Abstract
There are multiple mechanisms by which adenine nucleotides can be released into the extracellular space in brain. Adenine nucleotides are converted extracellularly to adenosine, which then acts on adenosine receptors to elicit physiological responses, but the rate at which this conversion takes place is unknown. In the present experiments, adenine nucleotides were applied to individual hippocampal neurons, and the subsequent activation of a postsynaptic K+conductance by adenosine A1 receptors was used to determine the rate of adenosine formation. None of the adenine nucleotides tested (cAMP, AMP, ADP, and ATP) activated A1 receptors directly at the concentrations tested (≤200 μm). AMP, ADP, and ATP were all rapidly converted to adenosine, with aT1/2 for ATP conversion to adenosine of ∼200 msec, and the last step in this pathway (transformation of AMP to adenosine by 5′-nucleotidase) seems to be the rate-limiting step. As we have reported previously, cAMP is converted to adenosine as well, but on a much slower time scale than any of the other nucleotides tested. These experiments demonstrate that fast, localized release of AMP, ADP, or ATP can result in a transient activation of adenosine receptors but that this is unlikely to occur with cAMP. The existence of a highly active ecto-nucleotidase pathway in brain provides a mechanism for the rapid generation of adenosine after the release of adenine nucleotides into the extracellular space.
Keywords: adenosine, adenine nucleotides, adenosine receptors, hippocampus, electrophysiology, 5′-nucleotidase, P1receptors
The existence in brain of multiple subtypes of extracellular adenosine receptors, physiological responses linked to activation of those receptors, the normal presence of adenosine in the extracellular space, and the marked increases in adenosine that can occur under various conditions (e.g., hypoxia, ischemia, seizures) all suggest that adenosine may play an important role as a modulator of neuronal activity (Dunwiddie, 1985; Greene and Haas, 1991). The source(s) of extracellular adenosine, however, and the factors that regulate its levels are not well understood. Biochemical studies have established that a potential source of extracellular adenosine is its formation in the extracellular space from adenine nucleotides. Vesicular release of ATP that is colocalized with transmitters such as ACh, norepinephrine, and 5-HT (Silinsky, 1975;Burnstock, 1986; Richardson and Brown, 1987), nucleotide release after activation of NMDA receptors (Craig and White, 1992, 1993), and activation of adenylyl cyclase (Gereau and Conn, 1994; Rosenberg et al., 1994; Rosenberg and Li, 1995) are all potential sources of extracellular adenine nucleotides. Once adenine nucleotides reach the extracellular space, they are subsequently converted to adenosine through the actions of ecto-enzymes (Zimmermann, 1992; Craig and White, 1993; Rosenberg et al., 1994; Ziganshin et al., 1994); however, the rate at which the conversion of nucleotides to adenosine can occur and the relative rate of each of the steps involved in the interconversion of nucleotides have not been well characterized in brain.
A second unresolved issue concerns the ability of adenine nucleotides to directly activate adenosine receptors. In many systems, adenine nucleotides elicit responses that are mediated via the activation of adenosine-specific receptors (Dunwiddie and Hoffer, 1980; Lee et al., 1981). These responses are blocked by competitive adenosine receptor antagonists, but they are also generally blocked by adenosine deaminase, which converts adenosine to the inactive metabolite inosine, suggesting that the nucleotides are converted to adenosine before activation of these receptors. It is unclear, however, whether it is arequirement that nucleotides be converted to adenosine before they can act on A1 receptors; the conversion processes may simply be so effective that the nucleotides are metabolized to adenosine before they have the opportunity to exert any direct actions. It has been reported that stable nucleotide analogs can mimic the effects of adenosine (Lee et al., 1981; Wiklund et al., 1985;von Kugelgen et al., 1992), suggesting that nucleotides might be able to activate these receptors directly, although there is substantial evidence to the contrary (Bruns, 1980; Pirotton and Boeynaems, 1993). Ligand binding studies have generally found that nucleotides have very low affinities for adenosine receptors (Schwabe and Trost, 1980;Ragazzi et al., 1991), although the interpretation of these experiments is not always straightforward.
To examine these issues, we have compared responses to the application of adenosine and adenine nucleotides directly to the cell bodies of hippocampal pyramidal neurons. Application of adenosine induces small, outward currents that are mediated via the inwardly rectifying G-protein-coupled K+ channels activated by adenosine in these neurons (Greene and Haas, 1985; Trussell and Jackson, 1987;Thompson et al., 1992). Responses to adenosine have latencies of a few hundred milliseconds; in the case of adenine nucleotides, any significant additional delays would reflect the necessity of some intervening process (e.g., metabolism) occurring before the response.
MATERIALS AND METHODS
Slice preparation. Hippocampal slices for extracellular recording experiments were obtained from 40- to 60-d-old, male Sprague Dawley rats (Sasco Animal Laboratories, Omaha, NE) using standard techniques (Dunwiddie and Lynch, 1978; Dunwiddie and Hoffer, 1980). For the whole-cell patch experiments, 18- to 24-d-old animals were decapitated, the brain was cooled and blocked, and 300 μm slices containing the hippocampus were cut in the coronal plane with a Pelco Series 1000 Vibratome. Slices were quickly transferred via a large-mouth pipette to a petri dish with fresh, oxygenated artificial CSF (aCSF), hemisected with a scalpel blade, and transferred to an incubation chamber maintained at 30–32°C. The incubation medium was a bicarbonate-buffered salt solution containing 124 mmNaCl, 3.3 mm KCl, 1.2 mmKH2PO4, 2.4 mmMgCl2, 1.5 mm CaCl2 mmCaCl2, 10 mmd-glucose, and 25.7 mm NaHCO3, pH 7.4. The buffer was gassed with humidified 95% O2/5% CO2to saturation. At least 1 hr after preparation, the slices were transferred to a submersion recording chamber mounted on the stage of an upright Nomarski microscope, anchored with platinum weights, and superfused continuously (2 ml/min) with aCSF.
Electrophysiological recordings. Extracellular electrophysiological recordings of the field EPSPs (fEPSPs) were made using glass microelectrodes (2–4 MΩ) filled with 3 mNaCl and placed under visual guidance in stratum radiatum of the CA1 region. Twisted bipolar nichrome wire stimulating electrodes were placed in stratum radiatum near the border of the CA1 and CA2 regions, and stimuli were delivered to the Schaffer and commissural afferents at 15 sec intervals. Responses were recorded using an AC amplifier, and a computer was used to digitize, analyze, and store the responses.
Whole-cell voltage-clamp experiments were performed using patch pipettes pulled from borosilicate glass (outer diameter 1.5 mm, inner diameter 0.86 mm, with filament) (Sutter Instrument Co., Novato, CA) on a Flaming/Brown Micropipette puller Model P-87 (Sutter). Electrodes had tip resistances of 6–10 MΩ when filled with a solution containing (in mm): potassium gluconate 130; HEPES 10; EGTA 10; CaCl2 1; MgCl2 2; ATP 2; GMP 0.3, pH adjusted to 7.2–7.4 with KOH, osmolarity adjusted to 280–295 mOsm.
Pyramidal cells were visually located in the CA1 somal layer using differential interference contrast videomicroscopy. To record from individual hippocampal neurons, pipettes were lowered into the slice under visual control while positive pressure was maintained on the micropipette to keep the tip clear. When the pipette touched the cell, suction was then applied to the micropipette to obtain a seal. When a satisfactory seal (>1 GΩ) was obtained, an increase in suction was applied until the membrane patch ruptured.
Cells were voltage-clamped at −65 mV (after correction for the liquid junction potential at the electrode tip) with an Axoclamp-2A amplifier (Axon Instruments, Foster City, CA) in the continuous single-electrode voltage-clamp mode. All responses were digitized at between 50 and 100 Hz with an R.C. Electronics ISC-16 analog-to-digital card, and responses were analyzed by computer with software developed in our laboratory. The membrane potential and holding current were monitored every 10 sec. The membrane resistance was determined from the current response to a 10 mV hyperpolarizing voltage command step every 30 sec.
Because of the small amplitude of the responses, signals were low-pass-filtered at 0.1–1.0 kHz, and averages of 10–30 individual responses were made under the various experimental conditions. To determine whether specific components of the evoked waveforms differed significantly under different experimental conditions, control averages were plotted with 95% confidence limits on the averaged waveform. Regions in which there was no overlap between the 95% limits for the control response, and the averaged evoked waveform under the test condition were considered to be statistically different.
Iterative curve fitting (Slide Write Plus version 4.0, Advanced Graphics Software) was used to estimate the latency and the on and off rates of responses to adenosine and adenine nucleotides. Responses were fit to the equation:
where I is the membrane current, τonand τoff correspond to the time constants for the onset and offset of the response, T0 is the latency after the pressure ejection of drug, and R is a scaling factor. Nearly all responses could be closely fit to this equation, with r2 values between 0.90 and 0.99.
All data were analyzed using the two-tailed Student’s ttest (or paired t test when appropriate) for statistical significance.
Drug application. At least 10–15 min of stable baseline responses were obtained in each experiment before drug applications began. Drugs to be superfused were made up at 20–2000 times the desired final concentration and added directly to the flow of the superfusion medium with a calibrated syringe pump to achieve the desired final concentration. For local application experiments, adenosine and adenine nucleotides were made up at concentrations from 100 to 500 μm in freshly gassed extracellular buffer, which was used to fill single- and double-barrel pipettes having tip diameters of ∼1–2 μm. Pipettes were positioned within 2–10 μm of the soma of the cell from which recordings were made (see Fig. 2), and drugs were ejected by brief application of pressure pulses (10–20 msec at 10 psi) using a Picospritzer II (General Valve Co., Fairfield, NJ) and delivered every 20 sec. Usually some adjustment of the pipette position was required to elicit an optimal response.
Fig. 2.
Local drug application protocol. This photomicrograph shows the relative positioning of the patch recording electrode (large arrow) and the drug application pipette (small arrow) during recording from a CA1 pyramidal neuron. After the initiation of whole-cell recording, the drug application pipette was lowered through the tissue under visual control while periodically testing for adenosine responses. Adenosine and adenine nucleotides were ejected by applying brief pressure pulses to the drug application pipette (typically 10 psi/10 msec). Responses could be obtained when the drug pipette was in close proximity to the neuron (2–10 μm), but they were generally undetectable when the pipette was moved farther than 20 μm from the cell being tested. Stable responses could be evoked with this protocol at 30 sec intervals for periods >2 hr. Despite the proximity of the drug pipette to the cell, pressure ejection artifacts were rarely encountered. Scale bar, 13 μm.
Chemicals. Adenosine, cAMP, AMP, ADP, ATP, GMP, α,β-methyleneadenosine 5′-diphosphate (AOPCP), theophylline, and adenosine deaminase were obtained from Sigma (St. Louis, MO).
RESULTS
Superfusion of slices with adenine nucleotides activates adenosine receptors
As has been observed previously (Dunwiddie and Hoffer, 1980; Lee et al., 1981), superfusion of brain slices with adenine nucleotides such as ATP, AMP, and cAMP elicited responses that were consistent with the activation of presynaptic adenosine A1 receptors. Adenine nucleotides, as well as adenosine itself, markedly inhibited excitatory transmission in the Schaffer collateral/commissural inputs to the CA1 pyramidal neurons, and these actions were blocked by the competitive adenosine receptor antagonist theophylline (Fig.1). Given that these nucleotides have been reported to have relatively low affinities (>100 μm) for adenosine receptors (Schwabe and Trost, 1980;Ragazzi et al., 1991), these observations suggest that the nucleotides act on adenosine receptors after their conversion to adenosine. The potencies of adenine nucleotides in eliciting these responses were all quite close to that for adenosine. EC50 values ± SEM in μm were AMP 30 ± 3.4 (n = 8), ATP 35 ± 3.7 (n = 8), cAMP 12 ± 0.8 (n = 9), and adenosine 20 ± 1.6 (n = 7) [data for cAMP and adenosine are from Brundege et al. (1997)], suggesting that the conversion of nucleotides to adenosine must be essentially quantitative. The hypothesis that responses to the nucleotides were mediated via adenosine is confirmed by the fact that these responses could be completely blocked by adenosine deaminase (Lee et al., 1981; Brundege et al., 1997), which can convert adenosine to inosine but has no effect on nucleotides. Thus, the requisite enzymes for the conversion of each of these nucleotides to adenosine must exist in the extracellular space. This conclusion is consistent with direct biochemical studies that have shown that brain slices can readily convert adenine nucleotides to adenosine (Craig and White, 1993; Rosenberg et al., 1994).
Fig. 1.
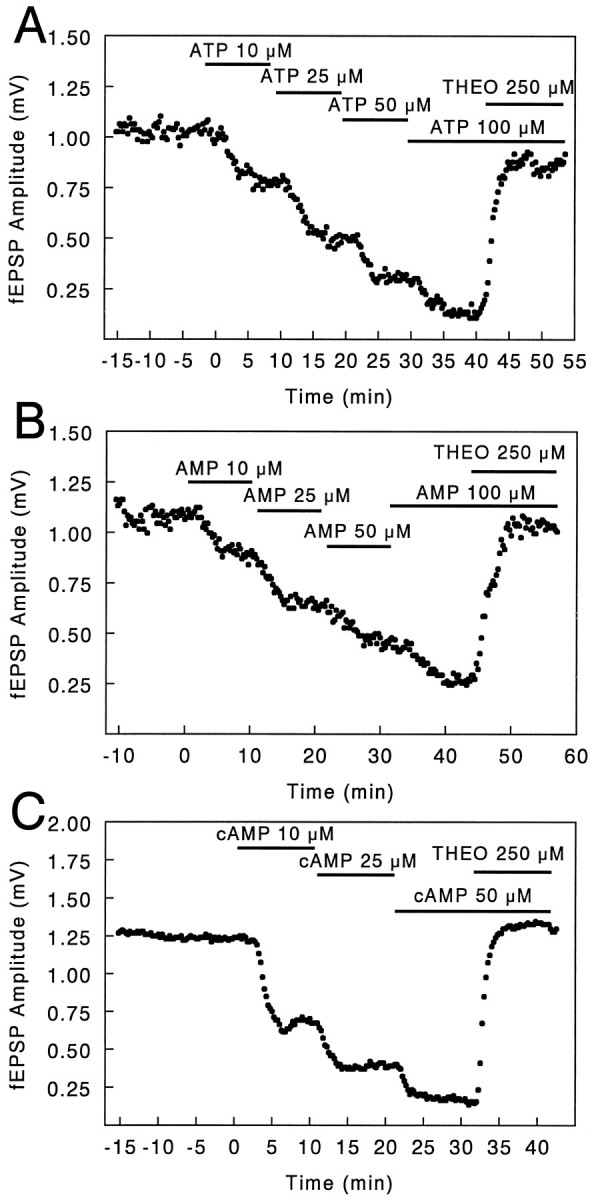
Effects of bath superfusion with adenine nucleotides on field potentials. Synaptic responses were evoked at 30 sec intervals, and the peak field EPSP (fEPSP) amplitude was plotted as a function of time. Superfusion of slices with increasing concentrations of adenine nucleotides inhibited the fEPSP in a dose-dependent manner. Adenosine A1 receptors show no desensitization under these conditions (Dunwiddie and Fredholm, 1984), so accurate cumulative dose–response curves can be obtained in this manner. The competitive adenosine receptor antagonist theophylline (THEO) completely reversed the effects of ATP, AMP, and cAMP (A, B, C, respectively), demonstrating that the inhibitory effect was in each case mediated via adenosine receptors. cAMP was significantly more potent than adenosine itself in eliciting this response, but its effects were completely blocked by adenosine deaminase (Brundege et al., 1997), indicating that it is converted to adenosine before it acts on the receptor.
Local application of adenosine produces a fast activation of postsynaptic A1 receptors
Because biochemical and bath superfusion experiments provide little information concerning the rate at which nucleotides can be converted to adenosine, a different protocol was developed to examine the kinetic aspects of adenosine generation from extracellular nucleotides. To do so, we characterized the rate at which postsynaptic adenosine receptors were activated after a very brief local pressure application of adenine nucleotides to individual CA1 pyramidal neurons. This postsynaptic response to adenosine reflects the activation of a G-protein-coupled K+ conductance mediated by A1 receptors (Trussell and Jackson, 1987; Gerber et al., 1989). Although the fast kinetics of the adenosine response have not been characterized in detail, they are probably quite similar to those of the GABAB receptor, which is also found on CA1 pyramidal neurons and shares the same postsynaptic transduction mechanisms (Nicoll, 1988; McCormick and Williamson, 1989), and the kinetics of which have been extensively characterized (50 msec absolute latency, ∼1–2 sec to peak response) (Sodickson and Bean, 1996).
The protocol that was used to compare the actions of adenosine and adenine nucleotides on single, visually identified pyramidal neurons is illustrated in Figure 2. To minimize the delay between drug application and activation of receptors, an adenosine-containing micropipette was lowered under visual guidance until it was within 2–10 μm of the cell from which recordings were made, and adenosine was ejected by brief application of pressure to the drug pipette (usually 10–20 msec at 10 psi). When 200 μmadenosine was applied in this manner, small outward currents were detected after the pressure ejection (Fig.3). Adenosine responses usually had latencies of several hundred milliseconds, reached peak amplitudes at 3–5 sec after adenosine application, and lasted from 10 to 20 sec. These responses were usually very well fit by the product of two exponential functions, one describing the onset of the response and the other corresponding to the decay (Fig. 3). The reversal potential of these responses was estimated to be −93 mV (not shown), and they could be blocked by Ba2+ (the mean response amplitude in 2 mm Ba2+ was 14 ± 5.4% of control;n = 4 cells), properties that are consistent with the K+-selective ion channel that is linked to A1 receptors in hippocampal pyramidal neurons (Gerber et al., 1989). Although very small pressure artifacts were sometimes observed with this application protocol (Fig.4D), these were very transient and clearly preceded the onset of the adenosine response. The peak amplitude of responses that could be obtained with optimal drug pipette localization and 200 μm adenosine was quite small (usually 2–12 pA). In comparison, bath superfusion with adenosine typically elicits substantially larger currents; 100 μmadenosine, which is a high but not saturating concentration, induced an outward current of 51 ± 6 pA (n = 34 cells). Although the stratum radiatum has a substantially higher density of A1 receptors compared with the pyramidal layer, direct application of adenosine to the dendrites of pyramidal neurons did not result in significantly larger responses (data not shown).
Fig. 3.
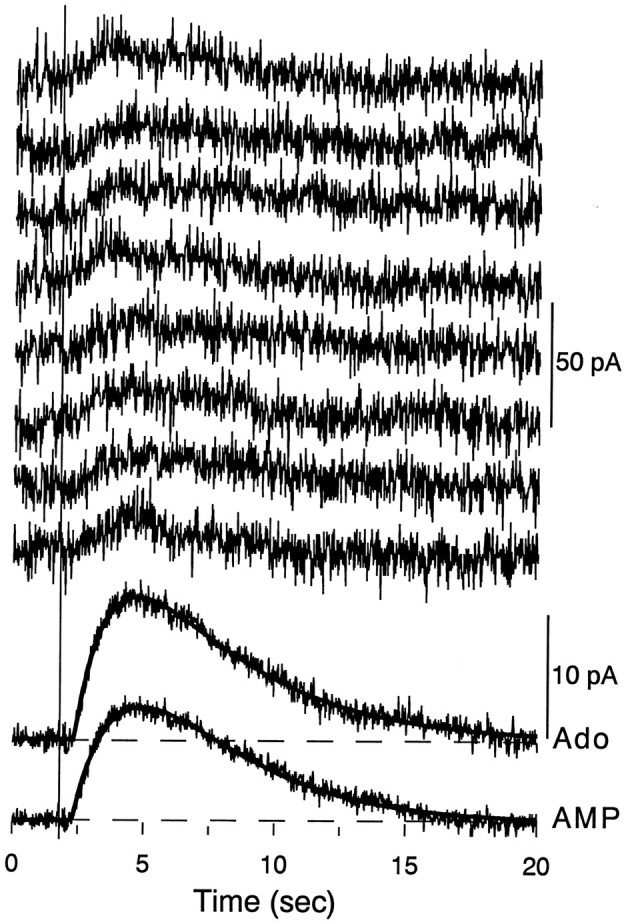
Outward currents evoked by local application of adenosine. Application of adenosine (Ado) (200 μm concentration in the drug pipette) to CA1 pyramidal neurons elicited slow outward currents that reached a peak within ∼2–3 sec and typically lasted between 10 and 20 sec. The eight records at the top are consecutive individual evoked responses, low-pass-filtered at 1 kHz, elicited by pressure application of adenosine (10 psi/10 msec), and the upper of the two records at the bottom is an average of 51 such responses shown at 5 × gain (calibration = 50 pA for individual responses, 10 pA for averages). The smooth line superimposed on the average is the best fit (r2 = 0.976) to the response using the double exponential equation described in Materials and Methods, with parameters r = 14.9 pA, τon = 2.38 sec, τoff = 4.18 sec, andT0 = 344 msec. The lowest record is a similar average of responses to local application of AMP, with the best fit line (r2 = 0.975) corresponding to parameters r = 39.3 pA, τon = 9.09 sec, τoff = 3.09 sec, and T0 = 280 msec. The thin vertical line on theleft indicates the time at which adenosine/AMP were applied, and T0 corresponds to the delay between drug application and the point at which the exponential function crosses the abscissa (i.e., the onset of the response). Thedashed lines at the bottom indicate the pre-response baseline.
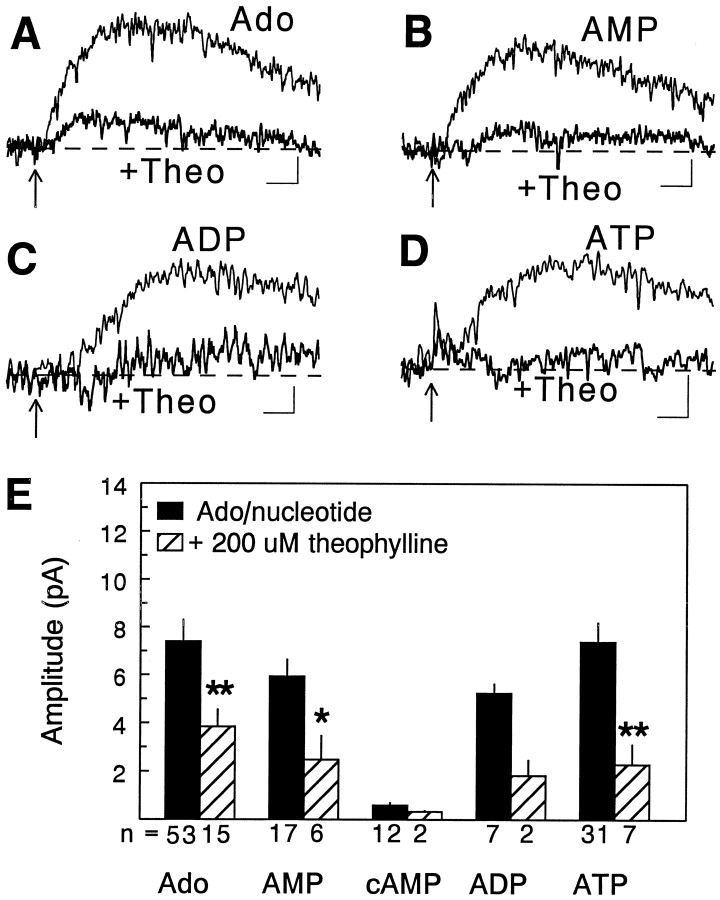
Fig. 4.
Theophylline reversibility of responses to adenosine, AMP, ADP, and ATP. Application of AMP, ADP, and ATP (B–D) (each at 200 μm in the drug pipette) elicited responses that were qualitatively and quantitatively similar to those elicited by 200 μm adenosine (A). Responses to adenosine as well as to the nucleotides were antagonized by bath superfusion with 200 μm theophylline (Theo), a competitive antagonist at adenosine receptors, but showed full recovery when theophylline was washed from the bath (not shown). In this and in subsequent figures, each response is the average of 5–20 responses low-pass-filtered at 1 kHz, and the time of drug application is denoted with an arrow. The theophylline antagonism of these responses in all slices tested for each of the nucleotides is summarized in E; numbers below thebars indicate the number of cells tested with each combination of drugs. A subset of the control data for adenosine, AMP, and cAMP has been published previously (Brundege et al., 1997). Calibration: 1 sec and 3 pA (A, B); 1.5 pA (C); and 1 pA (D). *p < 0.02; **p < 0.002.
Responses to local application of adenine nucleotides
When adenine nucleotides such as AMP, ADP, and ATP were applied to different neurons using an identical protocol (200 μm, 10–20 msec at 10 psi), responses to each nucleotide were observed that were nearly identical to those seen with adenosine (Figs. 3, 4). Both the magnitude and the time course of responses to the nucleotides were similar to those observed with adenosine. Responses to adenosine as well as adenine nucleotides were uniformly inhibited by bath superfusion with 200 μm theophylline (Fig. 4), which is a competitive antagonist at adenosine receptors, as well as by 8-cyclopentyl-1,3-dipropylxanthine (not shown), demonstrating that these effects were mediated via adenosine receptors of the A1 subtype. As we have shown previously (Brundege et al., 1997), cAMP was unable to elicit similar responses when applied using this protocol (Fig. 4E; also see Fig. 7B). Although responses to cAMP were sometimes detectable, on average they were <10% of those elicited by adenosine. The mean amplitudes of responses elicited by each of the nucleotides is illustrated in Figure4E. With the exception of cAMP, there were no significant differences in the magnitude of the responses elicited by any of the nucleotides or adenosine.
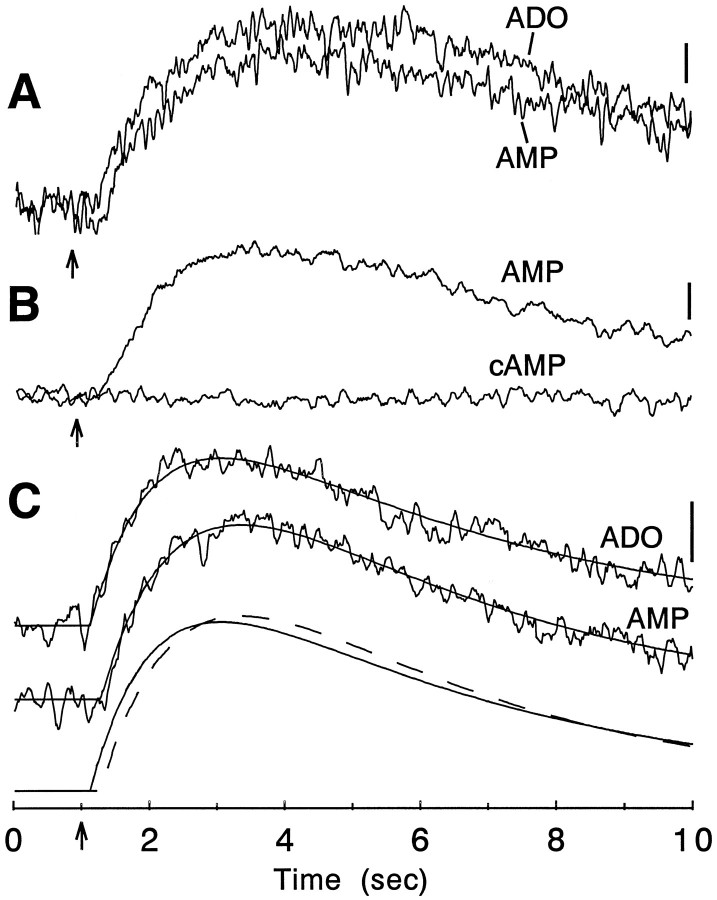
Fig. 7.
Comparison of paired applications of AMP or cAMP versus adenosine. When applied alternately from adjacent barrels of a drug pipette, very similar responses were elicited by AMP and adenosine (A). Both adenosine and AMP responses were blocked by superfusion with theophylline (not shown). In contrast, cAMP did not elicit a detectable current when it was applied alternately with AMP (B). C, Responses from another pyramidal neuron when adenosine and AMP were applied alternately from a two-barrel drug pipette. Both responses were well fit by the product of two exponential functions (solid lines superimposed on the averaged responses). At thebottom the fit lines are shown superimposed; thesolid line corresponds to the adenosine response, and the dashed line the AMP response. The fit parameters for the adenosine and AMP responses (respectively) werer = 18/25 pA, τon = 1.28/1.95 sec, τoff = 4.52/3.71 sec, and T0 = 110/260 msec. In this example, T0 was the only parameter that differed significantly for the two responses. Time of drug ejection is indicated by the vertical arrow, and the calibration bar in all cases is 3 pA. The time scaleat the bottom applies to all the records.
A potential concern in experiments using bath superfusion with theophylline was whether the ejection of a small volume of buffer containing adenosine (or a nucleotide) into the extracellular space might physically displace theophylline from the vicinity of the receptors and weaken its apparent potency as an antagonist. To determine whether this was the case, experiments were conducted with two-barrel drug pipettes, one barrel of which contained 200 μm adenosine and the other 200 μm adenosine + 200 μm theophylline. Comparison of responses to ejection of each of these solutions demonstrated that the presence of 200 μm theophylline in the pipette had no effect on the adenosine response (Fig. 5A). When responses to adenosine + theophylline were tested against bath-superfused theophylline, the degree of antagonism exerted by the superfused theophylline was identical to that observed against adenosine alone (Fig. 5B). This clearly rules out the possibility that the effectiveness of theophylline as an antagonist is reduced because it is being physically displaced or diluted by the ejection solution, because in the case of adenosine + theophylline ejection, the concentration of theophylline in the extracellular space would be completely unchanged. The observation that 200 μm theophylline has no antagonistic effect when ejected with adenosine, but when superfused can block the response to locally applied adenosine by as much as 80% (Fig. 4), emphasizes the fact that the agonist responses are not at equilibrium (see Discussion).
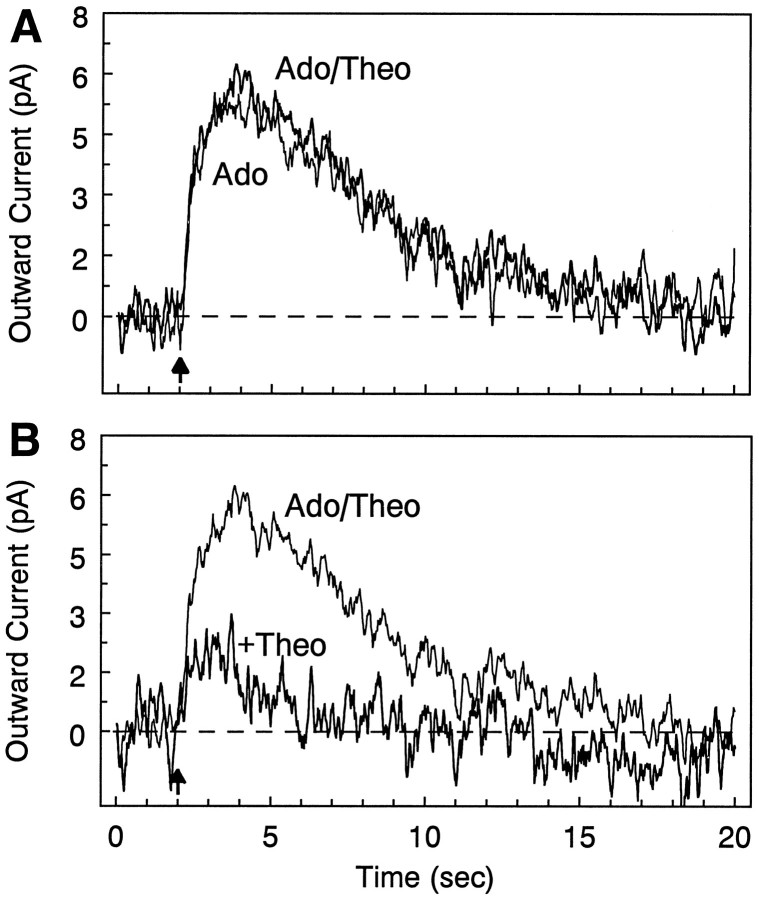
Fig. 5.
Effect of superfused theophylline versus theophylline in the drug application pipette. Double-barrel pipettes were filled with adenosine alone (200 μm) in one barrel and adenosine + theophylline (also 200 μm) in the other, and the two solutions were applied alternately to the same cell.A, The response to concurrently applied adenosine and theophylline (Ado/Theo) was virtually identical to the response to adenosine alone (Ado); i.e., there was no antagonism by theophylline, even though bath superfusion with this concentration of theophylline was sufficient to nearly abolish the adenosine response (compare Fig. 4A).B, The effect of bath superfusion of 200 μm theophylline on the adenosine + theophylline response from A. The degree of antagonism of the adenosine + theophylline response by bath-superfused theophylline (C) was equivalent to that observed with application of adenosine alone (not shown). This experiment was replicated in another cell with identical results.
Do responses to nucleotides require prior conversion to adenosine?
One way to determine whether adenine nucleotides must be converted to adenosine before they can interact with A1 receptors would be to inhibit the ecto-nucleotidase pathway responsible for the formation of adenosine. The last step on this pathway for all of the nucleotides is the removal of the terminal phosphate group of AMP via the enzyme 5′-nucleotidase. Although it has a relatively low potency, GMP is a competitive inhibitor of the ecto-5′-nucleotidase. Therefore, we tested the ability of GMP to block responses to adenosine and the adenine nucleotides. Bath superfusion with 2 mm GMP had no effect on the response to adenosine (Fig.6A), nor did it have a significant effect on the holding current (0.32 ± 3.92 pA;n = 11; p > 0.5) or the input resistance of cells (103.4 ± 3.4%; p > 0.5). It did antagonize, however, the effects of all the nucleotides tested, including AMP, ADP, and ATP (Fig. 6B–D), which is consistent with the hypothesis that the conversion of AMP to adenosine is a final common step in the transformation of each of the nucleotides to adenosine. Even responses to cAMP, which were extremely small to begin with (<1 pA), were reduced by GMP (Fig. 6E). In some cases, much-reduced responses to the nucleotides were observed after superfusion with GMP, and the time course of the response was substantially altered. In the example shown in Figure6D, the response to ATP was markedly reduced in amplitude, but also had a much slower rate of onset relative to the control response. To confirm that the effects of GMP were related to inhibition of 5′-nucleotidase, several experiments were conducted using AOPCP (250 μm), which is a more potent inhibitor of 5′-nucleotidase. As with GMP, AOPCP markedly inhibited the response to AMP but not to adenosine (Fig. 6E).
Fig. 6.
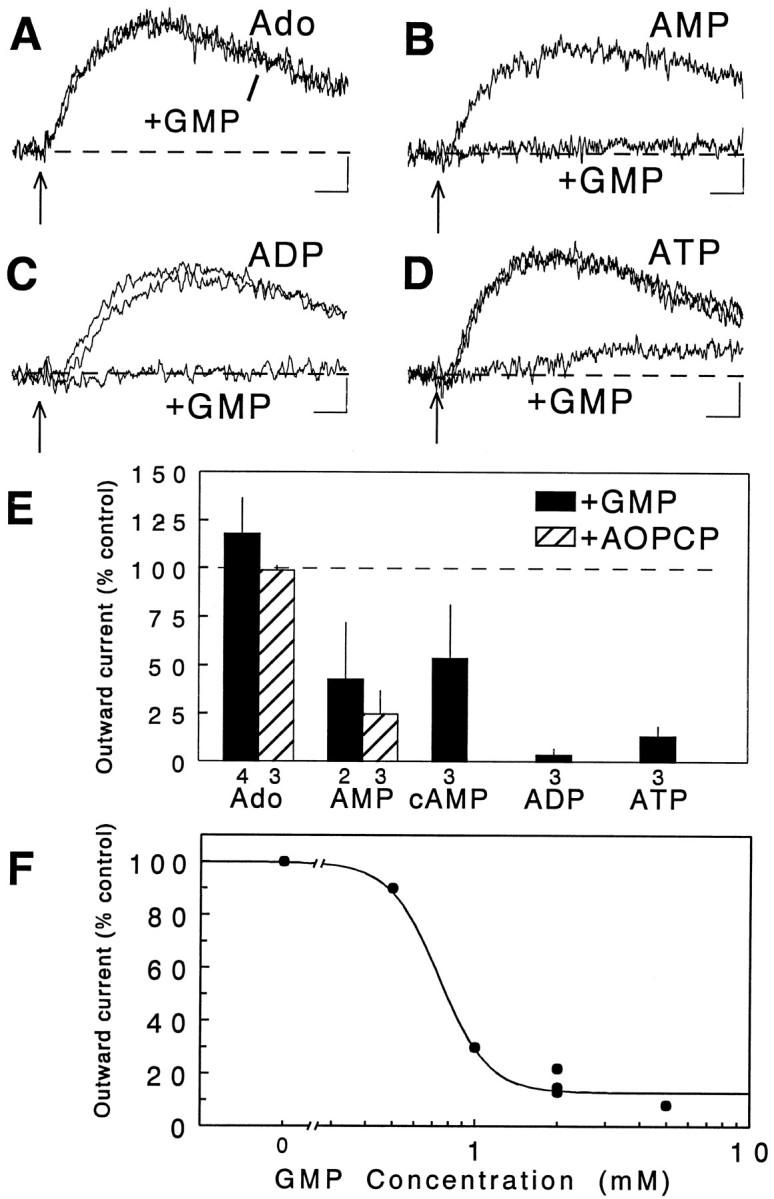
GMP and AOPCP antagonize responses to nucleotides but not adenosine. Responses to local application of adenosine (A) (200 μm) were completely insensitive to bath superfusion of the slice with 2 mmguanosine monophosphate (+GMP), which is an inhibitor of 5′-nucleotidase, the enzyme that converts AMP to adenosine. GMP concentrations up to 5 mm had no effect on the response to adenosine; however, responses to AMP (B), ADP (C), and ATP (D) (all at 200 μm) were blocked nearly completely by superfusion with 2 mm GMP. In C and D, separate averages of pre-GMP responses and responses obtained after GMP washout are illustrated and are virtually superimposable. InD, there was a small, very slow inward current response to ATP that persisted in 2 mm GMP, and this was abolished by increasing the GMP concentration to 5 mm (not shown). Calibration bars indicate 1 sec and 3 pA for each set of averages. Summary data for all slices tested with GMP are shown inE, as well as for slices tested with 250 μm AOPCP. The dose–response curve for bath-superfused GMP versus 200 μm ATP (local pressure ejection) is illustrated in F. Each point represents an individual slice tested with a single concentration of superfused GMP; the solid line represents the best fit to the points using the logistic equation, with an EC50 of 0.74 mm.
The preceding experiments demonstrated that the conversion of the nucleotides to adenosine is an obligatory step in eliciting responses to the nucleotides. The observation that the responses to nucleotides were not significantly different in amplitude from adenosine responses suggests that this conversion is essentially complete, i.e., that ejection of nucleotides leads to the formation of equimolar amounts of adenosine. To confirm that this is the case, however, it was necessary to demonstrate that the response to 200 μm adenosine applied in this manner was not saturating. If adenosine receptors were saturated, then a less than complete conversion of the nucleotide to adenosine might be able to elicit a response of similar magnitude by occupying essentially all the available adenosine receptors. To ensure that this was not occurring, several parallel experiments were run comparing responses to 100, 200, or 500 μm adenosine ejected from adjacent barrels of the same pipette. The mean response to 100 μm adenosine was 58 ± 5% of the response to 200 μm adenosine ejected from an adjacent barrel (n = 5 cells), and the response to 500 μmadenosine was 294 ± 20% of the response to 200 μm(n = 3 cells). These results demonstrate that the response to 200 μm adenosine was in a nearly linear portion of the dose–response curve, and if the nucleotides were not completely converted to adenosine, smaller responses to these agents would have been evident. Thus, the results indicate that the conversion of the nucleotides must have been nearly complete within a few hundred milliseconds of the pressure application to elicit responses of comparable magnitude and time course. In the case of cAMP, however, the conversion must occur slowly enough that the cAMP diffuses away from the site of release faster than it can be converted to AMP, resulting in essentially no response to this nucleotide (Fig.7B).
How rapidly are adenine nucleotides converted to adenosine?
The comparisons between nucleotides summarized in Figure4E were all made on different cells, and because of the intrinsic variability in such responses, it was difficult to determine whether the minor differences in latency, amplitude, and duration that were observed were differences between the nucleotides and adenosine or reflected cell-to-cell variation in the response. For that reason, parallel experiments were conducted using two-barrel drug application pipettes, so that direct comparisons might be made of the responses to various nucleotides and adenosine on the same cell. Figure7 illustrates this type of comparison between AMP and adenosine and between cAMP and AMP. In cells recorded with AMP and adenosine, responses to both purines were quite similar (Fig. 7A), confirming the similarity of the responses observed in the experiments on different cells (Fig. 4). On the other hand, cAMP produced little if any effect, even when ejected using identical conditions onto the same cell (Fig. 7B). As far as the time course of the AMP response was concerned, there was a small but consistent lag of several hundred milliseconds in the onset of the AMP response relative to adenosine. These observations were confirmed by fitting responses to AMP and adenosine with the product of two exponential functions (Fig.7C) and then comparing the estimates of the free parameters. There were no significant differences in the goodness of fit (r2), the maximum amplitude, τon, or τoff, but there was a significant difference in the estimated latency of the response; the onset of the AMP response was delayed by 230 ± 40 msec relative to the adenosine response (p < 0.05;n = 3 cells). Although the difference in the τon values was not statistically significant with the three cells tested, individual comparisons using double-barrel pipettes (Figs. 7, 8) clearly suggest that the responses to both AMP and ATP had slower rise times than the corresponding adenosine responses.
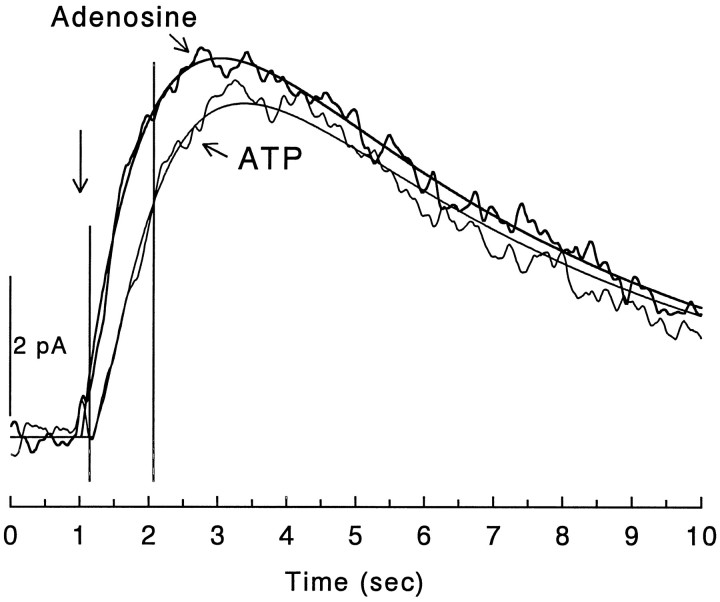
Fig. 8.
Comparison of the kinetics of adenosine and ATP responses. Application of ATP (200 μm) (light line) from one barrel of a drug pipette elicited outward current responses that were qualitatively and quantitatively similar to those elicited by the ejection of adenosine (also 200 μm) (heavy line) from the adjacent barrel. The only period during which there was a statistically significant difference between these two averaged response waveforms, as determined by lack of overlap in their associated 95% confidence limits, is during the segment bracketed by the vertical lines. For the adenosine response, the raw data were fit by the product of two exponential functions, as described in Materials and Methods. For the ATP response, a somewhat modified function was used. It was assumed that ATP had no direct effect on the receptor, that a constant fraction of the ATP that was present was converted to adenosine per unit time (i.e., first order kinetics), and that the time course of the response to adenosine formed from ATP was governed by the kinetic parameters corresponding to the best fit to the direct response to adenosine. When theT1/2 for the conversion of ATP to adenosine was allowed to vary as a free parameter, the best fit to the ATP response was obtained with a T1/2 of 170 msec. The fit lines corresponding to these two functions are thesmooth lines superimposed on the responses. Time of drug ejection is indicated by the vertical arrow.
The experiments with paired AMP/adenosine application demonstrated that there was a significant lag in the onset of responses to AMP relative to adenosine. These differences were characterized in two different ways. First, the latency to the half-maximal response was determined for each of the responses in all of the experiments involving application of drugs from double-barreled pipettes. In cells tested with both ATP and adenosine, the average latency at half-maximum for the ATP responses was 300 ± 52 msec longer than the adenosine responses (n = 14 cells). Responses to AMP were delayed slightly (albeit not significantly) longer relative to adenosine (340 ± 94 msec; n = 7 cells) than were ATP responses. The lack of a significant difference between ATP and AMP indicates that the metabolism of AMP to adenosine must be almost entirely responsible for the delay in both the AMP and ATP responses. Thus, 5′-nucleotidase must be the primary rate-limiting step in determining the time course of purine nucleotide responses.
The comparisons of the latencies with half-maximal responses suggested that adenine nucleotides were converted to adenosine within a few hundred milliseconds, but to obtain a more quantitative estimate for the rate at which ATP was converted to adenosine, the results illustrated in Figure 8 were fit to a kinetic model. In this experiment, adenosine and ATP were ejected from adjacent barrels of a double-barrel pipette. The response to adenosine alone was well fit (r2 = 0.982) by the product of two exponential functions, with τ values of 1.13 sec for the onset of the response and 5.53 sec for the decay (Fig. 8). The response to ATP was then fit by assuming that the additional delay in the ATP response and its slower rise time reflected the time required to form adenosine from the ATP (see legend to Fig. 8). The best fit to the ATP response was obtained when the time required to convert half of the ATP to adenosine (T1/2) was 170 msec. Similar analysis of another cell tested with both ATP and adenosine gave an estimated T1/2 of 204 msec. Although these are only estimates, T1/2 values for ATP in the extracellular space in this range would correspond to >96% of the ATP being converted to adenosine within 1 sec.
DISCUSSION
Adenine nucleotides are thought to be an important potential source of extracellular adenosine in the brain, because mechanisms exist for the release of nucleotides from neurons and glia, and the enzymes required to convert ATP, ADP, AMP, and cAMP to adenosine are found in brain with their catalytic moieties exposed to the extracellular space. Nevertheless, a number of issues concerning responses to adenine nucleotides remain unresolved, including their ability to directly interact with adenosine receptors and the absolute and relative rates at which various nucleotides can be converted to adenosine.
Ligand binding studies have suggested that nucleotides are relatively poor agonists at adenosine receptors (Schwabe and Trost, 1980; Ragazzi et al., 1991), although these studies are somewhat problematic in that even isolated membranes still maintain their ability to convert nucleotides to adenosine. Previous physiological studies have suggested that stable analogs of adenine nucleotides can produce adenosine-like responses, but these responses are at least partially blocked by adenosine deaminase (Lee et al., 1981; Wiklund et al., 1985; von Kugelgen et al., 1992). This latter observation suggests that these nucleotide analogs may act at least in part by an indirect mechanism, such as by altering purine metabolism so that extracellular adenosine levels are elevated; however, the present studies demonstrate that even relatively high concentrations of adenine nucleotides are unable to directly activate adenosine receptors. ATP, ADP, and AMP all produced robust activation of adenosine receptors, but responses to these agents were delayed in a statistically significant manner relative to adenosine, indicating that some intermediary process must occur before activation of adenosine receptors. GMP and AOPCP, which are competitive antagonists of 5′-nucleotidase, blocked responses to the nucleotides but had no significant effects on responses to adenosine itself. These results suggest that conversion of AMP to adenosine is an obligatory final common step in the conversion of each of these nucleotides to adenosine and that they must be converted to adenosine before they can act on adenosine receptors. Unlike all the other nucleotides, cAMP produced essentially no response when applied locally, suggesting that the metabolic conversion of cAMP to AMP by ecto-phosphodiesterases occurs slowly enough that the cAMP diffuses away from the site of release faster than it can be converted to adenosine (Brundege et al., 1997).
An important aspect of the present experiments is that because the purines were applied directly to visualized neurons across very short distances, the amounts of these agents ejected into the slice were very small relative to most local application studies. The functional significance of this is that these responses were quite sensitive to the rate at which various steps in the interconversion of nucleotides could take place. If adenosine could not be formed rapidly enough, the nucleotides would diffuse away from the site of application before significant amounts of adenosine could accumulate, as we have hypothesized happens with cAMP (above). The “rate sensitivity” of these responses also explains why GMP was able to successfully antagonize responses to the nucleotides, even though this concentration of GMP has no effect on responses to bath superfusion of nucleotides (data not shown), and in biochemical studies must be used in conjunction with other nucleotidase inhibitors to block the conversion of labeled nucleotides to adenosine (MacDonald and White, 1985; Craig and White, 1993; Pedata et al., 1993). In the protocol used in these experiments, it is not necessary to completely inhibit this process but simply to slow the rate of conversion to the point at which diffusion limits the response.
It is clear from these experiments that the conversion of AMP to adenosine by 5′-nucleotidase is the rate-limiting step in the extracellular conversion of nucleotides to adenosine. Although responses to all of the nucleotides had longer latencies than adenosine, there were no significant differences in the latencies of ATP, ADP, or AMP responses. The enzymes that convert ATP to AMP are not as well characterized as the 5′-nucleotidase, and potential pathways could include a direct conversion of ATP to AMP by apyrase (Rocha et al., 1990; Ziganshin et al., 1994; Battastini et al., 1995) or the successive formation of ADP and AMP via the action of an ATPase and an ADPase (James and Richardson, 1993; Ziganshin et al., 1994). Nevertheless, the formation of AMP does not seem to be rate-limiting in these responses. This is in contrast to other tissues, such as the heart, in which 5′-nucleotidase does not seem to be the rate-limiting step in the conversion of ATP to adenosine (Ragazzi et al., 1991). The largely extracellular localization of 5′-nucleotidase (Kreutzberg et al., 1978), and its highly specific distribution in different brain regions (Lee et al., 1986; Fastbom et al., 1987; Zimmermann et al., 1993), suggest that it may be a particularly important component of the nucleotide/adenosine signaling pathway in brain.
Although the rate of formation of adenosine from ATP can only be estimated from these experiments, it is clear that the majority of the ATP in the extracellular space is converted to adenosine in <1 sec. The physiological importance of this mechanism for the rapid enzymatic conversion of ATP to adenosine is unclear, but similarly rapid and efficient conversion of ATP occurs in other tissues, such as the lung, where the t1/2 for perfused ATP has been estimated to be 200 msec or less (Ryan and Smith, 1971). Although it is possible that parts of this nucleotidase pathway may play a role in terminating ATP responses mediated via P2 receptors, this would not explain the rapid conversion of AMP to adenosine, because both AMP and adenosine are inactive on P2 receptors. Perhaps the most likely explanation is that this provides a mechanism for the rapid activation of adenosine receptors. The release of adenosine via the facilitated diffusion transporter is likely to be rather slow, but the vesicular release of ATP colocalized with other transmitters (Silinsky, 1975;Burnstock, 1986; Richardson and Brown, 1987) or permeation of ATP through certain types of ion channels (Abraham et al., 1993) would lead to very rapid, localized release of ATP. A highly active nucleotidase pathway provides a means for generating high local concentrations of adenosine, but only if the conversion of ATP to adenosine occurs rapidly enough that the adenosine is formed before the ATP can diffuse away from the site of release.
Several issues related to the novel method of applying agonists in this study deserve comment. First, it is likely that the rate of rise of the outward current primarily reflects the rate at which adenosine reaches adenosine receptors, and the rate of decay reflects the amount of time that is required to clear the extracellular space of adenosine, rather than time constants related to receptor activation and deactivation. There have been no fast kinetic studies of A1 receptors, but similar analyses of G-protein-coupled K+ channels in other systems indicate that there is an intrinsic lag of ∼50–100 msec in the activation of this channel (Surprenant and North, 1988; Inomata et al., 1989; Sodickson and Bean, 1996), whereas the lag times in the present experiments were typically several hundred milliseconds. Similarly, the values for τon and τoff that were observed (e.g., 2.6 and 4.1 sec, respectively, for the adenosine response in Fig. 3) are considerably slower than corresponding values for the GABABreceptor (200–400 and 500–1000 msec) (Sodickson, Bean, 1996). Thus, it would seem unlikely that these rate constants provide much information concerning the rate of receptor activation and deactivation, but more likely reflect the time course of the adenosine concentration near the receptor.
The rapid application of high concentrations of agonists also resulted in some apparently paradoxical observations concerning the effects of the competitive receptor antagonist theophylline. The first of these was the finding that although bath application of 200 μm theophylline antagonized responses to all of the purines, this same concentration had no detectable effect when included in the pressure pipette with adenosine. The most likely explanation for this phenomenon is that with superfused theophylline, its off-rate from the receptor is slow enough that little if any theophylline is displaced by adenosine after local adenosine application. In this sense theophylline acts essentially as a noncompetitive inhibitor, and its effectiveness in antagonizing adenosine responses is determined only by the fraction of receptors that theophylline occupies at the time that the adenosine pulse is delivered.
In conclusion, these experiments clearly demonstrate that mechanisms exist for the rapid conversion of ATP, ADP, and AMP to adenosine in the extracellular space in hippocampus. After the local release of any of these agents, conversion to adenosine occurs within <1 sec, and the adenosine that is formed can then activate nearby A1 receptors. Direct activation of A1 receptors by adenine nucleotides does not occur at the concentrations tested in this study. Thus, the primary response to the liberation of these agents would seem to be mediated by adenosine receptors and to occur subsequent to their conversion to adenosine. As we have shown previously, cAMP is metabolized to adenosine at a much slower rate, suggesting that the maximum capacity of ecto-phosphodiesterases is considerably lower than that of 5′-nucleotidase (Brundege et al., 1997). Both superfusion with cAMP (Dunwiddie and Hoffer, 1980; Madison and Nicoll, 1986) (Fig. 1C) and the liberation of cAMP after activation of adenylyl cyclase (Gereau and Conn, 1994; Brundege et al., 1997) ultimately result in activation of adenosine receptors, but with a time course that is much slower than the responses observed with local application of nucleotides. These results suggest that the release of noncyclic adenine nucleotides may result in transient, localized increases in extracellular adenosine, whereas the release of cAMP may be associated with longer-term increases that take place over correspondingly larger areas.
Footnotes
This work was supported by Grant R01 NS 29173 from the National Institute of Neurological Disorders and Stroke and by the Veterans Administration Medical Research Service.
Correspondence should be addressed to Dr. Thomas V. Dunwiddie, Department of Pharmacology, Box C-236, University of Colorado Health Science Center, 4200 E. 9th Avenue, Denver, CO 80262.
REFERENCES
- 1.Abraham EH, Prat AG, Gerweck L, Seneveratne T, Arceci RJ, Kramer R, Guidotti G, Cantiello HF. The multidrug resistance (mdr1) gene product functions as an ATP channel. Proc Natl Acad Sci USA. 1993;90:312–316. doi: 10.1073/pnas.90.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battastini AM, Oliveira EM, Moreira CM, Bonan CD, Sarkis JJ, Dias RD. Solubilization and characterization of an ATP diphosphohydrolase (EC 3.6.1.5) from rat brain synaptic plasma membranes. Biochem Mol Biol Int. 1995;37:209–219. [PubMed] [Google Scholar]
- 3.Brundege JM, Diao LH, Proctor WR, Dunwiddie TV (1997) The role of cyclic AMP as a precursor of extracellular adenosine in the rat hippocampus. Neuropharmacology, in press. [DOI] [PubMed]
- 4.Bruns RF. Adenosine receptor activation by adenine nucleotides requires conversion of the nucleotides to adenosine. Naunyn Schmiedebergs Arch Pharmacol. 1980;315:5–13. doi: 10.1007/BF00504224. [DOI] [PubMed] [Google Scholar]
- 5.Burnstock G. The changing face of autonomic neurotransmission. Acta Physiol Scand. 1986;126:67–91. doi: 10.1111/j.1748-1716.1986.tb07790.x. [DOI] [PubMed] [Google Scholar]
- 6.Craig CG, White TD. Low-level N-methyl-d-aspartate receptor activation provides a purinergic inhibitory threshold against further N-methyl-d-aspartate-mediated neurotransmission in the cortex. J Pharmacol Exp Ther. 1992;260:1278–1284. [PubMed] [Google Scholar]
- 7.Craig CG, White TD. N-methyl-d-aspartate- and non-N-methyl-d-aspartate-evoked adenosine release from rat cortical slices: distinct purinergic sources and mechanisms of release. J Neurochem. 1993;60:1073–1080. doi: 10.1111/j.1471-4159.1993.tb03256.x. [DOI] [PubMed] [Google Scholar]
- 8.Dunwiddie TV. The physiological role of adenosine in the central nervous system. Int Rev Neurobiol. 1985;27:63–139. doi: 10.1016/s0074-7742(08)60556-5. [DOI] [PubMed] [Google Scholar]
- 9.Dunwiddie TV, Fredholm BB. Adenosine receptors mediating inhibitory electrophysiological responses in rat hippocampus are different from receptors mediating cyclic AMP formation. Naunyn Schmiedebergs Arch Pharmacol. 1984;326:294–301. doi: 10.1007/BF00501433. [DOI] [PubMed] [Google Scholar]
- 10.Dunwiddie TV, Hoffer BJ. Adenine nucleotides and synaptic transmission in the in vitro rat hippocampus. Br J Pharmacol. 1980;69:59–68. doi: 10.1111/j.1476-5381.1980.tb10883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunwiddie TV, Lynch GS. Long-term potentiation and depression of synaptic responses in the rat hippocampus: localization and frequency dependency. J Physiol (Lond) 1978;276:353–367. doi: 10.1113/jphysiol.1978.sp012239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fastbom J, Pazos A, Palacios JM. The distribution of adenosine A1 receptors and 5′-nucleotidase in the brain of some commonly used experimental animals. Neuroscience. 1987;22:813–826. doi: 10.1016/0306-4522(87)92961-7. [DOI] [PubMed] [Google Scholar]
- 13.Gerber U, Greene RW, Haas HL, Stevens DR. Characterization of inhibition mediated by adenosine in the hippocampus of the rat in vitro. J Physiol (Lond) 1989;417:567–578. doi: 10.1113/jphysiol.1989.sp017819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gereau RW, Conn PJ. Potentiation of cAMP responses by metabotropic glutamate receptors depresses excitatory synaptic transmission by a kinase-independent mechanism. Neuron. 1994;12:1121–1129. doi: 10.1016/0896-6273(94)90319-0. [DOI] [PubMed] [Google Scholar]
- 15.Greene RW, Haas HL. Adenosine actions on CA1 pyramidal neurones in rat hippocampal slices. J Physiol (Lond) 1985;366:119–127. doi: 10.1113/jphysiol.1985.sp015788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greene RW, Haas HL. The electrophysiology of adenosine in the mammalian central nervous system. Prog Neurobiol. 1991;36:329–341. doi: 10.1016/0301-0082(91)90005-l. [DOI] [PubMed] [Google Scholar]
- 17.Inomata N, Ishihara T, Akaike N. Activation kinetics of the acetylcholine-gated potassium current in isolated atrial cells. Am J Physiol. 1989;257:C646–650. doi: 10.1152/ajpcell.1989.257.4.C646. [DOI] [PubMed] [Google Scholar]
- 18.James S, Richardson PJ. Production of adenosine from extracellular ATP at the striatal cholinergic synapse. J Neurochem. 1993;60:219–227. doi: 10.1111/j.1471-4159.1993.tb05841.x. [DOI] [PubMed] [Google Scholar]
- 19.Kreutzberg GW, Barron KD, Schubert P. Cytochemical localization of 5′-nucleotidase in glial plasma membranes. Brain Res. 1978;158:247–257. doi: 10.1016/0006-8993(78)90672-8. [DOI] [PubMed] [Google Scholar]
- 20.Lee KS, Schubert P, Emmert H, Kreutzberg GW. Effect of adenosine versus adenine nucleotides on evoked potentials in a rat hippocampal slice preparation. Neurosci Lett. 1981;23:309–314. doi: 10.1016/0304-3940(81)90016-1. [DOI] [PubMed] [Google Scholar]
- 21.Lee KS, Schubert P, Reddington M, Kreutzberg GW. The distribution of adenosine A1 receptors and 5′-nucleotidase in the hippocampal formation of several mammalian species. J Comp Neurol. 1986;246:427–434. doi: 10.1002/cne.902460402. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald WF, White TD. Nature of extrasynaptosomal accumulation of endogenous adenosine evoked by K+ and veratridine. J Neurochem. 1985;45:791–797. doi: 10.1111/j.1471-4159.1985.tb04062.x. [DOI] [PubMed] [Google Scholar]
- 23.Madison DV, Nicoll RA. Cyclic adenosine 3′,5′-monophosphate mediates beta-receptor actions of noradrenaline in rat hippocampal pyramidal cells. J Physiol (Lond) 1986;372:245–259. doi: 10.1113/jphysiol.1986.sp016007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCormick DA, Williamson A. Convergence and divergence of neurotransmitter action in human cerebral cortex. Proc Natl Acad Sci USA. 1989;86:8098–8102. doi: 10.1073/pnas.86.20.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicoll RA. The coupling of neurotransmitter receptors to ion channels in the brain. Science. 1988;241:545–551. doi: 10.1126/science.2456612. [DOI] [PubMed] [Google Scholar]
- 26.Pedata F, Latini S, Pugliese AM, Pepeu G. Investigations into the adenosine outflow from hippocampal slices evoked by ischemia-like conditions. J Neurochem. 1993;61:284–289. doi: 10.1111/j.1471-4159.1993.tb03566.x. [DOI] [PubMed] [Google Scholar]
- 27.Pirotton S, Boeynaems JM. Evidence that ATP, ADP and AMP are not ligands of the striatal adenosine A2A receptors. Eur J Pharmacol. 1993;241:55–61. doi: 10.1016/0014-2999(93)90932-8. [DOI] [PubMed] [Google Scholar]
- 28.Ragazzi E, Wu SN, Shryock J, Belardinelli L. Electrophysiological and receptor binding studies to assess activation of the cardiac adenosine receptor by adenine nucleotides. Circ Res. 1991;68:1035–1044. doi: 10.1161/01.res.68.4.1035. [DOI] [PubMed] [Google Scholar]
- 29.Richardson PJ, Brown SJ. ATP release from affinity-purified rat cholinergic nerve terminals. J Neurochem. 1987;48:622–630. doi: 10.1111/j.1471-4159.1987.tb04138.x. [DOI] [PubMed] [Google Scholar]
- 30.Rocha JB, Mello CF, Sarkis JJ, Dias RD. Undernutrition during the preweaning period changes calcium ATPase and ADPase activities of synaptosomal fractions of weanling rats. Br J Nutr. 1990;63:273–283. doi: 10.1079/bjn19900114. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg PA, Li Y. Adenylyl cyclase activation underlies intracellular cyclic AMP accumulation, cyclic AMP transport, and extracellular adenosine accumulation evoked by β-adrenergic receptor stimulation in mixed cultures of neurons and astrocytes derived from rat cerebral cortex. Brain Res. 1995;692:227–232. doi: 10.1016/0006-8993(95)00668-g. [DOI] [PubMed] [Google Scholar]
- 32.Rosenberg PA, Knowles R, Knowles KP, Li Y. Beta-adrenergic receptor-mediated regulation of extracellular adenosine in cerebral cortex in culture. J Neurosci. 1994;14:2953–2965. doi: 10.1523/JNEUROSCI.14-05-02953.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan JW, Smith U. Metabolism of adenosine 5′-monophosphate during circulation through the lungs. Trans Assoc Am Physicians. 1971;84:297–306. [PubMed] [Google Scholar]
- 34.Schwabe U, Trost T. Characterization of adenosine receptors in rat brain by (−)[3H]N6-phenylisopropyladenosine. Naunyn Schmiedebergs Arch Pharmacol. 1980;313:179–187. doi: 10.1007/BF00505731. [DOI] [PubMed] [Google Scholar]
- 35.Silinsky EM. On the association between transmitter secretion and the release of adenine nucleotides from mammalian motor nerve terminals. J Physiol (Lond) 1975;247:145–162. doi: 10.1113/jphysiol.1975.sp010925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sodickson DL, Bean BP. GABAB receptor-activated inwardly rectifying potassium current in dissociated hippocampal CA3 neurons. J Neurosci. 1996;16:6374–6385. doi: 10.1523/JNEUROSCI.16-20-06374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Surprenant A, North RA. Mechanism of synaptic inhibition by noradrenaline acting at alpha2-adrenoceptors. Proc R Soc Lond [Biol] 1988;234:85–114. doi: 10.1098/rspb.1988.0039. [DOI] [PubMed] [Google Scholar]
- 38.Thompson SM, Haas HL, Gahwiler BH. Comparison of the actions of adenosine at pre- and postsynaptic receptors in the rat hippocampus in vitro. J Physiol (Lond) 1992;451:347–363. doi: 10.1113/jphysiol.1992.sp019168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trussell LO, Jackson MB. Dependence of an adenosine-activated potassium current on a GTP-binding protein in mammalian central neurons. J Neurosci. 1987;7:3306–3316. doi: 10.1523/JNEUROSCI.07-10-03306.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Kugelgen I, Spath L, Starke K. Stable adenine nucleotides inhibit [3H]-noradrenaline release in rabbit brain cortex slices by direct action at presynaptic adenosine A1-receptors. Naunyn Schmiedebergs Arch Pharmacol. 1992;346:187–196. doi: 10.1007/BF00165300. [DOI] [PubMed] [Google Scholar]
- 41.Wiklund NP, Gustafsson LE, Lundin J. Pre- and postjunctional modulation of cholinergic neuroeffector transmission by adenine nucleotides. Experiments with agonist and antagonist. Acta Physiol Scand. 1985;125:681–691. doi: 10.1111/j.1748-1716.1985.tb07771.x. [DOI] [PubMed] [Google Scholar]
- 42.Ziganshin AU, Hoyle CH, Burnstock G. Ecto-enzymes and metabolism of extracellular ATP. Drug Dev Res. 1994;32:134–146. [Google Scholar]
- 43.Zimmermann H. 5′-Nucleotidase: molecular structure and functional aspects. Biochem J. 1992;285:345–365. doi: 10.1042/bj2850345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zimmermann H, Vogel M, Laube U. Hippocampal localization of 5′-nucleotidase as revealed by immunocytochemistry. Neuroscience. 1993;55:105–112. doi: 10.1016/0306-4522(93)90458-r. [DOI] [PubMed] [Google Scholar]