Abstract
Neonicotinoid pesticides are applied to seeds and are known to cause lethal and sub-lethal effects in birds and mammals. Neonicotinoid-treated seeds could be available to wildlife through spillage or exposed seeds near or at the soil surface due to incomplete or shallow drilling. We quantified seed spills that may occur during loading or refilling the hopper at a landscape-scale using road-based surveys. We also quantified undrilled seeds in 1-m2 frames on the soil in the center and corner of fields to obtain estimates at the field scale. We broadcast seeds on the soil surface of a tilled field and left them for 0, 1, 2, 4, 8, 16, and 30 days to quantify neonicotinoid decrease under field conditions. Lastly, we documented wildlife at neonicotinoid-treated seed spills with trail cameras. We estimated the number of spills during planting to be 3,496 (95%CI: 1,855–5,138) and 2,609 (95%CI: 862–4,357) for corn, 11,009 (95%CI: 6,950–15,067) and 21,105 (95%CI: 6,162–36,048) for soybean, and 830 (95%CI: 160–1,500) and 791 (95%CI: 0–1,781) for wheat in 2016 and 2017, respectively. Exposed seeds were present at the soil surface in 35% of 71 fields. The probability that seeds were present on the soil surface was higher for soybeans (18.8 and 49.4% in the center and corners, respectively) than for corn (1.6 and 2.7%, respectively), and seed densities were also higher (1.04 vs 0.07 seeds/m2, respectively). Neonicotinoids decreased rapidly on seeds on the soil surface but persisted as long as 30 days. Over a dozen species of birds and mammals consumed seeds at simulated spills, with an average time for birds to find spills of 1.3 ± 1.5 days and an average time to consumption of 4.1 ± 3.4 days. Seeds are abundant on the soil surface for wildlife to consume during the spring planting season and should be considered in pesticide risk assessments.
Keywords: Agriculture, birds, mammals, Midwestern United States, pesticide, treated seeds
1. Introduction
Neonicotinoids, including imidacloprid (IMI), clothianidin (CLO), and thiamethoxam (TMX), comprise 25% of the global agricultural insecticide market, making them the most widely used pesticides worldwide, with imidacloprid comprising nearly half of this market (Jeschke et al., 2011; Mineau and Palmer, 2013; Goulson, 2013) until 2012 when thiamethoxam had the largest market share (Bass et al. 2015). Neonicotinoids are systemic pesticides that are commonly applied as seed treatments to important food crops like corn, soybeans, oilseed rape, sunflower, cereals, and beets. About 2–20% of the seed treatment is taken up by the plant as it grows and is distributed among the leaves, flowers, pollen, and nectar, at concentrations sufficient to control invertebrate pests (e.g., 5–10 μg per liter in sap; Sanchez-Bayo, 2014). Invertebrates are impacted at doses (0.82–88 ng active ingredient/insect) that are considered safe for vertebrates, because toxicity in vertebrates requires exposure to doses (14–5,000 mg active ingredient/kg body weight) that greatly exceed the levels that produce effects in invertebrates (Goulson, 2013). Neonicotinoids bind very specifically to invertebrate nicotinic acetylcholine receptors, and because they bind less strongly to vertebrate receptors and are not as persistent in the environment as organochlorines, they have been considered much less toxic to vertebrates than pesticide options that predated the early 1990’s (Tomizawa and Casida, 2005; Jeschke et al., 2011). This high specificity and systemic nature contributed to their widespread and rapid adoption beginning in 1994 with the registration of imidacloprid in the United States (FIFRA 1996).
Importantly, demonstrated impacts of neonicotinoids on non-target invertebrates have been documented over the last decade (Krupke et al., 2012; Sanchez-Bayo, 2014; Goulson et al., 2015). Concerns for incidental impacts on pollinators (e.g., through availability in nectar and pollen) led the European Union to ban or place a moratorium on use of IMI, CLO, and TMX on flowering crops in 2013. In May 2018, the moratorium was expanded to include all outdoor use of IMI, CLO, and TMX by the end of 2018, based on the threat that these chemicals pose to pollinators due to their persistence in soil, solubility in water, transport away from the site of application, and uptake by other plants (Krupke et al., 2012; Main et al., 2014; Bonmatin et al., 2015; Morrissey et al., 2015). However, these pesticides are widely used in North America, and elsewhere in the world. Recent studies are now also documenting adverse effects of neonicotinoids that reach beyond pollinators to include vertebrates (see reviews in Mineau and Palmer, 2013; Gibbons et al., 2014). In the United States, neonicotinoids are currently under registration review by the Environmental Protection Agency (EPA), with risks to both pollinators and non-pollinators, including birds and mammals, under consideration.
Vertebrate toxicity is expected to occur at doses that exceed the levels available in crop plants consumed by humans and livestock (FIFRA 1996). Wild birds and mammals are most likely to be exposed to large doses of neonicotinoids through ingestion of treated seeds (Goulson, 2013; Gibbons et al., 2014), although numerous other exposure mechanisms exist (e.g., soil, trophic transfer; SERA, 2005; Douglas et al., 2015). The Minnesota Department of Agriculture (2014) stated that, “Although neonicotinoids are less toxic to vertebrates than to arthropods, direct consumption of neonicotinoid treated seeds may expose birds and other taxa to acute or chronic doses.” Ingestion of a small number of neonicotinoid-treated seeds can be lethal to birds; for example, ingestion of a single treated corn kernel is lethal to a blue-jay sized (~ 85 g) bird (see reviews in Mineau and Palmer, 2013; Gibbons et al., 2014). However, toxicity varies by chemical and species, given differences in genetic and physiological factors including size, absorption, distribution, metabolic, and excretion processes (Bean et al. 2019). Differences among species in seed handling behavior could affect the ingested amount of chemical (Avery et al., 1997).
Sub-lethal effects in birds in the lab include hyporeactivity, lack of coordination, wing drop, immobility, disruption of migratory coordination, eggshell thinning, reduced egg hatching rate, impaired testicular function, and low weight in chicks (Cox, 2001; Lopez-Antia et al., 2013, and 2015; Tokumoto et al., 2013; Mineau and Palmer, 2013; Eng et al., 2017). Sub-lethal impacts in mammals include delayed sexual maturation, sperm deformities, premature deliveries, stillbirths, and offspring deformities (Rexrode et al., 2003; Anon, 2007). Yet, studies of neonicotinoid effects on vertebrates are overwhelmingly laboratory-based (91% of studies), which limits our ability to interpret the significance of findings in more natural settings (Gibbons et al., 2014).
Neonicotinoid-treated seeds could be available to wildlife through spillage during transport, reloading and refilling of the hopper or through seeds near or at the soil surface after planting (de Leeuw et al., 1995; Pascual et al., 1999; Lopez-Antia et al., 2016). The U.S. EPA estimated that ~1% of seeds remain accessible to granivores after planting (as reported by Goulson, 2013; Lopez-Antia et al., 2015). Higher densities of exposed seeds generally result in greater attraction of birds to fields (Murton et al., 1963; Feare et al., 1974). In Spain, 30 bird species were observed picking up treated seeds from cereal fields, and 3.1% of red-legged partridge (Alectoris rufa) gut contents collected by hunters tested positive for imidacloprid after planting of winter cereal crops despite insecticides not normally being used on winter cereal crops in the study area (Lopez-Antia et al., 2016). More recently in Texas, USA, 7 of 57 northern bobwhite (Colinus virginianus) livers had detectable concentrations of neonicotinoids (Ertl et al., 2018).
Given the toxicity to birds and mammals at the concentrations of neonicotinoids applied to treated seeds, consumption of treated seeds would be expected to produce lethal or sub-lethal effects in granivorous wildlife, yet poisoning incidents are infrequently reported. Dead and poisoned partridges have been found in agricultural fields in France following use of imidacloprid-treated seed (Berny et al., 1999, Mineau and Palmer 2013, Millot et al. 2017). A few other pesticide poisoning incidents have been detected (Greig-Smith, 1987; Fletcher et al., 1995; de Snoo et al., 1999), but carcasses can be scavenged quickly (Ponce et al., 2010), may not be localized or may be inconspicuous if effects are not immediate (de Snoo et al., 1999), and may not raise suspicion of pesticides as the cause of death (Millot et al., 2017). Thus, seed consumption or sub-lethal exposure may be easier to detect in field settings than mortalities.
Field studies conducted in Spain have focused on availability and consumption of winter cereals (wheat, oats, barley, and triticale seeds) planted in the fall (Lopez-Antia et al., 2016). We therefore conducted a study to estimate availability and document wildlife consumption of neonicotinoid-treated seeds during the spring planting season in the Midwestern USA. Birds are initiating nests, laying eggs, and incubating nests during the spring, and mammals give birth and raise young, so sub-lethal reproductive effects related to consumption of treated seeds during the breeding season might be particularly long-lasting. Furthermore, we examined an agricultural landscape dominated by corn, soybeans, and wheat, which provided 3 sizes of seeds that may be ingested by birds with varied beak sizes and bill types, as well as mammals that consume beans and grains. Almost all corn planted in the Midwestern USA has been treated with these pesticides (Stokstad, 2013); most soybean, wheat, and sunflower seeds are treated also; and neonicotinoids are applied as a foliar spray for several other crop types.
The overarching objective of our research was to determine whether wildlife may be exposed to potentially lethal or sub-lethal doses of neonicotinoids through treated seeds during the spring planting season. Specifically, we aimed to:
Quantify the rate of large seed spills during planting season at a landscape scale.
Quantify the availability of seeds on the soil surface in fields after planting.
Quantify the decrease of neonicotinoids (IMI, TMX, and CLO) on treated seeds left on the soil surface for up to 30 days.
Quantify the time for wildlife to find neonicotinoid-treated seed spills and determine whether wildlife consume treated seeds at simulated spills.
2. Materials and Methods
2.1. Study Area
We conducted our study in agricultural regions of western Minnesota. We quantified actual seed spills at the landscape-scale (Figure 1a), seeds on the soil surface at the field-scale, and seed consumption at simulated seed spills (Figure 1b) in the springs of 2016 and 2017.
Figure 1a.
Townships (9323.96 ha, n = 76) surveyed for seed spills during spring planting season in 2016 (dark gray), 2017 (light gray), and both years (light gray outlined with dark gray) in Minnesota, United States.
Figure 1b.
Location of fields where seeds were counted on the soil surface after planting (left) and where cameras were placed at simulated spills (right) in Minnesota, United States during 2016 and 2017. Fields are indicated as larger than their actual size to show their relative locations at a statewide scale; thus, some fields cannot be distinguished separately from other nearby fields. Generally, the same sites were used for both objectives, but some differences occurred related to the stage after planting during our visits and the ability to return to sites to remove cameras.
2.2. Quantifying Seed Spills at a Landscape Scale
In the United States, all chemically treated seeds (e.g., neonicotinoids, fungicides, other pesticides) are unnaturally colored, as mandated by the Federal Seed Act. Treated seeds are highly visible and easily identified by their unusual color (e.g., pink, blue, green, purple), which is used to prevent accidental feeding to livestock and humans. We quantified the frequency of actual seed spills on the landscape by inspecting fields with visual access from roads in agricultural areas. This approach allowed for landscape-level seed spill quantification without requiring landowner notification that might bias behavior and compromise results. Because most spills likely occur during seed transport to fields for planting or during refilling and overfilling hoppers near field access points by roads, quantification of seed spills from roads should be minimally biased by visual access from roads. However, this assumes that spill rates are similar for fields adjacent to roads and fields non-adjacent to roads, which also have field access points and privately-owned access roads.
We identified 211 townships (i.e., 36 mi2 or 9324 ha blocks in the U.S. Public Land Survey System) in the western third and southeastern part of the state of Minnesota, USA with ≥50 miles of roads and ≥50% of the area in corn, soybeans, and/or wheat production using the Minnesota Department of Transportation (MNDOT) Roads Layer (MNDOT, 2008) and 2014 Cropland Data Layer (USDA-NASS, 2015), respectively, in ArcGIS 10.2 (ESRI, 2015). These criteria were used to select townships with visual access to fields from roads while also not being so restrictive that the spatial distribution of the sample was constrained. We drew a spatially-balanced sample of 50 townships each year using a Generalized Random Tessellation Stratified (GRTS) design (Stevens and Olsen, 1999). However, we surveyed the 38 most western townships from the 50 selected each year, due to a later start to planting during the springs of 2016 and 2017, for a total of 76 townships surveyed during the 2 years of the study. We began in the southern townships and worked north as the soil warmed to temperatures suitable for planting during 18 April – 23 May 2016 and 23 April – 21 May 2017.
We recorded locations and approximate size (i.e., area) of seed spills near recently planted (i.e., based on row spacing and before the early seedling stage) fields with the DNRSurvey mobile computer application, a moving map software that allows digitization of aerial photography in real-time (Wright et al. 2015). Documenting only recently planted fields allowed for control in temporal variation in the timing of planting. For example, a field that has not been planted yet will not have a spill at the time of sampling, which is different from a spill not occurring during planting. Thus, by only including recently planted fields in our estimates, we measured spills during planting. We defined a “field” as a quarter of a quarter-section (i.e., 40 ac or 16.2 ha). We recorded each quarter of a quarter-section in agricultural row-crop production, whether any part of it was recently planted (i.e., before early seedling stage), documented the amount (number of seeds or approximate area) of spilled seed on the road, field edge, or visible in the field, and crop type (when known). When seed spills were accessible (e.g., along public roads and rights-of-way), we collected seeds to determine the proportion of accessible seed spills that contained neonicotinoid-treated seed. Seeds were sent to an analytical laboratory at Southern Illinois University Carbondale (SIUC) for quantitative determination of seven neonicotinoids: IMI, TMX, CLO, thiacloprid (THIA), dinotefuran (DIN), nitenpyram (NTP), and acetamiprid (ACE).
After our survey of recently planted fields was completed in May, we repeated the survey for the same townships to identify the crops that were growing in fields. This allowed us to quantify spill rates per crop type planted during the time of our survey. We also noted additional spills observed during the second pass in 2017, but these spills were not included in spill rate estimates because surveys were conducted too long after most fields were planted.
2.3. Quantifying Seeds on the Soil Surface After Planting
To estimate the amount of seed at the soil surface of fields after planting, we used a 1-m2 frame to define plots in recently planted fields and counted all treated seeds visible within the frame (Lopez-Antia et al., 2016). In each field, we sampled 5 plots in a randomly-selected corner and 5 plots in the center as estimated visually from field boundaries. Corner locations were randomly selected by flipping a coin twice. In each field corner, we paced 15 m and 30 m along each edge in an L-shape that had the field corner for a vertex to obtain a total of 5 measurements (i.e., 1 plot at a vertex, 2 plots at 15 m, and 2 plots at 30 m). We hypothesized that seed exposure would be greater at the end of rows where planters turn sharply than within rows. For field centers we paced 15 m in each cardinal direction to sample 5 plots, including the center. We counted all seeds on the soil surface within the frames, as well as documenting seeds observed on the soil surface while walking to plots, to get a better sense of whether our sampling intensity was sufficient to adequately characterize fields. We also recorded any seed spills that we observed in fields during our visits.
Fields included in our field-scale, post-planting surveys were comprised of 3 types; fields managed by the Department of Natural Resources (DNR) and farmed by DNR staff (hereafter, DNR fields), fields on lands managed by DNR but farmed by cooperating, private individuals in Cooperative Farming Agreements (CFAs), and privately-owned, privately-farmed fields (PVT). These surveys required permission to access privately-owned fields and thus private farmers were non-randomly selected by staff as individuals likely to cooperate with the study. We cannot exclude the possibility that farmers with prior knowledge of the study might have changed their seed stewardship behavior, but we attempted to minimize this through our selection of private farmers, and when landowner permission was not required (i.e., CFAs), participants were blind to the study. In 2016, we sampled plots in 10 DNR fields farmed by DNR staff, 36 CFA fields, and 2 PVT fields. In 2017, we sampled 6 CFAs and 17 PVT fields. In 4 cases, we included 2 PVT fields that were planted by the same farmer, but in 3 of these cases, the fields were planted to different crop types, with different planting equipment used for each crop type in cases where the equipment type used was known. Neonicotinoid-treated seed was no longer permitted on DNR-managed land beginning in 2017, but was not enforced in this initial year of implementation, so we continued to sample CFAs in 2017.
2.4. Quantifying Availability of Neonicotinoids on Treated Seeds on the Soil Surface
To estimate how long neonicotinoids may persist on seeds left on the soil surface, we broadcast hundreds of seeds on the soil surface of a tilled field by hand so that the seeds would experience UV, microbial factors, rainfall, and other ambient conditions in northern Minnesota. Experiments were conducted 5 May – 4 June 2016 and 4 May – 3 June in 2017. We exposed seeds to environmental conditions and collected 5–7 seeds of each type after environmental exposure for 0, 1, 2, 4, 8, 16, and 30 days to quantify the decrease of neonicotinoids. We noted daily precipitation and cloud cover during both years of the experiment, and measured exact rainfall amounts (mm) at the site of the experiment in 2017 with an Oregon Scientific RGR126N Wireless Rain Gauge. We conducted the experiment in 2016 and 2017 with 2 types of commercially available corn seed treatments (CLO and TMX) and commercially treated soybeans (IMI and CLO). After field collection, seeds were stored frozen (−18° C or colder) until shipment to SIUC for neonicotinoid analysis.
2.5. Time for Wildlife to Find Spills
We simulated treated seed spills in planted fields to estimate the time it takes for birds to discover spills and to identify wildlife species that consumed treated seeds. We selected CFAs on Wildlife Management Areas with a land cover composition similar to that of the surrounding landscape using the 2014 National Cropland Data Layer (USDA-NASS, 2015) in ArcGIS 10.2 (ESRI, 2015) and the available data on CFAs, which indicated there were 7,420 ac (3,003 ha) of row crops in 341 CFAs in southwest Minnesota and 2,431 ac (984 ha) of row crops in 66 CFAs in northwest Minnesota (M. Benage and J. Williams, respectively, pers. comm.). We prioritized this portion of the study in 2016 because farmers and managers were prohibited from planting neonicotinoid-treated seeds on DNR-managed lands beginning in 2017. In 2016, we placed cameras at simulated spills at 11 CFAs, 3 DNR-farmed fields, and 2 privately-owned fields where we had obtained permission. In 2017, we placed cameras at each simulated spill in 16 CFA fields and 21 privately-owned fields.
Spills were simulated with 1,000 treated corn, soybean, or wheat seeds. Seeds were counted with a SLY Automatic Seed Counter (Zhejiang, China), placed in separate bags, and stored away from sunlight. In 2016, we simulated 13 corn spills and 2 soybean spills. In 2017, we simulated 19 corn spills, 23 soybean spills, and 9 wheat spills. To simulate each spill, we buried a 25.4 × 50.8 cm seedling starter tray in the dirt, filled it with dirt, and placed the seeds in a thin layer on top of the dirt, so that we could account for any seeds that became submerged below the soil.
Camera locations at each site were selected along field edges to minimize risk of theft and to view a simulated seed spill in a recently planted field. Bushnell® Aggressor Trophy Cam HD Cameras (Overland Park, Kansas) were deployed to capture 1 minute of video when triggered by motion. We deployed cameras in each location for 3–6 weeks in 2016 and for 1–3 weeks in 2017, with weekly checks to replace batteries and data cards in 2017 after learning how quickly they needed to be replaced in 2016. Images were viewed to identify species at spills and document time until discovery of spills (i.e., when animals first arrived within 30 cm of a spill) and consumption of seeds by wildlife.
2.6. Analytical procedures for neonicotinoid measurement
Seed samples were ground into a powder and freeze-dried for 48 hours. Approximately 0.01–0.02 g of dry samples were extracted with a mixture of acetone and hexane (1:1; v/v) using sonication. Prior to extraction, a mixture of isotopically labelled surrogate standards, including thiamethoxam-d3, acetamiprid-d3, clothianidin-d3, imidacloprid-d4, and thiacloprid-d4 (purchased from CDN Isotopes, Quebec, Canada), was spiked with seed sample. The extraction was repeated three times (10 min each) and the resulting extracts were combined and concentrated to 20 mL. An aliquot of 1 mL of extract was cleaned through a gel permeation chromatography column (diameter: 1.5 cm; length: 40 cm) packed with 6 g of styrene divinylbenzene beads in a mixture of hexane and dichloromethane (1:1, v/v). The resulting extract was further purified through a 2-g Isolute ammonium silica cartridge. The cartridge was pre-conditioned with 10 mL of hexane and the concentrated extract was loaded and washed with 1.5 mL of hexane (discarded). Neonicotinoid analytes were then eluted with 12 mL of methanol/dichloromethane mixture (6:4, v/v). The final extract was concentrated and spiked with internal standard coumaphous-d10 (CDN Isotopes) prior to instrumental analysis.
Determination of neonicotinoids was conducted on an Agilent 1260 high performance liquid chromatography (HPLC) system interfaced with a 3200 QTrap triple quadrupole/linear ion trap MS (AB Sciex; Toronto, Canada). The HPLC was equipped with a ZORBAX Extended-C18 column (100 × 2.1 mm, 3.5 μm, 80 Å, Agilent Technologies). The mobile phase consisted of methanol (A) and water (B), both spiked with 0.1% formic acid (v/v). The mobile phase flow rate was 200 μL/min and the following gradient was employed: 10% B ramped to 70% B in 11 min (linear) and then ramped to 80% B in 6 min (linear), followed by a linear increase to 90% B in 2 min (held for 1 min) and then a change to 10% B in 1 min (held for 8 min). The MS was equipped with a TurboIonSpray® electrospray ionization (ESI) probe operated in the multiple reaction monitoring (MRM) mode.
2.7. Data analysis
To quantify seed spills at a statewide level, we first calculated the number of spills and the number of acres planted for each crop type in each surveyed township (i.e., a ratio estimator). We then calculated the ratio of sums across townships to calculate the mean Ȓ and variance of Ȓ (varȒ) for the surveyed townships. We scaled up to the statewide level by multiplying these estimates by the number of acres for each crop type (i.e., a constant, A) in Cropland Data Layers for 2016 and 2017 [National Agricultural Statistics Service (USDA-NASS, 2017 and 2018) National Agricultural Statistics Service], and the variance of the statewide estimates were calculated as varȒ*A2. All means are expressed a μ ± SD, except where noted otherwise. Confidence intervals (95%) were determined as μ ± 1.96(SD).
We examined predictors of exposed seeds on the soil surface in field plots after planting using the glmer function for generalized linear mixed models with field as a random effect. Our response variable was binomial (i.e., exposed seeds, or none) because our data were heavily zero-inflated with widely variable seed counts. However, we provide summary statistics of counts. We fit models for binomial responses using the R programming language (R Core Team, 2018) and the packages lme4, gplots, and AICcmodavg. Covariate predictors included seed type (corn or soybean), field type (i.e., DNR-farmed, private, CFA), field size, planting date, and plot location (i.e., field corner, center). We did not include wheat fields because our sample size was small (n = 3). Field size (in ha) was log transformed for a better distribution and planting date was rescaled to improve model convergence. To examine spills in the same fields, we used the glm function because we did not need to include a random effect for replicate plots when spill was the binomial response variable. (Spills were recorded anywhere in the field, not necessarily within plots). Similar predictors were included in models when the binomial response was ‘spill’ with the exception of plot location, which did not apply to spills.
3. Results
3.1. Quantifying Seed Spills at a Landscape Scale
We surveyed 429,269 ac (173,719 ha) in 2016 and 482,720 ac (195,350 ha) in 2017 during the spring planting season. Of the acres surveyed, 258,252 ac (60.2%) in 2016 and 112,389 ac (23.3%) in 2017 had been planted at the time of our surveys and could have had a spill. Planting in 2017 was later than in 2016 due to a very wet spring, with standing water in many fields during the planting season. At the time of our first pass of the road-based surveys in 2016, 79,752 acres (32,274 ha) of corn, 82,300 acres (33,306 ha) of soybeans, 73,205 acres (29,625 ha) of wheat, and 22,995 acres (9,306 ha) of other crops were planted. In 2017, 40,111 acres (16,232 ha) of corn, 23,556 ac (9,533 ha) of soybeans, 33,748 ac (13,657 ha) of wheat, and 14,973 ac (6,059 ha) of other crops were planted during our first pass of the survey. We observed 211 large seed spills that were visible from the road during surveys in 2016 and 117 spills in 2017. In 2016, we documented 33 corn, 120 soybean, and 46 wheat spills, and 4 spills of other crop types, and 8 spills that could not be identified during the first survey. In 2017, we documented 13 corn, 61 soybean, and 23 wheat spills, 3 spills of other crop types, and 1 unidentified spill during the first pass, and in the second pass we discovered 2 corn spills, 13 soybean spills, and 1 unidentified spill. However, spills from the second pass were not included in our spill rate estimates because most planting had been completed weeks prior to the survey. Spill rates in the areas surveyed were calculated as 4 spills/10,000 ac corn, 15 spills/10,000 ac soybeans, 6 spills/10,000 ac wheat, and 2 spills/10,000 ac other crop types in 2016. Spill rates of 3 spills/10,000 ac corn, 26 spills/10,000 ac soybean, 7 spills/10,000 ac wheat, and 2 spills/10,000 ac of other crop types planted were calculated for 2017.
Extrapolating statewide required the assumption that spill rates visible in fields adjacent to roads were representative of spill rates in fields located elsewhere. If spills near roads were more likely to be cleaned up than those less visible to passersby, then this assumption may not have been tenable. Yet, we did not observe spills being cleaned up or covered during our surveys. Furthermore, most spills likely occurred during hopper refilling, and this often occurs near field access points along roads (96% of spills were detected <60 m from field edges), although we detected spills as far as ~200 m from the road based on distances calculated with aerial photos. Thus we think our assumptions were reasonable. Applying our spill rates across the acres farmed statewide (8,450,000 acres of corn, 7,550,000 acres of soybeans, and 1,321,000 acres of wheat were planted in Minnesota during 2016 [National Agricultural Statistics Service (USDA-NASS 2016 Cropland Data Layer); last accessed 5 June 2017 National Agricultural Statistics Service], we estimated 3,496 (95% CI: 1,855 – 5,138) corn seed spills, 11,009 (95% CI: 6,950 –15,067) soybean seed spills, and 830 (95% CI: 160 – 1,500) wheat seed spills statewide in 2016. In 2017, 8,050,000 ac of corn, 8,150,000 acres of soybeans, and 1,160,000 acres of spring wheat were planted (USDA-NASS 2017 Cropland Data Layer; last accessed 5 March 2018 National Agriculture Statistics Service), which scaled up to 2,609 (95% CI: 862 – 4,357) corn seed spills, 21,105 (95% CI: 6,162 – 36,048) soybean seed spills, and 791 (95% CI: 0 – 1,781) wheat seed spills statewide during the planting season. Spills increased as we moved from south to north, and the proportion of fields planted during our surveys also increased as we moved south to north. Importantly, corn and soybeans are the most common crops in the southern part of the surveyed area, and soybeans and wheat are the most common crops in the north.
We collected samples from 107 actual spills of colored seeds on roadsides and right-of-ways, which were comprised of 26 corn, 58 soybean, 22 wheat, and 1 other bean spill. Of these spills, 77 (72%) tested positive for ≥1 neonicotinoid, with IMI being the most commonly detected (33%), followed by CLO (29%), and then TMX (26%). Corn was most commonly treated with TMX or CLO (50% and 62%, respectively), soybean with IMI (50%), TMX (19%), and/or CLO (26%), and wheat with IMI (19%) or TMX (19%). Fifteen spills contained seeds with 2 neonicotinoid treatments. When multiple seed treatments were applied to seeds in spills, TMX and IMI were most commonly applied together to soybean or wheat seeds. Other neonicotinoids (THIA, DIN, ACE, NTP) were not detected on seeds in spills. The geometric mean concentration on spilled seeds treated with each neonicotinoid and collected from roads and right-of-ways was 107.9 ± 7.6 μg/g for IMI (max: 890 μg/g), 85.9 ± 4.6 μg/g for TMX (max: 690 μg/g), and 209.6 ± 4.5 μg/g for CLO (max: 1,120 μg/g).
3.2. Quantifying Seeds on the Soil Surface
We documented exposed seeds on the soil surface in plots at 26 of the 71 fields (7 of 51 corn, 17 of 17 soybean, and 2 of 3 wheat fields) sampled in 2016 and 2017, and we observed spilled seed piles in 5 corn fields, 5 soybean fields, and 2 wheat fields. The average density of exposed seeds on the soil surface of centrally located plots was 0.04 (SE 0.03) corn seeds/m2 (range: 0 – 5, n = 255 center plots), 0.6 (SE 0.2) soybean seeds/m2 (range: 0 – 9, n = 85 center plots), and 7.8 (SE 5.0) wheat seeds/m2 (range: 0 – 69, n = 15 center plots). The density of exposed seeds on the soil surface in corner plots was 0.10 (SE 0.06) corn seeds/m2 (range: 0 – 15, n = 255 corner plots), and 1.5 (SE 0.3) soybean seeds/m2 (range: 0 – 15, n = 85 corner plots), and 8.4 (SE 4.2) wheat seeds/m2 (range: 0 – 51, n = 15 corner plots).
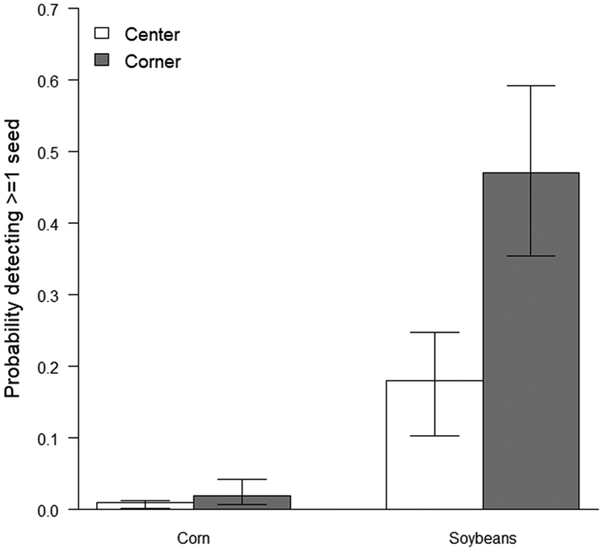
The most-supported model describing whether exposed seeds were detected on the soil surface in plots included additive effects of both field plot location (corner or center) and seed type (corn or soybean). No other competing models were identified with AICc < 2.0 (Table 1). The probability of exposed seeds on the soil surface after planting was higher for soybean fields than for corn fields, and plots in the field corners had a higher probability of seeds on the surface than plots in the center of fields (Figure 2).
Table 1.
Comparison of support for generalized linear mixed models of binomial counts of exposed seeds on the soil surface (response variable) with field as a random effect and field location (corner or center), seed type (corn or soybean), field type (Cooperative Farming Agreement, DNR-planted, or private), field size in ha (log transformed), and survey date as predictors. Seed type and survey date are not in the same model because soybeans are planted after corn. Field type and Log(field size) are not in the same model because private fields are larger than public fields managed for wildlife.
| Model | K | ΔAICc | Wt | Deviance |
|---|---|---|---|---|
| Location + Seed type | 4 | 0.00 | 0.79 | 300.2 |
| Location + Seed type + Field type | 6 | 2.67 | 0.21 | 298.8 |
| Seed type | 3 | 17.52 | 0.00 | 319.7 |
| Log(Field size) + Seed type | 4 | 19.39 | 0.00 | 319.6 |
| Location + Survey date | 4 | 39.13 | 0.00 | 339.3 |
| Location + Field type | 5 | 40.36 | 0.00 | 338.5 |
| Location | 3 | 51.03 | 0.00 | 353.3 |
| Field type + Survey date | 5 | 53.06 | 0.00 | 351.2 |
| Log(Field size) + Survey date | 4 | 54.31 | 0.00 | 354.5 |
| Survey Date | 3 | 56.58 | 0.00 | 358.8 |
| Field type | 4 | 58.01 | 0.00 | 358.2 |
| Log(Field size) | 3 | 67.80 | 0.00 | 370.0 |
Figure 2.
The predicted probability of detecting ≥1 seed exposed on the soil surface after planting in 5 1-m2 plots at the center and corners of corn and soybean fields in Minnesota, USA. Wheat fields were excluded due to small sample sizes (n = 3).
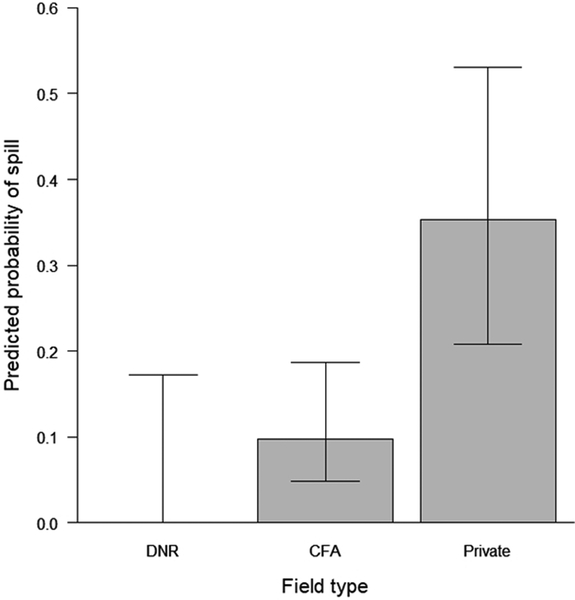
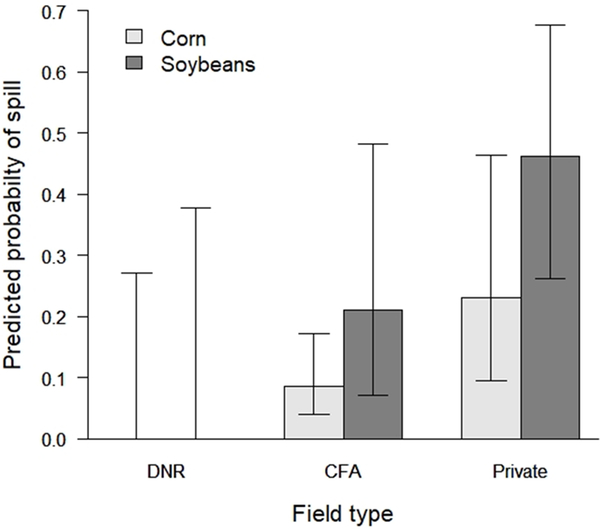
The most-supported model for predicting the probability of a seed spill in the same fields included field type (CFA, DNR, or private, Figure 3a), but 3 other models had AICc < 2.0 (Table 2). Two of these models contained field size, which was correlated with field type, because private fields were larger than fields that were farmed on publicly-owned land. Thus, we considered models with field size to be supported because they captured information already contained in the variable “field type.” The remaining model also contained seed type, but the estimate was imprecise despite seed type having a large effect size (Figure 3b).
Figure 3a.
The predicted probability of a seed spill of sufficient size to be visible from a distance occurring during farming operations in corn and soybean fields based on surveys after planting. Three field types were examined, 1) fields managed and farmed by staff of the Minnesota Department of Natural Resources (DNR), 2) publicly-owned fields farmed by private farmers with their own equipment in cooperative farming agreements (CFAs), and 3) privately-owned and privately-farmed fields.
Table 2.
General linear models of binomial counts of seed spills and the predictors: seed type (corn or soybean), field type (Cooperative Farming Agreement, DNR-planted, or private), field size (log transformed), and survey date as predictors. Because corn is planted earlier than soybeans, survey date and seed type do not occur in the same models. Additionally, because privately owned fields were larger than fields on wildlife areas (DNR<CFA<private), we did not include field type and field size in the same models.
| Model | K | ΔAICc | Wt | Deviance |
|---|---|---|---|---|
| Field type | 3 | 0.00 | 0.22 | 48.29 |
| Log(Field size) | 2 | 0.15 | 0.21 | 50.63 |
| Log(Field size) + Seed type | 3 | 0.21 | 0.20 | 48.50 |
| Field type + Seed type | 4 | 0.73 | 0.16 | 46.76 |
| Field type + Survey date | 4 | 2.17 | 0.08 | 48.20 |
| Log(Field size) + Survey date | 3 | 2.34 | 0.07 | 50.63 |
| Seed type | 2 | 2.83 | 0.05 | 53.31 |
| Survey date | 2 | 6.30 | 0.01 | 56.78 |
Figure 3b.
The predicted probability of a seed spill of sufficient size to be visible from a distance occurring during farming operations in corn and soybean fields based on surveys after planting. Three field types were examined, 1) fields managed and farmed by staff of the Minnesota Department of Natural Resources (DNR), 2) public fields farmed by private farmers with their own equipment in cooperative farming agreements (CFAs), and 3) privately-owned and privately-farmed fields.
3.3. Quantifying Availability of Neonicotinoids on Treated Seeds on the Soil Surface
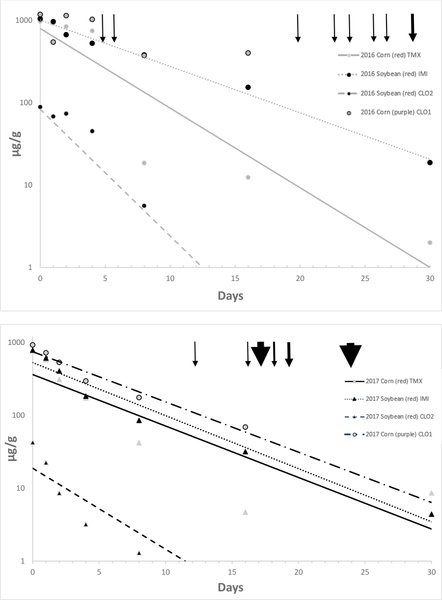
Neonicotinoids decreased on the surface of seeds quickly in both years, although initial concentrations were lower the second year after storage in an unheated outbuilding. The half-life of IMI was the longest, followed by TMX, and then CLO (Table 3). In 2016, rain fell on days 5, 6, 20, 23, 24, 26, 27, and 29 of the experiment, with sunny conditions dominating for 17 of the 30 days (Figure 4a). In 2017, rain fell on days 12, 13, 16, 17, 18, and 19, with sunny conditions dominating on 20 of the 30 days (Figure 4b). Concentrations exceeding 10 μg/g were present on all seeds after 16 days, and on IMI treated seeds after 30 days in 2016. We did not have a 30 day sample for CLO treated seeds in 2016 because no seeds remained on the soil surface, presumably due to wildlife consumption. In 2017, concentrations exceeding 10 μg/g were detected on IMI treated soybeans after 16 days and on CLO treated corn after 30 days. All treated seeds had low but detectable concentrations of neonicotinoids after 30 days in 2017, except CLO that was applied as a 2nd treatment on IMI-treated soybeans.
Table 3.
Half-lives (t1/2), empirical rate constants (k), and equations for changes in neonicotinoid concentrations on soybean treated with imidacloprid (IMI) as the primary treatment and clothianidin as a second treatment (CLO2), and corn with either thiamethoxam (TMX) or clothianidin (CLO1). Seeds were placed on the soil surface in the environment for 30 days during May – June in 2016 and 2017 in Minnesota, USA. Equations for CLO1 in 2016 are not provided because no seeds remained on the soil surface after 30 days.
| Chemical | Seed type | Seed color | t1/2 (days) |
k | Equation, R2 both years | Equation, R2 2016 |
Equation, R2 2017 |
|---|---|---|---|---|---|---|---|
| IMI | soybean | red | 4.7 | 0.149 | 729.66e−0.149× R2 = 0.88 | 1006.6e−0.13× R2 = 0.99 | 528.92e−0.168× R2 = 0.97 |
| TMX | corn | red | 3.6 | 0.193 | 536.89e−0.193× R2 =0.80 | 794.66e−0.223× R2 = 0.86 | 362.73e−0.163× R2 = 0.76 |
| CLO1 | corn | purple | 2.0 | 0.352 | 2195.8e−0.352× R2 = 0.85 | NA | 689.17e−0.138× R2 = 0.98 |
| CLO2 | soybean | red | 2.3 | 0.305 | 39.464e−0.305× R2 =0.86 | 82.923e−0.354× R2 = 0.86 | 18.782e−0.256× R2 = 0.96 |
Figure 4a,b.
Concentrations of neonicotinoid seed treatments on seeds left on the soil surface in northern Minnesota, United States for 0–30 days during May and early June 2016 (a, top panel) and 2017 (b, bottom panel). Clothianidin (CLO1) was a primary treatment on corn; imidacloprid (IMI) was a primary treatment on soybean, with clothianidin (CLO2) as a secondary treatment on the same seeds; and thiamethoxam (TMX) was a primary treatment on corn. Rainfall is indicated with arrows at the top, with drizzle and light rain indicated by a thin arrow and more substantial rain events indicated with a thick arrow. In 2017, we measured exact rainfall amounts (mm) and the amount of rainfall received (1–8 mm) is indicated by the size of the arrowhead. None of the rain events during the study would be expected to produce run-off.
3.4. Time for Wildlife to Find Spills
We reviewed images collected by trail cameras at simulated spills during spring 2016 (n = 12,602 videos) and 2017 (n = 39,653 videos). We documented ring-necked pheasants (Phasianus colchicus), Canada geese (Branta canadensis), American crows (Corvus brachyrhynchos), mourning doves (Zenaida macroura), wild turkeys (Meleagris gallapavo), blue jays (Cyanocitta cristata), brown thrasher (Toxostoma rufum), black-billed magpie (Pica hudsonia), rose-breasted grosbeak (Pheucticus ludovicianus), various species of sparrows (Emberizidae) and blackbirds (Icteridae), as well as white-tailed deer (Odocoileus virginianus), black bears (Ursus americanus), raccoons (Procyon lotor), rodents (mice and 3 species of squirrels), Eastern cottontails (Sylvilagus floridanus), white-tailed jackrabbits (Lepus townsendii), red fox (Vulpes vulpes), striped skunk (Mephitis mephitis), and domestic cat (Felis catus) consuming treated seeds. The average time for birds to find spills (observed within 30 cm of a spill, but not necessarily consuming seeds) was 1.5 days (range 0 – 8 days, n = 25 spills) for corn, 0.9 days (range 0 – 3 days, n = 18 spills) for soybean, and 0.9 days (range 0 – 3 days, n = 7 spills) for wheat spills. The average time after a spill was established that birds were first observed to consume seeds at spills was 4.9 days (range 1 – 11, n = 15 spills) for corn, 5.0 days (range 0 – 11, n = 6 spills) for soybean, and 1.8 days (range 0 – 7 days, n = 6 spills) for wheat. The average time after a spill was established that mammals were first observed to consume seeds was 1.9 days (range 0 – 6, n = 22) for corn, 2.5 days (range 0 – 9, n = 20) for soybean, and 2.0 days (range 0 – 8, n = 5) for wheat.
4. Discussion
We found that neonicotinoid-treated seed is common on the landscape during the spring planting season, both as seeds available on the soil surface and in seed spills. To our knowledge, this is the first study to document landscape-scale availability of neonicotinoid treated seed spills during the planting season, and the first to document availability of treated seeds on the soil surface after planting in North America. Furthermore, we also document that although neonicotinoids decrease rapidly under environmental conditions, wild birds and mammals find treated seeds at spills and consume the seeds within days, while chemical is still abundant on the seeds. Thus, wildlife may be exposed to doses of neonicotinoids that could potentially have sub-lethal or lethal effects. Our findings not only refute the idea that wild animals will not eat treated seeds, but unfortunately document that good seed stewardship practices were not always followed, despite clear warnings about dangers to wildlife on product labels.
Importantly, better seed stewardship could reduce the availability of neonicotinoid treated seeds on the landscape in the spring. We directly observed hundreds of large spills during our surveys, and estimated tens of thousands more spills occurring statewide, yet we never observed these spills being cleaned up or covered during our surveys. A seed spill large enough to be visible from the road is usually composed of thousands of seeds. Cleaning or covering spills could reduce the availability of seeds to wildlife. Chemical analysis of actual seed spills we found in our landscape-scale surveys had varying concentrations that would suggest that some spills had been left in fields for at least 5 days and not cleaned up or covered.
Outreach to farmers seems to indicate that many farmers do not read the product labels and are unaware of the dangers to wildlife (C. Roy, pers. comm.). This is borne out in our field-level sampling as well, with much higher spill rates in privately-owned, privately-farmed fields than publicly-owned fields managed by the DNR regardless of farmer (i.e., public staff or private citizen). Private farmers planting public lands with their own equipment had fewer spills than private farmers farming their own fields, which might indicate that wildlife awareness and a perceived expectation of seed stewardship may have impacted the number of spills left in fields (e.g., more careful hopper filling, refilling, or cleaning/covering spilled seed). Importantly, private farmers farming public land were blind to our study, but because we needed landowner permission to survey fields on private farms, farmers on private lands were aware of the study and our field visits. If this awareness influenced the number of seed spills on private farms, it was not obvious in the direction we predicted (i.e., fewer spills). However, private fields surveyed in our study were larger than fields on public lands, and thus activities that lead to spills (e.g., refilling hoppers) may have been necessary more often on these larger fields. Fields with spills tended to be larger than fields without spills but the difference was not statistically significant (155.6 ± 191.1 vs 72.3 ± 137.9 ha, t = −1.4, P = 0.09). Regardless, we suggest that educating farmers about the importance of good seed stewardship could produce meaningful reductions in the seed available on the landscape in the spring. No spills were left on fields farmed by DNR staff, but staff were acutely aware of the dangers to wildlife and prioritized wildlife over other objectives.
Seeds available on the soil surface after planting were also very common, yet less likely to be easily improved through better seed stewardship practices. Although our estimated seed densities on the soil surface were an order of magnitude lower for corn and soybeans than that reported for winter cereals by Lopez-Antia et al. (2016; 11.3 ± 1.2 seeds/m2 in field centers and 43.4 ± 5.5 seeds/m2 in the corners), they were still much higher than we anticipated with modern planting equipment for important row crops in the Midwestern United States. Winter cereal is normally standard drilled whereas corn and soybeans are precision drilled, and precision drilling produces fewer seeds on the soil surface (de Snoo and Luttik 2004).
The differences we found in seed availability between the corners and centers of fields would support the interpretation that the equipment is more efficient at drilling seeds into the soil when moving straight along rows than when turning along the field edges, just as Lopez-Antia et al. (2016) reported for winter cereals seeds (i.e. wheat, oats, barley, and triticale seeds). de Snoo and Luttik (2004) reported the percentage of seeds on the soil surface of headlands was 3.5 times higher than in field centers for 8 different crops in The Netherlands. Innovations or farming practices targeted toward more efficient drilling of seeds at the end of rows might reduce the availability of seeds for wildlife. Importantly, many edge-dependent wildlife species (e.g., ring-necked pheasants, turkeys, white-tailed deer) tend to concentrate their activities nearer to field edges where seed is more available than in the center of fields.
Seed type was supported in statistical models of seed availability on the soil surface, with soybean being much more probable at the soil surface than corn. Corn is seeded at a lower rate than soybean; optimal corn seeding rates in Minnesota are 34,000–36,000 seeds per acre (University of Minnesota Extension Corn Seeding Rates), whereas optimal soybean seeding rates are 140,000 seeds per acre in southern MN, and 140,000–170,000 seeds per ac in central and northwestern MN (University of Minnesota Extension Soybean Seeding Rates). Corn is also planted deeper than soybean seeds (University of Minnesota Crop Production). Corn can have poor nodal root development at shallow depths, but soybean does not require deep planting for proper root development. Differences in seeding rates and planting depth between crops likely explain the difference in availability on the soil surface after planting. Spring wheat is sown at still higher seeding rates (1,300,000–1,400,000 seeds per acre) and can be planted shallowly, with deep seeding producing problems with emergence and vigor (University of Minnesota Extension Small Grain Seeding Rates). For these reasons, we would expect high wheat seed availability on the soil surface relative to corn, and our limited sample size seemed to support this.
We suspect that the probability of seeds on the soil surface was also influenced by the type of equipment used for sowing (Lopez-Antia et al., 2016), although we did not collect data on the type of planting equipment used. Planting at high speeds can also impact seed placement in the soil and increase necessary seeding rates (University of Minnesota Extension Planting Cautions). Because improper seed placement in the soil also produces fewer plants, farmers, in addition to wildlife, also benefit from proper seed placement in the soil. Nevertheless, some wildlife species [e.g, sandhill cranes (Antigone canadensis), greater prairie-chickens (Tympanuchus cupido), ring-necked pheasants] have been observed digging or pecking near the soil surface for newly planted seeds and/or foraging on new seedlings with the seed still attached (unpublished reports, numerous DNR Wildlife). Therefore, even the best seed stewardship and planting practices will not eliminate all seeds available to wildlife.
Seeds collected from spills had highly variable concentrations of neonicotinoids. Importantly, some seeds had concentrations below the detection limit, indicating that the seeds had treatments other than neonicotinoids (e.g., fungicides) for which we did not test. Furthermore, in many cases concentrations on seeds were well below the usual application rate, indicating either that the chemicals had leached off the seed surface (Smalling et al. 2018), decreased after exposure to environmental conditions, or that seeds might have been stored after purchase in previous years; chemical concentrations on seeds decreased by more than 20–50% in cold storage during the year between our 30-day seed experiments. Leaching of chemicals off the seed surface might occur after heavy rainfall events, however, during our 30-day seed experiments to measure changes in concentrations of neonicotinoids, rainfall events (usually drizzle or a light rain, and rarely more substantial) often occurred in between sampling dates. We did not detect a strong reduction in chemical concentrations as a result of rainfall during our experiment, however, more frequent collection of seeds would allow more precise quantification of rainfall impacts on concentrations In summary, the factors affecting concentrations on seeds, in combination with seeds being exposed for varying amounts of time before discovery, makes it difficult to quantify doses that wildlife might ingest at actual seed spills.
Our study had several limitations that may have impacted our conclusions to some extent. First, we may have underestimated seed availability on the soil surface by not sampling at a high enough intensity. We observed many seeds on the soil surface in fields while walking to plots that were not captured by data from our 10 plots per field; 21% of surveyed fields had seeds detected incidentally outside plots but none detected within plots. We recommend future studies use a higher sampling intensity to obtain more precise estimates. Additionally, our landscape-level seed spill estimates assumed that spill rates in fields adjacent to public roads were similar to rates in fields non-adjacent to public roads. We believe this assumption was reasonable because fields non-adjacent to roads are commonly accessed through privately-owned access roads, and we would expect similar spill rates near these access points because hoppers need to be refilled in these fields as well. However, if this assumption was not reasonable, then we may have overestimated the number of spills at the landscape level.
A second possible limitation was that nearly half of our cameras were placed on Wildlife Management Areas, and although we tried to select sites that were similar in landscape composition to the surrounding landscape (e.g., small WMAs in an agricultural matrix), wildlife may have been using these fields more often than other fields. If true, wildlife may have found spills on public lands more quickly than they might elsewhere. To examine this possibility, we compared the time to find spills on public land to the time to find spills on private lands. All simulated spills at both public and private fields were discovered by wildlife. Birds did find spills (defined as approaching the spill to within ~30 cm) slightly sooner on public land than on private land (1.0 vs 1.6 days, Z = −2.1, P = 0.03), but the time to first consumption of seed was not different on public (3.8 days) and private land (4.5 days, Z = −0.6, P = 0.6, Wilcoxon-Mann-Whitney Test with package coin in R). Importantly, a delay in consumption shortly after a spill happens would make a much larger difference in the amount of neonicotinoid seed treatment remaining on seeds than a similar difference in the flatter part of the decrease curve (Figure 4). However, when consumed, seeds left on the soil surface still had large concentrations of chemicals that could affect wildlife.
Another important limitation of our study was that knowledge of our visits may have influenced seed stewardship behavior. However, farmers with prior knowledge of our visits to examine seeds on the surface after planting were more likely to have spills, which was opposite to our concern that they would be more careful. We think it unlikely that seed stewardship behavior would be modified in the direction of waste and noncompliance with the law (i.e., FIFRA 1996), even if these farmers were cooperative with the DNR. Thus we think that despite these limitations, our study provides an important first look at neonicotinoid-treated seed availability for wildlife in the Midwestern United States.
Most of the previous research concluding that these chemicals are safe for wildlife are based on captive studies, but bird behavior in captivity does not necessarily replicate or resemble bird behavior in the wild. For example, captive red-winged blackbirds (Agelaius phoeniceus) given a choice between imidacloprid-treated rice and untreated rice chose untreated seeds more often (Avery et al., 1994), similar to red-legged partridges given a choice between imidacloprid-treated wheat and untreated wheat (Lopez-Antia et al. 2014), but wild birds are not presented with a side-by-side choice of food items. When red-legged partridges were presented with more unpredictable situations (i.e., more feeders to search as is more similar to field situations), treated seeds were consumed at higher rates (Lopez-Antia et al. 2014). Furthermore, red-winged blackbirds presented rice with 3 different treatment doses avoided only the highest treatment doses, but did consume imidacloprid-treated seeds at levels that produced ataxia and temporary illness (Avery et al. 1994), which Lopez-Antia et al. (2014) suggested avoidance occurred through post-ingestion distress in their study. Ataxia and temporary illness (Avery et al., 1993, Avery et al., 1994) to treated seed consumption could impair a bird’s ability to escape predators and survive in the wild. Food availability and energy requirements are also unlikely to be similar between captive and field conditions, because wild animals must search for food, compete with conspecifics and heterospecifics, reproduce, and avoid predators. In one captive study where predators could attack but not reach red-winged blackbirds, the birds preferred to forage on treated seed nearer to cover than to forage on untreated seed farther from cover (Avery et al., 1994). In the same experiment, consumption of treated seeds was higher during colder temperatures, presumably due to increased food requirements (Avery et al., 1994). Wild birds likely have many additional factors influencing energetic requirements like reproductive behaviors, vigilance, and escape behavior that might impact their food choices.
Conclusions
This research provides evidence that treated seeds are consumed by wildlife, that seeds are always drilled below the soil surface and are thus not available for wildlife, and that packaging labels are sufficient to protect wildlife from seed spills. Seeds are abundant and widely available on the soil surface for wildlife consumption during the spring planting season. Soybeans were the most common seed available for consumption by wildlife on the soil surface and in seed spills, and is a seed type on which imidacloprid is still used in the United States. Imidacloprid is more toxic to vertebrate and invertebrate animals than other neonicotinoids. Corn and wheat spills were also documented in our study and due to their widespread agricultural importance and consumption by wildlife, may pose a substantial route of wildlife exposure to neonicotinoids. If the widespread-availability of treated seeds on the soil surface and in seed spills is not considered in pesticide risk assessments, they could pose a risk for sub-lethal and lethal effects to wildlife. We are exploring wildlife consumption of treated seeds in ongoing field research, but more field studies are needed.
Acknowledgments
Funding was provided by the Minnesota Environment and Natural Resources Trust Fund and the Minnesota Department of Natural Resources. We are thankful to the landowners that granted access to their crop fields after planting. We thank J. Markl, W. Schuna, R. Markl, N. Trauba, J. Stangel, S. Innvaer, C. Vacek, B. Olson, R. Baden, M. Palm, R. Prachar, E. Hutchins, D. Pietruszewski, J. Williams, and J. Parson for assisting with field planting information. We would like to thank Glacial Ridge National Wildlife Refuge, Talcot Lake Wildlife Management Area, and Roseau River Wildlife Management Area for accommodating technicians during field work. We would like to thank J. Markl, M. Palm, and A. Killian for acquiring seed. T. Fields, A. Mosloff, R. Kreb, and M. Zagorski surveyed for seed spills. R. Wright assisted with DNRSurvey. L. Gilbert provided clerical support. M. Larson provided comments that improved this manuscript.
This research does not reflect the official positions and policies of the United States Environmental Protection Agency (US EPA). Mention of products/trade names does not constitute recommendation for use by US EPA.
This work was funded by the Minnesota Department of Natural Resources Wildlife Restoration (Pittman-Robertson) Program Grant and the Minnesota Environment and Natural Resources Trust Fund.
References
- Anon 2007. Canadian water quality guidelines: imidacloprid. Canadian Council of Ministers of the Environment; ISBN 978-1-896997-71-1. [Google Scholar]
- Avery ML, Decker DG, Fischer DL, Stafford TR, 1993. Responses of captive blackbirds to a new insecticidal seed treatment. J. Wildl. Manage 57(3):652–656. [Google Scholar]
- Avery ML, Decker DG, Fischer DL, 1994. Cage and flight pen evaluation of avian repellency and hazard associated with imidacloprid-treated rice seed. Crop Protection 13(7):535–540. [Google Scholar]
- Avery ML, Fischer DL, Primus TM, 1997. Assessing the hazard to granivorous birds feeding on chemically treated seeds. Pestic. Sci 49:362–366. [Google Scholar]
- Bass C, Denholm I, Williamson MS, Nauen R, 2015. The global status of insect resistance to neonicotinoid insecticides. Pesticide Biochem Physiol. 121:78–87. [DOI] [PubMed] [Google Scholar]
- Bean TG, Gross MS, Karouna-Renier NK, Henry PFP, Schultz SL, Hladik ML, Kuivila KM, Rattner BA 2019. Toxicokinetics of imidacloprid-coated wheat seeds in Japanese quail (Coturnix japonica) and an evaluation of hazard. Environ. Sci Tech. DOI: 10.1021/acs.est.8b07062 [DOI] [PubMed] [Google Scholar]
- Berny PJ, Buronfosse F, Videmann B, Buronfosse T, 1999. Evaluation of the toxicity of imidacloprid in wild birds. A new high performance thin layer chromatography method for the analysis of liver and crop samples in suspected poisoning cases. J. Liq. Chromatogr. Relat. Technol 22:1547–1559. [Google Scholar]
- Bonmatin JM, Giorio C, Girolami V, Goulson D, Kreutzweiser DP, Krupke C, Liess M, Long E, Marzaro M, Mitchell EA, Noome DA, Simon-Delso N, Tapparo A 2015. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res 22:35–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Lydy M, Fell R, Anderson T, Chen D, 2014. An analytical method for detection of seven neonicotinoids in honey bees. Society for Toxicology and Chemistry 35th Annual Meeting, Nov 7–11, Vancouver, BC, Canada. [Google Scholar]
- Cox C 2001. Insecticide factsheet: imidacloprid. J. Pestic. Reform 21:15–21. [Google Scholar]
- de Leeuw J, Gorree M, de Snoo GR, Jamis WLM, van der Poll RJ, Luttik R, 1995. Risks of granules of treated seeds to birds on arable fields. GML report no. 118. Centre of Environmental Science, Leiden University, Leiden, ISSN 1381–1703. [Google Scholar]
- de Snoo GR, Scheidegger NMI, de Jong FMW, 1999. Vertebrate wildlife incidents with pesticides: a European survey. Pestic. Sci 55:47–54. [Google Scholar]
- De Snoo GR, Luttik R 2004. Availability of pesticide-treated seed on arable fields. Pest Manage Sci 60:501–506. [DOI] [PubMed] [Google Scholar]
- Douglas MR, Rohr JR, Tooker JF, 2015. Neonicotinoid insecticide travels through a soil food chain, disrupting biological control of non-target pests and decreasing soya bean yield. J. Appl. Ecol 52:250–260. [Google Scholar]
- Eng ML, Stutchbury BJM, Morrissey CA, 2017. Imidacloprid and chlorpyrifos insecticides impair migratory ability in a seed-eating songbird. Scientific Reports 7:15176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertl HM, Mora MA, Boellstorff DE, Brightsmith D, Carson K, 2018. Potential effects of neonicotinoid insecticides on northern bobwhites. Wildl. Soc. Bull 10.1002/wsb.921 [DOI] [Google Scholar]
- ESRI. 2015. ArcGIS Desktop: Release 10.3. Redlands, CA: Environmental Systems Research Institute. [Google Scholar]
- Feare CJ, Dunnet GM, Patterson IJ, 1974. Ecological studies of the rook (Corvus frugilegus L) in North-East Scotland: Food intake and feeding behavior. J. Appl. Ecol 11:867–896. [Google Scholar]
- Fletcher MR, Hunter K, Barnett EA, 1995. Pesticide poisoning of animals 1994: Investigations of suspected incidents in the United Kingdom, MAFF Publications, London, UK. [Google Scholar]
- Gibbons D, Morrissey C, Mineau P, 2014. A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environ. Sci. Pollut. Res. Int, 1–16 DOI 10.1007/s11356-014-3180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig-Smith PW 1987. Hazards to wildlife from pesticide seed treatments In: Martin T (ed.) Application to Seeds and Soil, British Crop Protection Council, Thornton Heath, pp. 127–134. [Google Scholar]
- Goulson D, 2013. An overview of environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol 50:977–987. [Google Scholar]
- Goulson D, Nicholls E Botías C, Rotheray EL, 2015. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347:1255957-1-9. [DOI] [PubMed] [Google Scholar]
- Jeschke P, Nauen R, Schindler M, Elbert A, 2011. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 59:2897–2908. [DOI] [PubMed] [Google Scholar]
- Krupke CH, Hunt GJ, Eitzer BD, Andino G, Given K, 2012. Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS One, 7, e29268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Antia A, Ortiz-Santaliestra ME, Mougeot F, Mateo R, 2013. Experimental exposure of red-legged partridges (Alectoris rufa) to seeds coated with imidacloprid, thiram, and difenoconazole. Ecotoxicol. 22:125–138. [DOI] [PubMed] [Google Scholar]
- Lopez-Antia A, Ortiz-Santaliestra ME, Mateo R, 2013. Experimental approaches to test pesticide-treated seed avoidance by birds under a simulated diversification of food sources. Sci. Total Environ. 496:179–187. [DOI] [PubMed] [Google Scholar]
- Lopez-Antia A, Ortiz-Santaliestra ME, Mougeot F, Mateo R, 2015. Imidacloprid-treated seed ingestion has lethal effect on adult partridges and reduces both breeding investment and offspring immunity. Environ. Research 136:97–107. [DOI] [PubMed] [Google Scholar]
- Lopez-Antia A, Feliu J, Camarero PR, Ortiz-Santaliestra ME, Mateo R, 2016. Risk assessment of pesticide seed treatment for farmland birds using refined field data. J. Appl. Ecol Doi: 10.1111/1365-2664.12668 [DOI] [Google Scholar]
- Main AR, Headley JV, Peru KM, Michel NL, Cessna AJ, Morrissey CA, 2014. Widespread use and frequent detection of neonicotinoid insecticides in wetlands of Canada’s Prairie Pothole Region. PLoS One 99(3):e92821 doi: 10.1371/journal.pone.0092821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millot F, Decors A, Mastain O, Quintaine T, Berny P, Voy D, Lasseur R, Bro E, 2017. Field evidence of bird poisonings by imidacloprid-treated seeds: a review of incidents reported by the French SAGIR network from 1995 to 2014. Environ Sci Pollut Res 24:5469–5485 doi: 10.1007/s11356-016-8272-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnesota Department of Agriculture. 2014. Scoping a review of neonicotinoid use, registration and insect pollinator impacts in Minnesota. Draft 12 pp. [Google Scholar]
- Minnesota Department of Transportation (DOT). 2008. Minnesota DOT Roads. hyperlink to DOT site http://www.dot.state.mn.us/maps/gisbase/html/datafiles.html.
- Mineau P, Palmer C, 2013. The impact of the nation’s most widely used insecticides on birds. American Bird Conservancy, USA. [Google Scholar]
- Morrissey C, Mineau P, Devries JH, Sanchez-Bayo F, Liess M, Cavallaro MC, Liber K, 2015. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environ. International 74:291–303. [DOI] [PubMed] [Google Scholar]
- Murton RK, Isaacson AJ, Westwood NJ, 1963. The feeding ecology of the woodpigeon. British Birds 503–517. [Google Scholar]
- Pascual JA, Hart ADM, Sanders PJ, McKay HV, Kilpatrick J, Prosser P, 1999. Agricultural methods to reduce the risk to birds from cereal seed treatments on fenlands in eastern England. I. Sowing depth. Agric. Ecosyst. Environ 72:59–73. [Google Scholar]
- Ponce C, Alonso JC, Argandona G, García Fernández A, Carrasco M, 2010. Carcass removal by scavengers and search accuracy affect bird mortality estimate at power lines. Anim. Conserv 13:603–612. [Google Scholar]
- R Core Team. 2018. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: R-project. [Google Scholar]
- Rexrode M, Barrett M, Ellis J, Gabe P, Vaughan A, Felkel J, Melendez J, 2003, EFED Risk Assessment for the seed treatment of Clothianidin 600FS on corn and canola. U.S. EPA, Washington, D. C. [Google Scholar]
- Sanchez-Bayo F, 2014. The trouble with neonicotinoids: chronic exposure to widely used insecticides kills bees and many other invertebrates. Science 346:806–807. [DOI] [PubMed] [Google Scholar]
- SERA., 2005. Imidacloprid-human health and ecological risk assessment-final report Report from Syracuse Environmental Research Associates to USDA, Forest Service. [Google Scholar]
- Smalling KL, Hladik ML, Sanders CJ, Kuivala KM 2018. Leaching and sorption of neonicotinoid insecticides and fungicides from seed coatings. J. Environ. Sci. Health, Pt B 53:176–183. 10.1080/03601234.2017.1405619 [DOI] [PubMed] [Google Scholar]
- Stevens DL, Olsen AR, 1999. Spatially restricted surveys over time for aquatic resources. J. Agric. Biol. Environ. Stat 4, 415–428. [Google Scholar]
- Stokstad E, 2013. How big a role should neonicotinoids play in food security? Science 340:675. [DOI] [PubMed] [Google Scholar]
- Tokumoto J, Danjo M, Kobayashi Y, Kinoshita K, Omotehara T, Tatsumi A, Hashiguchi M, Sekijima T, Kamisoyama H, Yokoyama T, Kitagawa H, Hoshi N, 2013. Effects of exposure to clothianidin on the reproductive system of quails. J. Vet. Med. Sci 75:755–760. [DOI] [PubMed] [Google Scholar]
- Tomizawa M, Casida JE, 2005. Neonicotinoid insecticide toxicology: mechanisms of selective action. Ann. Rev. Pharmacol. Toxicol 45:247–268. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture National Agricultural Statistics Service. 2014 Cropland Data Layer. 2015. Published crop-specific data layer [Online]. Available at (http://nassgeodata.gmu.edu/CropScape/).
- U.S. Department of Agriculture National Agricultural Statistics Service. 2016 Cropland Data Layer. 2017. Published crop-specific data layer [Online]. Available at (http://nassgeodata.gmu.edu/CropScape/).
- U.S. Department of Agriculture National Agricultural Statistics Service. 2017 Cropland Data Layer. 2018. Published crop-specific data layer [Online]. Available at (http://nassgeodata.gmu.edu/CropScape/).
- Wright RG, Haroldson BS, Pouliot C, 2015. DNRSurvey – Moving map software for aerial surveys. https://www.dnr.state.mn.us/mis/gis/DNRSurvey/DNRSurvey.html