Abstract
For chronic malignant and benign ureteral obstruction, the metal construction of the Resonance ureteral stent has been developed to maintain ureteral patency for up to 12 months, obviating the need for the frequent exchange required for conventional plastic ureteral stents. We report our experience placing tandem Resonance stents (TRS) in a single ureter of patients who failed management with a single Resonance stent. A retrospective review of patients who had TRS for management of ureteral obstruction between February 2014 and May 2017 was performed. Seven renal units from four patients with a median age of 62 years were managed with TRS. All but one renal unit was successfully managed with TRS initially. Hydronephrosis resolved in 80% of renal units at a median of 33 days, and creatinine reached its nadir a median of 38 days after placement, with a median improvement of 0.68 ng/mL. However, the median length of management with TRS was only 123.5 days with one exchange, and there was an overall success rate of 28.5% at 1 year. TRS placement is a feasible option for short-term management in a challenging population that would like to avoid nephrostomy and has failed other modalities.
Keywords: Chronic ureteral obstruction, malignant ureteral obstruction, Resonance stent, tandem ureteral stent
Chronic ureteral obstruction requires long-term drainage of the affected renal unit. Select patients with benign disease who are poor surgical candidates or those with malignant ureteral obstruction (MUO) may be managed chronically with indwelling double-J ureteral stents.1 The soft plastic construction of conventional indwelling stents is prone to failure due to encrustation and extrinsic compression. The metal alloy construction of the Resonance stent (Cook Medical, Bloomington, IN) has been developed to resist compression and maintain ureteral patency for up to 12 months,2 which allows for less frequent exchange and provides an alternative for internalized drainage after failed conventional stenting. Still, up to 37% of Resonance stents will fail due to obstruction.3 In these cases, management options are usually limited to percutaneous nephrostomy (PCN) drainage. The placement of tandem conventional ureteral stents in a single ureter has been described and appears safe and effective.4–6 To our knowledge, this technique has not been described using Resonance stents. We report our experience in a small series of patients whose ureteral obstruction was managed in this fashion due to failure of a single Resonance stent and declined placement of a PCN.
METHODS
A retrospective chart review of prospectively monitored patients who had tandem Resonance stents (TRS) for management of ureteral obstruction between February 2014 and May 2017 was performed. Patient demographics, perioperative outcomes, and follow-up data through the end of the study period were recorded.
Cystoscopically, a 0.035 hydrophilic guidewire is used to cannulate the affected ureteral orifice and is advanced into the renal pelvis under fluoroscopic guidance. The first Resonance introducer consisting of the 8.3 F transparent outer sheath and inner 6 F green open-ended pusher is advanced over the wire and, with the help of a retrograde pyelogram, positioned so the radiopaque marker delineating the end of the transparent sheath is proximal to the ureteropelvic junction (Figure 1a). The cystoscope is then removed, leaving the first introducer and wire in place.
Figure 1.
Tandem Resonance stent placement steps. (a) The introducers are positioned in tandem such that the radiopaque markers are seen overlying the renal pelvis. (b) The Resonance stents are advanced through the introducers simultaneously. (c) The distal ends of the stents are observed as the introducers are withdrawn with care not to advance the distal aspect of the stents into the ureter. (d) The introducers are completely removed allowing the distal aspects of the stents to curl within the bladder.
The cystoscope is reintroduced alongside the first introducer, and the affected ureteral orifice is cannulated with a second wire. The second introducer is then advanced over the wire and alongside the first introducer into the renal pelvis (Figure 1b). The first introducer can be stabilized by the surgeon or assistant, so that it is not displaced during placement of the second introducer. The stents must be placed simultaneously or the distal end of the first stent will be pushed into the ureter during placement of the second introducer (Figure 1c). When the second introducer cannot be advanced, the cystoscope should be removed, leaving both introducers in place. By pulling the first introducer back down the ureter 2 to 3 cm at a time and then advancing both introducers together 2 to 3 cm at a time, both introducers can be placed into a single ureter.
Once both introducers are in proper position, the inner 6 F green open-ended pushers and wires are removed, leaving the outer transparent sheaths in place. The metallic Resonance stents are then placed into the transparent outer sheath of the introducer and pushed using the green open-ended pushers until good curls of both proximal pigtails are seen overlying the renal pelvis on fluoroscopy (Figure 1d). Then, the outer sheaths are simultaneously removed over the pushers until good curls of the distal pigtails are seen overlying the bladder (Figure 2).
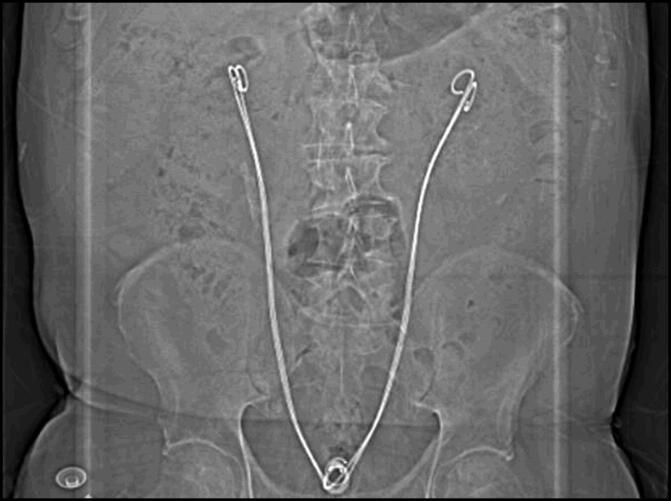
Figure 2.
Bilateral tandem Resonance stents.
RESULTS
Seven renal units from four patients with a median age of 62 years were managed with TRS. Two patients had MUO from metastatic colon cancer and two patients had benign disease that was not amenable to surgical repair. Patients’ clinical characteristics are summarized in Table 1. Patient dissatisfaction was cited as a factor for pursuing TRS in 100% of patients who had PCN prior to TRS. Hydronephrosis on follow-up imaging and renal failure were the most common reasons for failure of a single Resonance stent, with rates of 100% and 71.4%, respectively.
Table 1.
Characteristics of four patients who received tandem Resonance metallic double-J ureteral stents for salvage management of chronic ureteral obstruction
| Patient | Age (years) | Sex | Etiology | Renal units | Change in creatinine (ng/mL) | Resolved hydronephrosis | Number of exchanges | Duration of TRS (days) | Reason for failure | Management after failure |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45 | F | Retroperitoneal scarring | 2 | −0.2 | None prior (nephrostomy) | 2 | 188 | Recurrent fungal UTI | 8F plastic stent |
| 2 | 50 | F | Colon cancer | 2 | x | Yes | 1 | 59 | Hydronephrosis, renal failure | Nephrostomy |
| 3 | 74 | M | Ureteral stricture | 2 | −1.15 | Yes | 1 | 317 | NA | NA |
| 4 | 82 | M | Colon cancer | 1 | x | No | 0 | 6 | Flank pain, renal failure | Nephrostomy |
TRS indicates tandem Resonance stent; UTI, urinary tract infection.
One patient with benign disease had extensive stricture of his bilateral proximal ureters and renal pelvises due to intramural dystrophic calcifications. Malignancy was not identified on percutaneous resection of the renal pelvis for biopsy. Endoscopic management with endopyelotomy was attempted but failed due to progressive disease. He was deemed not to be a surgical candidate because of age, medical comorbidities, and lack of healthy ureter/renal pelvis to use in reconstruction. Another patient with benign disease had extensive retroperitoneal scarring secondary to necrotizing pancreatitis causing bilateral pan-ureteral stricture. She was managed with open abdomen and required colectomy and ileostomy formation for ischemic bowel. She required multiple takebacks for washout and debridement and developed several intra-abdominal abscesses, which required percutaneous drain placements. She was not offered endoscopic management or reconstruction due to the length of her strictures and hostile abdomen.
A total of 71.4% of renal units had hydronephrosis immediately prior to placement of TRS, which resolved in 80% of cases at a median of 33 days (range, 9–57) after placement. Creatinine reached nadir a median of 38 days (range, 32–44) after placement, with a median improvement of 0.68 ng/mL (range, 0.20–1.15). The median duration of TRS was 123.5 days (range, 6–373), with a median of one exchange (range, 0–2). The median follow-up was 363.5 days (range, 88–379).
Both cases of malignant obstruction (three renal units) were converted back to PCN due to hydronephrosis and renal failure, and one patient (two renal units) with benign disease failed due to recurrent urinary tract infection. The other patient (two renal units) with benign disease did not fail TRS during the study period, for an overall success rate of 28.5%.
DISCUSSION
Conventional ureteral stenting fails in approximately 40% of cases with MUO due to progressive disease and extrinsic compression.3,6 MUO portends a very poor prognosis, with overall survival frequently cited as <1 year.1,3,4,6,7 Benign chronic ureteral obstruction is typically amenable to definitive management of the underlying etiology, but patients with extensive medical comorbidities or extensive stricture may be reasonably managed with a chronic ureteral stent.1
The Cook Resonance stent is a reasonable option for internalized drainage after failure of a single plastic stent due to its relatively infrequent need for exchange. Despite its ability to resist 30 times more extrinsic force than its plastic counterpart, Resonance stents are still subject to failure from extrinsic compression.8 Of 41 Resonance stents placed after obstruction of a plastic stent, 37% failed due to obstruction at a median of 1.5 months, and all of these failures were due to MUO.3 In these cases, patients are typically relegated to externalized drainage of the collecting system with PCN.
The PCN’s externalized system is prone to dislodgement, and it too requires frequent exchange due to its plastic construction. PCN may not be a convenient option for patients with chronic ureteral obstruction who are otherwise ambulatory. In fact, all patients in this study cited dissatisfaction with PCN as a reason for further pursuit of internalized drainage.
Tandem ureteral stenting has been described with conventional plastic stents.4–6,9 Not only does the additional lumen resist compression and provide more space for intraluminal drainage, but the extraluminal drainage is also increased due to the space created between the two stents in parallel.5 Cystoscopically, using the unique Resonance stent delivery system as described in detail above, both 8.3 F clear outer plastic sheaths were placed into a single ureter and the Resonance stents were deployed simultaneously. Hydronephrosis improved in 80% of renal units and serum creatinine decreased by a median of 0.68 ng/mL, indicating good initial decompression. Similarly, in Liu and Hrebinko’s initial report of tandem ureteral stenting with conventional stents, three of four patients had resolution of flank pain and azotemia and all four patients had resolution of hydronephrosis.9 In our study, only one patient with metastatic colon cancer was never successfully managed with TRS. A PCN was replaced after just 6 days for flank pain, and he died 82 days later. Otherwise, the duration of TRS in this case series was a median of about 4 months (123.5 days) with one exchange and a median follow-up of just under 1 year. This compares similarly to the largest series of tandem stenting with plastic stents (34 patients), which showed a median stent duration of 129 days.4
The other patient with MUO due to metastatic colon cancer failed due to stent obstruction and renal failure. Still, she was managed with TRS for 59 days and died 277 days after TRS failure. Both patients in this series with MUO represent an extreme, because they both failed prior conventional and Resonance stenting. It is unknown whether other tumor origins (i.e., gynecologic or urologic) would be better managed with TRS.
Elsamra et al reported less tandem plastic stent failure and fewer repeat procedures in patients with benign ureteral obstruction vs MUO.4 Similar results were seen in the present study. The patient with extensive and progressive proximal ureteral stricture due to dystrophic intramural calcification was successfully managed with TRS through the end of the study period (373 days). The other patient with pan-ureteral stricture due to necrotizing pancreatitis had TRS success for 188 days but required two exchanges during that time and ultimately failed due to recurrent fungal urinary tract infection. Biofilm occlusion of the tight metal coil construction of the Resonance stent, through which urine must pass, has been reported and demonstrated with scanning electron microscopy.10 Interestingly, she has been subsequently managed with plastic stents, which she had previously failed due to extrinsic compression. It is possible that TRS passively dilated her dense pan-ureteral stricture.
The stiff nature of the Resonance introducer consisting of the 8.3 F transparent outer sheath over the 6 F green open-ended pusher allowed for successful TRS placement in all cases presented in this series without need for additional ureteral dilation. Elsamra et al reported requiring balloon dilation of the ureter in 39% of plastic tandem stent placements and one failure of placement.4 Perhaps TRS could be employed in cases of failed tandem plastic stent placement due to the unique delivery system of the Resonance stent.
Finally, TRS for chronic ureteral obstruction in this challenging patient population may result in a cost similar to that of current established methods for management. Taylor et al demonstrated a 61% cost savings when comparing a traditional polymer stent changed every 90 days to a single metal Resonance stent changed yearly.11 This margin would be diminished by the cost of an additional Resonance stent and need for more frequent Resonance stent exchanges as shown here in the present TRS population, so further cost analysis is warranted. This would also likely compare to PCN, because PCN has been shown to be more costly when compared with conventional stenting.12
In conclusion, TRS placement is a feasible option for short-term management of chronic ureteral obstruction in patients who would like to avoid PCN and have failed other modalities. Patients with benign disease may be better suited for this management strategy; however, study in a more diverse MUO population and direct comparison to tandem conventional plastic stenting is warranted.
References
- 1.Fiuk J, Bao Y, Calleary JG, Schwartz BF, Denstedt JD. The use of internal stents in chronic ureteral obstruction. J Urol. 2015;193:1092–1100. doi: 10.1016/j.juro.2014.10.123. [DOI] [PubMed] [Google Scholar]
- 2.Kang Q, Jiang F, Yu Y, Shen C, Lv H, Yang B. Application of Resonance metallic stents for malignant ureteral obstruction. Minim Invasive Ther Allied Technol. 2018:1–6. doi: 10.1080/13645706.2018.1443944. [DOI] [PubMed] [Google Scholar]
- 3.Modi AP, Ritch CR, Arend D, et al. Multicenter experience with metallic ureteral stents for malignant and chronic benign ureteral obstruction. J Endourol. 2010;24:1189–1193. doi: 10.1089/end.2010.0121. [DOI] [PubMed] [Google Scholar]
- 4.Elsamra SE, Motato H, Moreira DM, et al. Tandem ureteral stents for the decompression of malignant and benign obstructive uropathy. J Endourol. 2013;27:1297–1302. doi: 10.1089/end.2013.0281. [DOI] [PubMed] [Google Scholar]
- 5.Fromer DL, Shabsigh A, Benson MC, Gupta M. Simultaneous multiple double pigtail stents for malignant ureteral obstruction. Urology. 2002;59:594–596. [DOI] [PubMed] [Google Scholar]
- 6.Elsamra SE, Leavitt DA, Motato HA, et al. Stenting for malignant ureteral obstruction: tandem, metal or metal-mesh stents. Int J Urol. 2015;22:629–636. doi: 10.1111/iju.12795. [DOI] [PubMed] [Google Scholar]
- 7.Wang H-J, Lee TY, Luo HL, et al. Application of Resonance metallic stents for ureteral obstruction. BJU Int. 2011;108:428–432. doi: 10.1111/j.1464-410X.2010.09842.x. [DOI] [PubMed] [Google Scholar]
- 8.Gayed BA, Mally AD, Riley J, Ost MC. Resonance metallic stents do not effectively relieve extrinsic ureteral compression in pediatric patients. J Endourol. 2013;27:154–157. doi: 10.1089/end.2012.0263. [DOI] [PubMed] [Google Scholar]
- 9.Liu JS, Hrebinko RL. The use of 2 ipsilateral ureteral stents for relief of ureteral obstruction from extrinsic compression. J Urol. 1998;159:179–181. [DOI] [PubMed] [Google Scholar]
- 10.Qiao L, Yan W, Du Z, Cai Q, Chen S. Biofilm formation is a cause of ureteral Resonance metallic stent blockage: a case report. Int J Clin Exp Med. 2016;9:11919–11922. [Google Scholar]
- 11.Taylor ER, Benson AD, Schwartz BF. Cost analysis of metallic ureteral stents with 12 months of follow-up. J Endourol. 2012;26:917–921. doi: 10.1089/end.2011.0481. [DOI] [PubMed] [Google Scholar]
- 12.Ngweso S, O’Riordan J, Barrett T, Kuan M, Hayne D. Comparative radiation exposure and costs associated with percutaneous nephrostomy tube vs ureteric stent for emergency renal decompression. J Urol. 2018;199:e843. [Google Scholar]