Abstract
Spontaneous neuronal ensemble activity in the hippocampus is believed to result from a combination of preconfigured internally generated dynamics and the unique patterns of activity driven by recent experience. Previous research has established that preconfigured sequential neuronal patterns (i.e., preplay) contribute to the expression of future place cell sequences, which in turn contribute to the sequential neuronal patterns expressed post-experience (i.e., replay). The relative contribution of preconfigured and of experience-related factors to replay and to overall sequential activity during post-run sleep is believed to be highly biased toward the recent run experience, despite never being tested directly. Here, we use multi-neuronal sequence analysis unbiased by firing rate to compute and directly compare the contributions of internally generated and of recent experience-driven factors to the sequential neuronal activity in post-run sleep in naïve adult rats. We find that multi-neuronal sequences during post-run sleep are dominantly contributed by the pre-run preconfigured patterns and to a much smaller extent by the place cell sequences and associated awake rest multi-neuronal sequences experienced during de novo run session, which are weakly and similarly correlated with pre- and post-run sleep multi-neuronal sequences. These findings indicate a robust default internal organization of the hippocampal network into sequential neuronal ensembles that withstands a de novo spatial experience and suggest that integration of novel information during de novo experience leading to lasting changes in sequential network patterns is much more subtle than previously assumed.
Keywords: multi-neuronal sequences, neuronal ensembles, preplay, representations, sleep
1 ∣. INTRODUCTION
The hippocampus is crucially involved in memory encoding and consolidation (Eichenbaum, Dudchenko, Wood, Shapiro, & Tanila, 1999; Scoville & Milner, 1957; Squire, 1992). Despite remarkable progress, the neural ensemble mechanisms underlying memory formation have remained unclear. Traditional models of hippocampal function have focused on its role in memory consolidation and have largely assumed that prior to a novel spatial experience, the sequential network activity of the hippocampus shows random or poorly organized functional connectivity (Kudrimoti, Barnes, & McNaughton, 1999; Lee & Wilson, 2002; Silva, Feng, & Foster, 2015). Under this assumption, novel sequential spatial experiences drive de novo creation of place cell sequences (externally driven model), which are robustly replayed (Lee & Wilson, 2002; Silva et al., 2015; Skaggs & McNaughton, 1996; Wilson & McNaughton, 1994) during the entire or most of the subsequent sleep and rest to facilitate memory consolidation. This model dominated the field of research into hippocampal temporal and place cell sequence activity despite several contemporaneous reports indicating that, in the sensory neocortex, the stimulus-evoked activity is correlated with the preceding ongoing spontaneous activity (Arieli, Sterkin, Grinvald, & Aertsen, 1996; Kenet, Bibitchkov, Tsodyks, Grinvald, & Arieli, 2003; Luczak, Bartho, & Harris, 2009). Part of the reason for this dichotomy between the way sensory neocortex and hippocampus were believed to process information was likely based on the reasoning that sensory cortex invariably represents primary stimulus features across different contexts (e.g., orientation of a bar), mostly due to a more strongly hard-wired, less-plastic network compared with the hippocampus (McClelland, McNaughton, & O'Reilly, 1995). A direct prediction of this model of hippocampal function is that spontaneous temporal sequence activity during the sleep following a recent novel sequential spatial experience (i.e., post-run sleep) correlates stronger with and can be explained more accurately by the sequential neuronal activity during the novel run experience and its associated awake rest states than by the temporal sequence activity during the sleep preceding the novel experience, pre-run sleep (Figure 1, top).
FIGURE 1.
Diagram of two opposing models of hippocampal network structure generating multi-neuronal temporal sequences in post-run sleep. The externally driven model (top) illustrates the traditional model of exclusive experience-driven sequential dynamics observed in post-run sleep. There is no sequential activity structure related to future run activity in hippocampus during pre-run sleep. External stimuli encountered during a novel experience creates de novo sequential activity during run and post-run sleep reflects and consolidates this activity. The internally generated model (bottom) illustrates the pre-existing, largely stable sequential network structure during pre-run sleep recruited to express place cell sequences during de novo future run experience. Post-run sleep expresses multi-neuronal sequential structure that predominantly represents the default sequential network patterns and to a smaller extent incorporation of specific sequential activity expressed in the preceding run
More recent results that focused on the role of the hippocampus in memory encoding showed that hippocampal sequential network dynamics during the pre-run experience sleep are preconfigured into an internally generated repertoire of temporal sequences that can preplay the future sequence of place cells (Dragoi & Tonegawa, 2011, 2013a, 2013b) expressed during a novel sequential experience (internally generated model), see also (Grosmark & Buzsaki, 2016). In this scenario, rapid memory encoding is largely achieved by the selection and association of pre-existing temporal sequences with the external stimuli encountered during exploration (Dragoi & Tonegawa, 2014) rather than by a de novo creation of place cell sequences. This model, first demonstrated experimentally for hippocampal temporal–spatial sequences in novel environments in 2011 (Dragoi & Tonegawa, 2011), came in stark disagreement with the earlier (and later) work posing that activity during exposure to novel spatial environments is not preceded by correlated patterns of sequential activity in the preceding sleep (Kudrimoti et al., 1999; Lee & Wilson, 2002; Silva et al., 2015), and rather leads to decorrelated patterns of firing rate activity between the preceding and the following sleep sessions (Hirase, Leinekugel, Czurko, Csicsvari, & Buzsaki, 2001). Some of the earlier studies identified correlated patterns of hippocampal neuronal activity during the exploration of familiar environments and the preceding sleep (Kudrimoti et al., 1999; Nadasdy, Hirase, Czurko, Csicsvari, & Buzsaki, 1999). However, when explicitly tested, the sequence correlations had never been identified to occur before a novel spatial experience during sleep (Ji & Wilson, 2007; Kudrimoti et al., 1999; Lee & Wilson, 2002) and were generally implied to represent a replay of a previous similar experience when expressed before a now familiar environment (Kudrimoti et al., 1999; Nadasdy et al., 1999). Under the preconfigured network (internally generated) model (Dragoi, 2013; Dragoi & Tonegawa, 2014), sequential patterns of activity observed during sleep can be correlated with the future place cell sequence on a novel linear track. Therefore, the spontaneous temporal sequence activity observed during post-run sleep is predicted to correlate stronger with and be explained more accurately by the default temporal sequence activity during the pre-run sleep compared with the sequential activity that occurred during the exploratory run and awake rest states (Figure 1, bottom).
These two models (i.e., externally driven and internally generated) and their predictions as to what best explains the spontaneous network patterns of sequential activity during the post-run sleep in the hippocampus have never been tested and directly compared experimentally. This was primarily due to the almost exclusive focus of previous hippocampal research onto the relationship between sequential run experience and pre- and post-run sleep sequences and in part to a historical bias toward studying memory consolidation and replay, rather than memory encoding, within the hippocampal ensembles. Here, we directly compare the externally driven and internally generated models of temporal and spatial sequence generation in the hippocampus (Figure 1) and demonstrate that spontaneous multi-neuronal sequential activity during the post-run sleep, which includes replay, is much more strongly correlated with the one expressed in the pre-run sleep, which includes preplay, compared with the sequential activity expressed during awake exploratory and resting states in a novel environment.
2 ∣. MATERIALS AND METHODS
Animal handling and experimental procedures were approved by the IACUC at Yale University and in agreement with NIH guidelines for the ethical treatment of animals. Extracellular recordings were performed on six Long-Evans adult male rats. Animals were implanted under isoflurane anesthesia with either 32 independently movable tetrodes drive or two 64-channel 8-shank NeuroNexus linear silicone octatrodes. Craniotomy was performed above the area CA1 of the hippocampus as described earlier (Dragoi & Tonegawa, 2013b). The reference electrode was implanted posterior to lambda over the cerebellum. During the following several weeks of recovery, the tetrodes were advanced daily while animals slept and rested in a high-wall opaque sleeping box.
The experimental apparatus consisted of 150 × 150 cm rectangular elevated linear track maze with one arm open for exploration and access to the other arms blocked by 20 × 20 cm barriers (Dragoi & Tonegawa, 2013b). Experimental sessions were conducted while the animals explored for chocolate sprinkle rewards placed at the ends of the linear track. Neuronal activity was recorded in naive animals during the pre-run sleep sessions in the sleep box for 2–4 h, after which the linear maze was brought into the room and installed, followed by the recording of an additional ~2 h of pre-run sleep in the box. Subsequently, the animals were transferred onto the linear maze for the first time and allowed to explore one linear track. After the completion of experiments, the brains of all the rats were perfused, fixed, sectioned, and stained using Cresyl violet for electrode track reconstruction.
Electrophysiological data acquisition was performed using Neuralynx data acquisition system with Cheetah software. The raw signal was recorded at 30,000 Hz, digitally filtered between 1 and 6,000 Hz. Spikes were obtained by high pass filtering raw signal above 600 Hz and triggered by passing a threshold of 50 μV. The animal's position was monitored via two LEDs attached to the headstage by an overhead camera whose signal was recorded by the Cheetah software. Single cells were identified and isolated offline using the manual clustering program Xclust3 (Davidson, Kloosterman, & Wilson, 2009; Dragoi & Tonegawa, 2011) and cluster quality tests (isolation distance). Pyramidal cells were distinguished from interneurons based on spike width, average rate, and autocorrelations (Dragoi & Buzsaki, 2006).
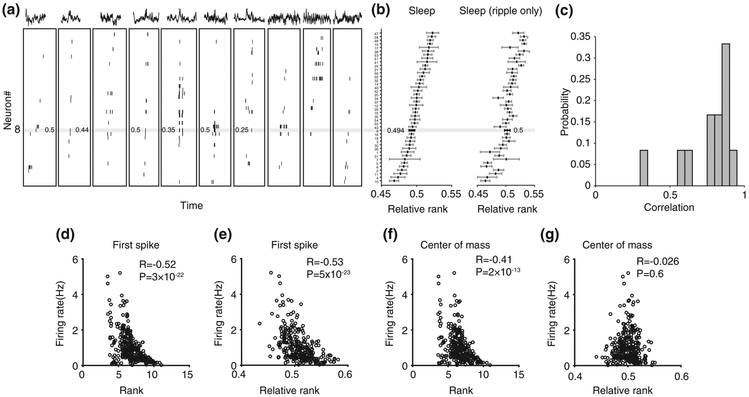
Spiking “frames” of activity were detected during slow-wave sleep periods in the sleep box (animal velocity < 1 cm/s and theta/delta ratio below 2, after Hilbert transform for 6–12 Hz for theta and 1–4 Hz for delta, and smooth with a 5 s Gaussian) and during awake resting period (animal velocity < 2 cm/s) at the ends of the linear track (Diba & Buzsaki, 2007; Dragoi & Tonegawa, 2013b). A spiking frame was defined as a transient increase in the multiunit firing activity of a population of at least four different pyramidal cells within a temporal window preceded and followed by at least 100 ms of silence that delimited the beginning and the end of the event (Dragoi & Tonegawa, 2011). Frames durations smaller than 80 ms or larger than 1.2 s were excluded from further analysis. The spike time center of mass was calculated for each cell in each frame and was used to compute cells' relative rank order in each spiking frame. Ripples were extracted from band-pass filtered (150–250 Hz) local field potential using Hilbert transform. Instantaneous power was computed and frames containing epochs above three standard deviations (SD) above the mean were detected and selected as ripple frames (Figure 2b,c). To analyze the neuronal activity restricted to sharp wave ripples instead of spiking frames, we extracted epochs of 240 ms duration (i.e., the average duration length of the original frames) centered by the time of sharp wave ripple peaks occurring during sleep in the box and during awake rest sessions on the track. This approach generated fewer epochs compared with the original frames (with five of our six animals passing a minimum of 80 epochs/session) and made the templates noisier.
FIGURE 2.
Generation of multi-neuronal sequence template from neuronal activity during sleep. (a) Examples of spiking frames detected during slow-wave sleep. Traces above spiking activity show the local field potential corresponding to each frame, some of which contain a ripple oscillation. Ticks and numbers within the horizontal grey line represent spikes and relative ranks of one cell (cell #8) across several frames of activity. (b) Example sleep template built from all (left) or ripple-containing (right) spiking frames by averaging the relative rank of each cell. Relative rank and cell position within the horizontal grey line correspond to cell #8 shown in (a). (c) Distribution of correlation values between all sleep frames-templates and ripple frames-templates. (d) Correlation between neuronal firing rate and rank estimated by first spike of each neuron. (e) Correlation between neuronal firing rate and neuronal relative rank estimated by first spike of each neuron. (f) Correlation between neuronal firing rate and rank estimated by center of mass of each neuron. (g) Correlation between neuronal firing rate and neuronal relative rank estimated by center of mass of each neuron
Place fields were computed as the ratio between the number of spikes and the time spent in 2 cm bins along the track, smoothed with a Gaussian kernel with an SD of 2 cm. Bins where the animal spent a total of <0.1 s and periods during which the animal's velocity was below 5 cm/s were excluded. Place field length and peak rate were calculated after separating the direction of movement and linearizing the trajectory of the animal. Linearized place fields were defined as areas with a localized increase in firing rate above 2 Hz for at least five contiguous bins (10 cm). The place field peak rate and location were given by the rate and location of the bin with the highest ratio between spike counts and time spent. Place field borders were defined as the points where the firing rate became <10% of the peak firing rate or 1 Hz (whichever was bigger) for at least 2 cm. Place cell sequences (i.e., run templates) were calculated by sorting primary place fields of putative pyramidal cells according to the temporal order in each they were “encountered” by the animals during their runs, always forward (Dragoi & Tonegawa, 2013b).
The sleep templates were generated according to the average relative rank of the center of mass (COM) of each putative pyramidal cell active during the sleep frames. Cells' COMs were rank ordered during each spiking frame in sleep. For example, if there were seven cells in a given spiking frame firing in the order BCDEAFG, cell B would receive rank = 1, cell C rank = 2, and so forth, using a method modified after (Stark, Roux, Eichler, & Buzsaki, 2015).
The relative rank of a cell in each frame was defined as , where ri is the relative rank of cell i, ki is the rank of cell i, and n is the number of cells in that frame. The relative rank of a cell active in the middle of the frame will be 0.5, that of a cell active near the beginning of the frame will be closer to 0 and the relative rank of a cell active near the end of the frame will be closer to 1.
The average relative rank of each cell across all spiking frames was calculated. The sleep template was calculated as the sequence in which cells were ordered according to cells' average relative rank in that sleep session. The ripple templates were estimated by using only the frames containing a ripple oscillation instead of all frames. The awake rest templates were estimated by using the frames during animal's resting on the linear track.
To evaluate the significance of correlations between the sleep template and the run template, shuffled sequences were generated by randomizing the order of cells without replacement in each run template. To start, one shuffled sequence was generated for each run template, Spearman's rank order correlation was calculated between the shuffled run templates and sleep templates and the average of correlation values across all six animals was taken. The shuffling and averaging steps were repeated 1,000 times and 1,000 average correlation values were obtained as a control distribution, which was compared with the corresponding average correlation between the real run templates and the sleep templates. The percentile of the real correlation value among the distribution of control shuffled correlations and the Wilcoxon signed rank test between the real correlation and the distribution of control shuffled correlations were performed to test for significance.
Analyses were performed using customized code written in MATLAB (R2015b; R2017b, MathWorks). We have used Spearman's correlation (rank order correlation) to compute correlations between any pair of sequences across different brain states and behavior (i.e., run, awake rest, pre-run, and post-run sleep). For the comparison between sample distributions and averages across two populations of different sample sizes, we used the Wilcoxon rank sum test, while populations of exact same sample sizes were tested pairwise when appropriate by Wilcoxon signed rank test. In all figures, * represents p < .05 and ** represents p < .01. Data are represented as mean ± standard error of the mean (SEM).
3 ∣. RESULTS
We recorded neuronal ensemble activity (29–76 putative pyramidal neurons/session, total of 309 neurons) from six experimentally naïve adult Long-Evans rats as they explored a linear track for the first time (de novo run), rested at the ends of the tracks between running laps (awake rest), and during the pre- and post-run sleep in a sleep box (pre-run and post-run sleep; see Methods). From the neuronal ensemble activity during the run on the linear track, we generated place cell spatial sequence templates, one for each of the two directions of run (Dragoi & Tonegawa, 2013b), by sorting the place cells according to the location of their place field peak along animal's trajectory (animal velocity > 5 cm/s). We segmented the neuronal ensemble activity during stationary (animal velocity < 2 cm/s) awake rest on the running track and sleep sessions in the sleep box (animal velocity < 1 cm/s) into “frames” of activity, which were defined as epochs of transient increases in multiunit activity contributed by at least four pyramidal neurons flanked by at least 100 ms epochs of neuronal silence (Dragoi & Tonegawa, 2011). For each of the awake rest and pre- and post-run sleep sessions, we generated multi-neuronal sequence templates (one for each session/state) by averaging the relative rank of the center of mass of the spikes of each cell (see Materials and Methods for a critical modification of (Stark et al., 2015) method) occurring during each of the awake rest, pre-run sleep, or post-run sleep frames, respectively (Figure 2a). We tested the impact of occurrence of 200 Hz ripple oscillations (Buzsaki, Horvath, Urioste, Hetke, & Wise, 1992) within specific sleep frames on the accuracy of multi-neuronal sequence template detection by comparing sequence templates computed using spikes emitted within all sleep frames with those computed using spikes emitted only within frames where at least one 200 Hz ripple oscillation co-occurred (Figure 2b). We found the correlation between all-frames and ripple-frames templates was very strong and highly significant within individual sleep session (R = 0.77 ± 0.05, p = 5 × 10−4, n = 12, Wilcoxon signed rank test, Figure 2c). This indicates that ripple-containing frames have similar multi-neuronal sequential organization compared with the overall sleep frames.
We investigated whether inhomogeneity in single cell excitability (i.e., firing rate) across the network had an impact on our estimation of multi-neuronal firing sequences (Peyrache, Benchenane, Khamassi, Wiener, & Battaglia, 2010). We found that neuronal firing rates of all pyramidal neurons (n = 309) were negatively correlated with their ranks (R = −0.52, p = 3 × 10−22; Pearson correlation) and relative ranks (R = −0.53, p = 5 × 10−23) within multi-neuronal sequences when they were constructed using neuronal first spike during sleep frames (Figure 2d,e) as in Stark et al. (2015). Similarly, firing rates of neuronal ensembles were negatively correlated with their ranks when they were constructed using neuronal center of mass (Figure 2f; R = −0.41, p = 2 × 10−13). When using these methods, high firing rate cells tended to fire at the beginning of sleep frames scoring low ranks, while the higher ranks at the end of frames were contributed primarily by the low firing rate cells, which were active during frames generally containing larger number of cells (Figure 2d-f). Frames with fewer neurons would tend to only contain high-firing neurons, which produced a bias by which high-firing neurons received lower average rank. In contrast, the relative ranks of neurons within multi-neuronal sequences calculated using the center of mass of neuronal activity within sleep frames were not affected by their firing rates during sleep (Figure 2g, R = −0.026, p = .65, Pearson correlation; n = 309). The rank and relative rank calculated using center of mass were positively correlated (r = 0.40, p = 8 × 10−13). This indicates that while the use of neuronal rank could still reveal temporal structure in neural activity, the influence of neuronal firing rate would need to be further evaluated. To further analyze the multi-neuronal sequence activity, we used exclusively the relative rank of neurons calculated from their center of mass activity.
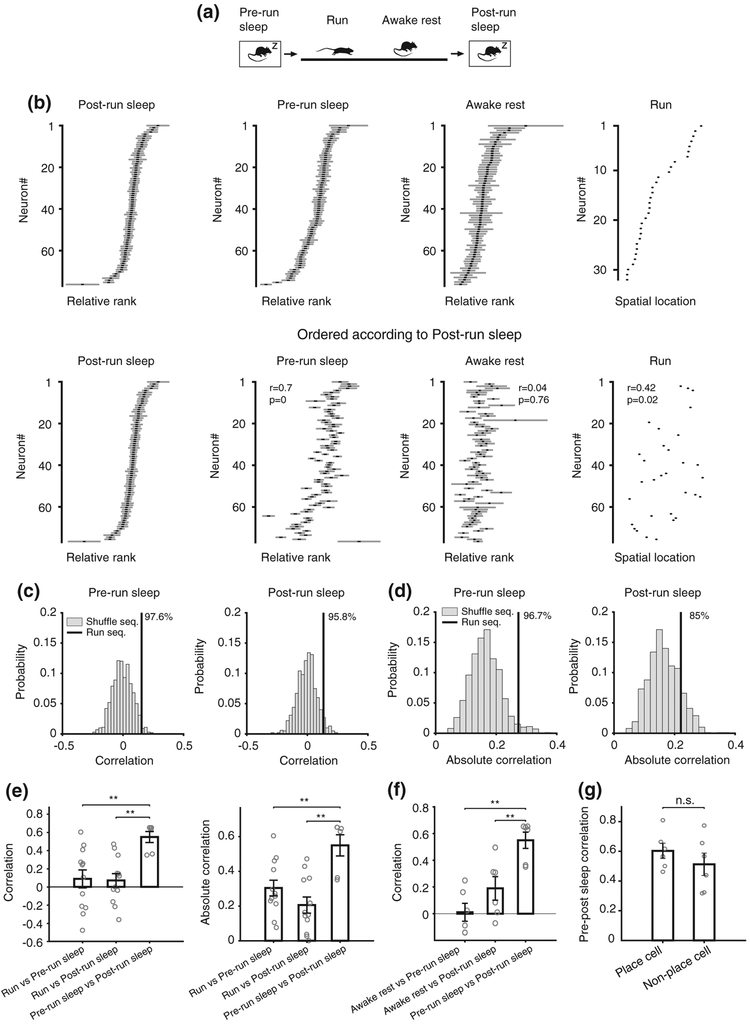
For each of the four behavioral and brain states during which we recorded neuronal ensemble activity (i.e., run, awake rest, pre-run, and post-run sleep, Figure 3a), we computed place-cell spatial sequence templates during run and individual multi-neuronal temporal sequences for each of the awake rest and sleep sessions, based on the relative rank order of the center of mass of cell firing (as in Figure 2g) within the corresponding frames (Figure 3b, top). To investigate the similarity between spatial–temporal sequences across different brain states and behavior and, most importantly, to assess and compare the contribution of pre-run sleep (internally generated model) versus awake rest and run (experience-driven model) to the spontaneous multi-neuronal sequences during the post-run sleep, for each state, we reordered the relative rank of all neurons according to their relative rank during the post-run sleep state (Figure 3b, bottom). While this operation did not appear to affect the correlations between the sequential organization of neurons during the pre- and post-run sleep (6 out of 6 animals had significant correlations, p < .05), it significantly reduced it during the corresponding preceding awake rest (3 out of 6 animals had significant correlations) and run sessions (only 1 out of 6 animals had significant correlations), as shown in Figure 3b, bottom.
FIGURE 3.
Internally generated multi-neuronal sequences predominantly contribute to sleep replay. (a) Diagram of the behavioral setup displaying the sequence of four brain and behavioral states. (b) Top: Example run template (built from place cells active during the run session) and awake rest on the track, pre-run and post-run sleep multi-neuronal sequence templates (all pyramidal cells active in those states) constructed by averaging relative rank order within frames of activity corresponding to individual brain and behavior states. Bottom: Example templates for all brain states, same as top row, but sorted by the cell order as expressed during post-run sleep. r represents the correlation of a template with the post-run sleep template. (c) Correlations between pre-run sleep and run (left) or post-run sleep and run (right) are higher than chance. Numbers at the top indicate the corresponding percentile of run sequence correlation among the correlations from shuffle sequences. (d) Absolute correlations between sleep template and run template. (e) Correlations (left) and absolute correlations (right) between run and sleep templates compared with correlations between pre- and post-run sleep templates. (f) Correlation between awake rest templates and sleep templates. (g) Correlations between sleep templates as a function of cell participation during the run experience (place cells vs silent cells). Error bars represent standard errors
To quantify this effect and, more generally, the similarity between neuronal sequences across brain states and behavior, we computed Spearman's rank–order correlations between neuronal sequence templates across all these states. We found that place cell sequences during run, but not shuffle run sequences, are significantly correlated with the multi-neuronal temporal sequences expressed during the preceding pre-run (i.e., preplay) and the following post-run sleep (i.e., replay) sessions (Figure 3c,d; percentiles of real correlation among shuffles: pre-run 97.6, post-run 95.8; percentiles of absolute correlation among shuffles: pre-run 96.7, post-run 85; real correlation significance, one-tailed test: pre-run p = .024, post-run p = .042, two-tailed: pre-run p = .048, post-run p = .084; absolute correlation significance, one-tailed: pre-run p = .033, post-run p = .15; n = 1,000 independent shuffles). Most importantly, the correlations between temporal sequences during pre-run and post-run sleep (Figure 3e, R = 0.57 ± 0.06, p < .008, Spearman's correlation) were found to be about one order of magnitude higher than those between run and pre-run and run and post-run sleep (pre-run to post-run vs pre-run to run: p = .0032; pre-run to post-run vs post-run to run: p = .0013, Wilcoxon rank sum test. N = 12, 12, 6). This effect was similar when sequences were constructed using putative pyramidal cells recorded from electrodes that did not also record putative inhibitory neurons and when using the pyramidal neurons with the highest (above 50th percentile) cluster quality measure (pre-run to post-run vs pre-run to run: p < .01; pre-run to post-run vs post-run to run: p < .01, for both conditions; data not shown). This relationship between neuronal sequences across brain states and behavior was maintained when the absolute values of all correlations (i.e., regardless of temporal sequences being forward versus reverse p/replay of place cell sequences) were considered (Figure 3e right; R = 0.29, p = .007 for pre-, and R = 0.22, p = .002 for post-run sleep). Furthermore, the correlations between place cell sequences during run and multi-neuronal temporal sequences during sleep were similar for pre-run (i.e., preplay) and post-run sleep (i.e., replay; Figure 3e, p > .05, Wilcoxon signed rank test). Altogether, these findings indicate that the expression of multi-neuronal temporal sequences during post-run sleep is predominantly the result of the default, pre-existing, network organization into temporal sequences expressed during pre-run sleep (internally generated model), rather than simply being the replay representation of place cell sequences expressed during the recent run experience (externally driven model).
One possible factor involved in the much stronger contribution of the sequential network dynamics expressed during pre-run sleep compared to those expressed during run to the sequential dynamics observed during post-run sleep could be the higher similarity in brain state and behavior between the two sleep sessions compared with run (i.e., primarily ripple oscillations and immobility during sleep versus primarily theta oscillations and movement during run). To control for this factor, we compared the correlations between the sequential activities expressed during awake rest (i.e., also during primarily ripple oscillations and immobility), when strong replay of run activity was shown to exist (Davidson et al., 2009; Diba & Buzsaki, 2007; Foster & Wilson, 2006; Karlsson & Frank, 2009), and that expressed during the pre- and post-run sleep, on one hand, with the sequence correlations between the two sleep sessions, on the other hand. Again, as in the case of run, the sequence correlations between awake rest and sleep were much weaker than those between the pre-run and post-run sleep (Figure 3f; p = .002 for pre-run sleep, p = .009 for post-run sleep correlation with awake rest compared with correlation between sleeps, Wilcoxon rank sum test), despite increased similarity between all these brain states and behaviors. These effects were maintained when the neuronal activity during ripple-centered epochs of sleep and awake rest instead of the spiking frames was used to compute multi-neuronal sequences (p < .05 for pre- and for post-run sleep correlations with awake rest compared with correlation between sleeps; data not shown). These results further strengthen the experimental support to the idea that temporal sequence patterns expressed during postrun sleep are predominantly internally generated and that place cell sequences are primarily selected from a pre-existing repertoire of motifs (Dragoi & Tonegawa, 2014) rather than being entirely created during the de novo run experience (Kudrimoti et al., 1999; Lee & Wilson, 2002; Silva et al., 2015).
With regard to a specific spatial run experience, the hippocampal pyramidal neurons that are active during sleep and awake rest are functionally organized into two groups: place cells (O'Keefe & Dostrovsky, 1971), also active during the run experience, and “silent” cells (Thompson & Best, 1989) that do not contribute to the spatial encoding during the specific run experience. We investigated whether the two groups of cells are similarly organized into multi-neuronal temporal sequences before and after a de novo run experience by computing correlation values between the pre-run and post-run sleep temporal sequences separately for both groups and comparing their strength. We found that despite directly participating in the encoding of de novo spatial run experience, the group of place cells had very high similarity between their temporal sequences during the pre- and post-run sleep, which was as strong as that of the silent cells group (Figure 3g; p = .31, Wilcoxon signed rank test, n = 6). This indicates that multi-neuronal temporal sequence organization in the CA1 is less dependent on the direct participation of individual neurons in a recent spatial experience and is not significantly affected by that experience, consistent with these temporal sequences being predominantly internally generated (Figure 1, bottom).
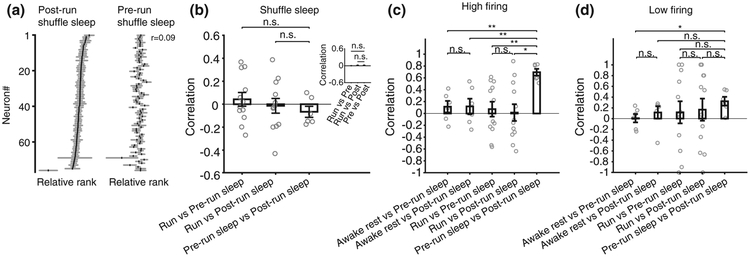
Next, we examined whether the correlations between multi-neuronal sequences of different sleep sessions were a byproduct of firing rate correlations. To address this, we generated shuffled sleep activity by shuffling the temporal order of neurons within each frame while maintaining their individual firing rates (Figure 4a). We found that the correlations between multi-neuronal sequences in pre- and post-run sleep using shuffled sleeps were decreased to chance levels and were similar with those between sleep and run (Figure 4b, p = .29 and p = .82 for one-time shuffle of all sleep frames, Wilcoxon rank sum tests, N = 12, 12, 6; see inset for averages of 30 independent all-frames sleep shuffles). These results further indicate that multi-neuronal sequence correlations (Figure 3) arise from the organization of neurons into sequences and not from inhomogeneities in neuronal firing rates across the network (Peyrache et al., 2010; Silva et al., 2015) or from potential firing rate correlations across sleep sessions (Hirase et al., 2001).
FIGURE 4.
Single cell excitability and multi-neuronal sequences. (a) Post-run sleep template and pre-run sleep template sorted according to post-run sleep template in an example animal. (b) Correlations between run and multi-neuronal sequences of shuffled-sleep compared with correlations between multi-neuronal sequences of pre- and post-run shuffled-sleep. Inset: The correlation when shuffle and correlation were repeated 30 times (p = .33, p = .89, Wilcoxon rank sum test). (c) Comparison of correlations using multi-neuronal sequences constructed from high-firing neurons only. (d) Comparison of correlations using multi-neuronal sequences constructed from low-firing neurons only
We have previously reported that the degree of long-term synaptic plasticity onto neurons correlates with their place field peak firing rates expressed during a spatial experience, with high-firing neurons being less susceptible to change and low-firing neurons being more plastic (Dragoi, Harris, & Buzsaki, 2003). More recently, this diversity in neuronal firing, measured at the overall average firing rate, was suggested to define the existence of subgroups of neurons in the CA1 whereby high-firing neurons are more rigid and contribute equally to sleep preplay and replay, whereas low-firing neurons are more plastic and contribute more to the sleep replay of a recent spatial experience (Grosmark & Buzsaki, 2016). Under this simplified scenario, multi-neuronal sequences composed of high-firing neurons are expected to exhibit pre- to post-run sleep correlations that are stronger than those between run/awake rest and post-run sleep, as depicted in Figure 1, bottom. At the same time, the evidence that low-firing cells are plastic should come from the correlation between post-run sleep with run (and with awake rest) being stronger compared with the correlation between pre-run sleep and run (and with awake rest), as depicted in Figure 1, top. Furthermore, the evidence that low-firing cells are more plastic than the high-firing cells should come from stronger post-run sleep to run (and awake rest) correlation values for low-firing cells compared with high-firing cells.
To explore these possibilities, we separated our neurons based on their average firing rate into high-firing (above the median average rate of all neurons) and low-firing neurons (below the median). We computed multi-neuronal sequences separately for each of the two neuronal subgroups and calculated their correlations across different states and behaviors (as in Figure 3). We found that the high-firing group maintained the stronger correlations between the pre-run and post-run sleep sessions compared with their sleep-run and sleep-awake rest correlations (Figure 4c, pre-run vs post-run sleep compared with: pre-run sleep vs awake p = .002; post-run sleep vs awake p = .004; pre-run sleep vs run, p = .002; post-run sleep vs run, p = .01; Wilcoxon ranksum test; comparison of pre-run sleep vs awake with post-run sleep vs awake, p = 1; pre-run sleep vs run with post-run sleep vs run, p = .62). At the same time, in the low-firing group, the correlation between pre-run sleep and run was not different from that between post-run sleep and run (p = 1, Wilcoxon rank sum test), while correlation of post-run sleep with run was not different in low-firing cells compared with high-firing cells (p = .57, Wilcoxon rank sum test; Figure 4d: p = .03; p = .2; p = .66; p = 1; p = .57; p = 1 for low-firing group results using same comparisons as for the high-firing group results shown in Figure 4c).
We conclude that high-firing cells do contribute more to the stability of hippocampal network across experiences; however, using multi-neuronal sequence analysis, we do not have experimental evidence to support the hypothesis that low-firing cells are plastic nor the hypothesis that low-firing cells are more plastic than the high-firing cells. As a possible interpretation for these findings, we suggest that, rather than being more plastic, the low-firing cells appear generally more variable across brain states and behaviors compared with the high-firing cells. We cannot exclude the possibility that other forms of plasticity, not tested in this study, might occur stronger in the low-firing cells compared with the high-firing ones (Grosmark & Buzsaki, 2016).
4 ∣. DISCUSSION
We have demonstrated that the CA1 hippocampal neurons are internally organized into robust multi-neuronal temporal sequences that can be rapidly recruited and edited to encode novel sequential spatial information and to later replay it during the subsequent awake rest and sleep epochs. Our data provide strong support for the internal generation and preconfiguration of these temporal sequences, likely achieved by synaptic connectivity with and within upstream areas CA3 and entorhinal cortex, as well as for their default expression even in the absence of explicit inputs from the external world, such as during slow-wave sleep (Dragoi & Tonegawa, 2014). Most importantly, we demonstrate that this sequential organization of neuronal ensembles during the sleep session preceding a de novo run experience can explain far better than the run experience the expression of multi-neuronal temporal sequences during the post-run sleep collectively known as replay. This effect is not simply due to the similarity in brain state and animal behavior between the pre- and post-run sleep, but rather to the fact that even the novel place cell sequences expressed during the run experience are contributed predominantly by a process of selection from a large internal repertoire of temporal sequence motifs expressed during the pre-run sleep (Dragoi & Tonegawa, 2014; Liu, Sibille, & Dragoi, 2018).
Several previous studies have demonstrated the existence of preplay temporal sequences during sleep (Dragoi & Tonegawa, 2011, 2013b; see also Grosmark & Buzsaki, 2016) and awake rest (Dragoi & Tonegawa, 2011) in the CA1 area of the hippocampus that correlate with future novel place cell sequences expressed during a subsequent spatial run experience. Additional studies have argued that while preplay is present in the network during pre-run sleep, the temporal sequence replay patterns expressed during post-run sleep can be more strongly correlated with the recently experienced place cell sequences than with preplay, particularly in pretrained animals (Dragoi & Tonegawa, 2013a; Grosmark & Buzsaki, 2016). The importance of preconfigured dynamics was also suggested by the strong relationship between the spontaneously organized patterns and the optogenetically induced events in the hippocampus during ripples (Stark et al., 2015). However, no study so far has directly compared the relative contributions of sequence preplay on the one hand and of a recent run and awake rest experience on the other hand to sequence replay in post-experience sleep. Our results point to a much stronger contribution of the preconfigured and preplay patterns during pre-run sleep to the replay patterns in post-run sleep when compared with the contribution of the run experience and awake rest replay. This finding is consistent with the smaller magnitude in the amount of change in space–time correlations from preplay to replay when compared with the overall level of preplay correlations (i.e., preplay >> replay minus preplay), as described earlier (Dragoi & Tonegawa, 2013a; also apparent in Grosmark & Buzsaki, 2016).
Most of the previous studies on replay and preplay have used correlation methods to compare whole session-average place cell sequence templates or run trajectories with instantaneous temporal sequences within individual frames of activity during sleep and awake rest (Davidson et al., 2009; Diba & Buzsaki, 2007; Dragoi & Tonegawa, 2011; Karlsson & Frank, 2009; Lee & Wilson, 2002). The resulting correlation values were often averaged over entire sleep or awake sessions and presented and compared as mean preplay and replay parameter values. Our current approach aims to directly compare temporal sequential organization of neurons across sleep sessions and therefore calculates and compares multi-neuronal temporal sequences computed over entire sleep sessions. The resulting parameters are representative for the entire sleep and awake rest sessions and allow for the simplest direct comparison of the strength of correlations between each and all of the four brain states and behavior defined earlier (i.e., run, awake rest, pre-run, and post-run sleep).
Our findings have relevance for the understanding of neuronal mechanisms underlying rapid memory formation as well as for understanding the role of the hippocampus in internally generated representations (Dragoi & Tonegawa, 2014) such as cognitive mapping (O'Keefe & Nadel, 1978), memory recall (Tulving, 1969), imagining (Hassabis, Kumaran, Vann, & Maguire, 2007), and planning (Miller, Botvinick, & Brody, 2017). The earlier studies on preplay have already established the existence of a preconfigured network of temporal sequence motifs in the experimentally-naïve hippocampus using template-matching and Bayesian decoding algorithms (Dragoi & Tonegawa, 2011; Dragoi & Tonegawa, 2013b). Here, we extend those findings using different methods and not only confirm the existence of such preconfigured sequential patterns, but also, most importantly, demonstrate their robustness and predominant role in the expression of sequence replay patterns in the hippocampus during post-experience slow-wave sleep. These temporal sequence patterns are not a simple byproduct of the inhomogeneity in firing rates across the CA1 network. Rather, they seem to reflect a more genuine temporal code (Dragoi, 2013) for encoding and representation of novel sequential information within hippocampal circuits.
ACKNOWLEDGMENTS
This work was supported by a Whitehall Foundation grant, a Brain and Behavior Foundation NARSAD Young Investigator fellowship and a Charles H. Hood Foundation award (G.D.). The reported data are archived on file servers at the Yale Medical School.
Funding information
Charles H. Hood Foundation; National Alliance for Research on Schizophrenia and Depression; Whitehall Foundation
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interests.
REFERENCES
- Arieli A, Sterkin A, Grinvald A, & Aertsen A (1996). Dynamics of ongoing activity: Explanation of the large variability in evoked cortical responses. Science, 273(5283), 1868–1871. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Horvath Z, Urioste R, Hetke J, & Wise K (1992). High-frequency network oscillation in the hippocampus. Science, 256(5059), 1025–1027. [DOI] [PubMed] [Google Scholar]
- Davidson TJ, Kloosterman F, & Wilson MA (2009). Hippocampal replay of extended experience. Neuron, 63(4), 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diba K, & Buzsaki G (2007). Forward and reverse hippocampal place-cell sequences during ripples. Nature Neuroscience, 10(10), 1241–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoi G (2013). Internal operations in the hippocampus: Single cell and ensemble temporal coding. Frontiers in Systems Neuroscience, 7, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoi G, & Buzsaki G (2006). Temporal encoding of place sequences by hippocampal cell assemblies. Neuron, 50(1), 145–157. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Harris KD, & Buzsaki G (2003). Place representation within hippocampal networks is modified by long-term potentiation. Neuron, 39(5), 843–853. [DOI] [PubMed] [Google Scholar]
- Dragoi G, & Tonegawa S (2011). Preplay of future place cell sequences by hippocampal cellular assemblies. Nature, 469(7330), 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoi G, & Tonegawa S (2013a). Development of schemas revealed by prior experience and NMDA receptor knock-out. eLife, 2, e01326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoi G, & Tonegawa S (2013b). Distinct preplay of multiple novel spatial experiences in the rat. Proceedings of the National Academy of Sciences of the United States of America, 110(22), 9100–9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoi G, & Tonegawa S (2014). Selection of preconfigured cell assemblies for representation of novel spatial experiences. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 369(1635), 20120522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Dudchenko P, Wood E, Shapiro M, & Tanila H (1999). The hippocampus, memory, and place cells: Is it spatial memory or a memory space. Neuron, 23(2), 209–226. [DOI] [PubMed] [Google Scholar]
- Foster DJ, & Wilson MA (2006). Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature, 440(7084), 680–683. [DOI] [PubMed] [Google Scholar]
- Grosmark AD, & Buzsaki G (2016). Diversity in neural firing dynamics supports both rigid and learned hippocampal sequences. Science, 351(6280), 1440–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Vann SD, & Maguire EA (2007). Patients with hippocampal amnesia cannot imagine new experiences. Proceedings of the National Academy of Sciences of the United States of America, 104(5), 1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirase H, Leinekugel X, Czurko A, Csicsvari J, & Buzsaki G (2001). Firing rates of hippocampal neurons are preserved during subsequent sleep episodes and modified by novel awake experience. Proceedings of the National Academy of Sciences of the United States of America, 98(16), 9386–9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, & Wilson MA (2007). Coordinated memory replay in the visual cortex and hippocampus during sleep. Nature Neuroscience, 10(1), 100–107. [DOI] [PubMed] [Google Scholar]
- Karlsson MP, & Frank LM (2009). Awake replay of remote experiences in the hippocampus. Nature Neuroscience, 12(7), 913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenet T, Bibitchkov D, Tsodyks M, Grinvald A, & Arieli A (2003). Spontaneously emerging cortical representations of visual attributes. Nature, 425(6961), 954–956. [DOI] [PubMed] [Google Scholar]
- Kudrimoti HS, Barnes CA, & McNaughton BL (1999). Reactivation of hippocampal cell assemblies: Effects of behavioral state, experience, and EEG dynamics. The Journal of Neuroscience, 19(10), 4090–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AK, & Wilson MA (2002). Memory of sequential experience in the hippocampus during slow wave sleep. Neuron, 36(6), 1183–1194. [DOI] [PubMed] [Google Scholar]
- Liu K, Sibille J, Dragoi G. 2018. Generative predictive codes by multiplexed hippocampal neuronal tuplets. Neuron 99(6):1329–1341 e6. [DOI] [PubMed] [Google Scholar]
- Luczak A, Bartho P, & Harris KD (2009). Spontaneous events outline the realm of possible sensory responses in neocortical populations. Neuron, 62(3), 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, & O'Reilly RC (1995). Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review, 102(3), 419–457. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Botvinick MM, & Brody CD (2017). Dorsal hippocampus contributes to model-based planning. Nature Neuroscience, 20(9), 1269–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadasdy Z, Hirase H, Czurko A, Csicsvari J, & Buzsaki G (1999). Replay and time compression of recurring spike sequences in the hippocampus. The Journal of Neuroscience, 19(21), 9497–9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, & Dostrovsky J (1971). The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Research, 34(1), 171–175. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, & Nadel L (1978). The hippocampus as a cognitive map. Oxford: Oxford University Press. [Google Scholar]
- Peyrache A, Benchenane K, Khamassi M, Wiener SI, & Battaglia FP (2010). Sequential reinstatement of neocortical activity during slow oscillations depends on cells' global activity. Frontiers in Systems Neuroscience, 3, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, & Milner B (1957). Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry, 20(1), 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva D, Feng T, & Foster DJ (2015). Trajectory events across hippocampal place cells require previous experience. Nature Neuroscience, 18(12), 1772–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, & McNaughton BL (1996). Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science, 271(5257), 1870–1873. [DOI] [PubMed] [Google Scholar]
- Squire LR (1992). Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review, 99(2), 195–231. [DOI] [PubMed] [Google Scholar]
- Stark E, Roux L, Eichler R, & Buzsaki G (2015). Local generation of multineuronal spike sequences in the hippocampal CA1 region. Proceedings of the National Academy of Sciences of the United States of America, 112(33), 10521–10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LT, & Best PJ (1989). Place cells and silent cells in the hippocampus of freely-behaving rats. The Journal of Neuroscience, 9(7), 2382–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E (1969). Retrograde amnesia in free recall. Science, 164(875), 88–90. [DOI] [PubMed] [Google Scholar]
- Wilson MA, & McNaughton BL (1994). Reactivation of hippocampal ensemble memories during sleep. Science, 265(5172), 676–679. [DOI] [PubMed] [Google Scholar]