Abstract
Inwardly rectifying potassium (K+) channels (Kir) in Müller cells, the dominant glial cells in the retina, are supposed to be responsible for the spatial buffering action of K+ ions. The molecular properties and subcellular localization of Müller cell Kir channels in rat and rabbit retinas were examined by using electrophysiological, molecular biological, and immunostaining techniques. Only a single population of Kir channel activity, the properties of which were identical to those of KAB-2/Kir4.1 expressed in HEK293T cells, could be recorded from endfoot to the distal portion of Müller cells. Consistently, Northern blot, in situ hybridization, and RT-PCR analyses indicated expression of Kir4.1 in Müller cells per se. The Kir4.1 immunoreactivity was distributed in clusters throughout Müller cell membrane. The Kir4.1 expression in Müller cells disappeared promptly after culturing. When the dissociated Müller cells were cultured on laminin-coated dishes in the presence of insulin, Kir4.1 immunoreactivity was detected in a clustered manner on the cell membrane. Because insulin and laminin exist in the surrounding of Müller cells in the retina, these substances possibly may be physiological regulators of expression and distribution of Kir4.1 in Müller cells in vivo.
Keywords: inwardly rectifying potassium channel, retinal (glial) Müller cell, clustered distribution, insulin, laminin, cell culture
Neural excitation causes an increase of extracellular potassium ions (K+) especially at synaptic sites in the CNS, including the retina, which if uncorrected would result in the loss of synaptic transmission by depolarizing the membrane. Glial cells, which surround neurons, are supposed to transport the accumulated K+ from proximal to distal portions of the cells. This regulatory function was proposed first as a spatial buffering mechanism of K+ for astrocytes in the optic nerves (Orkand et al., 1966) and also was termed the K+ siphoning mechanism for Müller cells of the retina (Newman et al., 1984).
Müller cells, which are the principal glial cells in the retina, have been used extensively to elucidate this function, because the structure of the retina has been well characterized, and dynamics of K+-movement could be characterized more easily there than in the brain. Moreover, Müller cells can be isolated and identified easily. Newman et al. (1984) actually showed a dissociated amphibian Müller cell to possess the capability of aspirating extracellular K+ from its distal end and secreting it from its proximal endfoot.
Isolated Müller cells also have been used in electrophysiological studies. Patch-clamp studies demonstrated the expression of inwardly rectifying K+ channels (Kir) in salamander Müller cells (Brew et al., 1986; Newman, 1987). In amphibia, >90% of the K+ conductance exists in the endfoot because of the higher density of Kir in this region. This characteristic distribution of Kir is supposed to be crucial for K+ siphoning in the retina of this species. In mammalian Müller cells, on the other hand, conductivity of K+ of the endfoot was relatively small compared with that of amphibian cells (Newman, 1987). Rabbit Müller cells have been reported to express at least three kinds of Kir channels, the properties and distributions of which are different from those of Kir in salamander Müller cells (Nilius and Reichenbach, 1988). These suggest that mammalian Müller cells perform K+spatial buffering differently from amphibian cells.
Recently, molecular biological dissection of Kir has demonstrated that this family is composed of >10 members. We previously have isolated one of these members, KAB-2/Kir4.1, which has a Walker-type A ATP-binding domain in its C terminus, from rat brain and found that Kir4.1 mRNA was expressed predominantly in mammalian glial cells (Bond et al., 1994; Takumi et al., 1995; Pessia et al., 1996). On the other hand, other Kir mRNAs are expressed mainly in neurons (Horio et al., 1996; Karschin et al., 1996). These data strongly suggest that Kir4.1 is responsible for the glial K+ spatial buffering.
We investigated Kir channels of mammalian retinal (glial) Müller cells with electrophysiology, molecular biology, and immunostaining techniques. We found that the predominant Kir in these cells was Kir4.1, which distributed in clusters on the Müller cell membrane. We further found on cultured retinal cells that insulin and laminin were indispensable factors to induce the expression and clustered distribution of the Kir channel. Thus the K+ buffering current in Müller cells may be regulated dynamically by hormones and extracellular matrices in the retina.
MATERIALS AND METHODS
Preparation of isolated cells. Müller cells were isolated from the eyes of Japanese white rabbits (Kitayama Rabess, Nagano, Japan) or Wister rats (Nippon Doubutsu, Kyoto, Japan). Animals were anesthetized by an overdose of intravenous (rabbit) or intraperitoneal (rat) pentobarbital injection (100 mg/kg body weight) and enucleated. Müller cells were isolated essentially as described by Newman (1987). Pieces of isolated retina were incubated with 0.0125% papain (Worthington, Freehold, NJ) in 2.5 mmEGTA, 2 mml-cysteine, and Ca2+/Mg2+-free HBSS for 20 min at 37°C and then rinsed in 1% bovine serum albumin and 300 U/ml DNase I (Takara, Kyoto, Japan) in DMEM (Nikken, Kyoto, Japan). Cells were triturated gently with a Pasteur pipette and collected by centrifugation (30 × g for 10 min). Then the cells were washed four times with DMEM. This final fraction contained ∼30% of Müller cells.
Cell culture. Dissociated Müller cells were seeded on glass coverslips (15 mm diameter) coated with poly-d-lysine or laminin of the Engelbreth-Holm-Swarm mouse tumor (Upstate Biotechnology, New York, NY), kept in a humidified environment of 95% air/5% CO2 at 37°C, and fed with DMEM containing 10% fetal calf serum (FCS) (Life Technologies, Gaithersburg, MD) and antibiotic–antimycotic (Life Technologies). Cells were cultured for 4 d and then used for experiments.
Northern blot hybridization. Total RNA was extracted from dissociated rabbit Müller cells enriched fraction with RNeasy (Invitrogen, San Diego, CA). Northern blot analysis was performed as described previously (Takumi et al., 1995). TheSacI-digested fragment of rat Kir4.1 cDNA was used as a probe. A high-stringency washing condition (0.1× SSC and 0.1% SDS at 65°C for 15 min each for two times) was introduced to identify rabbit Kir4.1 mRNA.
In situ hybridization histochemistry. In situ hybridization was performed with frozen sections of Wistar rat eyes. The BstXI–SacI fragment (0.37 kb) of rat Kir4.1 cDNA was used as a template. Sections (20 μm thick) were hybridized with [35S]-labeled antisense or sense cRNA probe and washed as described previously (Takumi et al., 1995).
PCR amplification of Kir4.1 cDNA. Total RNAs of cultured Müller cells and a dissociated single Müller cell, which was aspirated and transferred into a microcentrifuge tube with a glass tip such as that used for patch-clamp experiments under a microscope, were extracted, and cDNAs were synthesized as described by Sucher and Deitcher (1995). Because the Kir4.1 gene is an intronless gene (our unpublished observation), to eliminate contamination of genomic DNA, we treated RNA samples with DNaseI (Takara) before cDNA synthesis. Primers for PCR reaction were located in nucleotides 194–211 and nucleotides 848–865 of rat Kir4.1 cDNA (Takumi et al., 1995). PCR amplification was performed for 30 cycles at 94°C for 45 sec, at 55°C for 1 min, and at 72°C for 2 min and then at 72°C for 8 min. Because the products from the single and six Müller cells were not enough to be visualized, second PCR reactions (50 μl) using the same primers and amplification conditions were performed with 2 μl of the first PCR products. The products were electrophoresed on a 1% agarose gel. The amplified PCR products were confirmed to be rabbit or rat Kir4.1 DNA fragments by their nucleotide sequences. The nucleotide sequence of the amplified PCR products was performed with the dye-primer method and DNA sequencer (A-381, Perkin-Elmer, Foster City, CA) after TA cloning (Invitrogen).
Immunohistochemistry. The polyclonal antibody for Kir4.1 (anti-KAB-2C2) was raised in rabbit against a synthetic peptide corresponding to amino acid residues 366–379 (EKEGSALSVRISNV) in the C-terminal region of rat Kir4.1 (Ito et al., 1996). The antiserum was purified with protein A-Cellulofine (Seikagaku, Tokyo, Japan) and antigenic peptide-coupled Sulfolink resin (Pierce, Rockford, IL). Male Wister rats weighing ∼250 gm were anesthetized deeply with pentobarbital (100 mg/kg) and perfused with 100 ml of PBS and then with 250 ml of 4% paraformaldehyde in 0.1 m sodium phosphate (PA solution), pH 7.4. After perfusion, eyes were enucleated, fixed again with PA solution for 3–48 hr, dehydrated with sucrose, and frozen. Sections (10 μm) were cut on a cryostat and thaw-mounted on gelatin-coated slides. Cultured cells on glasses were rinsed with PBS and then fixed with PA solution. Samples were washed twice with PBS containing 0.1% Triton X-100 (PBST) for 5 min each, treated with 1% (w/v) bovine serum albumin in PBS at room temperature for 30 min, and then incubated with anti-KAB-2C2 (0.15 μg/ml) and mouse monoclonal anti-vimentin antibody (Zymed Laboratory, San Francisco, CA) in PBS at 4°C overnight. The sections were washed five times with PBS at room temperature for 15 min each and visualized with fluorescein isothiocyanate (FITC)-labeled anti-rabbit IgG (EY Laboratories, San Mateo, CA) and Texas Red-labeled anti-mouse IgG (Protos Immunoresearch, San Francisco, CA). The sections were examined with a confocal microscope (MRC-1024, Bio-Rad, Hertfordshire, England). For control experiments, anti-KAB-2C2 preabsorbed with excess antigenic peptide (3 μg/ml) was used.
Electron microscopy. The immunogold electron microscopic experiment was performed as described previously (Gotow et al., 1995). After fixation, the retinas were dehydrated in 2.3 msucrose containing 0.1 m sodium phosphate, pH 7.4, and frozen in liquid nitrogen. Cryothin sections were cut with a microtome equipped with a cryo attachment (OmU4, Reichert, Vienna, Austria) and collected on Formvar carbon-coated grids. The cryothin sections on grids were treated with 1% BSA in PBS and incubated with anti-KAB-2C2 and then goat anti-rabbit IgG coupled to 5-nm-colloidal-gold particles (Amersham, Buckinghamshire, England). The sections again were fixed with 2% glutaraldehyde and post-fixed with 1% OsO4, stained with 0.5% uranyl acetate, dehydrated in ethanol, and embedded in London Resin white.
Electrophysiological recordings. Whole-cell and single-channel currents of acutely isolated Müller cells and cultured cells were measured at room temperature by a patch-clamp amplifier (Axon 200A, Axon Instruments, Foster City, CA) and recorded on videocassette tapes with a PCM converter system (VR-10B, Instrutech, New York, NY). For analysis, data were reproduced, low-pass-filtered at 1 kHz (−3 dB) by an eight-pole Bessel filter (Frequency Devices, Haverhill, MA), sampled at 5 kHz, and analyzed off-line on a computer (Macintosh Quadra 700, Apple Computer, Cupertino, CA) with commercially available software (Patch Analyst Pro, MT Corporation, Nishinomiya, Hyogo, Japan). In whole-cell current recording, the bathing solution contained (in mm): 130 NaCl, 5 KCl, 1.8 CaCl2, 0.53 MgCl2, 5.5 glucose, and 5.5 HEPES-KOH, pH 7.4. The pipette solution contained (in mm): 150 KCl, 5 K2ATP, 1 MgCl2, 5 EGTA, and 5 HEPES-KOH, pH 7.3. In single-channel recording, the bathing solution contained (in mm): 150 KCl, 5 EGTA, and 5 HEPES-KOH, pH 7.4, and the pipette solution contained (in mm): 150 KCl, 1 MgCl2, 1 CaCl2, and 5 HEPES-KOH, pH 7.4. Data were expressed as mean ± SE.
RESULTS
Electrophysiological properties of Müller cell K+ channels
Whole-cell currents were examined in Müller cells isolated from rabbit retinas (Fig.1A). The isolated cells exhibited the typical morphological characteristics of the Müller cell, i.e., distal end, soma, proximal stalk, and endfoot, as shown in Figure 1E. These cells were stained by anti-vimentin antibody (see also Fig. 6); vimentin is an intermediate filament that is a marker of Müller cells (Dräger, 1983; Vaugham and Lasater, 1990). From these observations we concluded that these isolated cells were retinal Müller cells. The resting membrane potential of rabbit Müller cells was −70 ± 3 mV (n = 5). Under the whole-cell voltage-clamp condition, depolarizing voltage steps from the holding potential of −70 mV elicited outward K+ currents and hyperpolarizing steps induced inwardly flowing K+ currents (Fig.1Aa). The latter currents were inhibited completely by 100 μm Ba2+ added to the bathing solution (Fig. 1Ab), whereas the former were blocked by 10 mm 4-aminopyridine, an inhibitor of voltage-dependent K+ (KV) channels (data not shown). The Ba2+-sensitive component of the membrane current was inward at potentials more negative than −80 mV and became zero at approximately −70 mV. The Ba2+-sensitive outwardly flowing currents at potentials more positive than −70 mV were much smaller than the inward currents and became negligible at those >0 mV (Fig. 1Ac). Thus the Ba2+-sensitive current component in rabbit Müller cells is carried mainly by K+ ions and exhibits the classical inwardly rectifying property.
Fig. 1.
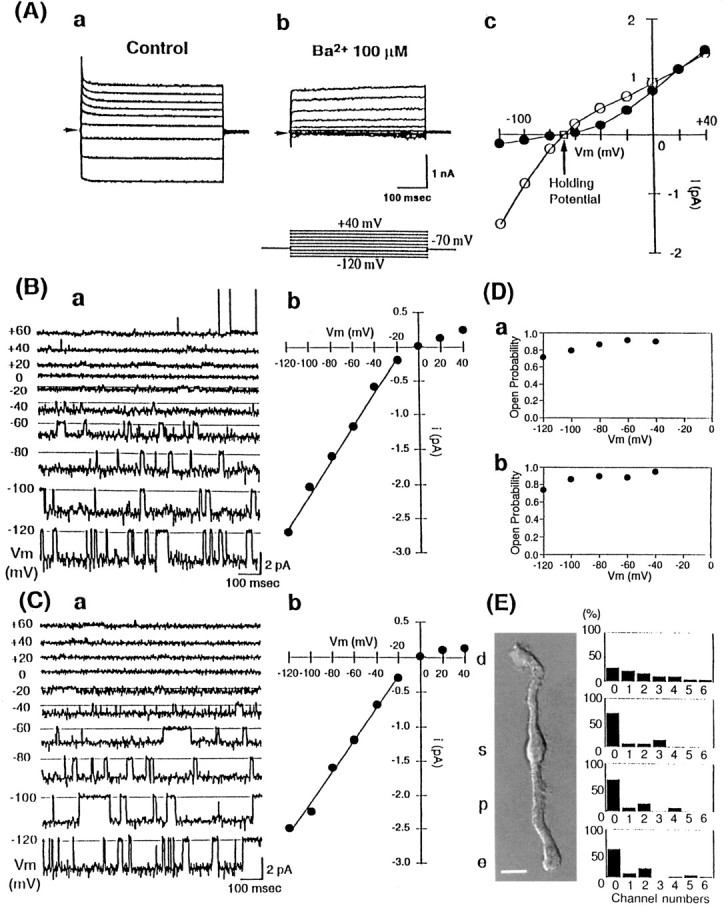
Functional expression of Kir4.1 in rabbit Müller cells. A, Whole-cell recordings of isolated rabbit Müller cells. The holding potential was −70 mV. Traces were recorded with voltage steps from −120 to +40 mV in 20 mV steps (inset). a, Control. b, Effect of 100 μm external Ba2+. c, Current–voltage relationship of the steady-state currents in the presence (•) or absence (○) of 100 μmBa2+. Ba2+ predominantly inhibited inward currents. B, Single-channel recordings from cell-attached membrane patches of isolated rabbit Müller cells. a, Membrane current traces were recorded at the membrane potential values indicated to the left of each trace. The patch contained a Kir channel dominantly, but at depolarized potentials, currents of K+ channels with a large conductance were recorded. b, Current–voltage relationship of the Kir channel of isolated rabbit Müller cells. The single-channel conductance of this Kir was 25 pS. C, Single-channel recordings from cell-attached membrane patches of HEK293T cells expressing rat Kir4.1 channel. a, Traces were elicited from the same potentials as Ba.b, Current–voltage relationship of rat Kir4.1. The single-channel conductance of rat Kir4.1 was 23 pS. D, Voltage dependence of open probabilities of the Kir on rabbit Müller cells (a) and rat Kir4.1 (b). Both channels showed very high open probabilities. E, Distribution of the Kir channel on isolated rabbit Müller cells. The isolated cells exhibited typical morphological characteristics of the Müller cell shown in the figure, i.e., distal end (d), soma (s), proximal stalk (p), and endfoot (e) (left panel). The histograms of Kir4.1 channel number per patch in these four different regions are shown (right panel). Each patch contains 0–6 Kir4.1 channels:x-axis, the number of channels in each patch;y-axis, the relative frequency (in percentage) of each number of Kir4.1 channels (the total number of cell-attached patch experiments = 32 in d, n = 12 in s, n = 14 in p, and n = 18 in e). Channels were distributed diffusely and not concentrated in the endfoot region. Scale bar, 10 μm.
Fig. 6.
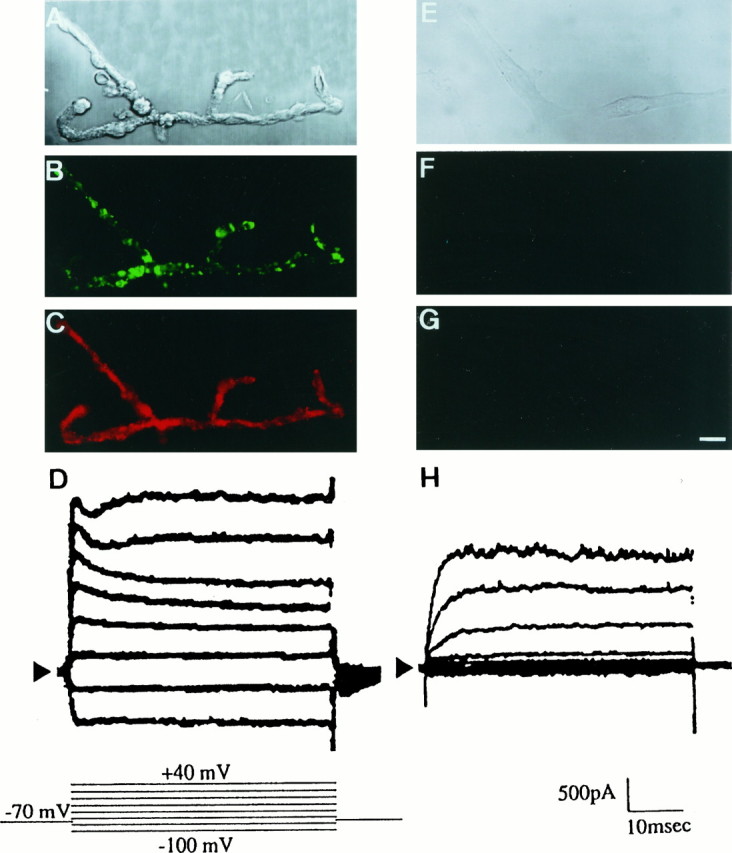
Immunostaining and whole-cell currents of Kir4.1 in acutely dissociated and 4 d cultured Müller cells from rat retina. A–D, Acutely dissociated rat Müller cells. E–H, Cells cultured for 4 d on cover glasses coated with poly-d-lysine. A, E, Nomarski images. Dissociated rat Müller cells aggregated with each other, which is different from rabbit Müller cells. InA, there were at least four cells. B, F, Immunostaining of Kir4.1 (green). C, G, Immunostaining of vimentin (red). D, H, Whole-cell currents were induced under voltage steps from −100 to +40 mV with 20 mV steps (inset). Cells were bathed in 5 mm Ko+. The holding potential was −70 mV. Inward currents of cultured cells were diminished. Scale bar (shown in G), 10 μm.
The properties of the inwardly rectifying K+ (Kir) channels in Müller cells also were examined by the cell-attached patch-clamp technique (Fig. 1B). The pipette solution contained 150 mm K+. In >200 trials of cell-attached patches, we could record only a single population of Kir channel currents from Müller cell membranes. Figure1Ba depicts single-channel currents of this Kir channel at various potentials. The currents through this channel flowed much more readily in the inward than in the outward direction, thus clearly exhibiting the inwardly rectifying property. At depolarized potentials, large-conductance (180 ± 10 pS, n = 20) K+ channel currents sometimes were recorded. This channel was identified as a Ca2+-activated K+ channel because its openings were increased dramatically when Ca2+ (1 μm) was added to the internal side of excised patch membranes (data not shown).
The unitary conductance of the Kir channel in the inward direction was 25 ± 3 pS (n = 40, Fig. 1Bb). The channel openings occurred in bursts. The open time histogram could be fit by a single exponential with a time constant of ∼100 msec at −100 to approximately −60 mV, and the closed time histogram was fit by a sum of two exponentials with the time constants of ∼3 and 20–30 msec (data not shown). The open probability (Po) of the channels was ∼0.8–0.9 at potentials between −100 and −40 mV (Fig. 1Da). These characteristics are very similar to those of Kir4.1 channel in its low-conductance state expressed in Xenopus oocytes (Takumi et al., 1995).
In Figure 1C we examined the properties of rat Kir4.1 channel heterologously expressed in human embryonic kidney (HEK) HEK293T cells. Although we observed two distinct conductances (21 and 36 pS with 150 mm Ko+) of rat Kir4.1 channel in Xenopus oocytes, only the low-conductance state of the channel activity was recorded in 50 patches of HEK cells. The single-channel currents at various potentials are depicted in Figure1Ca. The single-channel conductance was 23 ± 3 pS (n = 30, Fig. 1Cb). At potentials between −100 and −60 mV, the open time was ∼100 msec, the short closed time was 3 msec, and the long closed time was ∼20–30 msec (data not shown). The channel Po at potentials between −100 and −40 mV was ∼0.9 (Fig. 1Db). Thus the conductance and kinetic properties of Kir4.1 in HEK cells were identical to those of the Kir channel recorded in rabbit Müller cells.
In Figure 1E we show the distribution of Kir4.1 channel activity on rabbit Müller cell membrane. We were able to record Kir4.1 channel currents throughout the Müller cell membrane from its endfoot region to the distal end. Different from the salamander Müller cell, there was no clear accumulation of the channel activity at the endfoot. The channel activity could be recorded more frequently at the distal portion.
Expression of Kir4.1 mRNAs in Müller cells
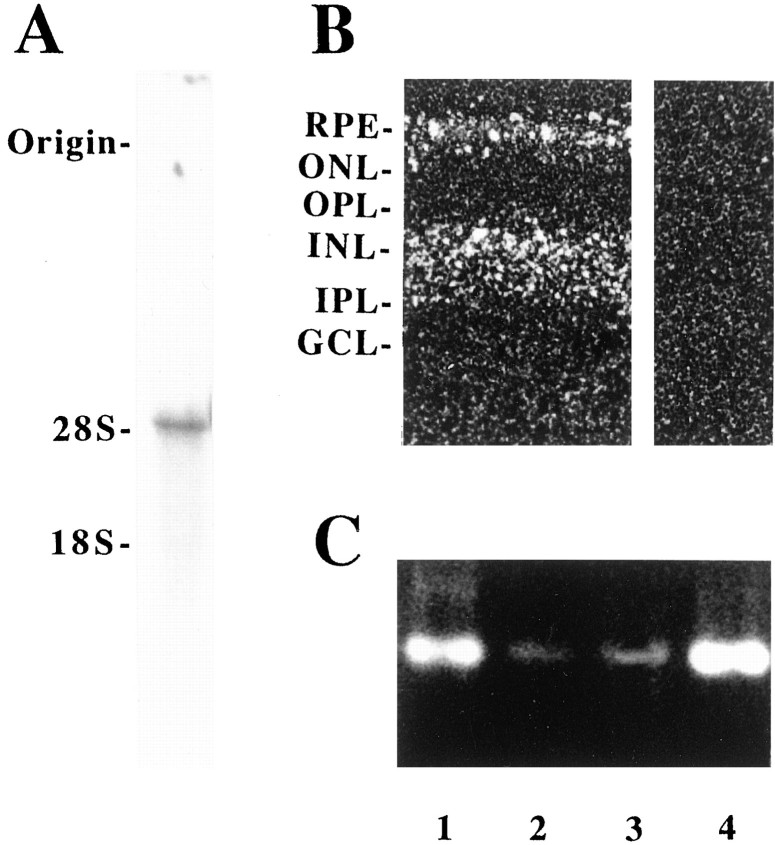
We next examined whether Kir4.1 mRNA is expressed in retinal Müller cells (Fig. 2). Northern blot analysis of a Müller cell enriched fraction prepared from rabbit retina showed a single band (4.8 kb) of rabbit Kir4.1 mRNA (Fig.2A). A single band of 5.5 kb also was obtained in rat (data not shown). The distribution of Kir4.1 mRNA in a slice of rat retina was examined by in situ hybridization (Fig.2B). Kir4.1 mRNA was detected in the inner nuclear layer (INL), where somata of Müller cells exist (Fig.2B, left panel). Retinal pigment epithelial cells also expressed Kir4.1 mRNA. These signals disappeared when a sense probe was used (Fig. 2B,right panel). To examine further whether Müller cells per se express Kir4.1 mRNA, we performed reverse transcriptase PCR (RT-PCR) of Kir4.1, using a dissociated single Müller cell from rabbit retina (Fig. 2C). A single rabbit Müller cell identified under an inverted microscope was aspirated with a glass capillary tube. RNA was extracted from the cell, as described by Sucher and Deitcher (1995). RT-PCR analyses of RNAs obtained from single or six Müller cells demonstrated 672 bp of rabbit cDNA fragment (Fig. 2C, lanes 2 and 3). Although nucleotide sequences of the amplified rabbit DNA fragment showed 88.2% identity with that of rat Kir4.1 cDNA, its deduced amino acid sequence showed 99.1% similarity, indicating that the amplified DNA fragment was rabbit Kir4.1 cDNA (data not shown). All of these data indicate that retinal Müller cells per se actually express Kir4.1 mRNA.
Fig. 2.
Expression of Kir4.1 mRNA in the retina.A, Northern blot analysis of rabbit Kir4.1 mRNA. Total RNA (20 μg) from a rabbit Müller cell enriched fraction was separated on 1% agarose gel containing formaldehyde and transferred to a nylon membrane. Rat Kir4.1 cDNA was used as a probe. B, Distribution of Kir4.1 mRNA. Frozen sections (20 μm) of rat eyes were fixed with 4% paraformaldehyde and hybridized with Kir4.1 cRNA antisense probe (left panel) or sense probe (right panel). After a high-stringency wash, sections were dipped in emulsion and developed for 2 weeks. Grains showing Kir4.1 mRNA were detected in the inner nuclear layer (INL), where cell bodies of Müller cells exist, and the retinal pigment epithelial layer (RPE). Retinal pigment epithelial cells also expressed Kir4.1. Labels are explained in Figure 3. C, PCR amplification of Kir4.1 cDNA from a single Müller cell. A single rabbit Müller cell was isolated by using a siliconized glass capillary. RNA was extracted from the cell, and cDNA was synthesized. After PCR reaction, products were electrophoresed on a 1% agarose gel. Kir4.1 fragments (672 bp) were amplified with cDNA/mRNAs from a Müller cell enriched fraction (lane 1), a single rabbit Müller cell (lane 2), six rabbit Müller cells (lane 3), and a control rat Kir4.1 cDNA (lane 4).
Confocal image and immunogold electron microscopy analyses of Kir4.1 immunoreactivity in retina
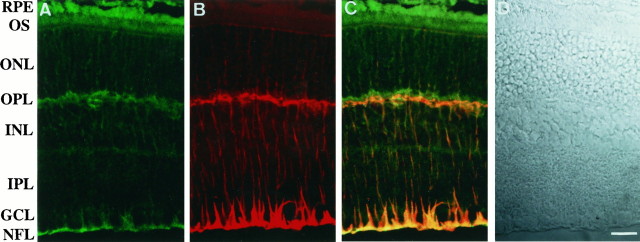
A polyclonal anti-Kir4.1 antibody (anti-KAB-2C2) was raised in rabbit against a synthetic peptide corresponding to amino acids 366–379 (EKEGSALSVRISNV) in the C terminus of rat Kir4.1 (Ito et al., 1996). Because this antibody recognized rat Kir4.1, but not rabbit Kir4.1, we used rat retinas in the following immunohistochemical experiments. In immunostaining of the rat retinal section, anti-KAB-2C2 stained the nerve fiber layer (NFL), the ganglion cell layer (GCL), the inner plexiform layer (IPL), the inner nuclear layer (INL), the outer plexiform layer (OPL), and the outer nuclear layer (ONL) (Fig. 3A). String-like stainings were observed in INL and ONL. These stainings disappeared when the antibody was preabsorbed with immunogenic peptide (data not shown). The staining pattern by anti-KAB-2C2 was quite similar to that of vimentin (Fig. 3B), a marker of Müller cells in the retina (Dräger, 1983; Vaugham and Lasater, 1990). The triangular stainings by anti-KAB-2C2 in NFL and GCL appeared to be endfeet of Müller cells. Strong immunoreactivity at OPL may be attributable to many fine filamentous processes of Müller cells (Reichenbach et al., 1989). As shown in Figure 3A, the apical membrane of retinal pigment epithelial cells also showed strong Kir4.1 immunoreactivity, where the vimentin was not detected. This is consistent with the expression of Kir4.1 mRNA in these cells detected in in situ hybridization (Fig.2B).
Fig. 3.
Immunohistochemical analysis of Kir4.1 in the retina. A sagittal section of rat retina (10 μm) was double-stained with affinity-purified rabbit anti-rat Kir4.1 antibody, followed by FITC-conjugated anti-rabbit IgG (A andC, in green), and monoclonal anti-vimentin antibody, followed by Texas Red-labeled anti-mouse IgG (B and C, in red).C, Double exposures of both images. D, Nomarski image of the same sagittal section asA–C. Kir4.1 was expressed in Müller cells and also in retinal pigment epithelial cells. Scale bar (shown in D), 10 μm. NFL, Nerve fiber layer; GCL, ganglion cell layer;IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer;ONL, outer nuclear layer; OS, outer segment of photoreceptor cell; RPE, retinal pigment epithelial cell layer.
Whole-mount immunohistochemistry of a rat retina was performed with anti-KAB-2C2 (Fig. 4). The distribution of Kir4.1 immunoreactivity was examined at various horizontal levels of the retina with a confocal microscope. The immunoreactivity was densely detected around photoreceptor cells in ONL (Fig. 4A), where the distal ends of Müller cells surrounded photoreceptor cells, as indicated by the vimentin staining (Fig. 4B). Kir4.1 immunoreactivity was detected in a scattered manner around ganglion cells in GCL (Fig.4D), although Müller cells wrapped ganglion cells in this region (Fig. 4E). Double staining of Kir4.1 and vimentin clearly indicated clustered and scattered distribution of Kir4.1 immunoreactivity on Müller cell membranes in the retina, especially at the level of GCL (Fig.4F).
Fig. 4.
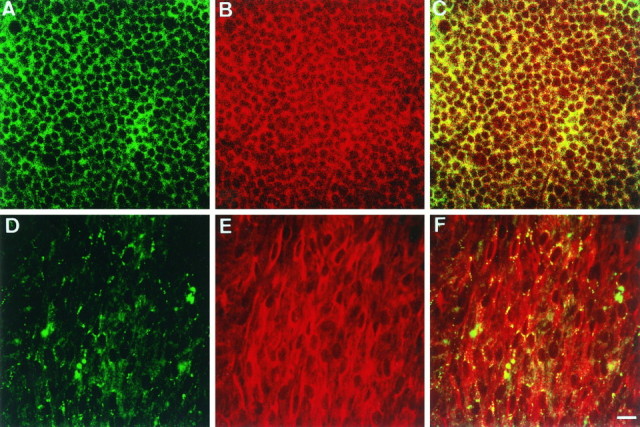
Whole-mount immunohistochemistry of Kir4.1 in the retina. Whole retina was fixed with 4% paraformaldehyde and stained with anti-Kir4.1 antibody (green) and anti-vimentin antibody (red), as described in Figure 3. The levels of ONL (A–C) and GCL (D–F) were analyzed with confocal microscopy. Note that the expression of Kir4.1 (green) was clustered around ganglion cells. Scale bar (shown in F), 10 μm.
Immunogold electron microscopic examination of retina with anti-KAB-2C2 showed that Kir4.1 proteins actually were expressed in the cell membranes of Müller cells (Fig.5). Figure 5A shows that gold particles existed on the filamentous processes at the distal ends of Müller cells. We also detected the expression of Kir4.1 channels in Müller cell membranes at the portions that were adjacent to endothelial cells and pericytes (Fig. 5B,C). In these regions Müller cells were always separated from the entire capillary complex by a continuous basement membrane (Carlson et al., 1988). At the endfoot region Kir4.1 also was expressed on the Müller cell membrane along the inner limiting (basement) membrane attached to the vitreous body (Fig. 5D).
Fig. 5.
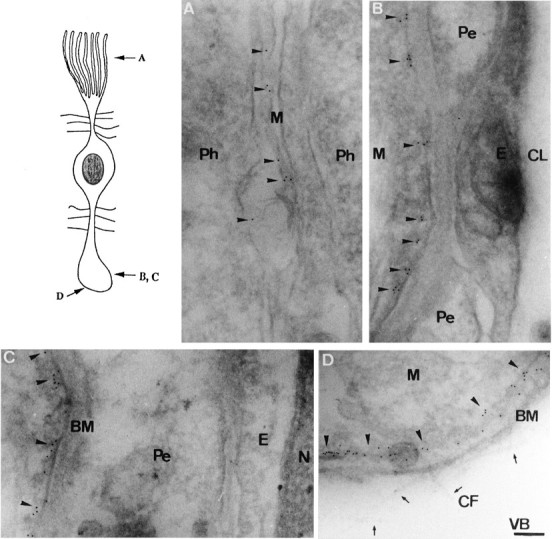
Immunogold electron microscopy of Kir4.1 in the retina. Ultra-thin sections were stained with anti-Kir4.1 antibody and anti-rabbit IgG coupled to colloidal gold particles.A–D, The electron microscopic images of the portions, as indicated in the top left schema. Positive gold particles were detected on the membranes of various regions of Müller cells. M, Müller cell;Ph, photoreceptor cell; Pe, pericyte;E, endothelial cell; CL, capillary lumen;BM, basement membrane; N, nucleus;CF, collagen fiber; VB, vitreous body;arrowheads, gold particles; arrows, collagen fibers. Scale bar (shown in D), 0.5 μm.
Clustering of Kir4.1 proteins in isolated Müller cells and its regulation by insulin and laminin
We examined the distribution of Kir4.1 immunoreactivity in acutely dissociated rat Müller cells (Fig.6A–D). Several Müller cells were aggregated in Figure 6A. Müller cells were stained with anti-KAB-2C2, not diffusely but in a clustered manner. Clustering of Kir4.1 immunoreactivity was detected, not only in the endfoot region but also in the soma, stalk, and filamentous processes (Fig.6B). The isolated Müller cells were stained diffusely with anti-vimentin antibody (Fig. 6C). In these acutely dissociated Müller cells from rat retina, the Ba2+-sensitive inwardly rectifying K+ channel current was recorded in the whole-cell (Fig. 6D) as well as in the cell-attached patch configurations (data not shown). The conductance and kinetic properties of the Kir4.1 channels in rat Müller cells were identical with those of Kir4.1 in rabbit Müller cells and also in HEK cells (data not shown). When dissociated cells were cultured on cover glasses coated with poly-d-lysine with DMEM containing 10% FCS for 4 d, their shapes became spindle-like (Fig. 6E). In these cells neither immunoreactivities of Kir4.1 nor of vimentin were detected (Fig. 6F,G), indicating that these characteristics of Müller cells were lost by culturing under this condition. Consistently, in these cultured cells the Kir current was completely lost from the whole-cell current recording, whereas a certain amount of the voltage-dependent outward K+current remained (Fig. 6H).
The above results suggest that some factors that were absent in the present condition of culturing may be essential for the differentiation of Müller cells, such as the expression and clustering of Kir4.1 channels. We examined the effects of hormones on the expression and distribution of Kir4.1 in cultured cells (Fig.7). Because insulin plays several important roles in the retina (Reichenbach et al., 1993;Hérnandez-Sánchez et al., 1995), cells were cultured for 4 d in the medium containing 1 μm insulin. We found that both Kir4.1 and vimentin were expressed in these cells (Fig.7Aa–c). However, the distribution of Kir4.1 was diffuse and not clustered. The double staining shown in Figure 7Acindicates that Kir4.1 immunoreactivity in large part overlaps with vimentin reactivity. Therefore, Kir4.1 proteins may localize in the cytosol, but not on the cell membrane, of the cultured cells. Consistently, no Kir currents were recorded in the cells cultured in this condition (n = 3) (Fig. 7Ad).
Fig. 7.
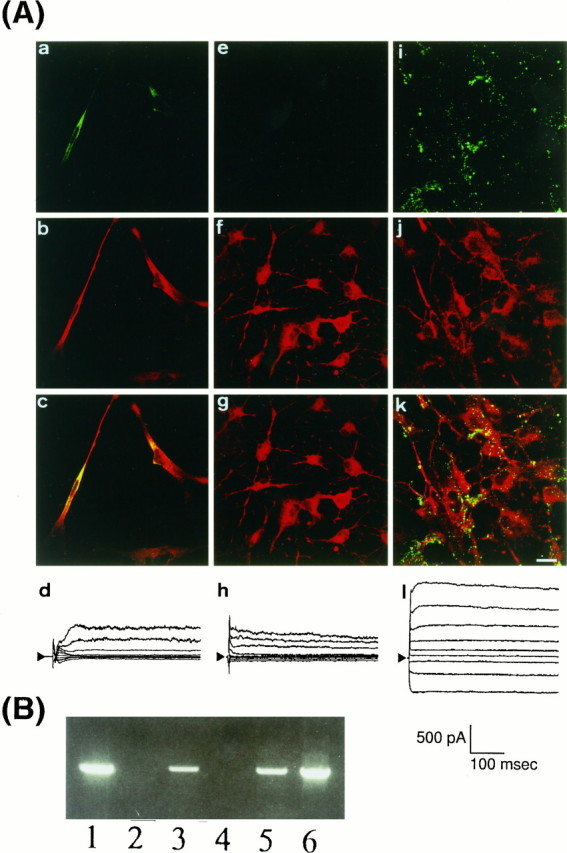
Expression and distribution of Kir4.1 in cultured cells from rat retina. A, Immunostaining of Kir4.1 (green) and vimentin (red) and whole-cell currents of cultured cells. Dissociated cells were cultured for 4 d on poly-d-lysine with 1 μminsulin (a–d), on laminin without insulin (e–h), and on laminin with 1 μm insulin (i–l). In i and k, Kir4.1 clustered on the membrane of cells. Scale bar (shown ink), 20 μm. Whole-cell current recordings were performed in these cells. The holding potential was −70 mV, and traces were elicited with voltage steps from −120 to +40 mV in 20 mV steps (inset). In l, large inward currents were recorded, whereas no inward currents were recorded in dand h. B, Expression of Kir4.1 mRNA in cultured cells. RT-PCR experiments of Kir4.1 were performed as shown in Figure 2C. Lane 1, Acutely dissociated Müller cells; lane 2, cells cultured on poly-d-lysine; lane 3, on poly-d-lysine in the presence of 1 μminsulin; lane 4, on laminin without insulin; lane 5, on laminin in the presence of 1 μm insulin;lane 6, control Kir4.1 cDNA. Insulin induced the expression of Kir4.1 mRNA.
We next examined the effect of glass disks coated with extracellular matrices. Because laminin, one of extracellular matrices, was reported to exist in the retina (Halfter and Fua, 1987), the isolated cells were cultured on laminin-coated glasses. After 4 d in culture, the cells possessed foot-like and spine-like structures. In these cells expression of vimentin was observed as assessed by immunostaining, whereas Kir4.1 immunoreactivity was not detected (Fig.7Ae–g). It also was confirmed that no Kir currents were recorded from these cells (n = 3, Fig. 7h). Then we combined insulin in the medium and laminin-coated glasses to culture the dissociated cells (Fig. 7Ai–l). After 4 d of culture these cells expressed both Kir4.1 (Fig.7Ai) and vimentin (Fig. 7Ak). Moreover, Kir4.1 immunoreactivity in these cells was clustered on the membrane in a similar manner to that observed in acutely dissociated Müller cells (Fig. 7Ak). Furthermore, a large Kir current was detected in the cells cultured in this condition (Fig.7Al). The Kir currents were inhibited by 100 μm Ba2+ (data not shown). At the single-channel level the 25 pS Kir4.1 channel openings were recorded in 11 different patches from these cells (data not shown).
In the cells cultured with the above three different conditions, the expression of Kir4.1 mRNA was examined further with the RT-PCR techniques shown in Figure 7B. Consistent with the immunohistochemical observations, on both poly-d-lysine-coated and laminin-coated glasses Kir4.1 mRNA was lost in the cells cultured in the absence of insulin but was expressed in the presence of insulin.
DISCUSSION
Kir4.1 channel and its distribution in mammalian Müller cells
The electrophysiological properties of Kir channels are suited to play a role in the K+ buffering action of Müller cells: i.e., the current flow through Kir channels is inward at potentials more negative than the equilibrium potential for K+ (EK) but reduced at those more positive than EK. Thus K+ ions that accumulate locally because of neural excitation would enter Müller cells through the channels wherever the local EK is more positive than the resting potential. The elevated intracellular K+ ions then would be shunted rapidly by current flow in the cell. At the regions in which the local EK would be more negative than the resting potential, in other words, in which the concentration of local Ko+ is low, K+ ions can be extruded through Kir channels. However, the efficiency of extrusion of K+ ions would depend on the magnitude of rectification of Kir channels. Because the Kir4.1 channel exhibits an intermediate magnitude of inward rectification (Fig. 1; also see Takumi et al., 1995; Kubo et al., 1996), the Kir4.1 channels can function for the extrusion as well as for the uptake of K+ions.
Immunocytochemical studies showed the following: (1) Kir4.1 channels were localized at IPL and OPL where Ko+ concentration increases prominently by light (Oakley and Green, 1976). These channels might be responsible for the uptake of K+ ions into Müller cells. (2) The channels also were localized adjacent to endothelial cells of vessels or vitreous body, where Kir4.1 may participate in extruding K+ ions to blood vessels or vitreous body. (3) On light flash, K+ ions decrease at ONL (Oakley and Green, 1976), where the Kir4.1 immunoreactivity was detected (Figs. 3, 4). In this region K+ ions may be supplied from Müller cells through Kir4.1 channels. Thus the distribution and function of the Kir channel proteins indicate that Kir4.1 channels are mainly responsible for both intrusion and extrusion of K+ ions across mammalian Müller cell membrane.
In isolated rabbit Müller cells, Nilius and Reichenbach (1988)electrophysiologically identified three kinds of K+channels: a strong inwardly rectifying K+ channel of 105 pS (with 140 mm Ko+), an intermediate inwardly rectifying K+ channel of 60 pS, and a nonrectifying K+ channel of 360 pS. They showed specific distributions of these channels suitable for K+ siphoning action. In contrast, in this study, we did not record any of these three channels in >200 cell-attached patches of rabbit Müller cells. Consistent with our results, a recent report by Kusaka and Puro (1997) indicated that monkey Müller cells possess a Kir channel with a conductance of 20 pS with 100 mm Ko+, which is equivalent to 25 pS with 150 mm Ko+. They showed that intracellular ATP was essential to maintain the 20 pS-Kir channel activity, which was also the case for the cloned Kir4.1 expressed inXenopus oocytes (Takumi et al., 1995). These results strongly suggest that the Kir channel reported by Kusaka and Puro (1997) is identical to Kir4.1.
In the Müller cells isolated from tiger salamander (Ambystoma tigrinum), Newman (1993) identified a single population of strong inwardly rectifying K+channels. This Kir has a unitary conductance of 28 pS with 98 mm Ko+ and is localized mostly in the endfoot region (Newman, 1993). We confirmed that, in isolated Müller cells from Akahara (Japanese) salamander (Cynops pyrrhogaster), a single population of strong inwardly rectifying K+ channels existed, the properties of which were different from those of Kir4.1 but almost the same as those of the Kir recorded in tiger salamander and which were localized mainly in its endfoot (our unpublished observation). These discrepancies may be attributable to the difference in the K+ buffering action of Müller cells in amphibian and mammalian retinas. In avascular retinas, such as amphibia, Müller cells are supposed to suck K+ ions and extrude them only to vitreous body (K+ siphoning action) (Newman et al., 1984). On the other hand, Müller cells of mammalian vascularized retinas, including less vascularized rabbit retina, can transport K+ ions not only to vitreous body but also to blood vessels (Fig. 5).
On rabbit Müller cell membrane we sometimes recorded large-conductance Ca2+-activated K+ channels (Fig. 1Ba), which also were recorded previously in amphibian Müller cells (Newman, 1985). Recent studies indicate that local elevation of Ko+ evokes intracellular Ca2+ waves in rat and salamander Müller cells (Keirstead and Miller, 1995;Newman and Zahs, 1997), which may cause activation of the Ca2+-activated K+ channels. Therefore, it is possible that the Ca2+-activated K+ channels also are involved in the redistribution of K+ ions surrounding Müller cells.
Insulin and laminin signals induced clustered expression of KAB-2
Recently, human Müller cells of several eye diseases, such as detachment of retina, were reported to lose their Kir currents (Francke et al., 1997). The electroretinographic b-wave, which is supposed to be associated with K+ ion movement in Müller cells (Miller and Dowling, 1970), also was clearly depressed in retinal injury. These facts suggest that the expression of Kir currents on Müller cell membrane is affected by surrounding conditions.
Insulin and IGF-I stimulated proliferation and differentiation of neuroretina as autocrine and/or paracrine hormones in embryonic eye (Hérnandez-Sánchez et al., 1995). Moreover, Müller cells were reported to express insulin receptor (Reichenbach et al., 1993). In this study we showed that insulin has the ability to induce the expression of both Kir4.1 and vimentin in cultured retinal cells. However, double staining of Kir4.1 and vimentin in insulin-treated cells suggested that Kir4.1 might exist diffusely in the cytosol, but not on the membrane. Consistently, we could not detect any Kir channel activity in these cells. Thus an additional factor or factors may be required for translocation of Kir4.1 channels to the cell membrane.
Laminin is one of the extracellular matrix proteins that plays several important roles in the retina, such as axon extension (Halfter and Fua, 1987) and differentiation of neurons in vitro (Campochiaro and Hackett, 1993; Frade et al., 1996). Integrins, which are laminin receptors, were reported to be expressed also in glial cells, such as type I astrocytes (Tawil et al., 1994). In our experiments laminin in the presence of insulin induced the clustered distribution of Kir4.1 immunoreactivity on the membrane. Furthermore, the inwardly rectifying Kir4.1 channel currents were recorded in these cells. Thus a laminin-evoked signal or signals may be essential for transporting and clustering functional Kir4.1 channel proteins on the cell membrane.
The mechanism of laminin-induced clustering of Kir4.1 channels is not known. Laminin interacted with talin through integrins and pp125FAK (for review, see Schwarz et al., 1995). Talin is one of the protein 4.1 family members. It was reported that protein 4.1 bound to SAP97/dlg, which is a member of the PSD-95/SAP90 anchoring proteins (Lue et al., 1994). We have shown previously that Kir4.1 bound to SAP97 and clustered on the membrane in HEK293T cells (Horio et al., 1997). Because SAP97 was expressed in Müller cells, the signaling mechanism including talin and SAP97 might be involved in clustering Kir4.1 on the Müller cell membrane. Further studies definitely are needed to clarify the molecular mechanisms underlying laminin- and insulin-induced clustering of Kir4.1 on the cultured retinal cell membrane.
Functional significance of clustered distribution of Kir4.1 on Müller cell membrane
Clustered distribution of Kir4.1 channels on Müller cell membranes may be functionally important for the K+buffering action of Müller cells. (1) Clustered distribution itself would allow for regional high K+ conductance on Müller cell membrane. If the clusters of Kir4.1 on Müller cells are localized in the vicinity of the outlet of K+ ions, which might be the voltage-dependent K+ (KV) channels in neurons, K+ ions released from neurons would be sucked into Müller cells more promptly and effectively. (2) Clustering would enhance the Kir4.1 channel activity itself. We have shown recently that coexpression of SAP97 with Kir4.1 enhanced the expressed functional Kir4.1 current by threefold (Horio et al., 1997). Because SAP97 was expressed in mammalian Müller cells, it is highly possible that clustering of Kir4.1 with SAP97 occurs in Müller cells in vivo, which thus increases the channel activity. This enhanced channel activity should be beneficial for the K+buffering action of Müller cells. To elucidate the physiological significance of clustering of Kir4.1 on Müller cell membrane, however, further studies are needed.
Footnotes
This work was supported partly by grants from the Ministry of Education, Culture, Sports, and Science of Japan, from Research for the Future Program (JSPS-RFTF96L00302) of The Japan Society for the Promotion of Science, from Ichiro Kanehara Foundation, and from Yamanouchi Foundation for Research on Metabolic Disorders. We thank Dr. Ian Findlay (Université de Tours, Tours, France) for critically reading this manuscript.
Correspondence should be addressed to Dr. Y. Kurachi, Department of Pharmacology II, Faculty of Medicine, Osaka University, 2-2 Yamadaoka, Suita, Osaka 565, Japan.
REFERENCES
- 1.Bond CT, Pessia M, Xia XM, Lagrutta A, Kavanaugh MP, Adelman JP. Cloning and expression of a family of inward rectifier potassium channels. Receptors Channels. 1994;2:183–191. [PubMed] [Google Scholar]
- 2.Brew H, Gray PTA, Mobbs P, Attwell D. Endfeet of retinal glial cells have higher densities of ion channels that mediate K+ buffering. Nature. 1986;324:466–468. doi: 10.1038/324466a0. [DOI] [PubMed] [Google Scholar]
- 3.Campochiaro PA, Hackett SF. Corneal endothelial cell matrix promotes expression of differentiated features of retinal pigmented epithelial cells: implication of laminin and basic fibroblast growth factor as active components. Exp Eye Res. 1993;57:539–547. doi: 10.1006/exer.1993.1158. [DOI] [PubMed] [Google Scholar]
- 4.Carlson EC, Audette JL, Swinscoe JC. Ultrastructural evidence for morphological specificity in isolated bovine retinal capillary basement membranes. J Ultrastruct Mol Struct Res. 1988;98:184–198. doi: 10.1016/s0889-1605(88)80910-8. [DOI] [PubMed] [Google Scholar]
- 5.Dräger UC. Coexistence of neurofilaments and vimentin in a neurone of adult mouse retina. Nature. 1983;303:169–172. doi: 10.1038/303169a0. [DOI] [PubMed] [Google Scholar]
- 6.Frade JM, Martínez-Morales JR, Rodríguez-Tébar A. Laminin-1 selectively stimulates neuron generation from cultured retinal neuroepithelial cells. Exp Cell Res. 1996;222:140–149. doi: 10.1006/excr.1996.0018. [DOI] [PubMed] [Google Scholar]
- 7.Francke M, Pannicke T, Biedermann B, Faude F, Wiedemann P, Reichenbach A, Reichelt W. Loss of inwardly rectifying potassium currents by human retinal glial cells in diseases of the eye. Glia. 1997;20:210–218. doi: 10.1002/(sici)1098-1136(199707)20:3<210::aid-glia5>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 8.Gotow T, Tanaka J, Takeda M. The organization of neurofilaments accumulated in perikaryon following aluminum administration: relationship between structure and phosphorylation of neurofilaments. Neuroscience. 1995;64:553–569. doi: 10.1016/0306-4522(94)00394-k. [DOI] [PubMed] [Google Scholar]
- 9.Halfter W, Fua CS. Immunohistochemical localization of laminin, neural cell adhesion molecule, collagen type IV, and T-61 antigen in the embryonic retina of the Japanese quail by in vivo injection of antibodies. Cell Tissue Res. 1987;249:487–496. doi: 10.1007/BF00217320. [DOI] [PubMed] [Google Scholar]
- 10.Hérnandez-Sánchez C, López-Carranza A, Alarcón C, De la Rosa EJ, De Pablo F. Autocrine/paracrine role of insulin-related growth factors in neurogenesis: local expression and effects on cell proliferation and differentiation in retina. Proc Natl Acad Sci USA. 1995;92:9834–9838. doi: 10.1073/pnas.92.21.9834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horio Y, Morishige K, Takahashi N, Kurachi Y. Differential distribution of classical inwardly rectifying potassium channel mRNAs in the brain: comparison of IRK2 with IRK1 and IRK3. FEBS Lett. 1996;379:239–243. doi: 10.1016/0014-5793(95)01519-1. [DOI] [PubMed] [Google Scholar]
- 12.Horio Y, Hibino H, Inanobe A, Yamada M, Ishii M, Tada Y, Satoh E, Hata Y, Takai Y, Kurachi Y. Clustering and enhanced activity of an inwardly rectifying potassium channel, Kir4.1, by an anchoring protein, PSD-95/SAP90. J Biol Chem. 1997;272:12885–12888. doi: 10.1074/jbc.272.20.12885. [DOI] [PubMed] [Google Scholar]
- 13.Ito M, Inanobe A, Horio Y, Hibino H, Isomoto S, Ito H, Mori K, Tonosaki A, Tomoike H, Kurachi Y. Immunolocalization of an inwardly rectifying K+ channel, KAB-2 (Kir4.1), in the basolateral membrane of renal distal tubular epithelia. FEBS Lett. 1996;388:11–15. doi: 10.1016/0014-5793(96)00502-9. [DOI] [PubMed] [Google Scholar]
- 14.Karschin C, Dißman E, Stühmer W, Karschin A. IRK(1–3) and GIRK (1–4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. J Neurosci. 1996;16:3559–3570. doi: 10.1523/JNEUROSCI.16-11-03559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keirstead SA, Miller RF. Calcium waves in dissociated retinal glial (Müller) cells are evoked by release of calcium from intracellular stores. Glia. 1995;14:14–22. doi: 10.1002/glia.440140104. [DOI] [PubMed] [Google Scholar]
- 16.Kubo Y, Miyashita T, Kubokawa K. A weakly inward rectifying potassium channel of the salmon brain. J Biol Chem. 1996;271:15729–15735. doi: 10.1074/jbc.271.26.15729. [DOI] [PubMed] [Google Scholar]
- 17.Kusaka S, Puro DG. Intracellular ATP activates inwardly rectifying K+ channels in human and monkey retinal Müller (glial) cells. J Physiol (Lond) 1997;500:593–604. doi: 10.1113/jphysiol.1997.sp022045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lue RA, Shirin MM, Branton D, Chishti AH. Cloning and characterization of hdlg: the human homologue of the Drosophila discs large tumor suppressor binds to protein 4.1. Proc Natl Acad Sci USA. 1994;91:9818–9822. doi: 10.1073/pnas.91.21.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller RF, Dowling JE. Intracellular responses of the Müller (glial) cells of mudpuppy retina: their relation to b-wave of the electroretinogram. J Neurophysiol. 1970;33:323–341. doi: 10.1152/jn.1970.33.3.323. [DOI] [PubMed] [Google Scholar]
- 20.Newman EA. Voltage-dependent calcium and potassium channels in retinal glial cells. Nature. 1985;317:809–811. doi: 10.1038/317809a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman EA. Distribution of potassium conductance in mammalian Müller (glial) cells: a comparative study. J Neurosci. 1987;7:2423–2432. [PMC free article] [PubMed] [Google Scholar]
- 22.Newman EA. Inward-rectifying potassium channels in retinal glial (Müller) cells. J Neurosci. 1993;13:3333–3345. doi: 10.1523/JNEUROSCI.13-08-03333.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman EA, Zahs KR. Calcium waves in retinal glial cells. Science. 1997;275:844–847. doi: 10.1126/science.275.5301.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newman EA, Frambach DA, Odette LL. Control of extracellular potassium levels by retinal glial cell K+ siphoning. Science. 1984;225:1174–1175. doi: 10.1126/science.6474173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilius B, Reichenbach A. Efficient K+ buffering by mammalian retinal glial cells is due to cooperation of specialized ion channels. Pflügers Arch. 1988;411:654–660. doi: 10.1007/BF00580862. [DOI] [PubMed] [Google Scholar]
- 26.Oakley B, II, Green DG. Correlation of light-induced changes in retinal extracellular potassium concentration with c-wave of the electroretinogram. J Neurophysiol. 1976;39:1117–1133. doi: 10.1152/jn.1976.39.5.1117. [DOI] [PubMed] [Google Scholar]
- 27.Orkand RK, Nicholls JG, Kuffler SW. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966;29:788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- 28.Pessia M, Tucker SJ, Lee K, Bond CT, Adelman JP. Subunit positional effects revealed by novel heteromeric inwardly rectifying K+ channels. EMBO J. 1996;15:2980–2987. [PMC free article] [PubMed] [Google Scholar]
- 29.Reichenbach A, Schneider H, Leibnitz L, Reichelt W, Schaaf P, Schümann R. The structure of rabbit retinal Müller (glial) cells is adapted to the surrounding retinal layers. Anat Embryol (Berl) 1989;180:71–79. doi: 10.1007/BF00321902. [DOI] [PubMed] [Google Scholar]
- 30.Reichenbach A, Stolzenburg JU, Eberhardt W, Chao TI, Dettmer D, Hertz L. What do retinal Müller (glial) cells do for their neuronal “small siblings”? J Chem Neuroanat. 1993;6:201–213. doi: 10.1016/0891-0618(93)90042-3. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 32.Sucher NJ, Deitcher DL. PCR and patch-clamp analysis of single neurons. Neuron. 1995;14:1095–1100. doi: 10.1016/0896-6273(95)90257-0. [DOI] [PubMed] [Google Scholar]
- 33.Takumi T, Ishii T, Horio Y, Morishige K, Takahashi N, Yamada M, Yamashita T, Kiyama H, Sohmiya K, Nakanishi S, Kurachi Y. A novel ATP-dependent inward rectifier potassium channel expressed predominantly in glial cells. J Biol Chem. 1995;270:16339–16346. doi: 10.1074/jbc.270.27.16339. [DOI] [PubMed] [Google Scholar]
- 34.Tawil NJ, Wilson P, Carbonetto S. Expression and distribution of functional integrins in rat CNS glia. J Neurosci Res. 1994;39:436–447. doi: 10.1002/jnr.490390411. [DOI] [PubMed] [Google Scholar]
- 35.Vaugham DK, Lasater EM. Glial and neuronal markers in bass retinal horizontal and Müller cells. Brain Res. 1990;537:131–140. doi: 10.1016/0006-8993(90)90349-g. [DOI] [PubMed] [Google Scholar]