Introduction.
Positron emission tomography (PET) is a powerful, quantitative imaging modality that has been used for decades to noninvasively investigate cardiovascular biology and physiology 1 The PET images are, however, affected by physiological patient motion which degrades the images qualitatively as well as quantitatively. Three distinct motion patterns can be observed in thoracic PET scans: cardiac contraction, respiratory motion, and patient repositioning during the acquisition. In this review, we discuss recent advances in cardiac and respiratory gating and provide an overview of the most promising recent developments in the field.
Clinical Background
Due to its superior sensitivity, spatial and temporal resolution compared to SPECT, PET has been considered a gold standard for non-invasive assessment of myocardial perfusion and viability 2,3 Its potential extends beyond the assessment of coronary artery disease (CAD) patients. PET facilitates early diagnosis of a broad range of cardiac conditions which affect the myocardium such as cardiomyopathies4,5, infiltrative myocardial disease including sarcoidosis6,7, and amyloidosis 8. Furthermore, PET plays a key role in the detection of endocarditis9 and inflammation related to implantable device infection 10. Recently cardiac PET-imaging is also undergoing clinical validation in the assessment of unstable coronary plaques, which are at high risk of rupture 11-14.
Modern PET systems
Aside from the development of novel tracers, the improvement in the clinical assessment of cardiac disease has partly been facilitated through the continuous improvement in the spatial resolution of the PET systems, which in current PET/CT systems offer resolutions of 2-5 mm at full-width at half maximum (FWHM) 15,16. Correction for point spread function as well as time-of-flight imaging has become standard in many modern PET systems, which offer improved localization of the annihilation event and, thus, improved spatial recovery of the tracer distribution17-20. The high-resolution PET systems, in theory, permit accurate delineation of abnormal areas with a precision similar to the PET scanner’s spatial resolution 11,12,21-23. Unfortunately, high-resolution imaging of the myocardium is hampered by motion during the acquisitions 24. The detrimental impact of motion during the PET-acquisition was recognized already in 1982 when it was proposed to divide the PET-data into motion-limited bins based on the respiratory/cardiac phase 25.
Since then several studies have investigated the effects of cardiac and respiratory motion 26-30. The most investigated has been the correction for the cardiac contraction, despite the fact that other motion patterns have equally detrimental effects on image quality 31,32. One reason for this is the potential need for additional equipment to track these motion patterns.
Cardiac gating:
Nowadays in the clinical setting usually 3-lead ECG is utilized 33. With the lead data being directly transferred to the scanner both retrospective and prospective gating of the acquired PET is feasible. The use of 3-lead electrocardiograms (ECG) is relatively easy, cheap and has been shown to be reproducible in many studies 34. Aside from the gating functionality it also serves to monitor the patient during the acquisition. The acquired ECG signal employs the R-wave as a reference to estimate the cardiac phase in which each coincidence was acquired, ultimately allowing the data to be sorted into near motion-free cardiac gates (Figure 1). Cardiac gating in most modern systems is performed retrospectively. Prospective gating is mainly used in older PET systems where listmode storage is not feasible and relies on defining phases in relation to the peak of the R wave. Such phases can be defined as preceding the R wave (backward gating) of occurring after it (forward gating). In both scenarios, the annihilation events can be sorted into predefined sinogram buffers and reconstructed once the acquisition is completed.
Figure 1.

Principle of ECG-gating, here shown using an 8-bin ECG-gating. The acquired PET-data for each R-R interval is divided into a user-specified number of phases of the cardiac contraction.
Cardiac gating can serve two functions: (1) it can be used for motion compensation of the myocardium 35 and (2) for functional assessment of the myocardium. The functional assessment provides both diagnostic and prognostic value in the clinical assessment of global cardiac function (left ventricular ejection fraction, LVEF), regional wall motion abnormalities and myocardial dyssynchrony 36,37. The motion-compensated images are mainly used for research purposes while the functional assessments are used in the clinical routine. Analyses of the functional parameters have shown that an increase in LVEF (from baseline to peak stress) is inversely related to the magnitude of ischemia and the extent of angiographic CAD 38,39. In patients with multivessel CAD, LVEF often shows a blunted response or can even drop on stress imaging. The change in LVEF during peak stress has been shown to have value for risk prediction 40,41. In addition, cardiac gating has been proven a strong tool as a first approach in the assessment in the coronary plaques as the coronary arteries can shift up to 26 mm during the cardiac cycle 32,42,43 (Figure 2).
Figure 2.
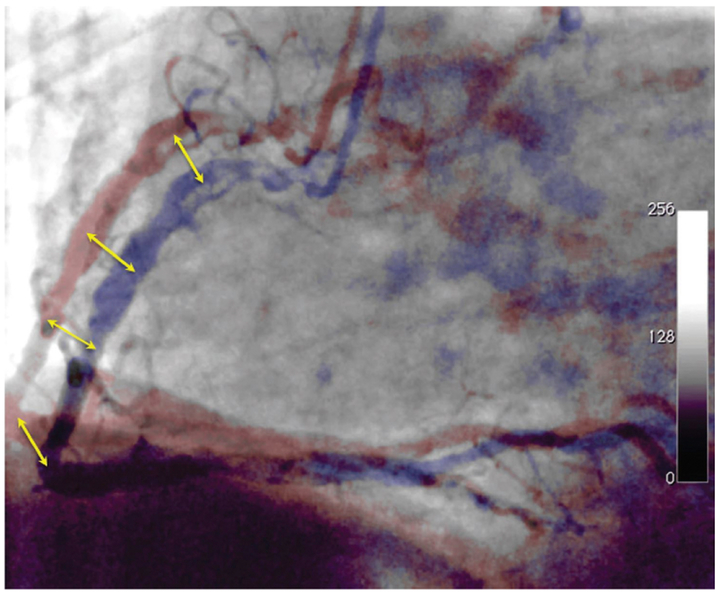
Displacement of the coronary arteries during the cardiac contraction. The coronary arteries are shifting 8-26 mm during cardiac contraction (yellow arrows).
Respiratory Gating
Respiratory gating is desired in the clinical settings to improve image quality but is not often utilized in many modern systems which only allow for one form of gating during the reconstruction (ECG or respiratory gating). In systems facilitating dual-physiological trigger events (cardiorespiratory signals), the respiratory signal may be extracted using external markers such as piezo-electric respiratory belts or infra-red systems24. Other solutions employing spirometers, and measurements of the nasal temperature/humidity have also been successfully tested44. Respiratory gating employing external markers has several drawbacks. These include: a time-consuming imaging setup 45, potential malfunction during the acquisition 46 and rather rigid monitoring of the respiratory signal which results in less robust monitoring in patients with changes in the respiratory baseline 47. Owing to the complex setups, the introduction of respiratory gating is still mainly a tool applied in research-studies primarily in centers with technical personnel who can maintain the systems.
To overcome the drawbacks of the external markers, the tendency in recent research is to replace the external markers using data-driven methods 34. The data-driven methods offer several benefits over the use of external markers: First, the data-driven methods do not require frequent calibrations as they extract the respiratory signal directly from the list files 45. Second, they allow for ad-hoc correction of all acquisitions acquired in listmode format, whereas careful planning is needed when using external markers. A third benefit is that the data-driven methods do not require the user to buy any additional hardware, which can be costly both to acquire and install.
In addition, the data-driven methods, in general, have the potential of facilitating accurate gating in patients with changes in the respiratory baseline, a frequent problem in myocardial perfusion imaging where stress scans are performed after administration of pharmaceutical agents. The common agents (Adenosine, Dipyridamole, and Regadenoson) all have short half-lives, which require optimized stressing protocols such that the maximum effect is obtained during the infusion of the PET-tracer. Given the fast roll-off effect of the pharmaceuticals, it is not uncommon to encounter changes in the respiratory baseline during the acquisition 48,49. If not corrected for, the change in the respiratory baseline might introduce a degradation of the gated images in comparison to the non-gated images 50. Here, data-driven gating approaches allow for tailored gating-approaches that fits the stress-imaging protocol and, thus, have the potential of outperforming the use of the external markers which often are calibrated to the respiratory baseline at the beginning of the acquisition 47,51.
Sensitivity based methods
The first attempts at extracting information of the respiratory motion directly from the PET-raw data (list files) were proposed by Bundschuh et al and He et al 52,53. These methods, in brief, are based on the heterogeneous sensitivity profiles that exist in all PET-systems. The sensitivity profile is partly introduced by the geometry of the system and partly by the detector materials used 54. Owing to the geometry in the PET system, the highest sensitivity is obtained in the center of the field of view. Heterogeneous objects moving in and out of the center field of view will result in changes in the obtained count rates equivalent to the motion in the scanners axial direction. For patient scans, the heterogeneous uptake rates are obtained through differences in the tracer distribution as well as differences in the linear attenuation coefficients in the lungs and diaphragm.
Center-of-Mass/centroid-of-mass based methods
The center-of-mass or centroid-of-mass (CoM) based approaches have gained substantial interest in imaging of organs with focal uptake and high contrast to background ratio, such as myocardial scans and in studies of non-small cell lung cancers. Several methods relying on this assessment have been proposed, using either the full field of view or through detection of localized motion vector fields 44,55. The CoM assessment, in brief, evaluates the centroid of the counts obtained in the region of interest using singleslice rebinned sinograms (SSRB)56, which are marked by the user in most cases. The SSRB algorithm, in short, is an algorithm that compresses the full 3D sinograms into a reduced 3D sinogram. By performing the compression, the noise is reduced and provides a more stable respiratory signal. This omits the varying sensitivity profiles and, thus, provides a more stable measurement of the respiratory cycle even for lesions slightly misplaced from the center of the systems field of view.
Sinogram Fluctuation model
The sinogram fluctuation model evaluates the fluctuations obtained in sinograms with short time duration (~500ms). Following their binning, the sinograms can be evaluated for the periodicity of the signal changes in each of the short time-duration datasets, thus, permitting extraction of the respiratory signal using only data with frequencies within the normal respiratory range (2-9 s periodicity) 57.
MR-based approaches
The introduction of the hybrid PET/MR systems has facilitated new methods for motion detection approach, in which accurate estimates of the respiratory signal can be extracted directly from the diaphragm in the PET-images 58-61. The respiratory signal can be obtained from either dedicated MR sequences that target the golden angle 58, or through tracking of the heart/diaphragm in standard MR-sequences 62. The resulting data can be used either for respiratory gating or for motion compensation during the image reconstruction 29,58. Despite the accurate tracking of both respiratory and cardiac motion through dedicated MR sequences, the MR-based approaches have some drawbacks. They can only be utilized in integrated PET/MR systems, and often require specific MR-sequences for motion detection, which can limit the time left for the acquisition of clinically important data 58.
Respiratory gating: Phase vs amplitude.
Once acquired, the respiratory signal can be used for gating in multiple ways where phase-based/time-based (similar to the ECG-based gating approaches) and amplitude-based gating are the two most common approaches (Figure 3) 51.
Figure 3.
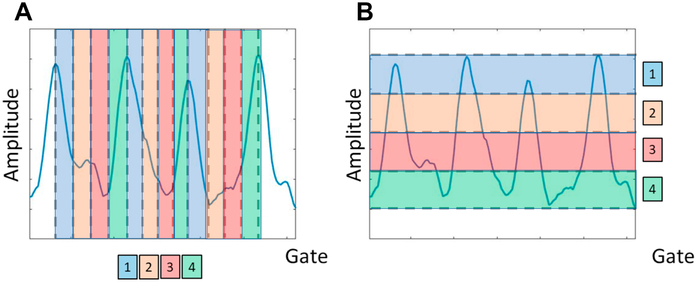
Time-based (phase-based) gating and amplitude-based gating techniques, here exemplified using 4 respiratory gates. (A) time-based method divides the data into equally time-divided bins that will have the same noise properties (equal time-duration). (B) amplitude based gating divides the data into bins with the same respiratory amplitude, and thereby pose the risk of having noise-variated gates in the following assessments, as can be observed for the third respiratory cycle which does not reach the same amplitude as the preceding cycles.
Time-based / Phase-based gating
The phase-based method is the most simplistic method of the two, where each respiratory phase is divided into a user-defined number of phases, each with equal time-duration 51. This ensures homogeneous noise-levels for all gates, which is beneficial in the subsequent analyses. Unfortunately, this method does not allow for differentiating between normal tidal breathing and sudden excessive in/expiratory breath-holds or changes in the respiratory baseline.
Amplitude-based gating
The amplitude-based gating offers more accurate gating than the time-based/phase-based gating approach. Despite the superiority in providing high spatial differentiation of data from different respiratory amplitudes, this technique also has its limitations. The highly dynamic range of respiratory signals often hampers its functionality and, thus, most often requires truncation or discarding of data outside the normal range to ensure enough counts to provide clinical image-qualities. In addition, the asynchronous respiratory cycle will often introduce inhomogeneities in the noise-characteristics in the resulting gated images with the best quality often obtained in the end-expiratory phase. Due to this, it has been proposed to use the optimal respiratory gate, which only employs data from the end-expiratory phase – known as the optimal respiratory phase, which typically can contain up to 35% of all image counts 63.
Dual gating
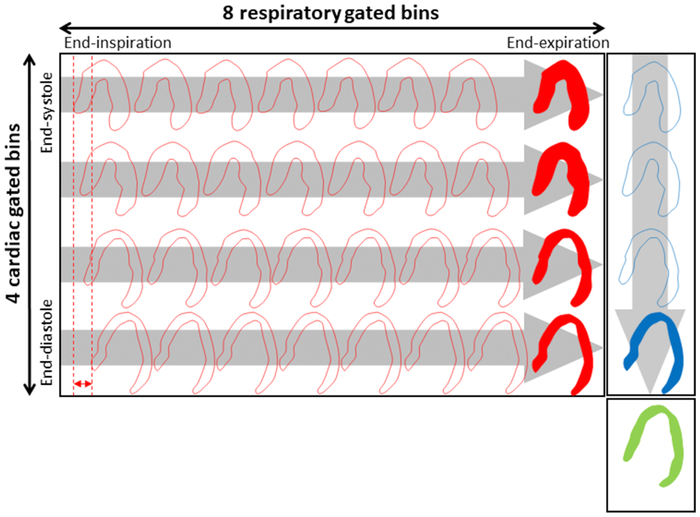
The single gating techniques have been proven suboptimal for many PET-scans as the non-corrected motion-pattern is still embedded in the resulting images. Dual-gating approaches, which combine the cardiorespiratory motion, have been proposed 26,64-66. The combination of the two gating techniques ensures virtually motion-free images, with only little intra-gate motion present (Figure 4). Unfortunately, this requires sufficient image quality (count rates per single gate) in the subsequent reconstructions, as often up to 16-64 gates are being utilized. The exact number depends on how many respiratory and cardiac gates are used (NRespiratory gates × Ncardiac gates) 67,68, with N being the number of the respective gates.
Figure 4.
Dual-gating scheme. Example of a dual gating scheme employing 4 ECG and 8 respiratory bins, which creates a total of 24 virtually motion-free images. Each of the gated images can be coregistered to obtain images with reduced noise properties when compared to the noise in each individual gate. This research was originally published in JNM. Slomka PJ, Rubeaux M, Le Meunier L, et al. Dual-Gated Motion-Frozen Cardiac PET with Flurpiridaz F18. J Nucl Med. 2015;56(12): 1876-1881. © SNMMI.
PET attenuation correction issues resulting from motion
Attenuation correction (AC) is an important prerequisite for absolute quantification in all PET imaging. Several attenuation correction techniques have been proposed, depending on the modality (PET-only, PET/CT or PET/MRI) 69-71. Several drawbacks and limitations have been described for the AC maps, disregarding the acquiring modality. The drawbacks include both physiological and technical aspects such as beam-hardening, misalignment, truncation as well as non-physiological artifacts. In the context of gating, especially the misalignment artifacts are particularly relevant. The remaining artifacts have a more general character and have been discussed thoroughly elsewhere 72,73.
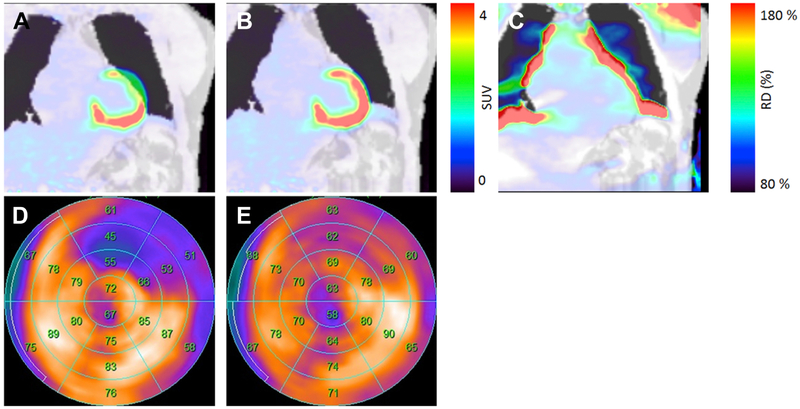
Misalignment of the PET-emission data and the AC-images can be classified either as repositioning events where the patient moves between the acquisition of the AC maps and the PET-emission data, differences between the respiratory-gated PET-images and the corresponding AC maps or as breathing during the AC acquisition (Figure 5) 74-76.
Figure 5.
Displacement of PET-emission data and the attenuation correction (AC) maps (A) can cause local changes of more than 80% in the quantitative assessment (B, C). Correction for the misalignment of the AC maps and PET-data reduced the extent and severity of the hypometabolic region (D, E). The displacements can be introduced through respiratory motion during the PET-acquisition or by patient repositioning between the acquisition of the AC map and the PET data. This figure was originally published in JNC under the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). Lassen ML, Rasul S, Beitzke D, et al.: Assessment of attenuation correction for myocardial PET imaging using combined PET/MRI. J Nucl Cardiol. 2017:1-12 © The Author(s) 2017.
Respiratory motion during the PET-images translates the heart up by up to a few centimeters (see section Respiratory gating). Due to the fast acquisition times of the AC images (a few seconds in CT, 30s in PET/MR systems), respiratory motion during the acquisition is often not a problem regardless whether a free-breathing or end-expiratory breath-hold acquisition protocol is utilized 77. Several optimizations of AC acquisitions have been proposed for both the PET/MR and PET/CT systems. Employing cine CT for PET/CT has been suggested 78. This allows reconstructing a respiratory-averaged AC map with the same displacements as obtained during the cine CT acquisition. In PET/MR systems, it has been proposed to acquire several AC maps in different respiratory positions as the AC images are acquired without the use of ionizing radiation.
New gating techniques and challenges ahead
Cardiorespiratory motion has been investigated by many researchers and several gating approaches have been proposed. The use of external markers has been used in the conventional assessment of the displacement during the acquisition. However, recent trends indicate that data-driven gating techniques are an emerging technology that will permit marker-less motion detection in clinical routine. These gating techniques mainly focus on respiratory motion detection, though cardiac gating might also be possible as demonstrated for the first time in 2009 34,44. While these established techniques might replace the conventional external marker methods, the potential of data-driven detection of patient repositioning events is another interesting field of research. A recent pilot study has shown that such techniques are feasible in coronary plaque studies, in which gross patient motion has a detrimental effect on the quantitative accuracy 79. Furthermore, it is believed that also tracer-kinetic studies for novel PET-tracers which scanning protocols can last up to one hour or more will benefit from patient repositioning detection, ultimately enabling triple-gating or application of sophisticated combinations of various gating techniques 80.
Moreover, the use of gated images is expected to be implemented in motion compensation techniques, either during or before image-reconstruction. This will improve the image quality of the static images, where accurate definition of pathophysiological changes can be difficult in the gated images due to the increased noise levels. By correcting for the motion during image-reconstruction, it is possible to obtain a fully motion compensated image with the spatial resolution similar to gated images, with the noise characteristics of the static image-acquisitions. In addition, the motion compensated images can also reduce the respiratory blur in the ECG-gated reconstructions and, thus, lead to improved quantification of left ventricular volumes for function assessments. Therefore, gating will become increasingly important in the future not only for the definition motion but also in the pursuit of accurate assessment of physiological parameters.
Summary:
In this article gating approaches for both cardiac and respiratory motion have been reviewed. Cardiac gating has enabled accurate heart and coronary imaging now respiratory gating has become an important frontier in PET imaging. With multiple limitations of currently utilized external marker methods and the increasing availability of list mode PET data, the field is now moving towards data-driven methods which emerge as a promising alternative. Ultimately dual gating encompassing both cardiac and respiratory motion or even triple gating which also takes into account gross patient motion effects shall lead to further improvements in image quality.
Key points.
Cardiac and respiratory motion have a detrimental effect on cardiovascular PET imaging and affect both quantitative and qualitative PET measures.
Gating can ameliorate the unfavorable impact of motion additionally enabling evaluation of left ventricular systolic function (ejection fraction) and wall motion abnormalities.
Cardiac gating is used in the clinical setting while respiratory motion gating remains a research tool.
Synopsis:
Cardiac Positron Emission Tomography (PET) provides high sensitivity and high negative predictive value in the diagnosis of coronary artery disease and cardiomyopathies. Cardiac, respiratory as well as bulk patient motion have detrimental effects on thoracic PET-imaging, hereunder cardiovascular PET imaging where the motion can affect the PET images quantitatively as well as qualitatively. Gating can ameliorate the unfavorable impact of motion additionally enabling evaluation of left ventricular systolic function. In this article, we review the recent advances gating approaches and highlight the advances in data-driven approaches which hold promise in motion detection without the need for complex hardware setup.
Footnotes
Disclosure
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bengel FM, Higuchi T, Javadi MS, Lautamäki R. Cardiac Positron Emission Tomography. J Am Coll Cardiol. 2009;54(1):1–15. doi: 10.1016/j.jacc.2009.02.065 [DOI] [PubMed] [Google Scholar]
- 2.Di Carli MF, Dorbala S, Meserve J, El Fakhri G, Sitek A, Moore SC. Clinical myocardial perfusion PET/CT. J Nucl Med. 2007;48(5):783–793. doi: 10.2967/jnumed.106.032789 [DOI] [PubMed] [Google Scholar]
- 3.Bateman TM, Dilsizian V, Beanlands RS, et al. American Society of Nuclear Cardiology and Society of Nuclear Medicine and Molecular Imaging Joint Position Statement on the Clinical Indications for Myocardial Perfusion PET. J Nucl Med. 2016;57(10): 1654–1656. doi: 10.2967/jnumed.116.180448 [DOI] [PubMed] [Google Scholar]
- 4.jung Kong E, hee Lee S, ho Cho I. Myocardial Fibrosis in Hypertrophic Cardiomyopathy Demonstrated by Integrated Cardiac F-18 FDG PET/MR. Nucl Med Mol Imaging (2010). 2013;47(3): 196–200. doi: 10.1007/s13139-013-0201-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bravo PE, Di Carli MF, Dorbala S. Role of PET to evaluate coronary microvascular dysfunction in non-ischemic cardiomyopathies. Heart Fail Rev. 2017;22(4):455–464. doi: 10.1007/s10741-017-9628-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skali H, Schulman AR, Dorbala S. 18F-FDG PET/CT for the assessment of myocardial sarcoidosis. Curr Cardiol Rep. 2013; 15(4):352. [PMC free article] [PubMed] [Google Scholar]
- 7.Hulten E, Aslam S, Osborne M, Abbasi S, Bittencourt MS, Blankstein R. Cardiac sarcoidosis-state of the art review. Cardiovasc Diagn Ther. 2016;6(1):50–63. doi: 10.3978/j.issn.2223-3652.2015.12.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorbala S, Vangala D, Semer J, et al. Imaging cardiac amyloidosis: A pilot study using 18F-florbetapir positron emission tomography. Eur J Nucl Med Mol Imaging. 2014;41(9): 1652–1662. doi: 10.1007/s00259-014-2787-6 [DOI] [PubMed] [Google Scholar]
- 9.Sarrazin JF, Philippon F, Tessier M, et al. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J Am Coll Cardiol. 2012;59(18): 1616–1625. doi: 10.1016/j.jacc.2011.11.059 [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Feller ED, Chen W, Liang Y, Dilsizian V. FDG PET/CT for Early Detection and Localization of Left Ventricular Assist Device Infection. Impact on Patient Management and Outcome. JACC Cardiovasc Imaging. 2018:1–8. doi: 10.1016/j.jcmg.2018.01.024 [DOI] [PubMed] [Google Scholar]
- 11.Dweck MR, Chow MWL, Joshi NV., et al. Coronary arterial 18F-sodium fluoride uptake: A novel marker of plaque biology. J Am Coll Cardiol. 2012;59(17): 1539–1548. doi: 10.1016/j.jacc.2011.12.037 [DOI] [PubMed] [Google Scholar]
- 12.Joshi NV, Vesey AT, Williams MC, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: A prospective clinical trial. Lancet. 2014;383(9918):705–713. doi: 10.1016/S0140-6736(13)61754-7 [DOI] [PubMed] [Google Scholar]
- 13.Cocker MS, Spence JD, Hammond R, et al. [18F]-NaF PET/CT Identifies Active Calcification in Carotid Plaque. JACC Cardiovasc Imaging. 2017;10(4):486–488. doi: 10.1016/j.jcmg.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 14.Hacker M Monitoring anti-inflammatory therapies in patients with atherosclerosis: FDG PET emerges as the method of choice. Eur J Nucl Med Mol Imaging. 2012;39(3):396–398. doi: 10.1007/s00259-011-2027-2 [DOI] [PubMed] [Google Scholar]
- 15.Rahmim A, Rousset O, Zaidi H. Strategies for Motion Tracking and Correction in PET. PET Clin. 2007;2(2):251–266. doi: 10.1016/j.cpet.2007.08.002 [DOI] [PubMed] [Google Scholar]
- 16.Cal-Gonzalez J, Rausch I, Sundar LKS, et al. Hybrid imaging: Instrumentation and data processing. Front Phys. 2018;6(May). doi: 10.3389/fphy.2018.00047 [DOI] [Google Scholar]
- 17.Conti M Focus on time-of-flight PET: The benefits of improved time resolution. Eur J Nucl Med Mol Imaging. 2011;38(6): 1147–1157. doi: 10.1007/s00259-010-1711-y [DOI] [PubMed] [Google Scholar]
- 18.Dasari PKR, Jones JP, Casey ME, Liang Y, Dilsizian V, Smith MF. The effect of time-of-flight and point spread function modeling on 82Rb myocardial perfusion imaging of obese patients. J Nucl Cardiol. 2018. doi: 10.1007/s12350-018-1311-y [DOI] [PubMed] [Google Scholar]
- 19.Slomka PJ, Pan T, Berman DS, Germano G. Advances in SPECT and PET Hardware. Prog CardiovascDis. 2015;57(6):566–578. doi: 10.1016/j.pcad.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 20.Armstrong IS, Tonge CM, Arumugam P. Impact of point spread function modeling and time-of-flight on myocardial blood flow and myocardial flow reserve measurements for rubidium-82 cardiac PET. J Nucl Cardiol. 2014;21(3):467–474. doi: 10.1007/s12350-014-9858-8 [DOI] [PubMed] [Google Scholar]
- 21.Rubeaux M, Doris MK, Alessio A, Slomka PJ. Enhancing Cardiac PET by Motion Correction Techniques. Curr Cardiol Rep. 2017;19(2). doi: 10.1007/s11886-017-0825-2 [DOI] [PubMed] [Google Scholar]
- 22.Blankstein R, Osborne M, Naya M, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63(4):329–336. doi: 10.1016/j.jacc.2013.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nensa F, Bamberg F, Rischpler C, et al. Hybrid cardiac imaging using PET/MRI: a joint position statement by the European Society of Cardiovascular Radiology (ESCR) and the European Association of Nuclear Medicine (EANM). Eur Radiol. 2018:1–16. doi: 10.1007/s00330-017-5008-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nehmeh SA, Erdi YE. Respiratory Motion in Positron Emission Tomography/Computed Tomography: A Review. Semin Nucl Med. 2008;38(3):167–176. doi: 10.1053/j.semnuclmed.2008.01.002 [DOI] [PubMed] [Google Scholar]
- 25.Ter-Pogossian MM, Bergmann SR, Sobel BE. Influence of cardiac and respiratory motion on tomographic reconstructions of the heart: implications for quantitative nuclear cardiology. J Comput Assist Tomogr. 1982;6(6): 1148–1155. http://www.ncbi.nlm.nih.gov/pubmed/6983534. [DOI] [PubMed] [Google Scholar]
- 26.Teras M, Kokki T, Durand-Schaefer N, et al. Dual-gated cardiac PET-Clinical feasibility study. Eur J Nucl Med Mol Imaging. 2010;37(3):505–516. doi: 10.1007/s00259-009-1252-4 [DOI] [PubMed] [Google Scholar]
- 27.Rubeaux M, Joshi NV., Dweck MR, et al. Motion Correction of 18F-NaF PET for Imaging Coronary Atherosclerotic Plaques. J Nucl Med. 2016;57(1):54–59. doi: 10.2967/jnumed.115.162990 [DOI] [PubMed] [Google Scholar]
- 28.Chander A, Brenner M, Lautamäki R, Voicu C, Merrill J, Bengel FM. Comparison of Measures of Left Ventricular Function from Electrocardiographically Gated 82 Rb PET with Contrast-Enhanced CT Ventriculography: A Hybrid PET/CT Analysis. J Nucl Med. 2008;49:1643–1650. doi: 10.2967/jnumed.108.053819 [DOI] [PubMed] [Google Scholar]
- 29.Manber R, Thielemans K, Hutton BF, et al. Joint PET-MR respiratory motion models for clinical PET motion correction. Phys Med Biol. 2016;61(17):6515. [DOI] [PubMed] [Google Scholar]
- 30.Thielemans K, Schleyer P, Marsden PK, et al. Data-driven dual-gating for cardiac PET. In: 2014 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC) ; 2014:1–4. doi: 10.1109/NSSMIC.2014.7430902 [DOI] [Google Scholar]
- 31.Dawood M, Büther F, Stegger L, et al. Optimal number of respiratory gates in positron emission tomography: a cardiac patient study. Med Phys. 2009;36(5):1775–1784. doi: 10.1118/1.3112422 [DOI] [PubMed] [Google Scholar]
- 32.Shechter G, Resar JR, McVeigh ER. Displacement and Velocity of the Coronary Arteries: Cardiac and Respiratory Motion. IEEE Trans Med Imaging. 2006;25(3):369–375. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2396264/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nekolla SG, Dinges J, Rischpler C. Clinical impact of cardiac-gated PET imaging. PET Clin. 2013;8(1):69–79. [DOI] [PubMed] [Google Scholar]
- 34.Kesner AL, Schleyer PJ, Büther F, Walter M a, Schäfers KP, Koo PJ. On transcending the impasse of respiratory motion correction applications in routine clinical imaging – a consideration of a fully automated data driven motion control framework. EJNMMI Phys. 2014;1(1):8. doi: 10.1186/2197-7364-1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Meunier L, Slomka PJ, Dey D, et al. Motion frozen 18F-FDG cardiac PET. J Nucl Cardiol. 2011;18(2):259–266. doi: 10.1007/s12350-010-9322-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pazhenkottil AP, Buechel RR, Nkoulou R, et al. Left ventricular dyssynchrony assessment by phase analysis from gated PET-FDG scans. J Nucl Cardiol. 2011;18(5):920–925. doi: 10.1007/s12350-011-9411-y [DOI] [PubMed] [Google Scholar]
- 37.AlJaroudi W, Alraies MC, Hachamovitch R, et al. Association of left ventricular mechanical dyssynchrony with survival benefit from revascularization: A study of gated positron emission tomography in patients with ischemic LV dysfunction and narrow QRS. Eur J Nucl Med Mol Imaging. 2012;39(10):1581–1591. doi: 10.1007/s00259-012-2171-3 [DOI] [PubMed] [Google Scholar]
- 38.Dorbala S, Vangala D, Sampson U, Limaye A, Kwong R, Di Carli MF. Value of vasodilator left ventricular ejection fraction reserve in evaluating the magnitude of myocardium at risk and the extent of angiographic coronary artery disease: a 82Rb PET/CT study. J Nucl Med. 2007;48(3):349–358. doi:48/3/349 [pii] [PubMed] [Google Scholar]
- 39.Brown TLY, Merrill J, Volokh L, Bengel FM. Determinants of the response of left ventricular ejection fraction to vasodilator stress in electrocardiographically gated 82rubidium myocardial perfusion PET. Eur J Nucl Med Mol Imaging. 2008;35(2):336–342. doi: 10.1007/s00259-007-0603-2 [DOI] [PubMed] [Google Scholar]
- 40.Lertsburapa K, Ahlberg A, Bateman T, et al. Independent and incremental prognostic value of left ventricular ejection fraction determined by stress gated rubidium 82 PET imaging in patients with known or suspected coronary artery disease. J Nucl Cardiol. 2008;15(6):745–753. doi: 10.1016/j.nuclcard.2008.06.168 [DOI] [PubMed] [Google Scholar]
- 41.Dorbala S, Hachamovitch R, Curillova Z, et al. Incremental Prognostic Value of Gated Rb-82 Positron Emission Tomography Myocardial Perfusion Imaging Over Clinical Variables and Rest LVEF. JACC Cardiovasc Imaging. 2009;2(7):846–854. doi: 10.1016/j.jcmg.2009.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dilsizian V, Bacharach SL, Beanlands RS, et al. ASNC Imaging Guidelines/SNMMI Procedure Standard for Positron Emission Tomography (PET) Nuclear Cardiology Procedures. Vol 23; 2016. doi: 10.1007/s12350-016-0522-3 [DOI] [PubMed] [Google Scholar]
- 43.Rubeaux M, Joshi N, Dweck MR, et al. Demons versus level-set motion registration for coronary 18 F-sodium fluoride PET. 2016:97843Y. doi: 10.1117/12.2217179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Büther F, Dawood M, Stegger L, et al. List mode-driven cardiac and respiratory gating in PET. J Nucl Med. 2009;50(5):674–681. doi: 10.2967/jnumed.108.059204 [DOI] [PubMed] [Google Scholar]
- 45.Giraud P, Houle A. Respiratory gating for radiotherapy: main technical aspects and clinical benefits. ISRN Pulmonol. 2013;2013. [DOI] [PubMed] [Google Scholar]
- 46.Liu C, Pierce LA II, Alessio AM, Kinahan PE. The impact of respiratory motion on tumor quantification and delineation in static PET/CT imaging. Phys Med Biol. 2009;54(24):7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lassen ML, Rasmussen T, Christensen TE, Kjaer A, Hasbak P. Respiratory gating in cardiac PET: Effects of adenosine and dipyridamole. J Nucl Cardiol. 2016. doi: 10.1007/s12350-016-0631-z [DOI] [PubMed] [Google Scholar]
- 48.Memmott MJ, Tonge CM, Saint KJ, Arumugam P. Impact of pharmacological stress agent on patient motion during rubidium-82 myocardial perfusion PET/CT. J Nucl Cardiol. 2017:1–10. doi: 10.1007/s12350-016-0767-x [DOI] [PubMed] [Google Scholar]
- 49.Watt A, Routledge P. Adenosine stimulates respiration in man. Br J Clin Pharmacol. 1985;20(5):503–506. doi: 10.1111/j.1365-2125.1985.tb05108.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gould KL. Optimizing quantitative myocardial perfusion by positron emission tomography for guiding CAD management. J Nucl Cardiol. 2016. doi: 10.1007/s12350-016-0666-1 [DOI] [PubMed] [Google Scholar]
- 51.Dawood M, Buther F, Lang N, Schober O, Schafers KP. Respiratory gating in positron emission tomography: A quantitative comparison of different gating schemes. Med Phys. 2007;34(7):3067–3076. doi: 10.1118/1.2748104 [DOI] [PubMed] [Google Scholar]
- 52.Bundschuh RA, Martínez-Moeller A, Essler M, et al. Postacquisition Detection of Tumor Motion in the Lung and Upper Abdomen Using List-Mode PET Data: A Feasibility Study. J Nucl Med. 2007;48(5):758–763. doi: 10.2967/jnumed.106.035279 [DOI] [PubMed] [Google Scholar]
- 53.He J, O’Keefe GJ, Gong SJ, et al. A Novel Method for Respiratory Motion Gated With Geometric Sensitivity of the Scanner in 3D PET. IEEE Trans Nucl Sci. 2008;55(5):2557–2565. doi: 10.1109/TNS.2008.2001187 [DOI] [Google Scholar]
- 54.Eriksson L, Townsend D, Conti M, et al. An investigation of sensitivity limits in PET scanners. Nucl Instruments Methods Phys Res Sect A Accel Spectrometers, Detect Assoc Equip. 2007;580(2):836–842. [Google Scholar]
- 55.Büther F, Ernst I, Dawood M, et al. Detection of respiratory tumour motion using intrinsic list mode-driven gating in positron emission tomography. Eur J Nucl Med Mol Imaging. 2010;37(12):2315–2327. doi: 10.1007/s00259-010-1533-y [DOI] [PubMed] [Google Scholar]
- 56.Daube-Witherspoon ME, Muehllehner G. Treatment of axial data in three-dimensional PET. J Nucl Med. 1987;28(11): 1717–1724. http://www.ncbi.nlm.nih.gov/pubmed/3499493. [PubMed] [Google Scholar]
- 57.Kesner AL, Kuntner C. A new fast and fully automated software based algorithm for extracting respiratory signal from raw PET data and its comparison to other methods. Med Phys. 2010;37(10):5550–5559. doi: 10.1118/1.3483784 [DOI] [PubMed] [Google Scholar]
- 58.Grimm R, Fürst S, Souvatzoglou M, et al. Self-gated MRI motion modeling for respiratory motion compensation in integrated PET/MRI. Med Image Anal. 2014;19(1): 110–120. doi: 10.1016/j.media.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 59.Munoz C, Kolbitsch C, Reader AJ, Marsden P, Schaeffter T, Prieto C. MR-Based Cardiac and Respiratory Motion-Compensation Techniques for PET-MR Imaging. PET Clin. 2016;11(2): 179–191. doi: 10.1016/j.cpet.2015.09.004 [DOI] [PubMed] [Google Scholar]
- 60.Munoz C, Neji R, Cruz G, et al. Motion-corrected simultaneous cardiac positron emission tomography and coronary MR angiography with high acquisition efficiency. Magn Reson Med. 2017;00. doi: 10.1002/mrm.26690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Munoz C, Kunze KP, Neji R, et al. Motion-corrected whole-heart PET-MR for the simultaneous visualisation of coronary artery integrity and myocardial viability : an initial clinical validation. 2018. doi: 10.1007/s00259-018-4047-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Munoz C, Neji R, Cruz G, et al. Motion-corrected simultaneous cardiac positron emission tomography and coronary MR angiography with high acquisition efficiency. Magn Reson Med. 2018;79(1):339–350. doi: 10.1002/mrm.26690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu C, Alessio A, Pierce L, et al. Quiescent period respiratory gating for PET/CT. Med Phys. 2010;37(9):5037–5043. doi: 10.1118/1.3480508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gigengack F, Ruthotto L, Burger M, Wolters CH, Jiang X, Schafers KP. Motion correction in dual gated cardiac PET using mass-preserving image registration. IEEE Trans Med Imaging. 2012;31(3):698–712. [DOI] [PubMed] [Google Scholar]
- 65.Lamare F, Le Maitre a., Dawood M, et al. Evaluation of respiratory and cardiac motion correction schemes in dual gated PET/CT cardiac imaging. Med Phys. 2014;41(7):072504. doi: 10.1118/1.4881099 [DOI] [PubMed] [Google Scholar]
- 66.Hyun MC, Gerlach J, Rubeaux M, Slomka PJ. Technical consideration for dual ECG/respiratory-gated cardiac PET imaging. J Nucl Cardiol. 2017;24(4):1246–1252. doi: 10.1007/s12350-016-0741-7 [DOI] [PubMed] [Google Scholar]
- 67.Slomka PJ, Rubeaux M, Le Meunier L, et al. Dual-Gated Motion-Frozen Cardiac PET with Flurpiridaz F18. J Nucl Med. 2015;56(12):1876–1881. doi: 10.2967/jnumed.115.164285 [DOI] [PubMed] [Google Scholar]
- 68.Martinez-Möller A, Zikic D, Botnar RM, et al. Dual cardiac-respiratory gated PET: Implementation and results from a feasibility study. Eur J Nucl Med Mol Imaging. 2007;34(9): 1447–1454. doi: 10.1007/s00259-007-0374-9 [DOI] [PubMed] [Google Scholar]
- 69.Chesler DA. 3-Dimensional activity distribution from multiple positron scintigraphs. In: Journal of Nuclear Medicine. Vol 12 ; 1971:347. [Google Scholar]
- 70.Kinahan PE, Hasegawa BH, Beyer T. X-ray-based attenuation correction for positron emission tomography/computed tomography scanners. Semin Nucl Med. 2003;33(3):166–179. doi: 10.1053/snuc.2003.127307 [DOI] [PubMed] [Google Scholar]
- 71.Martinez-Möller A, Souvatzoglou M, Delso G, et al. Tissue classification as a potential approach for attenuation correction in whole-body PET/MRI: evaluation with PET/CT data. J Nucl Med. 2009;50(4):520–526. [DOI] [PubMed] [Google Scholar]
- 72.Sureshbabu W, Mawlawi O. PET / CT Imaging Artifacts. J Nucl Med Technol. 2005;33(200218): 156–161. http://interactive.snm.org/docs/JNMT_Exam_PETCT_Imaging_Artifacts.pdf. [PubMed] [Google Scholar]
- 73.Keller SH, Holm S, Hansen AE, et al. Image artifacts from MR-based attenuation correction in clinical, whole-body PET/MRI. Magn Reson Mater Physics, Biol Med. 2013;26(1): 173–181. doi: 10.1007/s10334-012-0345-4 [DOI] [PubMed] [Google Scholar]
- 74.Martinez-Möller A, Souvatzoglou M, Navab N, Schwaiger M, Nekolla SG. Artifacts from Misaligned CT in Cardiac Perfusion Solutions. J Nucl Med. 2007;48(2):188–194. [PubMed] [Google Scholar]
- 75.Gould KL, Pan T, Loghin C, Johnson NP, Guha A, Sdringola S. Frequent diagnostic errors in cardiac PET/CT due to misregistration of CT attenuation and emission PET images: a definitive analysis of causes, consequences, and corrections. J Nucl Med. 2007;48(7): 1112–1121. doi: 10.2967/jnumed.107.039792 [DOI] [PubMed] [Google Scholar]
- 76.Lassen ML, Rasul S, Beitzke D, et al. Assessment of attenuation correction for myocardial PET imaging using combined PET/MRI. J Nucl Cardiol. 2017:1–12. doi: 10.1007/s12350-017-1118-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beyer T, Lassen ML, Boellaard R, et al. Investigating the state-of-the-art in whole-body MR-based attenuation correction: an intra-individual, inter-system, inventory study on three clinical PET/MR systems. Magn Reson Mater Physics, Biol Med. 2016;29(1):75–87. doi: 10.1007/s10334-015-0505-4 [DOI] [PubMed] [Google Scholar]
- 78.Pan T, Mawlawi O, Luo D, et al. Attenuation correction of PET cardiac data with low-dose average CT in PET/CT. Med Phys. 2006;33(10):3931–3938. doi: 10.1118/1.2349843 [DOI] [PubMed] [Google Scholar]
- 79.Lassen ML, Kwiecinski J, Cadet S, et al. Impact of gross patient motion in coronary 18F-NaF PET examinations. J Nucl Med. 2018;59(supplement 1):12. [Google Scholar]
- 80.Piccinelli M, Votaw JR, Garcia EV. Motion Correction and Its Impact on Absolute Myocardial Blood Flow Measures with PET. Curr Cardiol Rep. 2018;20(5). doi: 10.1007/s11886-018-0977-8 [DOI] [PubMed] [Google Scholar]