and Galea, 1996) have replicated and extended this finding (see Discussion), but the neurochemical nature of the female-specific system remains entirely unknown.
In addition to the role of sex, we and others have amassed considerable evidence pointing to the important role of genotype or genetic background in the modulation of exogenous and endogenous pain inhibition (for review, see Belknap and O’Toole, 1991; Mogil et al., 1996c). The recently developed techniques of molecular gene mapping have only now begun to be applied to pain-related traits, and quantitative trait loci (QTLs) have been identified for morphine analgesia (Belknap et al., 1995; Mogil et al., 1995) and basal nociceptive sensitivity (Mogil et al., 1997). Such approaches are very useful for generating novel hypotheses regarding physiological mediation of a trait, and we reasoned that a QTL mapping study of nonopioid SIA might serve to provide confirmatory and/or heuristic information regarding the neurochemical identity of this puzzling phenomenon. Furthermore, this experiment might be viewed as a direct attempt to identify the true nature of the female-specific SIA system without having to resort to the administration of numerous, arbitrarily chosen antagonists. In pilot data collected to these ends, we determined that 3 min swims in 15°C water produce nonopioid, non-NMDAergic SIA in females of both the DBA/2J (D2) and C57BL/6J (B6) strains (Mogil and Belknap, 1997). These strains were chosen for this study because of their progenitor status with respect to the BXD/Ty recombinant inbred (RI) strain set available at the Veterans Affairs Animal Research Facility. Like the Swiss– Webster mice used in the seminal studies, male B6 mice also displayed dizocilpine-sensitive nonopioid SIA. To our surprise, male D2 mice exhibited naloxone-reversible, opioid SIA after these swim stress parameters, indicating that the selective activation of neurochemically distinct SIA mechanisms is determined by both sex and genotype (Mogil and Belknap, 1997). The present study adds important evidence to this contention, because we have identified two female-specific QTLs that account for variability in nonopioid SIA magnitude between D2 and B6 mice.
Some of these data have been published previously (Mogil et al., 1997).
MATERIALS AND METHODS
Subjects. Mice used in this experiment were the same as those used in a previous QTL mapping experiment that considered basal sensitivity to hot-plate nociception (Mogil et al., 1997). Subjects were naïve, adult (8- to 12-week-old) mice of both sexes of the following populations: B6, D2, (B6xD2)F1hybrids (both reciprocals), (B6xD2)F2 hybrids (all reciprocals), and 24 BXD/Ty (BXD) RI strains (BXD-1 through BXD-32; strains BXD-3, -4, -7, -10, -17, -20, and -26 are no longer extant; the BXD-24 strain was unavailable). All mice were bred at the Veterans Affairs Animal Research Facility (Portland, OR) from breeding stock originally obtained from The Jackson Laboratory (Bar Harbor, ME) no more than three generations earlier. Mice were weaned at 22–25 d and housed with their same-sex littermates, two to five mice per cage, in a temperature-controlled (22°C) environment. Subjects were maintained on a 12 hr light/dark cycle (lights on at 6 A.M.), and all testing proceeded near mid-photophase to reduce circadian effects on pain sensitivity (Kavaliers and Hirst, 1983).
Algesiometric testing. Details of the hot-plate assay used have been described previously (Mogil et al., 1996a, 1997). Briefly, mice were removed from their home cages and placed on an aluminum surface maintained at 54.0 ± 0.2°C (Thermolyne Dri-Bath, Thermolyne, Dubuque, IA). Locomotion was limited by 15-cm-high Plexiglas walls to a 10 × 10 cm area. Latency to respond to the heat stimulus with a behavior indicative of nociception (sustained hindpaw lift, hindpaw lick, or hindpaw shake/flutter) was measured to the nearest 0.1 sec with a stopwatch by an observer blind to genotype. In the absence of any of these responses after 60 sec, mice were removed from the plate and assigned a cut-off latency of 60. Mice were tested for nociceptive sensitivity on the 54°C hot-plate test immediately before and 2 min after forced swims.
Swim SIA. SIA was produced by exposing mice to 3 min forced swims in 15°C (±1°C) water, as we have previously described (Marek et al., 1992; Mogil et al., 1993, 1996b; Mogil and Belknap, 1997). An experimenter maintained the water at the desired temperature by constant monitoring and the periodic addition of ice as needed. Mice were placed in a cylindrical plastic container 28 cm in diameter and 44 cm in height. The water level ranged from 30 to 35 cm high, so that escape was impossible. On completion of the 3 min swim, mice were towel-dried and placed in a paper towel-lined enclosure for 2 min to dry before being retested for nociceptive sensitivity on the hot plate.
SIA was expressed as the percentage of the maximum possible effect (%MPE), as calculated by the following formula:
The use of %MPE takes into account the cut-off latency and individual baseline latencies, so that these will not bias the quantification of analgesia. This transformation, however, has been criticized for imposing an arbitrary ceiling that may lead to distortions (Carmody, 1995). With this potential problem in mind, we also considered other possible indices of analgesic magnitude, including the raw change in hot-plate latency, the uncorrected percentage latency change, and the change in the nociception index (1/latency), as suggested by Carmody (1995). We found that these alternative indices were very highly correlated with each other (r = 0.80–0.91); we chose, therefore, to present and analyze %MPE data herein. In fact, ANOVAs were performed on all of these indices (not shown), and results were qualitatively the same in every case.
QTL mapping. A two-phase mapping strategy was used, as described in detail previously (Belknap et al., 1995). In the first phase, BXD RI strain distributions were collected by obtaining hot-plate latency means for each BXD strain (n = 6–11 per sex per strain, except for BXD-9 and BXD-22, in which only four males and three females, respectively, were available for testing). The hot-plate latency distributions were used to screen a database of strain distribution patterns of the allelic form (D2-derived or B6-derived) of >1200 polymorphic microsatellite DNA markers, naturally occurring stretches of DNA often consisting of a dinucleotide repeat (e.g., [CA]n), of known chromosomal location (Manly, 1993). Correlation coefficients were calculated for each comparison between the phenotype (mean %MPE) and genotype (0 if the strain exhibits a fixed homozygous B6 allele at that marker; 1 if the strain exhibits a fixed homozygous D2 allele) of the RI strain. Genomic regions found to be statistically associated with hot-plate latency atp < 0.01 were subjected to confirmation testing in the second phase, using mice of a (B6xD2)F2 intercross. The choice to use an α level of 0.01 in our preliminary genome screen was arbitrary; we wished to strike a balance between minimizing Type I errors (false positives), which calls for more stringent α levels, and minimizing Type II errors (false negatives), which calls for less stringent α levels. At the 0.01 level, our computer simulations show that approximately half of all provisionally identified QTLs are likely to be correct positives for the BXD data alone (Belknap et al., 1996).
In the second phase, 293 (B6xD2)F2 hybrid mice were bred and tested exactly as were the BXD RI mice used previously. Three or more new microsatellite markers known to be polymorphic in the D2 and B6 strains (Dietrich et al., 1996) were chosen to bracket each RI-implicated region, and some F2 hybrids were genotyped at each marker (see below). Each F2 animal is genetically unique and represents a new recombination of progenitor alleles. Because unlimited numbers of F2 mice can be bred and tested, this approach transcends the statistical power limitations inherent in the initial BXD RI screening phase. The testing of F2 mice is associated with two disadvantages, however: (1) phenotype measurement will be less accurate because the phenotypic value is a single datum as opposed to a strain mean, and (2) half of all F2 mice will have inherited heterozygous alleles at relevant loci, rendering them less informative. This latter limitation can be mitigated somewhat by using selective genotyping, in which only extreme responding F2 mice (both high and low tails of the distribution) are genotyped (Lander and Botstein, 1989). We used a selective genotyping paradigm in which 140 (79 male, 61 female) of 293 phenotyped mice were genotyped. This strategy reduces genotyping costs and effort by >50%, yet the power to detect QTLs is affected very little (Lander and Botstein, 1989).
Genomic DNA was isolated from spleen by a modification of the protein salting-out method (Miller et al., 1988), as described in Belknap et al. (1995). Microsatellites were amplified in 96-well microtiter plates using a modification of standard PCR procedures (Dietrich et al., 1992) with unlabeled commercially available marker primers (Research Genetics, Huntsville, AL). To each 200 ng genomic DNA sample (5 μl), 20 μl of PCR reactants was added (1 U Taq polymerase, 200 μm dNTPs, 0.66 μm forward and reverse primers, 1–3 mm MgCl2). The mixture was subjected to the following PCR program: 3 min at 94°C; 40 cycles of 94°C for 1 min, 56°C for 2 min, 72°C for 3 min; 7 min at 72°C; indefinite hold at 4°C. A 20% volume of bromophenol blue dye was added to PCR products, which were then separated by electrophoresis (4 hr/10 cm at 70 V) on high-resolution agarose gels (3% Metaphor or NuSieve; FMC Bioproducts, Rockland, ME), stained with ethidium bromide (1 μg/ml), and visualized with ultraviolet light. A DNA ladder and samples from B6 and D2 progenitors were run concurrently, so that the relevant band in every gel lane (each corresponding to an individual F2 mouse) could be unequivocally assigned a genotype and an arbitrary gene dosage: homozygous B6 (0), homozygous D2 (1), or heterozygous (0.5).
Statistical analysis. BXD RI data were first subjected to two-way ANOVA (strain x sex). Correlation coefficients for each marker were calculated on the combined BXD RI data and on data from each sex separately. Because there are only two genotypic classes possible per marker in the BXD RI strains, the calculated r value yields the same p value as a one-way ANOVA or t test between strains bearing each allele. For F2 data, the MAPMAKER program (Lander et al., 1987) was used to construct a primary linkage map for the markers tested in each RI-implicated region (MAPMAKER/EXP) and to assess the presence of a QTL within this framework (MAPMAKER/QTL). The advantages of MAPMAKER include the use of interval mapping using maximum likelihood estimation to increase statistical power, and a built-in error checking routine. MAPMAKER is not presently amenable to RI data, so only F2 data were analyzed in this way. F2 data were subjected to MAPMAKER analysis only if the marker showing the highest correlation displayedp < 0.05; otherwise the QTL was considered disconfirmed.
The two phases of this QTL mapping effort can be considered independent experiments testing the same hypothesis. As such, the pvalues obtained for a given marker in the two experiments (BXD RI and F2) were combined using the conservative method of Fisher (Sokal and Rohlf, 1981). To assess the significance of linkage, we used the stringent criteria recommended by Lander and colleagues (Lander and Schork, 1994; Lander and Kruglyak, 1995): RI,p = 0.00002 [logarithm of the odds (LOD) = 3.9]; F2 (1 df; additive effects only),p = 0.0001 (LOD = 3.3). Because we are pooling results from these two types of experiments, we used the mean of the RI and F2 p value recommendations, orp = 0.00006 (LOD = 3.5). LOD scores were estimated from p values on the basis of the asymptotic distribution of LOD as χ2 (df = 1) using the expression LOD = 1/2(log10e)χ2 for an additive (df = 1; no dominance) model.
RESULTS
BXD RI strain distribution
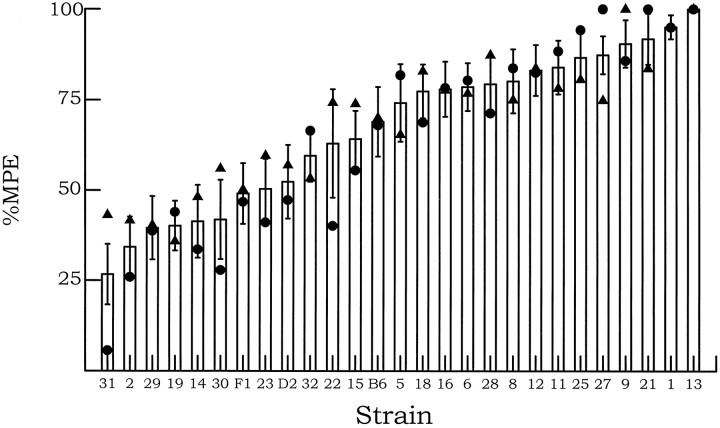
Raw pre-swim and post-swim hot-plate latency data, for all mice combined and separately by sex, are presented in Table1. The baseline hot-plate sensitivity data have been analyzed and considered elsewhere (Mogil et al., 1997). Baseline nociceptive sensitivity and SIA magnitude were found to be significantly but modestly correlated (r = 0.26). The distribution of SIA magnitude in B6, D2, and (B6xD2)F1hybrids and BXD RI strains is shown in Figure1. First, it is important to note that no significant differences were observed between the progenitor strains (B6 and D2), either considered together (t = 1.15;df = 28; p = 0.26) or when separated by sex (male: t = 0.51, df = 13,p = 0.62; female: t = 1.34,df = 13, p = 0.20). The finding of nonsignificant progenitor strain differences is quite uncommon for a QTL mapping study; usually such differences are the basis for the mapping attempt. We originally decided to test BXD RI strains because they were readily available to us at the Veterans Affairs Animal Research Facility, and it should be noted that progenitor strain differences are not required for successful QTL mapping (Gora-Maslak et al., 1991). We would expect in this situation to uncover some positively correlated QTLs (in which the D2 allele confers increased SIA magnitude) and some negatively correlated QTLs (in which the B6 allele confers increased SIA magnitude). The genes that these QTLs represent should sort independently in the BXD RI strains, such that some strains would display more extreme responses than either of the progenitor strains or their F1 hybrid.
Table 1.
Raw data from all genetic populationsa
| Strain | All mice | Female mice | Male mice | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | BL | PS | n | BL | PS | n | BL | PS | |
| B6 | 13 | 19.1 ± 1.2 | 46.5 ± 4.3 | 7 | 20.5 ± 1.4 | 46.7 ± 5.0 | 6 | 17.5 ± 1.9 | 46.2 ± 7.7 |
| D2 | 17 | 24.3 ± 1.6 | 42.5 ± 3.9 | 8 | 22.2 ± 2.4 | 39.1 ± 4.5 | 9 | 26.1 ± 2.0 | 45.6 ± 6.3 |
| (B6xD2)F1 | 26 | 18.0 ± 0.9 | 38.5 ± 3.6 | 8 | 18.8 ± 2.2 | 37.5 ± 6.7 | 18 | 17.7 ± 0.8 | 39.0 ± 4.4 |
| BXD-1 | 14 | 22.6 ± 0.9 | 58.2 ± 1.2 | 7 | 21.4 ± 0.6 | 58.1 ± 1.9 | 7 | 23.7 ± 1.7 | 58.3 ± 1.7 |
| BXD-2 | 15 | 19.4 ± 1.6 | 32.5 ± 3.8 | 7 | 20.0 ± 2.4 | 29.6 ± 3.2 | 8 | 18.9 ± 2.1 | 35.1 ± 6.6 |
| BXD-5 | 15 | 18.3 ± 1.7 | 48.2 ± 4.8 | 8 | 17.1 ± 2.3 | 51.6 ± 5.9 | 7 | 19.8 ± 2.5 | 44.2 ± 8.0 |
| BXD-6 | 18 | 19.8 ± 1.8 | 50.6 ± 3.0 | 9 | 19.9 ± 2.9 | 51.3 ± 4.4 | 9 | 19.7 ± 2.2 | 49.9 ± 4.4 |
| BXD-8 | 17 | 28.6 ± 2.1 | 52.1 ± 3.5 | 10 | 27.3 ± 2.8 | 54.2 ± 3.3 | 7 | 30.5 ± 3.2 | 49.1 ± 7.2 |
| BXD-9 | 12 | 19.8 ± 2.1 | 55.5 ± 3.1 | 8 | 16.3 ± 2.2 | 53.2 ± 4.4 | 4 | 26.6 ± 1.9 | 60 ± 0 |
| BXD-11 | 16 | 18.1 ± 1.7 | 52.5 ± 3.6 | 9 | 14.9 ± 1.7 | 54.5 ± 3.8 | 7 | 22.1 ± 2.6 | 49.8 ± 7.0 |
| BXD-12 | 14 | 24.5 ± 1.7 | 55.1 ± 2.1 | 7 | 26.0 ± 2.8 | 55.4 ± 3.3 | 7 | 23.0 ± 2.1 | 54.7 ± 2.7 |
| BXD-13 | 14 | 23.9 ± 2.3 | 60 ± 0 | 7 | 21.3 ± 2.2 | 60 ± 0 | 7 | 26.6 ± 4.0 | 60 ± 0 |
| BXD-14 | 13 | 24.6 ± 2.2 | 35.8 ± 4.6 | 6 | 18.7 ± 1.2 | 30.0 ± 7.5 | 7 | 29.7 ± 2.8 | 40.9 ± 5.5 |
| BXD-15 | 19 | 18.3 ± 1.6 | 43.9 ± 3.6 | 10 | 16.5 ± 2.7 | 39.4 ± 5.7 | 9 | 20.3 ± 1.6 | 48.8 ± 3.8 |
| BXD-16 | 15 | 15.1 ± 1.5 | 49.6 ± 3.6 | 8 | 15.9 ± 0.6 | 50.2 ± 5.1 | 7 | 14.1 ± 3.2 | 48.8 ± 5.5 |
| BXD-18 | 18 | 25.9 ± 1.7 | 51.5 ± 3.0 | 7 | 23.6 ± 2.7 | 47.7 ± 6.2 | 11 | 27.4 ± 2.1 | 53.9 ± 3.1 |
| BXD-19 | 17 | 16.1 ± 1.5 | 33.1 ± 3.1 | 9 | 15.2 ± 2.3 | 34.0 ± 4.4 | 8 | 17.0 ± 1.9 | 32.1 ± 4.5 |
| BXD-21 | 14 | 20.9 ± 1.5 | 56.4 ± 3.2 | 7 | 20.7 ± 2.7 | 60 ± 0 | 7 | 21.0 ± 1.4 | 52.7 ± 6.3 |
| BXD-22 | 9 | 24.6 ± 3.2 | 44.2 ± 6.5 | 3 | 17.3 ± 5.5 | 31.7 ± 14.2 | 6 | 28.2 ± 3.2 | 50.5 ± 6.0 |
| BXD-23 | 16 | 15.9 ± 2.1 | 37.0 ± 4.0 | 8 | 13.5 ± 2.1 | 31.5 ± 5.5 | 8 | 18.2 ± 3.5 | 42.6 ± 5.5 |
| BXD-25 | 18 | 24.2 ± 2.0 | 54.9 ± 2.6 | 8 | 19.5 ± 2.1 | 57.1 ± 2.9 | 10 | 27.9 ± 2.8 | 53.2 ± 4.1 |
| BXD-27 | 18 | 20.4 ± 1.8 | 54.5 ± 2.3 | 9 | 21.3 ± 1.9 | 60 ± 0 | 9 | 19.5 ± 3.3 | 48.9 ± 3.8 |
| BXD-28 | 18 | 18.3 ± 1.3 | 50.7 ± 3.3 | 9 | 17.4 ± 1.7 | 47.1 ± 4.4 | 9 | 19.2 ± 1.9 | 54.3 ± 4.8 |
| BXD-29 | 19 | 17.9 ± 1.3 | 33.4 ± 4.0 | 9 | 16.9 ± 1.9 | 33.4 ± 5.2 | 10 | 18.8 ± 1.8 | 33.5 ± 6.2 |
| BXD-30 | 14 | 18.9 ± 1.1 | 34.2 ± 5.0 | 7 | 18.0 ± 1.6 | 28.0 ± 6.1 | 7 | 19.9 ± 1.6 | 40.1 ± 7.7 |
| BXD-31 | 16 | 13.0 ± 1.1 | 24.6 ± 4.2 | 7 | 12.6 ± 1.3 | 14.7 ± 2.4 | 9 | 13.3 ± 1.7 | 32.3 ± 6.2 |
| BXD-32 | 21 | 13.6 ± 1.2 | 41.4 ± 3.3 | 10 | 12.4 ± 1.3 | 44.4 ± 4.6 | 11 | 14.7 ± 2.0 | 38.6 ± 4.8 |
The 54°C hot-plate latencies (in seconds) of all genetic populations immediately before [baseline (BL)] and 2 min after [post-swim (PS)] 3 min forced swims in 15°C water. Analgesic magnitudes expressed as %MPE = [(PS − BL)/(60 − BL)] × 100 were calculated from these data (in BXD strains only) for purposes of QTL analysis (see Fig. 1).
Fig. 1.
15°C swim SIA in B6, D2, and (B6xD2)F1 hybrid and 24 BXD RI strains. Mice were tested for nociceptive sensitivity on the 54°C hot-plate test immediately before and 2 min after a 3 min swim in 15°C water. Error bars represent mean ± SEM percentage of the maximum possible effect (%MPE) (see Materials and Methods) for all mice.Triangles represent mean of male mice only;circles represent mean of female mice only.
A two-way ANOVA (strain × sex; excluding B6, D2, and F1 hybrid mice) revealed a significant main effect of strain (F23,332 = 7.64; p < 0.001). The main effect of sex (F1,332 = 1.55;p = 0.21) and the strain × sex interaction (F23,332 = 1.09; p = 0.35) were not significant. Nonetheless, it is clear from an inspection of Figure1 that some strains but not others exhibited sex differences, suggesting the potential existence of sex-specific QTLs. A subsequent one-way ANOVA revealed a significant effect of strain (F23,356 = 7.43; p < 0.001). The frequency distribution of these strains was not significantly different from normal, implying polygenic control of the phenotype in support of our previous contention (Mogil et al., 1992). Narrow sense heritability (h2) of this phenotype can be estimated by comparing the between-strain variance with the total variance and was found to be 0.32 in the RI set. This value implies that 32% of the total trait variance is attributable to genetic factors and is comparable to that of other behavioral traits to which QTL mapping has been applied successfully.
BXD RI QTL analysis
The QTL analysis of SIA magnitude in BXD RI strains is shown in Table 2. Six chromosomal regions were found to be associated with SIA magnitude at p < 0.01 (uncorrected) when data from both sexes were combined. A reanalysis of this data set by sex revealed disparities in the apparent strength of the correlation in males versus females in one of these QTL regions, the D8Rik78 region of chromosome 8 (Table3). None of these QTL regions were similar to those identified previously for baseline hot-plate nociception (Mogil et al., 1997) or morphine analgesia (Belknap and Crabbe, 1992; Belknap et al., 1995).
Table 2.
Correlation coefficients (r) of significantly associated marker loci (p < 0.01 for all mice) for 15°C swim SIA in 24 BXD RI strainsa
| Marker | Location | r | Present status |
|---|---|---|---|
| Ms15-6 | Chromosome 5; 42 cM | −0.6182-160 | Disconfirmed |
| Brp1 | Chromosome 6; 32 cM | 0.559* | Putative2-b |
| D8Rik78 | Chromosome 8; 55–56 cM | 0.6432-165 | Confirmed for females only2-b |
| D11Ncvs69 | Chromosome 11; 16 cM | −0.5752-160 | Disconfirmed |
| Cbg | Chromosome 12; 51 cM | 0.524* | Disconfirmed |
| D17Mit22 | Chromosome 17; 10 cM | 0.6302-160 | Disconfirmed |
Only the marker showing the highest correlation (for all mice) among several closely linked markers is shown. The centiMorgan (cM) locations represent estimated map distances from the centromere (Mouse Genome Database athttp://www.informatics.jax.org/mgd.html). For all marker loci, a genotypic score of 0 was assigned to the B6 allele and a score of 1 to the D2 allele; negative correlations thus indicate that the B6 allele at that locus is associated with higher SIA magnitude. Attempts were made to confirm all six QTL regions in a (B6xD2)F2 hybrid population; four of these attempts were unsuccessful, indicating that the loci were likely false positives.
See Table 3 for details.
p < 0.01;
F2-160: p < 0.005;
F2-165: p < 0.0001.
Table 3.
Statistical analysis of two putative QTLs for 15°C swim SIA
| QTL | Sex | BXD experiment3-a | F2 hybrid experiment3-b | Combined data3-c | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | LOD | p | LOD | p | LOD | ||
| 8 | F | 0.732 | 4.8 × 10−5 | 3.6 | 1.6 × 10−4 | 3.1 | 1.2 × 10−7 | 6.13-150 |
| M | 0.452 | 0.026 | 1.1 | 0.274 | 0.3 | 0.038 | 0.9 | |
| 6 | F + M | 0.559 | 0.0084 | 1.5 | 0.019 | 2.0 | 0.002 | 2.1 |
Calculated at marker showing peak correlation among several closely linked markers.
Calculated by MAPMAKER/QTL using interval mapping. LOD scores are given for an additive model (df= 1). Because all QTL effects in RI data are additive, for the sake of consistency we looked only at additive effects in the F2hybrid experiment.
p values from the two independent experiments were combined using the conservative method of Fisher, −2Σln p = χ2,df = 2t, where t is the number of experiments (Sokal and Rohlf, 1981).
F3-150: This value far exceeds criterion levels of significance proposed byLander and Kruglyak (1995).
(B6xD2)F2 QTL confirmation
As described in Materials and Methods, all six regions implicated from the BXD RI data at the p < 0.01 level were subjected to confirmation using F2 hybrids of both sexes and at least four markers bracketing the QTL region (including one marker mapping within 1 cM of the location showing the highest statistical association). Four of the six putative QTL regions were disconfirmed, that is, found to be apparent false positives, indicated by a lack of p < 0.05 correlation at any marker in the region (data not shown). The present finding of four of six false positives obtained from a BXD RI QTL screen is only slightly higher than predicted by our computer simulations (i.e., half false positives) (Belknap et al., 1996). It remains possible, of course, that these regions truly are associated with 15°C swim SIA and that we simply lack the statistical power to demonstrate their linkage with the limited number of F2 mice used presently. This is especially likely for QTLs with small effects on the trait.
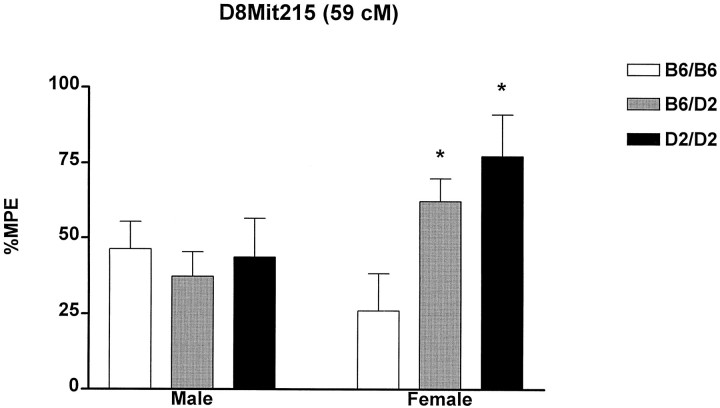
In one of the remaining QTL regions, the Ms6–4 toD6Rik58 region of chromosome 6 (30–39 cM), we obtained some further evidence supporting the existence of a QTL using F2mice, although the overall LOD score so far obtained (LOD = 2.1) (Table 3) is not significant. This LOD score may yet reach statistical significance once the sample size is increased, and efforts are currently underway to do so. We have an independent reason to believe that this region is a true QTL: Tarricone and colleagues (1995) have identified this same region as a QTL for hyperlocomotion after restraint stress. In contrast, strong evidence was obtained, even with the limited number of F2 mice tested, showing that the QTL region on chromosome 8 is significantly associated with 15°C swim SIA. In addition, an unequivocal sex-specificity was observed in the F2 data. The combined BXD RI and F2 hybrid significance levels (p = 0.00000012; LOD = 6.1) far exceed the criterion values for significant linkage proposed by Lander and Kruglyak (1995) for female mice alone. By contrast, combined BXD RI and F2 hybrid significance levels for male mice alone (p = 0.038; LOD = 0.9) show no evidence of the existence of a QTL (Table 3). The difference inp values between the two sexes was significant (p < 0.01; diffuse test) (Woolf, 1986). We thus propose that this QTL be named Fsia1, because it seems to mediate SIA in female mice only. This QTL has large effects, accounting for between 53.5 and 82.2% of the genetic variance and between 17.1 and 26.3% of the total trait variance in female mice of these strains (estimated from BXD RI data and F2 data, respectively). The location of the QTL within a 95% confidence interval (CI) as estimated by MAPMAKER/QTL (using a 1 LOD drop-off) is somewhere within 52 cM of the centromere and the distal end of the chromosome (84 cM). A 95% CI of similar size is estimated by the formula of Darvasi and Soller (1997): CI = (530/[n·ν]) = 39 cM, where ν = proportion of trait variance explained by the QTL. The resolving power to localize this QTL can be improved markedly by increasing the sample size (Darvasi and Soller, 1997), and this effort is underway in the laboratory of the first author. As can be seen in Figure2, female mice possessing homozygous D2 alleles at markers located in this region of chromosome 8 exhibit SIA magnitudes approximately double those of female mice possessing homozygous B6 alleles. In contrast, inheritance of D2 alleles at these markers confers no increase in SIA magnitudes in male mice.
Fig. 2.
15°C swim SIA (measured in %MPE) in male and female (B6xD2)F2 hybrid mice homozygous for the B6 allele (B6/B6), heterozygous (B6/D2), or homozygous for the D2 allele (D2/D2) of the microsatellite marker D8Mit215, located ∼59 cM from the centromere on mouse chromosome 8. Inheritance of either allele is irrelevant to SIA magnitude in male F2 mice. In contrast, inheritance of the D2 allele confers significantly greater SIA in female F2 mice. Female D2/D2 mice remain on the 54°C hot plate 20 sec longer than female B6/B6 mice. These data strongly suggest that a gene responsible for the majority of genetic variance in this trait in females is located in the vicinity ofD8Mit215. Two-way ANOVA revealed a significant gene dosage × sex interaction (F2,122 = 3.44; p = 0.038). A subsequent one-way ANOVA in female mice revealed a significant simple main effect of gene dosage (F2,51 =4.60; p < 0.015). *p < 0.05 versus B6/B6 by Tukey’spost hoc test.
DISCUSSION
The present study identifies a large QTL associated with variability in swim SIA in mice, which we name Fsia1 for female-specific SIA, because the QTL is significantly associated with variability in this trait in female but not male mice. This QTL is located at the distal portion of mouse chromosome 8, in a region showing syntenic conservation with human chromosomal region 16q22. The existence of female-specific QTLs was predicted by our demonstration several years ago that female mice possess a sex-specific SIA mechanism (Mogil et al., 1993). These data provide direct support for the growing appreciation that genetic background and sex are important influences on pain and pain inhibition.
Candidate genes and future directions
It is important to realize that QTL mapping efforts such as the present one do not identify the genes underlying the mapped traits; they identify only the approximate genomic locations of such genes. The microsatellites found to be statistically associated with SIA variance in female mice on chromosome 8 are typically noncoding stretches of DNA likely to have no effect on the trait in question. These microsatellites merely serve as markers. Because they are genetically linked (and thus cosegregate) with the genes that aretrait-relevant, they are useful for localizing such genes; however, the >30 cM region representing the 95% CI containing the gene(s) representing the QTL contains hundreds or even thousands of genes, most of which remain unknown at the present time. We have had considerable success in the recent past identifying candidate genes residing in the QTL region for morphine analgesia (Oprm: encoding the μ-opioid receptor; Htr1b: encoding the serotonin 5-HT1B receptor) (Belknap et al., 1995; Mogil et al., 1995) and basal sensitivity to thermal nociception (Oprd1: encoding the δ-opioid receptor) (Mogil et al., 1997). In each case we were able to provide pharmacological evidence supporting the contention that the candidate gene truly represented the QTL. For instance, the whole-brain homogenate Bmax (receptor density) for naloxone, using concentrations expected to be largely μ specific, was found also to map to the same chromosomal region (chromosome 10; 0–10 cM) as Oprm and the QTL for morphine analgesia. With respect to Htr1b, we have observed a correlation between analgesic sensitivity to morphine and to the serotonin-1B agonist CGS 12066 and greatly altered morphine dose–response relationships in transgenic knock-out mice lacking functional expression of this gene (Mogil et al., 1995).
In the present study, however, we can find no candidate genes of obvious relevance to swim SIA in this region. A few genes of possible relevance include Zfp1 and Zfp4, which encode zinc finger proteins that also act as transcriptional activators (chromosome 8; 54–55 cM), and Tat, which encodes tyrosine aminotransferase, a metabolic enzyme for tyrosine that is induced by stress via activation of the glucocorticoid receptor (chromosome 8; 55 cM) (Alexandrova, 1994). Formal genetic confirmation of a candidate gene for a QTL requires the study of additional strains, fine-structure mapping to test whether the candidate gene and the QTL are recombinationally inseparable, and ultimately, allelic substitution for the candidate gene in transgenic mice.
It is likely that the gene (or genes) truly representingFsia1 have not yet been cloned and mapped. A number of strategies exist to directly identify genes, including positional cloning (i.e., high-resolution mapping) and subtractive hybridization (e.g., genetically directed representational difference analysis) (Crabbe et al., 1994). The first step toward either of these ends would likely be the use of classic genetic approaches to transfer the gene of interest from a donor strain (D2) onto a background strain (B6). The resultant congenic lines would be genetically identical except for a small region surrounding the target gene, serving as a confirmation of the role of the QTL in the trait and permitting further localization of the gene. We have previously constructed such congenic lines for the region of chromosome 9 surrounding the Htr1b/d locus that we have implicated in morphine analgesic sensitivity, and these lines exhibit altered analgesic responses compared with wild-type littermates (H. Hain, J. S. Mogil, and J. K. Belknap, unpublished observations).
Sex-specific QTLs
To our knowledge, this is only the fourth demonstration of a sex-specific QTL residing on an autosome. Melo and colleagues (1996) recently reported the identification of a male-specific (Alcp1) and a female-specific (Alcp2) QTL associated with alcohol preference in the mouse, another trait with well documented sex differences. In their study, which used backcrosses between the ethanol-preferring B6 mouse and the ethanol-avoiding D2 mouse, parent-of-origin effects were observed for Alcp2, which the authors explained in terms of genomic imprinting. We did not keep track of the grandparental strains of the segregating F2 mice used for the QTL analysis, so we cannot presently evaluate either genomic imprinting or the possible existence of an X-linked locus with complementary activity to Fsia1. Clark and colleagues (1996) recently identified two QTLs associated with hypertension in male rats only, using an F2 cross between the normotensive Wistar–Kyoto rat and the stroke-prone spontaneously hypertensive rat.
We recently reported the possible existence of a male-specific QTL associated with basal sensitivity to acute, thermal nociception as measured on the hot-plate assay (Mogil et al., 1997). B6 and D2 mice are known to display divergent baseline hot-plate latencies (D2 > B6, implying that B6 mice are more sensitive to this type of nociception), and a QTL analysis of the baseline data presented in Table 1 revealed a QTL on chromosome 4 that was significantly associated with hot-plate latency in male but not female mice.
In that study a viable candidate gene was identified: theOprd1 gene that encodes the murine δ-opioid receptor. Supporting the potential role of Oprd1 in the mediation of thermal nociceptive sensitivity, we found that the δ-receptor antagonist naltrindole and the δ2 antagonist naltriben (but not the δ1 antagonist 7-benzylidenenaltrexone, lowered baseline hot-plate latencies in a strain- and sex-dependent manner predicted by the mapping data: D2 male > B6 male > D2 female > B6 female. In female mice of both strains, neither naltrindole nor naltriben produced significant alterations in nociceptive sensitivity.
Sex-specific mechanisms of swim SIA
It is now widely appreciated that important sex differences exist in pain sensitivity and sensitivity to analgesics in humans, even when sociocultural factors are accounted for pimprivate (for review, seeFillingim and Maixner, 1995; Unruh, 1996; Berkley, 1997). In general, when differences are found, females of many species are more sensitive to and less tolerant of pain than are males, and also less sensitive to opioid and nonopioid analgesic manipulations pimprivate (Lipsitt and Levy, 1959; Beatty and Beatty, 1970; Bodnar et al., 1988; Feine et al., 1991; Kepler et al., 1991; Kiefel and Bodnar, 1991; Kavaliers and Innes, 1992; Aloisi et al., 1994; Menendez et al., 1994; Cicero et al., 1996; but see Gear et al., 1996).
We (Mogil et al., 1993) and others (Wong, 1987; Romero et al., 1988) have also observed qualitative sex differences in the mediation of opioid and nonopioid pain inhibition, implying differential neurochemical mediation of similar phenomena in each sex. We demonstrated that the naloxone-insensitive SIA displayed by Swiss–Webster mice after 3 min swims in 15°C water was significantly attenuated by a low dose of dizocilpine (MK-801, 0.075 mg/kg) in males but not females (Mogil et al., 1993). Thus, the nonopioid SIA produced by these swim parameters was NMDAergic in male mice only. The fact that the SIA was equipotent in both sexes but undiminished in females treated with naloxone and dizocilpine led us to propose the existence of a female-specific SIA mechanism. In this same study we demonstrated that ovariectomy unmasked the “male” pattern of dizocilpine antagonism, and that expression of the “female” system was reinstated in these animals by estrogen replacement (Mogil et al., 1993). We have determined that intact female mice remain dizocilpine-insensitive throughout their estrous cycle (Sternberg et al., 1994). This novel, female-specific, nonopioid swim SIA mechanism has subsequently been found to be dependent for its expression on the absence of testosterone during ontogeny (Sternberg et al., 1996), to be expressed only after puberty but to persist after estropause (W. F. Sternberg, unpublished observations), and to vary with circannual reproductive status such that it is expressed only in female deer mice (Peromyscus maniculatus) maintained in a photoperiod-induced state of cyclicity (Kavaliers and Galea, 1996). Intriguingly, it has also been reported that female mice have much lower dizocilpine binding in the forebrain after acute swim stress than do males (Akinci and Johnston, 1993) and that estradiol can regulate NMDA binding in the rat hippocampus (Weiland, 1992). Very recently, Kavaliers and Choleris (1997) have reported that exposure to predator (weasel) odor produces SIA that is completely reversed by the competitive NMDA antagonist NPC 12626 in male mice, whereas it produces equipotent SIA in female mice that is completely unaffected by such antagonism. In the same study these authors show that the analgesic effects of the κ-opioid agonist U69,593 display the same sexually dimorphic pattern of antagonism. These data are important because they show that this phenomenon is not specific to dizocilpine, swim stress, or even SIA, but rather that the female-specific analgesic mechanism may have broad relevance.
The present data are unsatisfying in that they provide no direct insight into the neurochemical basis of SIA in either male or female mice. This problem is likely to be temporary for two reasons: (1) the phenotyping and genotyping of additional F2 mice, and of congenic mice, will increase statistical power to further localize the QTL on chromosome 8 and the putative QTL on chromosome 6, and (2) neurochemically relevant genes that reside in these regions will be identified by others, providing candidate genes for these QTLs in the future. Although the exact nature of Fsia1 remains elusive at the present time, its discovery provides additional and compelling evidence for the existence of female-specific mechanisms of nociceptive modulation in the rodent. Should such sexually dimorphic pain-modulatory systems be shown to exist in humans as well, it would not be unduly speculative to propose that qualitatively different analgesic strategies may one day be applied to each sex.
Footnotes
This research was supported by a National Research Service Award Fellowship from National Institutes of Health to J.S.M. and a Veterans Affairs Merit Review Program to J.K.B. We thank Dr. Nicholas Grahame for his helpful comments.
Correspondence should be addressed to Dr. Jeffrey S. Mogil, Department of Psychology, University of Illinois at Urbana–Champaign, 603 E. Daniel Street, Champaign, IL 61820.
REFERENCES
- 1.Akinci MK, Johnston GAR. Sex differences in acute swim stress-induced changes in the binding of MK-801 to the NMDA subclass of glutamate receptors in mouse forebrain. J Neurochem. 1993;61:2290–2293. doi: 10.1111/j.1471-4159.1993.tb07472.x. [DOI] [PubMed] [Google Scholar]
- 2.Alexandrova M. Stress induced tyrosine aminotransferase activity via glucocorticoid receptor. Horm Metab Res. 1994;26:97–99. doi: 10.1055/s-2007-1000781. [DOI] [PubMed] [Google Scholar]
- 3.Aloisi AM, Albonetti ME, Carli G. Sex differences in the behavioural response to persistent pain in rats. Neurosci Lett. 1994;179:79–82. doi: 10.1016/0304-3940(94)90939-3. [DOI] [PubMed] [Google Scholar]
- 4.Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 5.Beatty WW, Beatty PA. Hormonal determinants of sex differences in avoidance behavior and reactivity to electric shock in the rat. J Comp Physiol Psychol. 1970;16:413–417. doi: 10.1037/h0030216. [DOI] [PubMed] [Google Scholar]
- 6.Belknap JK, Crabbe JC. Chromosome mapping of gene loci affecting morphine and amphetamine responses in BXD recombinant inbred mice. Ann NY Acad Sci. 1992;654:311–323. doi: 10.1111/j.1749-6632.1992.tb25977.x. [DOI] [PubMed] [Google Scholar]
- 7.Belknap JK, O’Toole LA. Studies of genetic differences in response to opioid drugs. In: Harris RA, Crabbe JC, editors. The genetic basis of alcohol and drug actions. Plenum; New York: 1991. pp. 225–252. [Google Scholar]
- 8.Belknap JK, Mogil JS, Helms ML, Richards SP, O’Toole LA, Bergeson SE, Buck KJ. Localization to proximal chromosome 10 of a locus influencing morphine-induced analgesia in crosses derived from C57BL/6 and DBA/2 mouse strains. Life Sci. 1995;57:PL117–124. doi: 10.1016/0024-3205(95)02040-p. [DOI] [PubMed] [Google Scholar]
- 9.Belknap JK, Mitchell SR, O’Toole LA, Helms ML, Crabbe JC. Type I and Type II error rates for quantitative trait loci (QTL) mapping studies using recombinant inbred mouse strains. Behav Genet. 1996;26:149–160. doi: 10.1007/BF02359892. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Eliyahu S, Page GG, Marek P, Kest B, Taylor AN, Liebeskind JC. The NMDA receptor antagonist MK-801 blocks non-opioid stress induced analgesia and decreases tumor metastasis in the rat. Proc West Pharmacol Soc. 1993;36:293–298. [PubMed] [Google Scholar]
- 11.Berkley KJ (1997) Sex differences in pain. Behav Brain Sci, in press. [DOI] [PubMed]
- 12.Bodnar RJ, Merrigan KP, Sperber ES. Potentiation of cold-water swim analgesia and hypothermia by clonidine. Pharmacol Biochem Behav. 1983;19:447–451. doi: 10.1016/0091-3057(83)90118-1. [DOI] [PubMed] [Google Scholar]
- 13.Bodnar RJ, Romero M-T, Kramer E. Organismic variables and pain inhibition: roles of gender and aging. Brain Res Bull. 1988;21:947–953. doi: 10.1016/0361-9230(88)90032-9. [DOI] [PubMed] [Google Scholar]
- 14.Cannon JT, Prieto GJ, Lee A, Liebeskind JC. Evidence for opioid and non-opioid forms of stimulation-produced analgesia in the rat. Brain Res. 1982;243:315–321. doi: 10.1016/0006-8993(82)90255-4. [DOI] [PubMed] [Google Scholar]
- 15.Carmody JJ. Avoiding fallacies in nociceptive measurements. Pain. 1995;63:136. doi: 10.1016/0304-3959(95)90018-7. [DOI] [PubMed] [Google Scholar]
- 16.Chance WT. The role of brain and spinal cord norepinephrine in autoanalgesia. Ann NY Acad Sci. 1986;467:309–330. doi: 10.1111/j.1749-6632.1986.tb14637.x. [DOI] [PubMed] [Google Scholar]
- 17.Cicero TJ, Nock B, Meyer ER. Gender-related differences in the antinociceptive properties of morphine. J Pharmacol Exp Ther. 1996;279:767–773. [PubMed] [Google Scholar]
- 18.Clark JS, Jeffs B, Davidson AO, Lee WK, Anderson NH, Bihoreau M-T, Brosnan MJ, Devlin AM, Kelman AW, Lindpaintner K, Dominiczak AF. Quantitative trait loci in genetically hypertensive rats: possible sex specificity. Hypertension. 1996;28:898–906. doi: 10.1161/01.hyp.28.5.898. [DOI] [PubMed] [Google Scholar]
- 19.Coderre TJ, Rollman GB. Stress analgesia: effects of PCPA, yohimbine, and naloxone. Pharmacol Biochem Behav. 1984;21:681–686. doi: 10.1016/s0091-3057(84)80002-7. [DOI] [PubMed] [Google Scholar]
- 20.Crabbe JC, Belknap JK, Buck KJ. Genetic animal models of alcohol and drug abuse. Science. 1994;264:1715–1723. doi: 10.1126/science.8209252. [DOI] [PubMed] [Google Scholar]
- 21.Darvasi A, Soller M. A simple method to calculate resolving power and confidence interval of QTL map location. Behav Genet. 1997;27:125–132. doi: 10.1023/a:1025685324830. [DOI] [PubMed] [Google Scholar]
- 22.Dietrich W, Katz H, Lincoln SE, Shin H-S, Friedman J, Dracopoli NC, Lander ES. A genetic map of the mouse suitable for typing intraspecific crosses. Genetics. 1992;131:423–427. doi: 10.1093/genetics/131.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dietrich WF, Miller J, Steen R, Merchant MA, Damron-Boles D, Husain Z, Dredge R, Daly MJ, Ingalls KA, O’Connor TJ, Evans CA, DeAngelis MM, Levinson DM, Kruglyak L, Goodman N, Copeland NG, Jenkins NA, Hawkins TL, Stein L, Page DC, Lander ES. A comprehensive genetic map of the mouse genome. Nature. 1996;380:149–152. doi: 10.1038/380149a0. [DOI] [PubMed] [Google Scholar]
- 24.Feine JS, Bushnell MC, Miron D, Duncan GH. Sex differences in the perception of noxious heat stimuli. Pain. 1991;44:255–262. doi: 10.1016/0304-3959(91)90094-E. [DOI] [PubMed] [Google Scholar]
- 25.Fillingim RB, Maixner W. Gender differences in the responses to noxious stimuli. Pain Forum. 1995;4:209–221. [Google Scholar]
- 26.Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. Kappa-opioids produce significantly greater analgesia in women than in men. Nature Med. 1996;2:1248–1250. doi: 10.1038/nm1196-1248. [DOI] [PubMed] [Google Scholar]
- 27.Gogas KR, Hough LB. Inhibition of naloxone-resistant antinociception by centrally administered H2-antagonists. J Pharmacol Exp Ther. 1989;248:262–267. [PubMed] [Google Scholar]
- 28.Gogas KR, Hough LB, Glickl SD, Su K. Opposing actions of cimetidine on naloxone-sensitive and naloxone-insensitive forms of footshock-induced analgesia. Brain Res. 1986;370:370–374. doi: 10.1016/0006-8993(86)90496-8. [DOI] [PubMed] [Google Scholar]
- 29.Gora-Maslak G, McClearn GE, Crabbe JC, Phillips TJ, Belknap JK, Plomin R. Use of recombinant inbred strains to identify quantitative trait loci in psychopharmacology. Psychopharmacology. 1991;104:413–424. doi: 10.1007/BF02245643. [DOI] [PubMed] [Google Scholar]
- 30.Kavaliers M. Brief exposure to a natural predator, the short-tailed weasel, induces benzodiazepine-sensitive analgesia in white-footed mice. Physiol Behav. 1988;43:187–193. doi: 10.1016/0031-9384(88)90236-3. [DOI] [PubMed] [Google Scholar]
- 31.Kavaliers M, Choleris E (1997) Sex differences in NMDA involvement in kappa opioid and non-opioid predator-induced analgesia in mice. Brain Res, in press. [DOI] [PubMed]
- 32.Kavaliers M, Colwell DD. Sex differences in opioid and non-opioid mediated predator-induced analgesia in mice. Brain Res. 1991;568:173–177. doi: 10.1016/0006-8993(91)91394-g. [DOI] [PubMed] [Google Scholar]
- 33.Kavaliers M, Galea LAM. Sex differences in the expression and antagonism of swim stress-induced analgesia in deer mice vary with the breeding season. Pain. 1996;63:327–334. doi: 10.1016/0304-3959(95)00063-1. [DOI] [PubMed] [Google Scholar]
- 34.Kavaliers M, Hirst M. Daily rhythms of analgesia in mice: effects of age and photoperiod. Brain Res. 1983;279:387–393. doi: 10.1016/0006-8993(83)90216-0. [DOI] [PubMed] [Google Scholar]
- 35.Kavaliers M, Innes DGL. Sex differences in the effects of neuropeptide FF and IgG from neuropeptide FF on morphine- and stress-induced analgesia. Peptides. 1992;13:603–607. doi: 10.1016/0196-9781(92)90096-l. [DOI] [PubMed] [Google Scholar]
- 36.Kelly DD (ed) (1986) Stress-induced analgesia. Ann NY Acad Sci 467.
- 37.Kepler KL, Standifer KM, Paul D, Kest B, Pasternak GW, Bodnar RJ. Gender effects and central opioid analgesia. Pain. 1991;45:87–94. doi: 10.1016/0304-3959(91)90168-W. [DOI] [PubMed] [Google Scholar]
- 38.Kiefel JM, Bodnar RJ. Roles of gender and gonadectomy in pilocarpine and clonidine analgesia in rats. Pharmacol Biochem Behav. 1991;41:153–158. doi: 10.1016/0091-3057(92)90075-q. [DOI] [PubMed] [Google Scholar]
- 39.Lander ES, Botstein D. Mapping Mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics. 1989;121:185–199. doi: 10.1093/genetics/121.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lander ES, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- 41.Lander ES, Schork NJ. Genetic dissection of complex traits. Science. 1994;265:2037–2048. doi: 10.1126/science.8091226. [DOI] [PubMed] [Google Scholar]
- 42.Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L. MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1987;1:174–171. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- 43.Lewis JW, Cannon JT, Liebeskind JC. Opioid and non-opioid mechanisms of stress analgesia. Science. 1980;208:623–625. doi: 10.1126/science.7367889. [DOI] [PubMed] [Google Scholar]
- 44.Lipsitt LP, Levy N. Electrotactual threshold in the neonate. Child Dev. 1959;30:547–554. doi: 10.1111/j.1467-8624.1959.tb04961.x. [DOI] [PubMed] [Google Scholar]
- 45.Manly KE. A Macintosh program for storage and analysis of experimental genetic mapping data. Mamm Genome. 1993;4:303–313. doi: 10.1007/BF00357089. [DOI] [PubMed] [Google Scholar]
- 46.Marek P, Page GG, Ben-Eliyahu S, Liebeskind JC. NMDA receptor antagonist MK-801 blocks non-opioid stress-induced analgesia. I. Comparison of opiate receptor-deficient and opiate receptor-rich strains of mice. Brain Res. 1991;551:293–296. doi: 10.1016/0006-8993(91)90943-p. [DOI] [PubMed] [Google Scholar]
- 47.Marek P, Mogil JS, Sternberg WF, Panocka I, Liebeskind JC. N-methyl-d-aspartic acid (NMDA) receptor antagonist MK-801 blocks non-opioid stress-induced analgesia. II. Comparison across three swim stress paradigms in selectively bred mice. Brain Res. 1992;578:197–203. doi: 10.1016/0006-8993(92)90248-8. [DOI] [PubMed] [Google Scholar]
- 48.Mayer DJ, Frenk H. The role of neuropeptides in pain. In: Nemeroff CB, editor. Neuropeptides in psychiatric and neurological disorders. Johns Hopkins UP; Baltimore: 1988. pp. 199–280. [Google Scholar]
- 49.Melo JA, Shendure J, Pociask K, Silver LM. Identification of sex-specific quantitative trait loci controlling alcohol preference in C57BL/6 mice. Nat Genet. 1996;13:147–153. doi: 10.1038/ng0696-147. [DOI] [PubMed] [Google Scholar]
- 50.Menendez L, Andres-Trelles F, Hidalgo A, Baamonde A. Gender and test dependence of a type of kappa mediated stress-induced analgesia in mice. Gen Pharmacol. 1994;25:903–908. doi: 10.1016/0306-3623(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 51.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mogil JS, Belknap JK. Sex and genotype determine the selective activation of neurochemically-distinct mechanisms of swim stress-induced analgesia. Pharmacol Biochem Behav. 1997;56:61–66. doi: 10.1016/S0091-3057(96)00157-8. [DOI] [PubMed] [Google Scholar]
- 53.Mogil JS, Marek P, Sternberg WF, Spence MA, Liebeskind JC. A genetic analysis of swim stress-induced analgesia in selectively bred mice. Soc Neurosci Abstr. 1992;18:686. [Google Scholar]
- 54.Mogil JS, Sternberg WF, Kest B, Marek P, Liebeskind JC. Sex differences in the antagonism of swim stress-induced analgesia: effects of gonadectomy and estrogen replacement. Pain. 1993;253:17–25. doi: 10.1016/0304-3959(93)90050-Y. [DOI] [PubMed] [Google Scholar]
- 55.Mogil JS, Sadowski B, Belknap JK. The role of the serotonin-1B receptor in genetic sensitivity to opiate analgesia in mice. Soc Neurosci Abstr. 1995;21:1415. [Google Scholar]
- 56.Mogil JS, Kest B, Sadowski B, Belknap JK. Differential genetic mediation of sensitivity to morphine in genetic models of opiate antinociception: influence of nociceptive assay. J Pharmacol Exp Ther. 1996a;276:532–544. [PubMed] [Google Scholar]
- 57.Mogil JS, Sternberg WF, Balian H, Liebeskind JC, Sadowski B. Opioid and non-opioid swim stress-induced analgesia: a parametric analysis in mice. Physiol Behav. 1996b;59:123–132. doi: 10.1016/0031-9384(95)02073-x. [DOI] [PubMed] [Google Scholar]
- 58.Mogil JS, Sternberg WF, Marek P, Sadowski B, Belknap JK, Liebeskind JC. The genetics of pain and pain inhibition. Proc Natl Acad Sci USA. 1996c;93:3048–3055. doi: 10.1073/pnas.93.7.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mogil JS, Richards SP, O’Toole LA, Helms ML, Mitchell SR, Belknap JK. Genetic sensitivity to hot-plate nociception in DBA/2J and C57BL/6J inbred mouse strains: possible sex-specific mediation by δ2-opioid receptors. Pain. 1997;70:267–277. doi: 10.1016/s0304-3959(97)03333-2. [DOI] [PubMed] [Google Scholar]
- 60.Rodgers RJ, Randall JI. Benzodiazepine ligands, nociception and “defeat” analgesia in male mice. Psychopharmacology. 1987;91:305–315. doi: 10.1007/BF00518182. [DOI] [PubMed] [Google Scholar]
- 61.Rodgers RJ, Shepherd JK. Prevention of the analgesic consequences of social defeat in male mice by 5-HT1A anxiolytics, buspirone, gepirone and ipsapirone. Psychopharmacology. 1989;99:374–380. doi: 10.1007/BF00445561. [DOI] [PubMed] [Google Scholar]
- 62.Rodgers RJ, Shepherd JK, Randall JI. Highly potent inhibitory effects of 5-HT3 receptor antagonist, GR38032F, on non-opioid defeat analgesia in male mice. Neuropharmacology. 1990;29:17–23. doi: 10.1016/0028-3908(90)90078-6. [DOI] [PubMed] [Google Scholar]
- 63.Romero M-T, Kepler KL, Bodnar RJ. Gender determinants of opioid mediation of swim analgesia in rats. Pharmacol Biochem Behav. 1988;29:705–709. doi: 10.1016/0091-3057(88)90191-8. [DOI] [PubMed] [Google Scholar]
- 64.Sokal RR, Rohlf FJ. Biometry. Freeman; San Francisco: 1981. [Google Scholar]
- 65.Sternberg WF, Mogil JS, Pilati ML, Boun C, Wong SK, Liebeskind JC. Neurochemical quality of nonopioid stress-induced analgesia is not altered by estrous phase in female mice. Proc West Pharmacol Soc. 1994;37:141–143. [PubMed] [Google Scholar]
- 66.Sternberg WF, Mogil JS, Kest B, Page GG, Leong Y, Yam V, Liebeskind JC. Neonatal testosterone exposure influences neurochemistry of swim stress-induced analgesia in adult mice. Pain. 1996;63:321–326. doi: 10.1016/0304-3959(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 67.Tarricone BJ, Hingtgen JN, Belknap JK, Mitchell SR, Nurnberger J. Quantitative trait loci associated with the behavioral response of BXD recombinant inbred mice to restraint stress: a preliminary communication. Behav Genet. 1995;25:489–495. doi: 10.1007/BF02253378. [DOI] [PubMed] [Google Scholar]
- 68.Terman GW, Shavit Y, Lewis JW, Cannon JT, Liebeskind JC. Intrinsic mechanisms of pain inhibition: activation by stress. Science. 1984;226:1270–1277. doi: 10.1126/science.6505691. [DOI] [PubMed] [Google Scholar]
- 69.Tierney G, Carmody JJ, Jamieson D. Stress analgesia: the opioid analgesia of long swims suppresses the non-opioid analgesia induced by short swims in mice. Pain. 1991;46:89–95. doi: 10.1016/0304-3959(91)90038-Y. [DOI] [PubMed] [Google Scholar]
- 70.Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 71.Watkins LR, Mayer DJ. The organization of endogenous opiate and nonopiate pain control system. Science. 1982;216:1185–1192. doi: 10.1126/science.6281891. [DOI] [PubMed] [Google Scholar]
- 72.Watkins LR, Wiertelak EP, Grisel JE, Silbert LH, Maier SF. Parallel activation of multiple spinal opiate systems appears to mediate “non-opiate” stress-induced analgesias. Brain Res. 1992;594:99–108. doi: 10.1016/0006-8993(92)91033-b. [DOI] [PubMed] [Google Scholar]
- 73.Weiland NG. Estradiol selectively regulates agonist binding sites on the N-methyl-d-aspartate receptor complex in the CA1 region of the hippocampus. Endocrinology. 1992;131:662–668. doi: 10.1210/endo.131.2.1353442. [DOI] [PubMed] [Google Scholar]
- 74.Wong C-L. Sex difference in naloxone antagonism of swim stress induced antinociception in mice. Methods Find Exp Clin Pharmacol. 1987;9:275–278. [PubMed] [Google Scholar]
- 75.Woolf FM. Meta-analysis: quantitative methods for research synthesis. Sage Publications; Beverly Hills, CA: 1986. [Google Scholar]