Abstract
This case report describes the first endoscopic submucosal dissection performed at Baylor University Medical Center for high-grade dysplasia of the stomach. The patient, a 76-year-old man with multiple medical comorbidities, presented with 4 months of abdominal pain. Endoscopy found a mass on the lesser curvature, and endoscopic ultrasound confirmed that it did not invade the submucosa. A complete endoscopic resection was performed, alleviating the need for surgical intervention. Endoscopic submucosal dissection offers a minimally invasive treatment for premalignant lesions and early stage cancers of the stomach. Endoscopic resection is as effective as gastrectomy, with less morbidity in select patients with early stage lesions.
Keywords: Endoscopic submucosal dissection, gastric cancer, high-grade dysplasia, upper endoscopy
Gastric cancer is the second leading cause of cancer-related mortality worldwide.1,2 Incidence rates vary widely by gender, race, and geographic distribution. Gastric cancer has historically been discovered late, leaving gastrectomy as the only potentially curative treatment. Countries with a high incidence of gastric cancers, such as Japan, implemented screening programs for earlier detection of the disease, as well as high-risk lesions such as high-grade dysplasia.3 This led to the exploration of less aggressive treatment options for high-risk lesions and early stage gastric cancer confined to the mucosa. Endoscopic submucosal dissection (ESD), the most accurate procedure to remove these lesions, was first described 17 years ago.4 This is a description of the first ESD performed at Baylor University Medical Center at Dallas.
CASE DESCRIPTION
A 76-year-old man with coronary artery disease, chronic obstructive pulmonary disease, ischemic cardiomyopathy, iron-deficiency anemia, and diabetes mellitus initially presented to an outside facility 4 months earlier due to epigastric pain. An upper gastrointestinal series detected a gastric filling defect. Subsequent endoscopy found a 2-cm polyploid mass on the lesser curvature. Biopsy revealed high-grade dysplasia without involvement of the submucosa. An endoscopic ultrasound was performed that showed no invasion into the deep mucosa, and the lesion was staged as T1A. Given the patient’s significant medical comorbidities, an endoscopic approach was attempted.
The patient was taken to the operating room for ESD, because the lesion was too large to undergo an endoscopic mucosal resection. Endoscopic mucosal resection is an injection-assisted, cap-assisted, or band-assisted resection of superficial lesions (generally <1 cm), whereas ESD is a free-hand endoscopic dissection of the submucosa to obtain an en bloc resection of larger lesions. There is a risk of perforation with ESD. Due to this risk, this case was performed in the operating room so that anesthesiologists and surgeons would have all of their tools at their disposal should this occur, despite this patient being a poor surgical candidate.
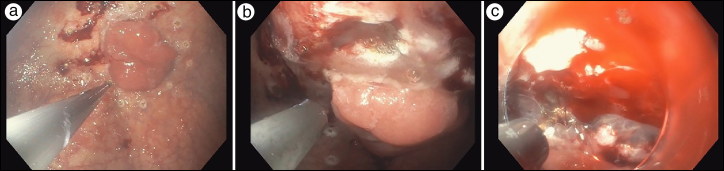
The first step of the ESD was a diagnostic upper endoscopy, where contents of the esophagus and stomach were irrigated and suctioned clear. The tumor was visualized on the lesser curvature and outlined with cautery (Figure 1a). A mixture of saline, methylene blue, and epinephrine was injected into the submucosal space using an endoscopic needle to separate the mucosal lesion from the gastric muscle by expanding the submucosal space. A mucosotomy was performed just proximal to the lesion, outside the previous marks that were outlined with cautery (Figure 1b) using the cut function on the energy device.
Figure 1.
(a) Outlining the lesion with cautery. (b) Mucosotomy. (c) Submucosal tunneling.
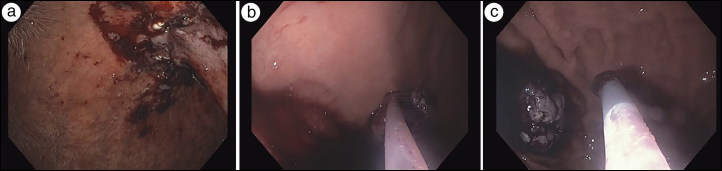
With the aid of a dissection cap, the endoscope was maneuvered through the mucosotomy and into the submucosal space (Figure 1c). Electrocautery with a triangle-tip knife was used to create a longitudinal tunnel between the mucosa and the muscularis propria circumferentially so that the lesion would be resected in its entirety (Figure 2a). To provide countertension, a dual-channel scope was used to insert a second endoscopic tool, which retracted the lesion away from the gastric muscle. Once the lesion was resected, a Roth net was inserted to remove the lesion in its entirety (Figure 2b). Given the patient’s previous epigastric pain, we approximated the mucosal using a combination of clips and figure-of-eight sutures (Figure 2c). The patient was kept on twice-daily proton pump inhibitor, nightly H2 blocker, and three-times-daily sucralfate for a month to prevent gastric ulcer formation. On postoperative day 1, the patient tolerated clear liquids and was discharged home. Histopathologic examination of the specimen revealed a lesion with high-grade dysplasia and negative margins. The patient returned 6 months later for an upper endoscopy, which revealed no recurrence.
Figure 2.
(a) Complete resection of the lesion. (b) Removal via Roth net. (c) Closure.
DISCUSSION
Resection is the only potentially curative treatment for gastric cancer and high-risk lesions associated with it. This has historically been carried out via gastrectomy. However, gastrectomy carries risks such as leaks, infection, and strictures. Our patient was also a poor candidate for surgery due to his medical comorbidities. ESD was determined to be the ideal first-line treatment due to decreased morbidity and mortality. High-risk lesions and early stage gastric cancer are amenable to endoscopic resection because there is little risk of lymphatic spread given the depth of invasion.
A highlight of ESD is its use of the submucosal tunneling technique. This involves the creation of a mucosal flap both proximal to and slightly offset from the lesion. The mucosal flap offers a considerable technical advantage; it allows for improved visual identification of blood vessels, submucosal tumors, and the layers of the stomach. The mucosal flap further enhances the safety of the surgery because its design inherently minimizes the risk of perforation and resultant fluid and air leakage into the peritoneum.5
Total and partial gastrectomies can be associated with prolonged hospital stays.6 In contrast, the patient was discharged 1 day after his ESD and has had no complications related to his procedure. In conclusion, ESD offers a minimally invasive treatment for premalignant lesions and early stage cancers of the stomach. Endoscopic resection can offer superior perioperative outcomes and decreased lengths of hospital stay compared to traditional surgical gastrectomy in select patients.
References
- 1.Bray F, Ren JS, Masuyer E, et al. . Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132:1133–1145. doi: 10.1002/ijc.27711. [DOI] [PubMed] [Google Scholar]
- 2.Karimi P, Islami F, Anandasabapathy S, et al. . Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isobe Y, Nashimoto A, Akazawa K, et al. . Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer. 2011;14:301–316. doi: 10.1007/s10120-011-0085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirao M, Masuda K, Asanuma T, et al. . Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264–269. doi: 10.1016/s0016-5107(88)71327-9. [DOI] [PubMed] [Google Scholar]
- 5.Kobara H, Mori H, Rafiq K, et al. . Submucosal tunneling techniques: current perspectives. Clin Exp Gastroenterol. 2014;7:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qi J, Zhang P, Wang Y, et al. . Does total gastrectomy provide better outcomes than distal subtotal gastrectomy for distal gastric cancer? A systematic review and meta-analysis. PLoS One. 2016;11:e0165179. doi: 10.1371/journal.pone.0165179. [DOI] [PMC free article] [PubMed] [Google Scholar]