Abstract
The right intraparietal sulcus (rIPS) is a key region for the endogenous control of selective visual attention in the human brain. Previous studies suggest that the rIPS is especially involved in top-down control and spatial distribution of attention across both visual hemifields. We further explored these attentional functions using transcranial direct current stimulation (tDCS) of the rIPS to modulate behavioral performance in a partial report task. Performance was analyzed according to the theory of visual attention (TVA) (Bundesen, 1990), which provides a computational framework to investigate different parameters of visuo-attentional processing such as top-down control, attentional weighting, capacity of visual short term memory, and processing speed. We investigated the effects of different tDCS current strengths (1 mA and 2 mA) in two experiments: 1 mA tDCS (anodal, cathodal, sham) did not affect any of the TVA parameters, but cathodal 2 mA stimulation significantly enhanced top-down control as evidenced by a reduction of the α parameter of TVA, regardless of hemifield. This differential impact on the top-down control component of attentional processing suggests that the horizontal rIPS is mainly involved in attentional selection as none of the spatial or resource variables of TVA were altered. Furthermore, the data add evidence to previous work highlighting (1) the importance of using appropriate current strength in stimulation protocols, and (2) that the often reported inhibitory effect of cathodal stimulation in e.g., motor tasks might not extend to cognitive paradigms.
Introduction
Selective visual attention, i.e., focusing on relevant while ignoring irrelevant stimuli, is a key capacity of the human brain. Endogenous control and spatial distribution of selective attention are attributed to the parietal cortex (Corbetta et al., 2008; Vandenberghe and Gillebert, 2009). In particular, the intraparietal sulcus is involved in establishing attentional priority maps and calibrating attentional weights (Molenberghs et al., 2007). Most theories, empirical findings from brain-damaged patients, and functional imaging studies argue for a right-hemispheric dominance of attentional control. Hence, one prominent theory proposes that right parietal cortex controls attention in both visual hemifields, whereas the left hemisphere attends to contralateral hemispace only (Mesulam, 1999).
However, visual selection is not a unitary process. The theory of visual attention (TVA) (Bundesen, 1990) provides a computational framework to disentangle its subcomponents: top-down control, attentional weighting, capacity of visual short-term memory (VSTM), and processing speed. TVA is based on a race model of selection and recognition, in which the competing elements are encoded into the VSTM depending on their subjective biases and sensory evidence (Bundesen, 1990; Kyllingsbaek, 2006). TVA has been successfully applied in both healthy subjects and patients with parietal damage suffering from neglect (Duncan et al., 1999; Habekost and Bundesen, 2003; Finke et al., 2005; Peers et al., 2005; Habekost and Rostrup, 2007). To date, however, a mapping of specific TVA parameters to neural correlates has not yet been achieved.
Noninvasive brain stimulation techniques, such as transcranial direct current stimulation (tDCS), provide valuable insights into neural mechanisms underlying cognitive processing. TDCS allows for polarity-dependent facilitatory (anodal) or inhibitory (cathodal) stimulation (Nitsche et al., 2008) resulting in effects that outlast the stimulation itself. These so-called “after-effects” are thought to be mediated by changes of membrane polarization thresholds, effects exerted upon glutamatergic synapses, and the involvement of intracortical interneurons (Stagg and Nitsche, 2011). Variation of stimulation parameters (i.e., current strength) results in differential outcomes that must be carefully considered when refining tDCS protocols (Nitsche et al., 2008). TDCS applied over the parietal cortex has been shown to rebalance line bisection biases of neglect patients and to enhance visual detection in healthy subjects depending upon current polarity and stimulated hemisphere (Sparing et al., 2009).
This study aimed at investigating the differential effects of parietal tDCS on visual attention as assessed with TVA. Due to the known dependency of tDCS effects on current strength, we ran two consecutive experiments with different tDCS current strengths (1 mA and 2 mA). We hypothesized that tDCS applied over horizontal rIPS would affect visual attention in a polarity-specific manner. As the horizontal rIPS is a key region for endogenous top-down control and spatial distribution of attention, we expected to alter the TVA parameters α (top-down control) and attentional weights (spatial distribution).
Materials and Methods
Participants
Twenty right-handed healthy volunteers without CNS-acting medication or recreating drugs (mean age 25.86 years, 10 males) participated in the study. The experiments were performed in accordance with the declaration of Helsinki and had been approved by the local ethics committee. All subjects gave written informed consent and received monetary allowance compensational for their participation.
Design and procedure
Partial report paradigm.
Subjects performed a partial report paradigm (Shibuya and Bundesen, 1988; Bundesen, 1990; Duncan et al., 1999; Finke et al., 2005) in which they were asked to detect and verbally report predefined target letters (subtending 0.5° visual angle), while ignoring irrelevant distractor letters. Target letters were defined by their color in different experimental blocks. When target letters were defined by the color red, the distractors were green and vice versa. Letters for targets and distractors were randomly chosen from the set ABEFHJKLMNPRSTWXYZ. The stimuli were presented on a black background in the corners of a virtual square (4.7 × 4.7°) centered upon a fixation cross.
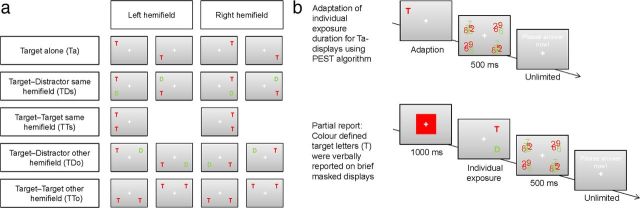
Stimulus arrays contained either one or two letters, arranged in rows or columns, resulting in five different experimental conditions. (1) One target was presented alone [Target-alone (Ta)]. (2) A target was accompanied by a distractor in the same hemifield [Target-Distractor-same-hemifield (TDs)]. (3) Two targets were presented in the same hemifield [Target-Target-same-hemifield (TTs)]. (4) A target was accompanied by a distractor in the opposite hemifield [Target-Distractor-other-hemifield (TDo)]. (5) Two targets were presented in opposite hemifields [Target-Target-other-hemifield (TTo)].
Hence, when also taking the hemifield (left vs right) into account, the four conditions in which two stimuli were presented (conditions 2–5) could be described in terms of a 2 × 2 × 2 design, with the factors: hemifield (left vs right), display (same vs opposite hemifield), and relevance of the second stimulus (target vs distractor). In sum, there were 16 different display conditions: Four Ta conditions, eight TD conditions, and four TT conditions (Fig. 1a). It is noteworthy that the TT other-hemifield conditions for left and right hemifields are virtually identical, but were analyzed separately with respect to both target positions (e.g., the TT other-hemifield condition for right targets indicates the percentage of correctly reported items in the right hemifield only). Therefore, the design is not a simple factorial one. In total, 288 trials were presented per experimental session.
Figure 1.
Display conditions and design. a, The Partial Report paradigm consisted of different display conditions (T, target; D, distractor). b, Experimental design.
Figure 1b is an illustration of the stimulus sequences presented to the subjects. Participants were instructed to keep fixation on a small white cross (0.3°) during the entire experiment.
A square presented for 1000 ms around the fixation indicated the relevant target color (which changed every 16 trials) to the subjects. Then, one of the stimulus arrays (compare Fig. 1a) appeared in randomized order. Stimuli were followed by a mask (0.7 × 0.7°) of superimposed red and green digits (0.5°). Participants were instructed to verbally report the target letters and to avoid guessing. In the two target conditions (TTs and TTo), both letters were chosen independently from the predefined letter set (two identical letters were allowed). There was no time limit for answering and the order of the report was arbitrary. The experimenter entered the answers in a keyboard and started the next trial by pressing a button.
Procedure.
The study consisted of two experiments conducted in the same sample of participants. Each experiment contained different sessions involving different types of stimulation (see Transcranial direct current stimulation). Each session included an introductory practice session, followed by the calibration of the individual stimulus exposure duration, the direct current stimulation, and the partial report task.
The general procedure was identical for all stimulation sessions. Subjects were seated in front of a monitor (19′', flat screen, 1280 × 1024 resolution, viewing distance 85 cm) in a darkened and sound-proof cabin. Subjects placed their head on a chin rest to stabilize their head position and eye movements were monitored using an infrared eye tracker (see Eye tracking).
Each session started with a short practice version of the task. Next, each subject's individual stimulus exposure duration was determined for target alone (Ta) displays. Beginning with an exposure duration below the perceptual threshold (50 ms, lower than t0 in terms of TVA), exposure durations were adapted to achieve a detection performance of 70%. The 70% threshold was determined using the PEST algorithm (Taylor, 1967). Subsequently, the tDCS stimulation (see Transcranial direct current stimulation) was delivered and followed by the actual partial report task. The task lasted on average 15.27 min. As the exposure duration was adapted individually and time for answering was unlimited, variation between subjects occurred.
Estimation of TVA parameters.
The individual performance of each subject (i.e., the number of correctly reported letters in each trial) was used for computing the TVA parameters. Using a trial-by-trial maximum likelihood fitting procedure (LIBTVA) (Kyllingsbaek, 2006; Dyrholm et al., 2011), mean values for the TVA parameters K, t0, w, α, and C were obtained. Attentional weights of targets and distractors w, as well as top-down control α values were computed for all four display positions. One K-value for the VSTM-capacity and one C-value (i.e., processing speed) were computed. K is the number of elements which can maximally be stored in VSTM. Because a maximum of two stimuli were presented in the current version of the partial report task, K was set at ≤2. t0 is the minimal exposure duration for conscious perception. Displays presented with shorter exposure than t0 were not reported by the subject. If only one exposure duration for stimulus displays is used as in this experiment, t0 is usually assumed to be zero. To be more precise, we used the data of the exposure duration estimation session with the PEST algorithm to estimate t0 values from the data of each individual subject. As the PEST algorithm uses different exposure durations to find the 70% benchmark, an exponential curve can be fitted to the individual performance (number of correctly reported items) as a function of exposure duration. LIBTVA uses a maximum likelihood fitting procedure to fit this exponential curve to find t0: the value between the largest exposure duration a subject does not report anything and the shortest exposure where the subject reports for the first time. This estimation of t0 provides a closer fit to each subject's actual t0 than assuming t0 to be zero, and consequently results in more precise fits (Dyrholm et al., 2011).
Attentional weights of targets w (i.e., the sensory evidence that an element is relevant) were computed for all four possible display positions and reflect the spatial distribution of attention. The parameter α reflects attentional top-down control and is defined as the ratio of distractor weights to target weights. Hence, values close to zero indicate perfect selection, whereas values close to one indicate no selection (and values larger than one indicate that distractors were perceived more than targets). The processing capacity C, measured in elements per second, describes the velocity of attentional processing in general. It is not measured for each position individually, but for the whole display.
For a detailed mathematical description of the computation of all values, we refer to the relevant literature (Bundesen, 1990; Kyllingsbaek, 2006; Dyrholm et al., 2011).
Transcranial direct current stimulation.
A constant current stimulator (neuroConn) generated a current that was transferred through a pair of surface rubber electrodes, covered with electrode cream. The exact anatomical location of electrode placement was specified with stereotactical neuronavigation, using a Polaris infrared tracking device and Brainsight software 2.1 (Rogue Research). For anatomical images at high resolution, a T1-weighted magnetization-prepared, rapid gradient echo (MP-RAGE) pulse sequence was used with the following parameters: FoV read 256 mm, FoV phase 100%, slice thickness 1.00 mm, TR = 2250 ms, TE = 3.03 ms, distance factor 50%, orientation sagittal, flip angle 9°, 176 slices. The subject's individual T1 weighted MR brain image was coregistered to anatomical surface landmarks of the subject's head. The stimulation electrode (35 cm2) was centered on the horizontal part of the right intraparietal sulcus and aligned parallel to the mid-sagittal plane. The reference electrode (96 cm2) was fixed contralaterally above the left orbit. To avoid any adverse effects of the reference electrode, its size was increased to reduce current densities and make this stimulation functionally inert (Nitsche et al., 2007). Furthermore, to keep the itching sensation at the beginning of the stimulation to a minimum, the current was faded in and faded out over 8 s at the start and end of stimulation.
Experiment 1 consisted of three different stimulation sessions (anodal, cathodal, and sham) with a current strength of 1 mA for 20 min (current density: 0.0286 mA/cm2). To allow a successful blinding of participants, sham stimulation was performed in the same way as active stimulation, but the current was turned off after 30 s so that subjects could realize the itching sensation at the beginning of the stimulation. Subsequent to Experiment 1, Experiment 2 was conducted in the same sample of subjects. Experiment 2, however, included two sessions only, in which cathodal and sham stimulation were performed with a constant current of 2 mA for 20 min (current density: 0.0571 mA/m2). Anodal stimulation was not included in the second experiment because of missing effects at the behavioral level in Experiment 1. Both stimulation protocols complied with current safety guidelines (Nitsche et al., 2003, 2008). The different stimulation sessions were pseudo-randomized for each experiment (in the first experiment no perfect counterbalancing for session order could be achieved, as only 20 subjects participated in the study. Seven subjects started with anodal stimulation, 5 with cathodal stimulation, and 8 with sham stimulation. In the second experiment the order was counterbalanced). The sessions were separated by at least 48 h to avoid any putative carry over effects of the previous session (Nitsche et al., 2008).
Eye tracking.
Eye movements were monitored and recorded using a high speed eye tracking device (IVIEW XTM Hi-Speed, SMI), with a sampling rate of 1000 Hz. Subjects were instructed to concentrate on a fixation cross during the entire experiment. Eye movements were monitored online and the subjects were given feedback during and after the training. ILAB (Gitelman, 2002) was used to analyze eye movement datasets.
Statistical analysis.
Individual mean performance scores (i.e., the percentage of correctly reported target letters) were calculated for each of the five display conditions, separately for left and right hemifields.
Statistical analyses were performed using IBM SPSS statistics version 19. Data were analyzed with repeated measures ANOVAs and are reported at a significance level of p < 0.05 for all analyses. Where appropriate, degrees of freedom were Greenhouse–Geisser corrected.
We investigated the effect of stimulation on the attentional parameters measured with TVA. To accomplish this, 3 × 4 (Experiment 1) [or 2 × 4 (Experiment 2)] ANOVAs with the factor stimulation [1 mA: anodal, cathodal, sham (first experiment) | 2 mA: cathodal, sham (second experiment)] and position [left upper, left lower, right upper, right lower] were conducted for the α values and attentional weights of targets. As attentional weights are measured on ratio scales with arbitrary units, it is not possible to compare weights between stimulation conditions. Therefore, we normalized the attentional weights for targets within each stimulation condition and used the normalized weights within the ANOVAs. For the position-independent variables, K, C, and t0 estimates were calculated and analyzed for both experiments in repeated measure one-way ANOVAs with the factor stimulation (1 mA: anodal, cathodal, sham; or 2 mA: cathodal, sham, respectively).
To compare the results from Experiment 1 and 2, data from both experiments were combined to test for the effect of current strength of cathodal stimulation. The factor current strength (1 mA, 2 mA) was added, resulting in a 2 × 2 × 4 ANOVA for α values and attentional weights of targets, and a 2 × 2 × 1 ANOVA, for the K, C, and t0, respectively.
To investigate whether or not tDCS influenced raw performance scores (i.e., the number of correctly reported letters) in the two-item conditions, separate ANOVAs were conducted with the factors: Stimulation (dependent on the experiment), hemifield (targets in the left or right hemifield), display (the second item is presented in the same or other hemifield), and relevance (the second item is a distractor or a second target). For the first experiment, comprising three experimental sessions, the factor stimulation contained the 1 mA anodal, cathodal, and sham data, leading to a 3 × 2 × 2 × 2 ANOVA. Furthermore, we conducted two additional 2 × 2 × 2 × 2 ANOVAs with two types of stimulation (anodal/sham or cathodal/sham, respectively) to find the most effective stimulation. For the 2 mA experiment, the 2 × 2 × 2 × 2 ANOVA contained data from the two stimulation sessions, 2 mA cathodal and 2 mA sham. As the target-alone condition did not fit into our quasi-factorial design, we calculated and compared performance scores in separate 3 × 2 ANOVA with the factors stimulation (1 mA: anodal, cathodal, sham) and hemifield (left, right) for the first experiment and a 2 × 2 ANOVA with the factor stimulation (2 mA: cathodal, sham) and hemifield (left, right) for the second experiment.
To assure that session order did not affect our results (as it was not perfectly counterbalanced within Experiment 1), we also conducted an additional ANOVA with the factors session order, hemifield, display, and relevance, for the first experiment. There were no significant effects of session order.
Results
Eye-tracking
During the critical time interval of stimulus presentation, subjects maintained central fixation in >90% of all trials for all stimulation sessions (stimulation sessions in percentage ± STD: anodal 1 mA: 96% ± 3%; cathodal 1 mA: 93% ± 6%; sham 1 mA: 97% ± 3%; cathodal 2 mA: 96% ±5% sham 2 mA: 95% ±6%). We excluded all trials in which subjects did not fixate from further analysis. On average, 6% of all trials were discarded (∼17 trials per session).
Performance patterns
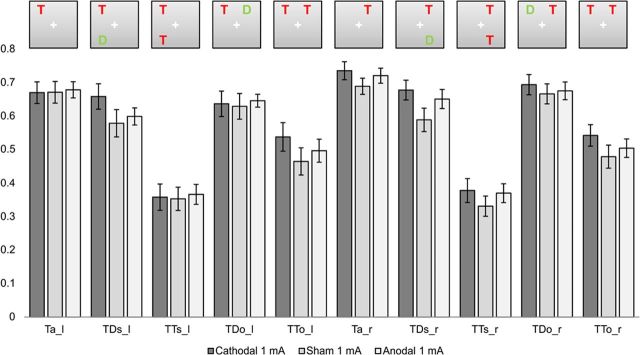
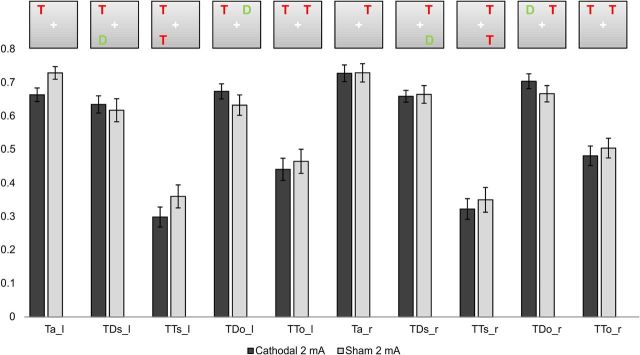
Inspection of the raw performance data (the percentage of correctly reported letters in the different experimental conditions; compare Figs. 2 and 3), regardless of tDCS stimulation, revealed the expected pattern of results in the partial report task: Performance was highest in the Ta condition when only one target stimulus was present. Performance was lowest when two targets were present, particularly when both targets were presented in the same hemifield.
Figure 2.
Experiment 1. Performance (percentage correctly reported items) (±SEM) for left and right hemifields in the five display conditions.
Figure 3.
Experiment 2. Performance (percentage correctly reported items) (±SEM) for left and right hemifields in the five display conditions.
This differential effect of accompanying target versus distractor stimuli generates the basis for the α parameter of the TVA as an indicator of top-down control of selective attention.
Alpha
Table 1 depicts the values for α in the 5 different stimulation conditions for the 4 stimulus positions.
Table 1.
Alpha for each position and stimulation condition
| Top-down control alpha for stimulation sessions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Display position | Experiment 1 |
Experiment 2 |
||||||||
| Cathodal 1 mA |
Sham 1 mA |
Anodal 1 mA |
Cathodal 2 mA |
Sham 2 mA |
||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Left upper | 0.350 | 0.085 | 0.532 | 0.107 | 0.477 | 0.137 | 0.211 | 0.043 | 0.540 | 0.209 |
| Left lower | 0.384 | 0.076 | 0.408 | 0.103 | 0.598 | 0.121 | 0.329 | 0.088 | 0.632 | 0.138 |
| Right upper | 0.486 | 0.101 | 0.363 | 0.070 | 0.346 | 0.063 | 0.240 | 0.066 | 0.327 | 0.065 |
| Right lower | 0.446 | 0.144 | 0.778 | 0.269 | 0.455 | 0.094 | 0.253 | 0.069 | 0.544 | 0.194 |
Alpha ranges from 0, perfect selection, to 1, no selectivity.
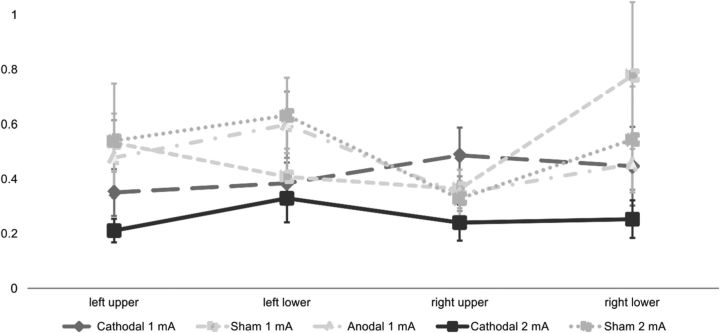
The 3 × 4 ANOVA with the factors stimulation (1 mA: anodal, cathodal, sham) and position (left upper, right upper, left lower, right lower) revealed no significant effects for the 1 mA experiment (all main effects and interactions F < 1.300, p > 0.300) (see also Fig. 4).
Figure 4.
The TVA parameter α (±SEM) for each stimulus location in the two experiments. Smaller values reflect better top-down control.
For Experiment 2, the 2 × 4 ANOVA with the factors stimulation (2 mA: cathodal, sham) and position (left upper, right upper, left lower, right lower) revealed a significant main effect of stimulation (F(1,19) = 6.505, p = 0.020), but no main effect of position (F(2.305, 43.802) = 0.929, p = 0.414) and no stimulation × position interaction (F(2.206, 41.921) = 0.382, p = 0.705). This result reflects a reduction of the α parameter (i.e., an improvement of top-down control) that was independent of the position of the stimuli.
Attentional weights
To analyze whether or not the stimulation had a position-dependent effect on normalized attentional weights of targets, we computed a 3 × 4 ANOVA, with the factor stimulation (1 mA: anodal, cathodal, sham) and position (left upper, right upper, left lower, right lower) for Experiment 1 (Table 2) and a 2 × 4 ANOVA with the factor stimulation (2 mA: cathodal, sham) and position (left upper, right upper, left lower, right lower) for Experiment 2. This analysis revealed a significant main effect of position in both experiments (F > 3.500, p < 0.04) but no stimulation × position interaction (F < 1.1, p > 0.360) position.
Table 2.
Normalized attentional weights for each position and stimulation condition
| Normalized attentional weights for stimulation sessions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Display position | Experiment 1 |
Experiment 2 |
||||||||
| Cathodal 1 mA |
Sham 1 mA |
Anodal 1 mA |
Cathodal 2 mA |
Sham 2 mA |
||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Left upper | 0.286 | 0.119 | 0.261 | 0.107 | 0.282 | 0.093 | 0.288 | 0.122 | 0.266 | 0.116 |
| Left lower | 0.208 | 0.122 | 0.223 | 0.146 | 0.200 | 0.105 | 0.165 | 0.093 | 0.182 | 0.081 |
| Right upper | 0.290 | 0.143 | 0.305 | 0.132 | 0.320 | 0.102 | 0.332 | 0.139 | 0.314 | 0.084 |
| Right lower | 0.217 | 0.093 | 0.212 | 0.114 | 0.198 | 0.083 | 0.215 | 0.128 | 0.238 | 0.108 |
In each column, the sum of the weights equals 1. Due to this, values are comparable across stimulation positions. Larger values indicate higher amounts of attentional resources on that position.
The impact of stimulation on the TVA parameters VSTM-capacity K, processing speed C, and t0 is summarized in Table 3.
Table 3.
Units for the individual parameters are VSTM-capacity K (elements), processing speed C (elements/s), and minimal exposure duration t0 (ms)
| Attentional performance of stimulation sessions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Experiment 1 |
Experiment 2 |
||||||||
| Cathodal 1 mA |
Sham 1 mA |
Anodal 1 mA |
Cathodal 2 mA |
Sham 2 mA |
||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| K | 1.954 | 0.190 | 1.969 | 0.063 | 1.945 | 0.170 | 1.930 | 0.230 | 1.907 | 0.197 |
| C | 11.969 | 5.907 | 12.322 | 5.828 | 13.967 | 6.712 | 13.716 | 8.357 | 13.598 | 8.489 |
| t0 | 68.620 | 32.178 | 68.064 | 28.289 | 78.788 | 30.598 | 63.239 | 25.362 | 61.290 | 16.426 |
VSTM-capacity K
We used only one or two-item displays. Per definition, it is impossible to estimate K-values larger than 2 with this paradigm. Across all stimulations, K was very close to two elements and there was no significant effect of stimulation detected by a one-way ANOVA of the effects of stimulation (1 mA: anodal, cathodal, sham) or (2 mA: cathodal, sham) on the estimates of K (all F < 1.3, p > 0.250).
Processing speed C
Processing speed C is measured in items per second and indicates the rate of processing. Larger values indicate a higher processing speed. We conducted a one-way ANOVA of the effects of stimulation (1 mA: anodal, cathodal, sham) on the estimates of C which revealed no significant effects (F(1.706, 32.420) = 1.904, p = 0.183). In the 2 mA experiment, the analog 2 × 1 ANOVA of the estimates did not reveal a significant effect of stimulation (F(1,19) = 0.005, p = 0.944).
Perceptual thresholds t0
The 3 × 1 ANOVA with the factor stimulation (1 mA: anodal, cathodal, sham) on the estimates of t0 revealed no significant main effect of stimulation (F(1.959, 37.229) = 1.323, p = 0.278).
There was no effect of stimulation on the estimates of t0 in the second experiment (2 × 1 ANOVA, stimulation (F(1,19) = .168, p = 0.687).
Performance scores
To investigate the effect of stimulation in the raw performance data (Fig. 2), we calculated an ANOVA with the factors stimulation (1 mA: anodal, cathodal, sham), hemifield (left, right), display (same, other) and relevance (target, distractor) on the percentage of correctly reported items in each condition.
The 3 × 2 × 2 × 2 repeated-measures ANOVA for the first experiment contained data of the anodal, cathodal, and sham 1 mA stimulation. The three-way interaction of stimulation × display × relevance was significant (F(1.994, 37.880) = 3.961, p = 0.027). Further testing showed that this effect was mainly driven by cathodal 1 mA stimulation: Subsequent 2 × 2 × 2 × 2ANOVAs for anodal and cathodal stimulation, compared with sham stimulation, revealed this interaction only when contrasting cathodal and sham stimulation (F(1,19) = 7.610, p = 0.012). In contrast, anodal versus sham stimulation revealed neither a significant main effect nor an interaction involving the factor stimulation (F(1,19)= 0.614, p = 0.443).
The significant stimulation × display × relevance interaction resulted from a differential impact of cathodal 1 mA compared with sham 1 mA tDCS on TDs and TTo conditions, when taking both hemifields into account. There was a significant difference between TDs cathodal and sham conditions, with better performance scores for cathodal tDCS [post hoc t test TDs (left and right hemifield) cathodal vs sham p = 0.025]. A similar pattern was found for TTo conditions (left and right hemifield). Here there was a trend for cathodal 1 mA stimulation to increased performance scores when compared with sham stimulation (post hoc t test p = 0.052). This effect was absent for TDo and TTs conditions, where no significant differences were found (post hoc t tests p > 0.440). Comparing anodal and cathodal stimulations, the effects did not reach significance [post hoc t test TDs (left and right hemifield) cathodal vs anodal p = 0.107; post hoc t test TTo (left and right hemifield) cathodal vs anodal p = 0.106].
No significant differences were found for the comparison of anodal and sham 1 mA conditions (all post hoc t test p values >0.190).
Furthermore, for the Ta condition a 3 × 2 ANOVA with the factor stimulation and hemifield was conducted. There was no significant main effect or interaction of the factor stimulation [main effect stimulation (F(1.767, 33.569)= 0.282, p = 0.729, stimulation × hemifield (F(1.924, 36.557) = 0.943, p = 0.396].
Anodal stimulation caused no significant differences compared with sham stimulation and was therefore not regarded as an efficient stimulation technique to alter attentional processing. Accordingly, further investigation focused on cathodal stimulation only and current strength was enhanced in the second experiment specifically for cathodal stimulation.
The 2 × 2 × 2 × 2 repeated-measures ANOVA comparing 2 mA cathodal and sham stimulation revealed a significant stimulation × relevance interaction (F(1,19) = 7.816, p = 0.012) (Fig. 3). Post hoc t tests revealed that this effect could be attributed to the differential impact on TD compared with TT conditions. Cathodal 2 mA stimulation caused a significant decrease in performance scores in TT conditions (TTs and TTo for left and right hemifields cathodal vs sham 2 mA: p = 0.031) and a trend toward an increase in performance scores for TD conditions (post hoc t test: TDs and TDo for left and right hemifields cathodal vs sham 2 mA: p = 0.158).
For Ta conditions, the 2 × 2 ANOVA with the factors stimulation and hemifield revealed a significant stimulation × hemifield interaction (F(1,19) = 5.261, p = 0.033), but no main effect of stimulation (F(1,19) = 2.214, p = 0.153). Performance was worse after cathodal 2 mA stimulation compared with sham stimulation in the left hemifield (two-sided t test, p = 0.019), but not in the right hemifield (two-sided t test, p = 0.956) (Fig. 3).
Comparison of current strengths (1 mA vs 2 mA)
Alpha.
The 2 × 2 × 4 ANOVA of the estimates of α with the factors strength (1 mA, 2 mA), stimulation (cathodal, sham) and position (left upper, left lower, right upper, right lower) revealed a significant main effect of stimulation (F(1,19) = 7.268, p = 0.014) but no main effect of strength or any other significant interaction (all F values <1.500, p > 0.200).
Attentional weights of targets, VSTM-capacity K, processing speed C, and perceptual thresholds t0.
None of the other TVA parameters (normalized attentional weights of targets, VSTM capacity K, processing speed C, perceptual thresholds t0) were differentially affected by current strength (all p values >0.1).
Performance scores.
The 2 × 2 × 2 × 2 × 2 repeated-measures ANOVA with the factors current strength (1 mA, 2 mA), stimulation (cathodal, sham), hemifield (left, right), display (same, other), and relevance (target, distractor) revealed a significant strength × relevance interaction (F(1,19) = 7.953, p = 0.011). This effect resulted from a differential impact of current strength on TT and TD conditions. Post hoc t tests revealed a significant difference between 1 mA and 2 mA stimulation for TT conditions (p = 0.028) with worse performance after 2 mA cathodal stimulation.
For Ta-conditions, the analogous 2 × 2 × 2 ANOVA with the factors strength (1 mA, 2 mA), stimulation (cathodal, sham), and hemifield (left, right) revealed a significant main effect of strength (F(1,19) = 39.820, p = 0.000), strength × stimulation interaction (F(1,19) = 12.172, p = 0.002), strength × hemifield interaction (F(1,19) = 13.739, p = 0.001), stimulation × hemifield interaction (F(1,19) = 20.535, p = 0.000), and strength × stimulation × hemifield interaction (F(1,19) = 15.353, p = 0.001). Post hoc t test showed that cathodal 2 mA stimulation caused a significant increase of performance in the right hemifield compared with the left hemifield (Ta_r cathodal 2 mA vs Ta_l cathodal 2 mA p = 0.039). Cathodal 1 mA led to a decrease of performance scores in the left hemifield compared with the right hemifield (Ta_r cathodal 1 mA vs Ta_l cathodal 2 mA: p = 0.060).
To summarize, 2 mA cathodal stimulation reduced the TVA parameter α (i.e., the ratio of distractor weights to target weights, thereby indicating increased selection) compared with sham stimulation. Anodal stimulation did not change any of the attentional parameters or performance scores. Normalized attentional weights of targets, K, C, and t0, were not affected by tDCS. Current strength distinctly affected performance scores.
Discussion
The present study aimed at investigating the effects of tDCS applied over the horizontal rIPS on visual attention. A partial report paradigm was used to enable a formal quantification of specific attentional parameters within the framework of the TVA (Bundesen, 1990). Attentional top-down control (reflected in a reduction of the α parameter of the TVA) was enhanced by cathodal tDCS when applied with a current strength of 2 mA (compared with 2 mA sham stimulation), while other parameters of attentional processing remained unaffected by tDCS.
Overall, the performance pattern in the partial report task in both experiments was in good accordance with prior studies (Bundesen, 1990; Duncan et al., 1999; Hung et al., 2005). Introducing a second stimulus to the display reduced the subjects' ability to detect the target, particularly when this stimulus was presented in the same hemifield. The differential impact of adding a second target versus a distractor illustrates the competition for limited attentional resources and reflects the brain's ability to selectively attend to relevant information while ignoring irrelevant aspects of the visual scene. Within the TVA framework, this is more formally expressed as the top-down control parameter α. The 2 mA cathodal stimulation augmented the brain's ability to selectively attend to the target: adding a distractor no longer impaired performance (Fig. 3, TD conditions). In contrast, the presence of a second target induced stronger competition; performance was impaired compared with sham stimulation (Fig. 3, TT conditions). The differential impact of 2 mA cathodal stimulation on the processing of targets and distractors suggests a specific improvement of attentional selection resulting from tDCS applied over right IPS.
At first sight, this facilitatory effect of 2 mA cathodal stimulation may seem surprising, as most previous tDCS studies report a polarity-specific impact of stimulation with inhibitory effects resulting from cathodal stimulation. Note, however, to date most conclusions regarding the effects of tDCS (and its underlying physiology) have been drawn from studies stimulating the primary motor cortex. At least in principle, stimulation effects might differ for nonmotor areas. Consistent with this hypothesis, a recent meta-analytic review showed that polarity-dependent effects of tDCS occur frequently in motor studies, but are less consistently observed in cognitive studies (Jacobson et al., 2012). One might speculate that the 2 mA cathodal stimulation in our experiment enhanced the signal-to-noise ratio and facilitated target detection in TD displays via a decrease of global excitation and thus a decrease in the amount of suprathreshold activations caused by targets. Such a mechanism was proposed to focus perception after cathodal stimulation applied over V5 (Antal et al., 2004).
Furthermore, a facilitatory effect of the 2 mA cathodal stimulation can be attributed to different physiological mechanisms: the applied current strength determined the current density in the targeted brain region, with higher densities associated with higher current strengths (Nitsche and Paulus, 2000; Miranda et al., 2009). This might explain why the 2 mA stimulation induced different results compared with1 mA stimulation: Higher current strengths are likely to influence different populations of neurons, as they affect more deeply layered neurons (Miranda et al., 2009) and white matter. Another possibility could be that the same group of neurons are affected distinctly by different current strengths so that different current strengths may result in differential physiological alterations at the cellular level. For example, a current strength-dependent modulation of voltage-dependent ion-channels may effect plasticity (Lisman and McIntyre, 2001) by long-term potentiation, long-term depression (Malenka and Nicoll, 1999; Malenka and Bear, 2004), or by influencing the homeostatic state (Bienenstock et al., 1982; Siebner et al., 2004; Thirugnanasambandam et al., 2011). Importantly, our study is not the first to find facilitatory effects after 2 mA cathodal stimulation (Monti et al., 2008; Batsikadze et al., 2011). In these studies, the facilitatory effects were explained by the inhibition of inhibitory interneurons (Monti et al., 2008), a calcium-dependent process, or the involvement of deeper layered neurons.
In summary, previous findings together with our data suggest that the 2 mA cathodal stimulation influenced the neurons of rIPS distinctly compared with sham and 1 mA stimulations, because it reached other populations of neurons or modulated other channels.
One putative confound of the current study is that the left frontal reference electrode may, at least in principle, have contributed to the observed results. However, a study investigating the effects of frontal tDCS with higher current densities than applied in our experiment on cognitive functions (verbal fluency and global processing) did not find any effect (Iyer et al., 2005). Nevertheless, an alternative explanation of the improvement of top-down control in both visual hemifields could be that the frontal electrode delivered an active (anodal) stimulation in the current setup. This theoretical hypothesis could pose the starting point for a systematic investigation of frontal cortex contribution to attentional processing measured with TVA.
Furthermore, our data allow refining current models of the role of rIPS (and parietal cortex) in attentional selection. The use of the formal model of TVA enabled us to qualify and quantify subcomponents of attentional selection, which were altered with tDCS. Using the TVA model we could show that 2 mA cathodal tDCS of the horizontal rIPS improved the top-down control value α in both visual hemifields. First, this implies an improvement of the subject's ability to differentiate between relevant and irrelevant display elements, regardless of their spatial position. As only α (the ratio of the attentional weight of a distractor to the attentional weight of a target at the same location) was altered, but not the spatial distribution of the attentional weights for targets or for distractors, the second important conclusion is that the horizontal rIPS selectively modulates top-down control and not the distribution of spatial attention across the visual scene. The latter conclusion is supported by the finding that the improvement was observed in both visual hemifields. This effect differs from stimulation effects of posterior parietal cortex, which have been shown to differ between hemifields in the partial report paradigm (Hung et al., 2005). The result that tDCS applied over right IPS leads to changes in attentional selection regardless of stimulus location is in accordance with attentional theories postulating a bilateral control of attention by right parietal cortex (Mesulam, 1981, 1999). It is also consistent with recent combined TMS/fMRI data showing bilateral modulation of visual cortex activity after stimulation of the right (in contrast to the left) IPS (Ruff et al., 2008; Blankenburg et al., 2010).
Other studies investigating visuospatial attention provided evidence for a functional specialization of parietal cortex: while the superior parietal lobule mediates spatial shifting, the IPS is involved in endogenous attentional control (Molenberghs et al., 2007). In good accordance with the latter, we observed that modulating rIPS indeed only altered attentional control (α), without changing spatially selective attention (the spatial distribution of attentional weights). Moreover, another study using the TVA-approach with spatially precise rTMS on right posterior parietal cortex (rPPC) found diminished top-down control in the contralateral hemifield but enhanced top-down control in the ipsilateral hemifield (Hung et al., 2005), supporting the hypothesis of a functional specialization of parietal cortex and a spatiotopic organization in parts thereof.
Evidence for a facilitatory effect of cathodal tDCS was also found in a study investigating the effect of cathodal tDCS of rPPC on attentional abilities with a flanker task (Weiss and Lavidor, 2012). Cathodal stimulation enabled flanker processing even in high loaded visual scenes. The authors suggested that cathodal stimulation enhanced attentional resources. With TVA an alternative explanation is plausible: as in that study the flanker fulfilled the top-down criteria for a target, altered top-down control due to cathodal tDCS may have resulted in a prioritized processing of the flanker.
To summarize, our findings strongly support the hypothesis of a functional specialization of parietal cortex and a spatiotopic organization in parts thereof. Our data suggest that the horizontal part of rIPS is selectively involved in mediating top-down control in both visual hemifields, whereas previous evidence suggests that more posterior parts of parietal cortex modulate top-down control differentially in the two hemifields (Hung et al., 2005) and that the superior parietal lobule mediates spatial components of visual attention (Molenberghs et al., 2007).
In conclusion, 2 mA cathodal tDCS of the rIPS lead to an improvement in attentional selection, as measured with TVA. Importantly, our work suggests putative mechanisms for a facilitatory effect of cathodal tDCS (like e.g., an enhancement of the signal-to-noise ratio of target-associated activity in parietal cortex). Future studies are needed to further explore the exact physiological mechanisms underlying the observed tDCS effects to improve the use of tDCS as both a neuroscientific and putatively also therapeutic tool. The data also strongly support the hypothesis of a functional specialization of parietal cortex and a spatiotopic organization of its parts, as well as complement findings from neuroimaging and patient studies (for review, see Vandenberghe and Gillebert, 2009). Finally, the data suggest that horizontal rIPS serves as a central mediator of attentional top-down control for both visual hemifields without affecting the spatial distribution of attention.
Footnotes
S.V. (Vo 1733/1-1) and R.W. and G.R.F. (We4299/3-1) are supported by the Deutsche Forschungsgemeinschaft (DFG). We are grateful to our colleagues for valuable support and discussions. We would also like to thank Mads Dyrholm and Signe Vangkilde from the Center for Visual Cognition, Department of Psychology, University of Copenhagen for their support concerning the model fitting in TVA and Jessie England for valuable comments on this manuscript.
References
- Antal A, Nitsche MA, Kruse W, Kincses TZ, Hoffmann KP, Paulus W. Direct current stimulation over V5 enhances visuomotor coordination by improving motion perception in humans. J Cogn Neurosci. 2004;16:521–527. doi: 10.1162/089892904323057263. [DOI] [PubMed] [Google Scholar]
- Batsikadze G, Kuo MF, Moliadze V, Paulus W, Nitsche M. P19.18 intracortical and corticospinal effect of 2 mA direct current stimulation. Clin Neurophysiol. 2011;122(Suppl 1):S145. http://www.sciencedirect.com/science/article/pii/S1388245711605191. [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenburg F, Ruff CC, Bestmann S, Bjoertomt O, Josephs O, Deichmann R, Driver J. Studying the role of human parietal cortex in visuospatial attention with concurrent TMS–fMRI. Cereb Cortex. 2010;20:2702–2711. doi: 10.1093/cercor/bhq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundesen C. A theory of visual attention. Psychol Rev. 1990;97:523–547. doi: 10.1037/0033-295x.97.4.523. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Bundesen C, Olson A, Humphreys G, Chavda S, Shibuya H. Systematic analysis of deficits in visual attention. J Exp Psychol. 1999;128:450–478. doi: 10.1037//0096-3445.128.4.450. [DOI] [PubMed] [Google Scholar]
- Dyrholm M, Kyllingsbaek S, Espeseth T, Bundesen C. Generalizing parametric models by introducing trial-by-trial parameter variability: The case of TVA. J Math Psychol. 2011;55:416–429. [Google Scholar]
- Finke K, Bublak P, Krummenacher J, Kyllingsbaek S, Müller H J, Schneider WX. Usability of a theory of visual attention (TVA) for parameter-based measurement of attention I: evidence from normal subjects. J Int Neuropsychol Soc. 2005;11:832–842. doi: 10.1017/s1355617705050976. [DOI] [PubMed] [Google Scholar]
- Gitelman DR. ILAB: a program for postexperimental eye movement analysis. Behav Res Methods Instrum Comp. 2002;34:605–612. doi: 10.3758/bf03195488. [DOI] [PubMed] [Google Scholar]
- Habekost T, Bundesen C. Patient assessment based on a theory of visual attention (TVA): subtle deficits after a right frontal-subcortical lesion. Neuropsychologia. 2003;41:1171–1188. doi: 10.1016/s0028-3932(03)00018-6. [DOI] [PubMed] [Google Scholar]
- Habekost T, Rostrup E. Visual attention capacity after right hemisphere lesions. Neuropsychologia. 2007;45:1474–1488. doi: 10.1016/j.neuropsychologia.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Hung J, Driver J, Walsh V. Visual selection and posterior parietal cortex: effects of repetitive transcranial magnetic stimulation on partial report analyzed by Bundesen's theory of visual attention. J Neurosci. 2005;25:9602–9612. doi: 10.1523/JNEUROSCI.0879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer MB, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann EM. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology. 2005;64:872–875. doi: 10.1212/01.WNL.0000152986.07469.E9. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Koslowsky M, Lavidor M. tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Exp Brain Res. 2012;216:1–10. doi: 10.1007/s00221-011-2891-9. [DOI] [PubMed] [Google Scholar]
- Kyllingsbaek S. Modeling visual attention. Behav Res Methods. 2006;38:123–133. doi: 10.3758/bf03192757. [DOI] [PubMed] [Google Scholar]
- Lisman JE, McIntyre CC. Synaptic plasticity: a molecular memory switch. Curr Biol. 2001;11:R788–R791. doi: 10.1016/s0960-9822(01)00472-9. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation—a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Ann Neurol. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999;354:1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda PC, Faria P, Hallett M. What does the ratio of injected current to electrode area tell us about current density in the brain during tDCS? Clinical Neurophysiology. 2009;120:1183–1187. doi: 10.1016/j.clinph.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P, Mesulam MM, Peeters R, Vandenberghe RR. Remapping attentional priorities: differential contribution of superior parietal lobule and intraparietal sulcus. Cereb Cortex. 2007;17:2703–2712. doi: 10.1093/cercor/bhl179. [DOI] [PubMed] [Google Scholar]
- Monti A, Cogiamanian F, Marceglia S, Ferrucci R, Mameli F, Mrakic-Sposta S, Vergari M, Zago S, Priori A. Improved naming after transcranial direct current stimulation in aphasia. J Neurol Neurosurg Psychiatr. 2008;79:451–453. doi: 10.1136/jnnp.2007.135277. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clinical Neurophysiology. 2003;114:2220–2222. doi: 10.1016/s1388-2457(03)00235-9. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Doemkes S, Karaköse T, Antal A, Liebetanz D, Lang N, Tergau F, Paulus W. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2007;97:3109–3117. doi: 10.1152/jn.01312.2006. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, Pascual-Leone A. Transcranial direct current stimulation: State of the art 2008. Brain Stim. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Peers PV, Ludwig CJ, Rorden C, Cusack R, Bonfiglioli C, Bundesen C, Driver J, Antoun N, Duncan J. Attentional functions of parietal and frontal cortex. Cereb Cortex. 2005;15:1469–1484. doi: 10.1093/cercor/bhi029. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Bestmann S, Blankenburg F, Bjoertomt O, Josephs O, Weiskopf N, Deichmann R, Driver J. Distinct causal influences of parietal versus frontal areas on human visual cortex: evidence from concurrent TMS–fMRI. Cereb Cortex. 2008;18:817–827. doi: 10.1093/cercor/bhm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya H, Bundesen C. Visual selection from multielement displays: Measuring and modeling effects of exposure duration. J Exp Psychol Percept Perform. 1988;14:591–600. doi: 10.1037//0096-1523.14.4.591. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, Rothwell JC. Preconditioning of Low-Frequency Repetitive Transcranial Magnetic Stimulation with Transcranial Direct Current Stimulation: Evidence for Homeostatic Plasticity in the Human Motor Cortex. J Neurosci. 2004;24:3379–3385. doi: 10.1523/JNEUROSCI.5316-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparing R, Thimm M, Hesse MD, Küst J, Karbe H, Fink GR. Bidirectional alterations of interhemispheric parietal balance by non-invasive cortical stimulation. Brain. 2009;132:3011–3020. doi: 10.1093/brain/awp154. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- Taylor MM. PEST: efficient estimates on probability functions. J Acoust Soc Am. 1967;41:782. [Google Scholar]
- Thirugnanasambandam N, Sparing R, Dafotakis M, Meister IG, Paulus W, Nitsche MA, Fink GR. Isometric contraction interferes with transcranial direct current stimulation (tDCS) induced plasticity: evidence of state-dependent neuromodulation in human motor cortex. Restor Neurol Neurosci. 2011;29:311–320. doi: 10.3233/RNN-2011-0601. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Gillebert CR. Parcellation of parietal cortex: convergence between lesion-symptom mapping and mapping of the intact functioning brain. Behav Brain Res. 2009;199:171–182. doi: 10.1016/j.bbr.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Weiss M, Lavidor M. When less is more: evidence for a facilitative cathodal tDCS effect in attentional abilities. J Cogn Neurosci. 2012:1–8. doi: 10.1162/jocn_a_00248. [DOI] [PubMed] [Google Scholar]