Abstract
Preterm-born children commonly experience motor, cognitive, and learning difficulties that may be accompanied by altered brain microstructure, connectivity, and neurochemistry. However, the mechanisms linking the altered neurophysiology with the behavioral outcomes are unknown. Here we provide the first physiological evidence that human adolescents born preterm at or before 37 weeks of completed gestation have a significantly reduced capacity for cortical neuroplasticity, the key overall mechanism underlying learning and memory. We examined motor cortex neuroplasticity in three groups of adolescents who were born after gestations of ≤32 completed weeks (early preterm), 33–37 weeks (late preterm), and 38–41 weeks (term) using a noninvasive transcranial magnetic brain stimulation technique to induce long-term depression (LTD)-like neuroplasticity. Compared with term-born adolescents, both early and late preterm adolescents had reduced LTD-like neuroplasticity in response to brain stimulation that was also associated with low salivary cortisol levels. We also compared neuroplasticity in term-born adolescents with that in term-born young adults, finding that the motor cortex retains a relatively enhanced neuroplastic capacity in adolescence. These findings provide a possible mechanistic link between the altered brain physiology of preterm birth and the subsequent associated behavioral deficits, particularly in learning and memory. They also suggest that altered hypothalamic–pituitary–adrenal axis function due to preterm birth may be a significant modulator of this altered neuroplasticity. This latter finding may offer options in the development of possible therapeutic interventions.
Introduction
Up to 50% of preterm children without cerebral palsy experience motor and cognitive difficulties that significantly affect their motor development, academic progress, and social adjustment (Bracewell and Marlow, 2002). This is not confined to the very preterm but includes late preterm children [i.e., those born at gestational age (GA) 33–37 weeks] (Chyi et al., 2008; Morse et al., 2009; Woythaler et al., 2011), who experience greater morbidity in infancy and childhood than their term-born peers (Escobar et al., 2006; Khashu et al., 2009) and have lower levels of education and income in adulthood (Lindström et al., 2007).
These functional impairments are accompanied by alterations in neuroanatomy that persist in late adolescence and include the major motor control areas (Peterson et al., 2003; Mullen et al., 2011). In particular, preterm birth interrupts cortical microstructural and functional connectivity development within and between brain regions important for motor and sensory function, auditory and visual processing, and language (Kapellou et al., 2006; Counsell et al., 2008; Kesler et al., 2008; Lodygensky et al., 2010; Smyser et al., 2010, 2011). Emerging evidence also suggests abnormalities in neurochemical and hormone production and responsiveness, although this has yet to be examined in late preterm children. For example, early preterm birth is associated with long-term alterations in cortisol secretion and stress responsiveness (Grunau et al., 2007; Fernandez et al., 2008; Sullivan et al., 2008), and exposure of extremely preterm infants to stressors in the neonatal intensive care unit is associated with regional changes in brain size and functional connectivity (Smith et al., 2011).
What remains elusive is the physiological mechanism(s) linking the anatomical and neurochemical/hormonal changes with the behavioral deficits commonly observed. However, the substantial evidence of a range of functional learning and memory difficulties strongly suggests that preterm birth reduces the brain's capacity for neuroplastic reorganization. In addition, these children's abnormal cortisol secretion is likely to impact on their neuroplastic capacity since exogenously administered corticosteroids and endogenous cortisol have been shown to influence both long-term potentiation (LTP) and long-term depression (LTD) in adults (Sale et al., 2008; Henckens et al., 2011; Tse et al., 2012).
Here we used noninvasive transcranial magnetic stimulation (TMS) to examine LTD-like motor neuroplasticity in preterm- and term-born adolescents. Salivary cortisol was also sampled to establish whether there are persistent alterations in its levels following preterm birth and, if so, whether this influences neuroplasticity in adolescence. Lastly, we compared neuroplasticity in term-born adolescents and adults.
Preterm birth probably influences neuroplasticity in many brain regions, but we targeted the motor system for several reasons. The motor and sensory areas appear particularly sensitive to events in late pregnancy. Their growth is rapid between 20 and 37 weeks GA and they are the most common sites of perinatal brain damage (Eyre, 2003; Kapellou et al., 2006). We have also reported preliminary data (Pitcher et al., 2009) showing significant associations between preterm birth, corticomotor excitability, and cognitive development, suggesting that developmental abnormalities in the motor cortex might be reflective of more widespread changes.
Materials and Methods
Twenty-eight adolescents (15 females) participated [age: 13.8 ± 0.5 years (mean ± SD)]. GA ranged from 26 to 41 completed weeks (34.0 ± 3.9 weeks) and was recorded as a continuous variable (i.e., weeks GA) and as a categorical variable. The categories were based on the World Health Organization groupings for preterm birth and included adolescents born early preterm (≤32 weeks GA; N = 11, 29.9 ± 2.0 weeks GA), late preterm (33–36 weeks GA; N = 10, 35.2 ± 0.9 weeks GA), and at term (37–41 weeks GA; N = 7, 38.5 ± 1.6 weeks GA). GA (completed weeks) was obtained from each child's hospital perinatal records as this was a retrospective cohort. Determination was performed by the obstetrician, and neonatologist where appropriate, using a combination of methods including the days from last menstrual period (if known), antenatal morphometric ultrasounds (if available), and Dubowitz score at birth (Dubowitz et al., 1970). Nine term-born adults (GA determined by maternal report) were also studied (24.0 ± 5.4 years). All participants completed a TMS safety questionnaire before enrolment and gave informed, written consent (parents/guardians for adolescents). The study was approved by the Child, Youth and Women's Health Service and University of Adelaide Human Research Ethics Committees. All procedures were performed in the afternoon and in accordance with the Declaration of Helsinki (1998).
Birth weight centile.
To differentiate influences of suboptimal fetal growth from those of a shortened gestation, the birth weight centile (BW%) was calculated for each adolescent using Gestation Related Optimal Weight software (Gardosi and Francis, 2006). Birth and maternal information were obtained, with consent, from obstetric records. The coefficients for calculating the BW% were derived from the Australian database of ∼12,000 ultrasound-dated deliveries (Gardosi and Francis, 2006).
Cortisol.
Saliva samples were obtained from each adolescent immediately before TMS baseline measures using a Salivette (Sarstedt) and standardized subject preparation procedures. Samples were stored at −20°C until assayed. Twenty-five microliters of saliva was assayed in duplicate for cortisol by ELISA (HS-Cortisol; Salimetrics).
TMS and electromyography.
Surface electromyography (EMG) recordings were obtained from the right first dorsal interosseous muscle using a muscle belly–metacarpophalangeal joint montage. EMG signals were amplified (×1000; 1902 amplifier; CED), bandpass filtered (20 Hz–1 kHz), and digitized at 5 kHz (1401 interface; CED). All data were stored offline for later analysis.
Continuous theta burst stimulation (cTBS) uses repetitive TMS to induce a lasting LTD-like neuroplastic change in the motor cortical representation of, in this case, an index finger abduction muscle. The magnitude of the LTD-like change is quantified by comparing the preneuroplasticity and postneuroplasticity induction amplitudes of the motor-evoked potentials (MEPs) by activating the corticomotor representation with single-pulse TMS. Single-pulse TMS was applied to the left motor cortex hand area using a Magstim 2002 stimulator (Magstim) with a figure-of-eight coil (9 cm diameter). The coil was placed in the optimal position to evoke a response in the relaxed right first dorsal interosseous muscle. The coil was held tangentially to the scalp with the handle pointing posteriorly and 45° from the midline to produce a posterior–anterior current in the cortex.
Resting motor threshold (RMT) was determined as the minimum stimulus intensity required to elicit an MEP ≥ 50 μV in at least five of 10 consecutive trials. Active motor threshold (AMT) was defined as the TMS intensity (as a percentage of the maximum stimulator output) required to elicit an MEP ≥ 200 μV in at least five of 10 consecutive trials when the participant was performing a voluntary contraction of 10% of their maximum.
Corticomotor excitability was examined before and following the cTBS. Stimulator intensity was adjusted to evoke baseline MEPs of ∼1 mV peak to peak amplitude. This stimulator intensity was then used throughout the experiment for evoking test MEPs. Stimuli were delivered every 6 s with ±10% variance in blocks of 15. MEP recordings were obtained before and at 0, 5, 10, 20, 30, 45, and 60 min after cTBS. An air film coil connected to a Magstim Rapid2 stimulator (Magstim) was used to deliver cTBS. As previously described (Huang et al., 2005), cTBS consisted of bursts of three pulses at 50 Hz every 200 ms for 40 s (total of 600 pulses) with the stimulation intensity set at 80% of AMT.
Data and statistical analysis.
All MEPs were recorded at high gain and any with obvious EMG activity in the 50 ms before the TMS stimulus were discarded online. Individual MEPs were also analyzed offline and any trials contaminated in the 50 ms before TMS with EMG activity greater than ±2 SD of the root mean squared background EMG were discarded. The amplitude of the baseline MEP (in mV) was recorded for each participant and the mean baseline MEP amplitude calculated for each GA group. Average MEP amplitudes were calculated in each participant for each block at each time point. The mean MEP amplitude for each time point was then normalized to the amplitude of the baseline MEP. Normal distribution and homogeneity of variance of the data were assessed using the Kolmogorov–Smirnov test and Levene's statistic, respectively, with GA group as the factor.
Data were analyzed with repeated-measures ANOVA (rm-ANOVA) with polynomial contrasts and Bonferroni's correction for multiple comparisons. The between-subjects factor was GA Group (up to 3 levels; early preterm, late preterm, or term) or Age Group (2 levels; adolescent or adult) and the within-subjects factor was Time (up to 7 levels; 0, 5, 10, 20, 30, 45, and 60 min post-cTBS). Post hoc analysis was performed where appropriate using Tukey's HSD for comparisons between GA Groups at specific time points.
Separate rm-ANOVA for each GA Group and Age Group were conducted on the raw MEP data with the factor Time (8 levels; pre-cTBS, 0, 5, 10, 20, 30, 45, and 60 min post-cTBS). Paired-samples t tests were performed post hoc where appropriate to compare the post-cTBS and baseline MEP amplitudes. The influence of birth weight centile and salivary cortisol level were determined with covariate analyses. Linear regression analyses were used to examine relationships between GA (in weeks), responses to cTBS, and the influence of other explanatory variables including birth weight centile, handedness, sex, and cortisol level.
Results
The participant characteristics for adolescents (separated by GA group) and adults are summarized in Table 1. There was no difference in RMT or AMT between the three GA groups, nor was there any difference in the stimulator intensity used to evoke test MEPs or the amplitude (in mV) of the baseline MEP before cTBS.
Table 1.
Study Participant Characteristics. Adolescents are grouped by GA group. Data are group means and standard deviations
| Early preterm (N = 11) | Late preterm (N = 10) | Term (N = 7) | Adults (N = 9) | |
|---|---|---|---|---|
| Study participant characteristics | ||||
| Age (years) | 13.6 ± 0.5 | 14.0 ± 0.9 | 13.9 ± 0.4 | 23.6 ± 5.2 |
| GA (weeks) | 29.9 ± 2.0*# | 35.2 ± 0.9*# | 38.5 ± 1.6 | 40.3 ± 0.7 |
| BW% | 32.7 ± 32.4* | 28.1 ± 26.9* | 76.5 ± 17.9 | NA |
| Cortisol (nmol/l) | 3.24 ± 1.1 | 4.1 ± 1.9 | 4.7 ± 1.31 | NA |
| Resting motor threshold (% stimulator output) (figure eight coil) | 49.8 ± 14.5 | 43.7 ± 7.7 | 43.9 ± 3.7 | 45.1 ± 5.5 |
| Active motor threshold (% stimulator output) (rTMS coil) | 46.5 ± 10.9 | 42.0 ± 9.0 | 45.1 ± 5.0 | 51.4 ± 9.6 |
| cTBS Intensity (% stimulator output) | 37.2 ± 8.6 | 33.7 ± 7.2 | 36.1 ± 3.9 | 41.1 ± 7.7 |
| Baseline MEP amplitude (mV) | 0.92 ± 0.34 | 1.0 ± 0.16 | 1.16 ± 0.10 | 1.3 ± 0.24 |
| Mean MEP amplitude post cTBS (% baseline) | 96.3 ± 23.3* | 92.0 ± 21.4* | 55.8 ± 23.8 | 79.7 ± 12.6* |
*p ≤ 0.05 when compared with term group.
#p ≤ 0.05 when compared with adults.
LTD-like neuroplasticity is reduced in preterm adolescents
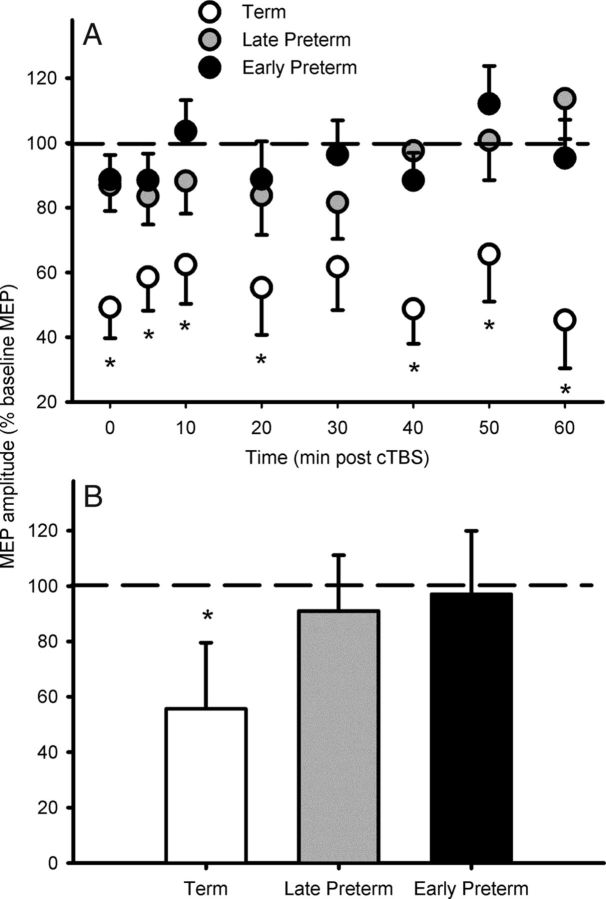
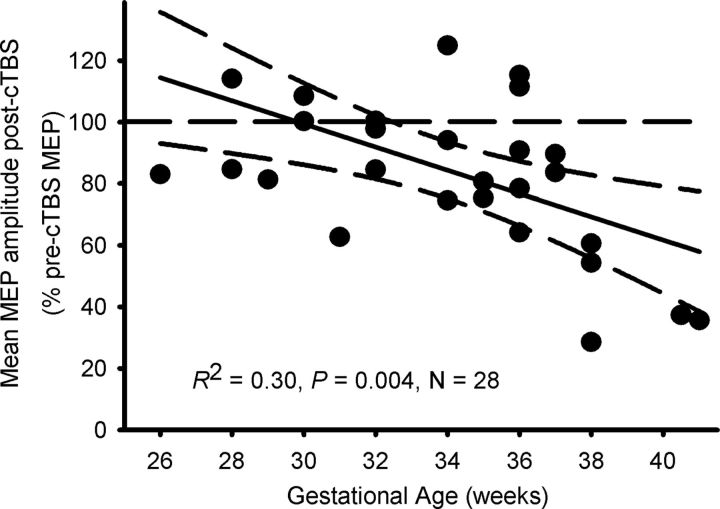
Across all participants, MEP amplitude was suppressed following cTBS (F(8,200) = 3.1, p = 0.003), indicating LTD-like neuroplasticity had been induced. However, the magnitude of the neuroplastic response differed between the GA groups (F(2,25) = 6.9, p = 0.004), as did the time course of the effects (F(16,200) = 2.1, p = 0.01). While the term-born adolescents demonstrated a robust reduction in MEP amplitude at every time point following cTBS (F(8,48) = 4.90, p ≤ 0.0001; Fig. 1), in the late preterm adolescents, MEP reduction was evident only at 5, 10, and 30 min post-cTBS. In the early preterm adolescents, cTBS did not alter MEP amplitude at any time point. The mean MEP amplitude for each GA group (relative to baseline) for the 60 min of follow-up is shown in Figure 1B. The average post-cTBS MEP reduction was greater in term-born adolescents than in both the late preterm (mean difference = 35.3%, p = 0.009) and early preterm (mean difference = 41.4%, p = 0.002) groups. Regression analysis revealed that for every week of GA, MEP suppression increased 3.2% (R2 = 0.30, F(1,27) = 10.1, p = 0.004; Fig. 2): Mean MEP suppression [average of all post-cTBS time points (expressed as a percentage of test MEP amplitude)] = 212.3 − 3.8 (GA).
Figure 1.
Average MEP responses of adolescents to cTBS. A, Compared with both groups of preterm adolescents, term-born adolescents responded with greater and more persistent suppression of MEP amplitude at all time points following cTBS. B, There was no difference between the two preterm groups at any time point, or when the mean MEP response for the entire 60 min follow-up was compared. Data are group mean ± SD, *p ≤ 0.05 when compared with both preterm groups.
Figure 2.
LTD-like neuroplastic changes were smaller with decreasing GA at birth. Data are individual mean MEP amplitudes (expressed relative to baseline i.e., 100%) during the 60 min follow-up after cTBS.
Low salivary cortisol is associated with a reduced LTD-like neuroplastic response
Useable saliva samples were obtained from 23 adolescents. Five samples (2 early preterm, 1 late preterm, and 2 term-born) were contaminated with blood and excluded from the analysis. Given the well characterized diurnal variations in human cortisol levels, it was important to ensure there were no systematic differences in the time of assessment across the three GA groups. There was no difference in the time of day (mean = 13:53 h) at which the cTBS experiments were performed across the three GA groups and no effect of time of day on any of the analyses.
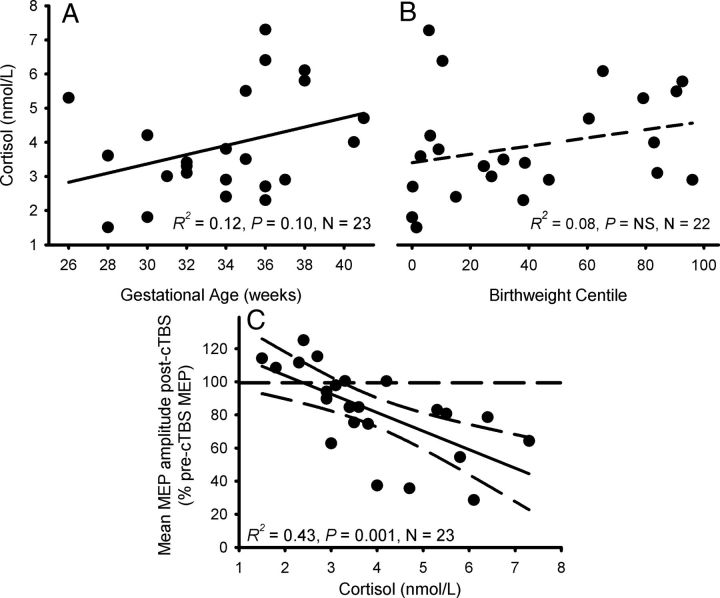
Salivary cortisol levels immediately before cTBS ranged between 1.4 and 7.3 nmol/l. Fewer than 30% of subjects returned salivary cortisol levels >4 nmol/l and all but two of these subjects were term-born. Oskis and colleagues (2012) have reported salivary cortisol levels ranging between 4 and 7 nmol/l in adolescent females for the corresponding time of day, suggesting that the levels in our preterm subjects were abnormally low. There was a weak trend for preterm birth to be associated with lower cortisol levels (R2 = 0.12, F(1,22) = 2.8, p = 0.1; Fig. 3A) but no association was detected between cortisol level and BW% (Fig. 3B; Table 1).
Figure 3.
The influence of salivary cortisol level on MEP suppression after cTBS. A, B, Cortisol levels tended to be lower with reduced GA (A), but there was no association with BW% (B). Data are results from individual subjects. C, Adolescents with low cortisol levels immediately before cTBS showed very little neuroplastic change in mean MEP following cTBS. Data are individual mean MEP amplitudes (expressed relative to baseline; i.e., 100%) during the 60 min follow-up after cTBS.
To investigate whether altered cortisol levels were influencing the GA-dependent changes in cTBS response, cortisol level was included as a covariate in the ANOVA examining the influence of GA group on the average post-cTBS response. Low cortisol levels were associated with a smaller response to cTBS (F(1,19) = 15.6, p = 0.001) and slightly strengthened the main effect of GA group on cTBS response (F(2,19) = 9.3, p = 0.002). Regression analysis showed that for every nanomole per liter of cortisol, MEP suppression increased 11.2% (R2 = 0.43, F(1,23) = 15.8, p = 0.001; Fig. 3C): Mean MEP suppression [average of all post-cTBS time points (expressed as a percentage of test MEP amplitude)] = 126.1 − 11.2 (Cortisol, in nmol/l).
cTBS response in term-born adolescents and adults
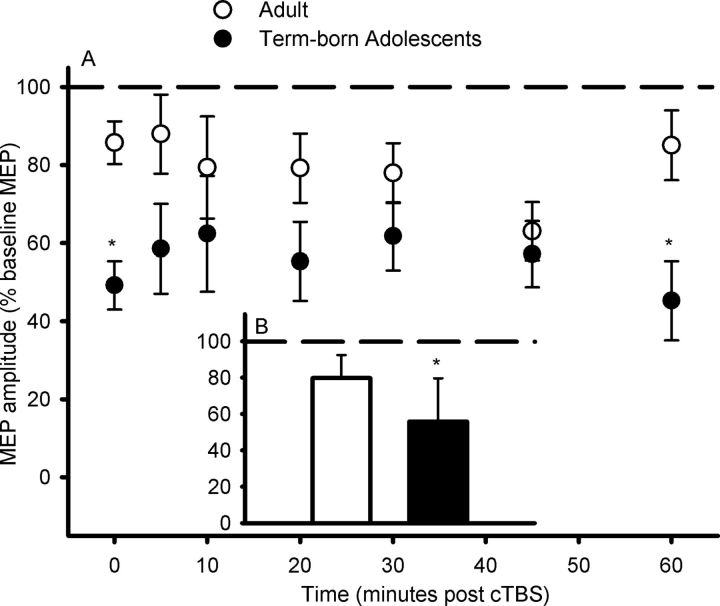
The response to cTBS in the seven term-born adolescents described above was compared with that in nine term-born young adults (Table 1). The response to cTBS was greater in the term-born adolescents than in the adults (F(1,16) = 8.59, p = 0.01; Fig. 4B) and the time course of effects differed between the two groups (F(1,16) = 8.59, p = 0.01) due to the adolescents having a larger response to cTBS than the adults at 0 and 60 min post-cTBS (Fig. 4A).
Figure 4.
MEP responses to cTBS in term-born adolescents and adults. A, B, The adolescents showed greater LTD-like MEP suppression than adults at several time points during the 60 min follow-up after cTBS (A) and when the age group mean response for the entire 60 min of follow-up was compared (B). Data are group mean ± SD, *p ≤ 0.05 when adolescents and adults are compared.
Discussion
A normally functioning cortex is critical in motor, cognitive, and sensory processes that rely on activity-dependent learning. Both LTP and LTD have been strongly implicated as key underlying cellular mechanisms of cortical plasticity, and factors altering their normal induction have been associated with learning impairments (for review, see Feldman, 2009). This is the first study to provide physiological evidence in humans that preterm birth is associated with impairments in LTD-like neuroplasticity that are apparent in adolescence. In contrast to term-born adolescents whose response to cTBS was characterized by a robust and long-lasting suppression of MEP amplitude, even very modest levels of prematurity were associated with a blunted neuroplastic response. In addition, cortisol levels were low in the preterm adolescents and these low levels were strongly associated with a reduced cTBS response. Lastly, the cTBS response in term-born adolescents was greater than that in young term-born adults, suggesting that the motor cortex remains highly responsive to neuroplastic interventions in early adolescence, but only in individuals born at term.
Perhaps the most surprising finding was that even very modest preterm birth was associated with a reduced response to cTBS. The mechanisms underlying this are not clear and, in particular, it is not clear whether cortisol levels have a direct effect on the response to cTBS, or whether altered cortisol and the reduced response to cTBS are separate manifestations of altered development of circadian rhythmicity as a consequence of preterm birth. The main conclusions of this study rely on the assumption that the response to cTBS is due to short-term LTD-like neuroplastic mechanisms and it should be noted that at present it is impossible to conclusively prove the involvement of such mechanisms. Indeed, it is likely that the response to cTBS can be influenced by modifications in many elements of cortical circuitry. However, there are a number of features of the response to cTBS that suggest involvement of synaptic neuroplastic mechanisms (for review, see Hoogendam et al., 2010). Both LTD and LTP are often thought of as NMDAR-dependent processes and there is some additional pharmacological evidence that the effects induced by cTBS task are also due to NMDAR-dependent processes. For example, prior administration of NMDAR antagonists blocks the response to cTBS in healthy humans (Huang et al., 2007). In addition, the partial NMDAR-dependent agonist d-cycloserine modulates the response to the similar, but facilitatory, intermittent theta burst stimulation (iTBS) paradigm (Teo et al., 2007). Also, dopamine is known to exert influence on neuroplasticity and D2 receptor blockade with sulpiride has been shown to block the response to both cTBS and iTBS (Monte-Silva et al., 2011). Therefore, although not conclusively proven, we suggest the most likely mechanism responsible for the response to cTBS is a NMDAR-dependent short-term LTD-like process.
Only very limited conclusions can be drawn regarding the influence of cortisol levels on the response to cTBS based on a single sample. In addition, in the present study, we provide no evidence for a causal relationship between cortisol and the altered response to cTBS. However, given the strength of the association between cortisol levels and the magnitude of the neuroplastic response, we suggest some speculation is warranted. Both fetal growth restriction (evident as low BW%) and preterm birth have been implicated in programming altered responsiveness of the hypothalamic–pituitary–adrenal axis postnatally and in adulthood (Sullivan et al., 2008). Further, animal studies have demonstrated that increased or decreased corticosterone levels not only have bidirectional effects on the induction of NMDAR-mediated hippocampal LTP and LTD, but exposure to abnormal levels (high or low) of corticosterone alters both the function and subunit composition of NMDARs, even in adult animals, and hence neuroplasticity (for review, see Tse et al., 2012). The effects of corticosterone on LTP and LTD differ, with the relationship between corticosterone level and NMDAR-mediated LTP induction being an inverted U-shape (Diamond et al., 1992; Rey et al., 1994), whereas increased corticosterone levels facilitate NMDAR-mediated LTD (Yang et al., 2004; Chaouloff et al., 2008). Our findings show that, compared with their term-born peers, adolescents born preterm tend to have low levels of cortisol and this is associated with a limited capacity for induction of NMDAR-mediated LTD-like motor cortical neuroplasticity in these individuals.
The term-born adolescents exhibited a greater response to cTBS than the young adults. The magnitude and time course of the response in the young adults was comparable to that previously reported in older adults (Huang et al., 2005). In contrast, MEPs continued to be suppressed in term-born adolescents at 60 min, suggesting the after-effects of cTBS are more enduring in adolescents than adults. It is interesting to note that the LTD induction threshold is increased in sensory, motor, and visual cortices of adult animals compared with juveniles (Sawtell et al., 2003; Sizemore and Perkel, 2011). In humans, immaturity is generally associated with enhanced neuroplasticity, even outside the perinatal periods of increased synaptogenesis and pruning, and there is evidence that various regions of cortex retain enhanced neuroplastic capacities into adolescence. Therefore, the enhanced cTBS response seen in term-born adolescents may reflect the greater neuroplastic capabilities of the still-developing motor cortex.
The differences in neuroplasticity induction between the preterm and term-born adolescents (or the term-born adolescents and adults) are unlikely to be due to technical factors such as individual variations in the baseline MEP amplitude or cTBS stimulus intensity used. Although the influence of factors including stimulus intensity and baseline response amplitude has not been examined in human studies, Dudek and Bear (1993) showed in rat hippocampus slice experiments that the larger the amplitude of the baseline response, the greater the subsequent depression in the slope of the EPSP (i.e., LTD) induced. However, in the current study, there were no significant differences in motor thresholds, baseline MEP amplitudes, or the stimulus intensities used for cTBS across the different GA groups or between the adolescents and adults.
There are several limitations to this study that warrant mention. The most obvious is that we have no evidence as to whether the response to a facilitatory brain stimulation intervention (such as intermittent theta burst stimulation), which is thought to be due to LTP-like processes, is similarly affected. However, induction of facilitatory changes with repetitive TMS increases cortical excitability and hence, the risk of seizure induction. While no study participant had any history of seizures or epilepsy, we chose to err on the side of caution and use only an intervention that suppresses cortical excitability. While not necessarily a limitation, we studied adolescents partly because young children have high thresholds to TMS and partly because the adolescents were all members of a larger, previously studied cohort and not naive to TMS. This latter point is important in terms of minimizing stress for a study in which cortisol levels were assessed. The fact that we could demonstrate a difference in cTBS response between these preterm and term-born individuals indicates that the altered cortical function with preterm birth remains at least into adolescence. While there was adequate power in this study to reliably detect differences due to GA, BW%, and cortisol, the sample size was too small to detect any influence of subject sex or exposure to prenatal corticosteroids. Also, a single daytime cortisol sample may give some indication of altered hypothalamic–pituitary–adrenal axis and/or suprachiasmatic nucleus function, but only very limited conclusions can be drawn. A more comprehensive diurnal profile is required to further confirm our speculations.
No magnetic resonance imaging data were available for the adolescents studied. For inclusion in this study, children had to have had no history of perinatal brain injury based upon neonatal cerebral ultrasounds and clinical assessments. We also excluded children in whom ipsilateral responses to TMS were evident. While ipsilateral responses can be developmental, they may also indicate the presence of a corticospinal tract lesion (Eyre et al., 2001). Despite this, it is possible that mild brain injury might have been present in some participants and that different mechanisms were responsible for the reduced response to cTBS in subjects with and without brain lesions. However, up to 25% of preterm children with apparently normal MRI/diffusion tensor imaging results exhibit motor and cognitive dysfunction (Miller et al., 2005; Ludeman et al., 2008), suggesting that most current clinical imaging modalities have limitations in detecting the microstructural abnormalities associated with otherwise neurologically normal preterm birth. A final limitation is that we had no measure of attention during the cTBS procedure. It has been shown previously that attention can modulate of the response to similar brain stimulation paradigms (Stefan et al., 2004). Therefore, it is possible that differences in attention between term-born and preterm adolescents, at baseline and/or during cTBS, might contribute to the results.
In summary, this study has provided novel evidence for impairments in the response to cTBS in humans born preterm that remains evident in adolescence. This impairment is clearly evident in individuals born only mildly preterm, with no known history of perinatal brain lesions and who, apart from their prematurity, were considered clinically normal at birth. We suggest that this reduced cTBS response reflects limitations in neuroplasticity that may underlie some of the motor and cognitive impairments reported in early and late preterm children and adolescents. Finally, if the reduced neuroplastic response to cTBS reflects, at least in part, limitations in the structural matrix of neurons and their synapses, there might be major consequences later in life, not only in terms of limitations to recovery from brain injury where neuroplastic reorganization is required (e.g., recovery from stroke), but also in maintaining memory of motor and cognitive functions.
Footnotes
This work was supported by grants from the National Health and Medical Research Council (NHMRC; Grant 565344) and the Women's and Children's Hospital Research Foundation (Adelaide). J.B.P. is an M. S. McLeod Trust Research Fellow. M.C.R. holds a NHMRC Senior Research Fellowship (ID 519313). S.H.D. is an NHMRC Peter Doherty Research Fellow (ID 1013320).
The authors declare no competing financial interests.
References
- Bracewell M, Marlow N. Patterns of motor disability in very preterm children. Ment Retard Dev Disabil Res Rev. 2002;8:241–248. doi: 10.1002/mrdd.10049. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Hémar A, Manzoni O. Local facilitation of hippocampal metabotropic glutamate receptor-dependent long-term depression by corticosterone and dexamethasone. Psychoneuroendocrinology. 2008;33:686–691. doi: 10.1016/j.psyneuen.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Chyi LJ, Lee HC, Hintz SR, Gould JB, Sutcliffe TL. School outcomes of late preterm infants: special needs and challenges for infants born at 32 to 36 weeks gestation. J Pediatr. 2008;153:25–31. doi: 10.1016/j.jpeds.2008.01.027. [DOI] [PubMed] [Google Scholar]
- Counsell SJ, Edwards AD, Chew AT, Anjari M, Dyet LE, Srinivasan L, Boardman JP, Allsop JM, Hajnal JV, Rutherford MA, Cowan FM. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008;131:3201–3208. doi: 10.1093/brain/awn268. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Dubowitz LM, Dubowitz V, Goldberg C. Clinical assessment of gestational age in the newborn infant. J Pediatr. 1970;77:1–10. doi: 10.1016/s0022-3476(70)80038-5. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J Neurosci. 1993;13:2910–2918. doi: 10.1523/JNEUROSCI.13-07-02910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar GJ, Clark RH, Greene JD. Short-term outcomes of infants born at 35 and 36 weeks gestation: we need to ask more questions. Semin Perinatol. 2006;30:28–33. doi: 10.1053/j.semperi.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Eyre JA. Developmental plasticity of the corticospinal system. In: Boniface S, Ziemann U, editors. Plasticity in the human brain: investigations with transcranial magnetic brain stimulation. Cambridge: Cambridge UP; 2003. pp. 62–89. [Google Scholar]
- Eyre JA, Taylor JP, Villagra F, Smith M, Miller S. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology. 2001;57:1543–1554. doi: 10.1212/wnl.57.9.1543. [DOI] [PubMed] [Google Scholar]
- Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez EF, Montman R, Watterberg KL. ACTH and cortisol response to critical illness in term and late preterm newborns. J Perinatol. 2008;28:797–802. doi: 10.1038/jp.2008.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardosi J, Francis A. Customised centile calculator—GROW-centile v5.1. Gestation Network. 2006. www.gestation.net.
- Grunau RE, Haley DW, Whitfield MF, Weinberg J, Yu W, Thiessen P. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. J Pediatr. 2007;150:151–156. doi: 10.1016/j.jpeds.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJ, Pu Z, Hermans EJ, van Wingen GA, Joëls M, Fernández G. Dynamically changing effects of corticosteroids on human hippocampal and prefrontal processing. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendam JM, Ramakers GM, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010;3:95–118. doi: 10.1016/j.brs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Chen RS, Rothwell JC, Wen HY. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007;118:1028–1032. doi: 10.1016/j.clinph.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Kapellou O, Counsell SJ, Kennea N, Dyet L, Saeed N, Stark J, Maalouf E, Duggan P, Ajayi-Obe M, Hajnal J, Allsop JM, Boardman J, Rutherford MA, Cowan F, Edwards AD. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med. 2006;3:e265. doi: 10.1371/journal.pmed.0030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Reiss AL, Vohr B, Watson C, Schneider KC, Katz KH, Maller-Kesselman J, Silbereis J, Constable RT, Makuch RW, Ment LR. Brain volume reductions within multiple cognitive systems in male preterm children at age twelve. J Pediatr. 2008;152:513–520. 520.e1. doi: 10.1016/j.jpeds.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khashu M, Narayanan M, Bhargava S, Osiovich H. Perinatal outcomes associated with preterm birth at 33 to 36 weeks' gestation: a population-based cohort study. Pediatrics. 2009;123:109–113. doi: 10.1542/peds.2007-3743. [DOI] [PubMed] [Google Scholar]
- Lindström K, Winbladh B, Haglund B, Hjern A. Preterm infants as young adults: a Swedish national cohort study. Pediatrics. 2007;120:70–77. doi: 10.1542/peds.2006-3260. [DOI] [PubMed] [Google Scholar]
- Lodygensky GA, Vasung L, Sizonenko SV, Hüppi PS. Neuroimaging of cortical development and brain connectivity in human newborns and animal models. J Anat. 2010;217:418–428. doi: 10.1111/j.1469-7580.2010.01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludeman NA, Berman JI, Wu YW, Jeremy RJ, Kornak J, Bartha AI, Barkovich AJ, Ferriero DM, Henry RG, Glenn OA. Diffusion tensor imaging of the pyramidal tracts in infants with motor dysfunction. Neurology. 2008;71:1676–1682. doi: 10.1212/01.wnl.0000304084.59964.e2. [DOI] [PubMed] [Google Scholar]
- Miller SP, Ferriero DM, Leonard C, Piecuch R, Glidden DV, Partridge JC, Perez M, Mukherjee P, Vigneron DB, Barkovich AJ. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005;147:609–616. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Monte-Silva K, Ruge D, Teo JT, Paulus W, Rothwell JC, Nitsche MA. D2 receptor block abolishes θ burst stimulation-induced neuroplasticity in the human motor cortex. Neuropsychopharmacology. 2011;36:2097–2102. doi: 10.1038/npp.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse SB, Zheng H, Tang Y, Roth J. Early school-age outcomes of late preterm infants. Pediatrics. 2009;123:e622–e629. doi: 10.1542/peds.2008-1405. [DOI] [PubMed] [Google Scholar]
- Mullen KM, Vohr BR, Katz KH, Schneider KC, Lacadie C, Hampson M, Makuch RW, Reiss AL, Constable RT, Ment LR. Preterm birth results in alterations in neural connectivity at age 16 years. Neuroimage. 2011;54:2563–2570. doi: 10.1016/j.neuroimage.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskis A, Clow A, Thorn L, Loveday C, Hucklebridge F. Differences between diurnal patterns of salivary cortisol and dehydroepiandrosterone in healthy female adolescents. Stress. 2012;15:110–114. doi: 10.3109/10253890.2011.582529. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Anderson AW, Ehrenkranz R, Staib LH, Tageldin M, Colson E, Gore JC, Duncan CC, Makuch R, Ment LR. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics. 2003;111:939–948. doi: 10.1542/peds.111.5.939. [DOI] [PubMed] [Google Scholar]
- Pitcher JB, Schneider LA, Higgins RD, Burns NR, Nettelbeck TA, Ridding MC, Haslam RR, Robinson JS. Motor cortex development and specific cognitive outcomes in children born 25–41 weeks gestation; preliminary findings of the PREMOCODE study. Acta Paediatr. 2009;98:210. [Google Scholar]
- Pitcher JB, Schneider LA, Burns NR, Drysdale JL, Higgins RD, Ridding MC, Nettelbeck TA, Haslam RR, Robinson JS. Reduced corticomotor excitability and motor skills development in children born preterm. J Physiol. 2012 doi: 10.1113/jphysiol.2012.239269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey M, Carlier E, Talmi M, Soumireu-Mourat B. Corticosterone effects on long-term potentiation in mouse hippocampal slices. Neuroendocrinology. 1994;60:36–41. doi: 10.1159/000126717. [DOI] [PubMed] [Google Scholar]
- Sale MV, Ridding MC, Nordstrom MA. Cortisol inhibits neuroplasticity induction in human motor cortex. J Neurosci. 2008;28:8285–8293. doi: 10.1523/JNEUROSCI.1963-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawtell NB, Frenkel MY, Philpot BD, Nakazawa K, Tonegawa S, Bear MF. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 2003;38:977–985. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- Sizemore M, Perkel DJ. Premotor synaptic plasticity limited to the critical period for song learning. Proc Natl Acad Sci U S A. 2011;108:17492–17497. doi: 10.1073/pnas.1104255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GC, Gutovich J, Smyser C, Pineda R, Newnham C, Tjoeng TH, Vavasseur C, Wallendorf M, Neil J, Inder T. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann Neurol. 2011;70:541–549. doi: 10.1002/ana.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 2010;20:2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Snyder AZ, Neil JJ. Functional connectivity MRI in infants: exploration of the functional organization of the developing brain. Neuroimage. 2011;56:1437–1452. doi: 10.1016/j.neuroimage.2011.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Wycislo M, Classen J. Modulation of associative human motor cortical plasticity by attention. J Neurophysiol. 2004;92:66–72. doi: 10.1152/jn.00383.2003. [DOI] [PubMed] [Google Scholar]
- Sullivan MC, Hawes K, Winchester SB, Miller RJ. Developmental origins theory from prematurity to adult disease. J Obstet Gynecol Neonatal Nurs. 2008;37:158–164. doi: 10.1111/j.1552-6909.2008.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo JT, Swayne OB, Rothwell JC. Further evidence for NMDA-dependence of the after effects of human theta burst stimulation. Clin Neurophysiol. 2007;118:1649–1651. doi: 10.1016/j.clinph.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Tse YC, Bagot RC, Wong TP. Dynamic regulation of NMDAR function in the adult brain by the stress hormone corticosterone. Front Cell Neurosci. 2012;6:9. doi: 10.3389/fncel.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woythaler MA, McCormick MC, Smith VC. Late preterm infants have worse 24-month neurodevelopmental outcomes than term infants. Pediatrics. 2011;127:e622–e629. doi: 10.1542/peds.2009-3598. [DOI] [PubMed] [Google Scholar]
- Yang CH, Huang CC, Hsu KS. Behavioral stress modifies hippocampal synaptic plasticity through corticosterone-induced sustained extracellular signal-regulated kinase/mitogen-activated protein kinase activation. J Neurosci. 2004;24:11029–11034. doi: 10.1523/JNEUROSCI.3968-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]