Abstract
NG2 cells are equipped with transmitter receptors and receive direct synaptic input from glutamatergic and GABAergic neurons. The functional impact of these neuron–glia synapses is still unclear. Here, we combined functional and molecular techniques to analyze properties of GABAA receptors in NG2 cells of the juvenile mouse hippocampus. GABA activated slowly desensitizing responses in NG2 cells, which were mimicked by muscimol and inhibited by bicuculline. To elucidate the subunit composition of the receptors we tested its pharmacological properties. Coapplication of pentobarbital, benzodiazepines, and zolpidem all significantly increased the GABA-evoked responses. The presence of small tonic currents indicated the presence of extrasynaptic GABAA receptors. To further analyze the subunit expression, single cell transcript analysis was performed subsequent to functional characterization of NG2 cells. The subunits α1, α2, β3, γ1, and γ2 were most abundantly expressed, matching properties resulting from pharmacological characterization. Importantly, lack of the γ2-subunit conferred a high Zn2+ sensitivity to the GABAA receptors of NG2 cells. Judging from the zolpidem sensitivity, postsynaptic GABAA receptors in NG2 cells contain the γ2-subunit, in contrast to extrasynaptic receptors, which were not modulated by zolpidem. To determine the effect of GABAA receptor activation on membrane potential, perforated patch recordings were obtained from NG2 cells. In the current-clamp mode, GABA depolarized the cells to approximately −30 mV, indicating a higher intracellular Cl− concentration (∼50 mm) than previously reported. GABA-induced depolarization in NG2 cells might trigger Ca2+ influx through voltage-activated Ca2+ channels.
Introduction
Glial cells express a similar set of ion channels and receptors as neurons (Verkhratsky and Steinhäuser, 2000). Recently, NG2 cells have emerged as a separate type of neuroglia. While in white matter the majority of NG2-positve cells differentiate into oligodendrocytes, many NG2 cells in gray matter keep their phenotype throughout postnatal life (Dimou et al., 2008). NG2 cells receive direct synaptic input from glutamatergic and GABAergic neurons, a property whose physiological impact is not yet understood (Nishiyama et al., 2009; Bergles et al., 2010). While the AMPA receptors of NG2 cells (previously termed complex cells, immature astrocytes, or GluR cells; for review, see Bergles et al., 2010) have been investigated in much detail (Seifert and Steinhäuser, 1995; Matthias et al., 2003; Seifert et al., 2003), less information is available about properties and identity of GABAA receptors expressed by these cells.
GABAA receptors are ionotropic pentameric receptors composed of two α-, two β-, and one γ-subunit, with the latter being replaced by a δ- or ε-subunit in some cells (Olsen and Sieghart, 2008). Fourteen GABAA receptor subunits (6α, 3β, 3γ, δ, ε) are known to form receptors with distinct functional properties. Cell type-specific differences in subunit composition can be distinguished by using modulators and blockers that bind at the numerous allosteric ligand binding sites of GABAA receptors, e.g., benzodiazepines, barbiturates, and Zn2+ (Hevers and Lüddens, 1998; Mehta and Ticku, 1999; Sieghart, 2006). GABAA receptors form Cl−-permeable and HCO3−-permeable anion channels. In neurons, a developmental switch from a depolarizing to a hyperpolarizing action of GABA occurs during the first two postnatal weeks, due to an increasing expression of the KCl cotransporter, KCC2 (Rivera et al., 1999; Stein et al., 2004). Immunostaining suggested expression of KCC2 by oligodendrocytes of the optic nerve (Malek et al., 2003), while no evidence is available for the presence of this transporter in gray matter NG2 cells. Accordingly, activation of GABAA receptors in these cells is thought to entail depolarization.
In this study we combined functional and molecular techniques to analyze properties of GABAA receptors in NG2 cells and their potential impact on neuron–glia synapses in the juvenile hippocampus. Of particular interest was the question whether the γ2-subunit is expressed by NG2 cells, because γ2 is a main constituent of neuronal GABAA receptors and essential for its clustering and anchoring at the scaffolding protein gephyrin, a prerequisite for proper function of postsynaptic receptors (Essrich et al., 1998; Kneussel and Betz, 2000; Alldred et al., 2005). We report that despite cell type-specific differences in receptor desensitization, NG2 cells are similarly endowed with subunits enabling postsynaptic clustering of GABAA receptors.
Materials and Methods
Preparation of hippocampal slices and acutely isolated cells.
Transgenic hGFAP/EGFP mice (Nolte et al., 2001; Matthias et al., 2003) of either sex, aged postnatal days 9–12 were anesthetized and decapitated. Brains were cut in frontal orientation into 200-μm-thick slices or in horizontal orientation into 300-μm-thick slices (stimulation experiments) in cold (4°C) oxygenated solution consisting of the following (in mm): 87 NaCl, 2.5 KCl, 1.25 NaH2PO4, 7 MgCl2, 0.5 CaCl2, 25 NaHCO3, 25 glucose, and 75 sucrose (347 mOsm). Slices were incubated for 15 min in the same solution at 35°C and transferred into artificial CSF (aCSF) containing the following (in mm): 126 NaCl, 3 KCl, 2 MgSO4, 2 CaCl2, 10 glucose 1.25 NaH2PO4, and 26 NaHCO3 after cooling-down to 25°C. Solutions were oxygenated with carbogen (5% CO2/95% O2).
For acute cell isolation (Seifert and Steinhäuser, 1995), brains were cut into 300-μm-thick slices in Ca2+ free, oxygenated solution containing the following (in mm): 150 NaCl, 5 KCl, 2 MgSO4, 1 Na-pyruvate, 10 glucose, and 10 HEPES, pH 7.4; and stored in this solution at 6°C. The tissue was incubated in oxygenated aCSF containing papain (28 U/ml) and cysteine (0.75 mg/ml) (10 min, room temperature). After wash in aCSF, the CA1 region of the hippocampus was dissected and cells were isolated using Pasteur pipettes.
Electrophysiology, drug application.
Slices were transferred to a recording chamber and constantly perfused with HEPES-buffered oxygenized solution containing the following (in mm): 150 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES. For stimulation experiments and recording of tonic currents, aCSF was used. Hippocampal NG2 cells of the CA1 region were identified based on their low EGFP fluorescence, expression of time- and voltage-dependent whole-cell currents, and morphological properties as previously reported (Matthias et al., 2003; Jabs et al., 2005). The cells were clamped at –70 mV if not stated otherwise. In the current-clamp mode, voltage signals were amplified with a DPA 2F amplifier (npi electronic). Signals were digitized with an ITC 16 (HEKA Elektronik). Whole-cell recordings were obtained at 25°C using an EPC800 amplifier (HEKA). Patch pipettes were fabricated from borosilicate capillaries (Hilgenberg; Science Products) with a resistance between 3 and 6 MΩ. Recordings were monitored with TIDA software (HEKA), filtered at 3 kHz or 10 kHz and sampled at 6 kHz or 30 kHz. Series and membrane resistance were checked in constant intervals with Igor Pro 5.03 software (WaveMetrics). Cells were selected with a microscope equipped with water-immersion/infrared DIC optics (Eclipse E600 FN; Nikon). Whole-cell recordings from isolated cells were obtained at room temperature using an EPC7 amplifier and an inverted microscope equipped with epifluorescence (Axiovert 135; Zeiss). Pulse sequences were generated with an AM-Systems isolation pulse stimulator (model 2100) in the constant voltage mode. Postsynaptic currents were evoked (ePSCs) by near-field stimulation (Jabs et al., 2005). A low resistance (<1 MΩ), aCSF-filled glass capillary was used as a monopolar stimulation electrode. It was positioned close to the patch electrode and moved under optical control to optimize PSC recordings.
Focal pressure application in situ was performed with an Octaflow system (ALA Scientific Instruments). To analyze receptor kinetics, we used freshly isolated cells and used a rapid concentration-clamp technique for drug application, allowing solution exchanges within 1 ms (Seifert and Steinhäuser, 1995).
The pipette solution for whole-cell recordings consisted of the following (in mm): 130 KCl, 2 MgCl2, 0.5 CaCl2, 3 Na2-ATP, 5 BAPTA, and 10 HEPES. For determination of receptor current reversal potentials, perforated patch recordings were performed with the following pipette solution (in mm): 125 K-gluconate, 20 KCl, 3 NaCl, 2 MgCl2, 0.5 EGTA, and 10 HEPES, pH 7.25. In these experiments, to block voltage-activated K+-, Na+-, and Ca2+-currents and AMPA receptor-mediated currents, bath solutions with 135 mm NaCl supplemented with the following (in mm): 10 BaCl2, 4 4-aminopyridine, 0.03 CdCl2, 0.001 tetrodotoxin (TTX), and 0.025 CNQX (300 mOsm) were used. Voltage was corrected for liquid junction potential (13 mV for K-gluconate solution vs blocking solution). Zolpidem, diazepam, DMCM, and SNAP 5114 were dissolved in dimethylsulfoxide (DMSO; final DMSO concentration ≤0.1%). Gramicidin A solution (20 mg/ml in DMSO) was freshly prepared every day and added to the pipette solution at a final concentration of 40–60 μg/ml. Tonic GABAA receptor currents were recorded using the following CsCl-based pipette solution (in mm): 130 CsCl, 2 MgCl2, 3 Na2-ATP, 0.5 CaCl2, 5 BAPTA, and 10 HEPES. Substances were purchased from Sigma and Tocris Bioscience.
Single cell reverse transcription-PCR.
After recording, the cytoplasm of individual cells was harvested under microscopic control and reverse transcription (RT) was performed (Matthias et al., 2003). A multiplex two-round single-cell PCR was performed with primers for α, β, and γ GABAA receptor subunits, respectively (Berger et al., 1998; Table 1). The first PCR was performed after adding PCR buffer, MgCl2 (2.5 mm), primers (200 nm each), and Taq polymerase (3.5 U; Invitrogen) to the RT product (final volume 50 μl). Forty-five cycles were performed (denaturation at 94°C, 25 s; annealing at 49°C, 2 min for the first five cycles, and 45 s for the remaining cycles; extension at 72°C, 25 s; final elongation at 72°C, 7 min). An aliquot (2 μl) of the PCR product was used as a template for the second PCR (35 cycles; annealing at 54°C, first five cycles: 2 min, remaining cycles: 45 s) using nested, subunit-specific primers (Table 1). The conditions were the same as described for the first round, but dNTPs (4 × 50 μm) and Platinum Taq polymerase (2.5 U; Invitrogen) were added. Products were identified by gel electrophoresis using a molecular weight marker. Specificity of primers was tested with total RNA prepared from freshly isolated mouse brain (p20). For optimization, a two-round RT-PCR was performed with 2 ng of total RNA and primers as described above. Subsequent gel analysis did not detect unspecific products. The primers for the targets were located on different exons to prevent amplification of genomic DNA. Omission of the RT enzyme and substitution of template by bath solution served as negative controls for RT and PCR amplification. To discriminate between expression of β2- and β3-subunits, the second PCR (β2/3) was repeated. The PCR product was purified (MinElute PCR Purification Kit; Qiagen) and dissolved in 30 μl of H2O. Seven microliters of the PCR product was incubated in restriction enzyme (10 U, 6 h, 37°C). cDNA fragments were analyzed by electrophoresis (2% agarose 1000; Invitrogen) using a 50 bp ladder as a length marker. The PCR products for β2/3-subunits were digested by PstI (specific to β2) and BanI (cleaving specifically β3). PstI cut the PCR product (307 bp) into 169 and 138 bp fragments, while BanI produced fragments of 202 and 105 bp length.
Table 1.
Primer for single cell RT-PCR
| Gene | Sequence | Product length | Position | GenBank accession number |
|---|---|---|---|---|
| Alpha | se 5′-TGGACTCCTGATACNTTYTT | 590 bp | 361, 364, 439, 382, 385 | |
| (Berger et al., 1998) | as 5′-GCHATRAACCARTCCATGGC | 931, 934, 1009, 952, 955 | ||
| Alpha | se 5′-AACATGACMAYGCCMAAYAAGCT | 554 bp | 409, 412, 487, 430, 433 | |
| as 5′-GCATARCAGACAGCWATRAACCA | 940, 943, 1018, 961, 964 | |||
| α1 (nested) | se 5′-CCAGCCCGTTCAGTGGTTGTA | 180 bp | 601 | NM_010250 |
| as 5′-GCACGGCAGATATGTTTGAAT | 760 | |||
| α2 (nested) | se 5′-TTACAATGCTTCTGACTCCGTTCA | 306 bp | 597 | NM_008066 |
| as 5′-CGRGCACTGATRCRWARGGT | 883 | |||
| α3 (nested) | se 5′-CTTGGGAAGAACAAATCTGTGGA | 305 bp | 673 | NM_008067 |
| as 5′-CGRGCACTGATRCRWARGGT | 958 | |||
| α4 (nested) | se 5′-ACCAAAGGCCCTGAGAAGTCA | 308 bp | 613 | NM_010251 |
| as 5′-CGRGCACTGATRCRWARGGT | 901 | |||
| α5 (nested) | se 5′-GCTGGAGGATGATGGCACACTTCT | 208 bp | 462 | NM_176942 |
| as 5′-GTTGAGCCTGGAGCCATCTTCTG | 647 | |||
| Beta | se 5′-CTGGATGARCAAAACTGYAC | 443 bp | 508, 505, 508 | |
| (Berger et al., 1998) | as 5′-ACAAAGACAAARCAWCCCAT | 931, 928, 931 | ||
| β1 (nested) | se 5′-ATGGAGGAGAGGGAGCAGTAACT | 358 bp | 581 | NM_008069 |
| as 5′-CAGCCCATGAGATAGATGTCAATC | 915 | |||
| β2/3 (nested) | se 5′-GGCGYGGCGRTGACAAKGC | 307 bp | 575, 578 | NM_008070 |
| as 5′-TCCCGRAGGTGRGTGTTGAT | 862, 865 | NM_008071 | ||
| Gamma | se 5′-TAGACAGCAAYATGGTGGG | 481 bp | 404, 407, 353 | |
| (Berger et al., 1998) | as 5′-TTGATCCAAAADGACACCCAGG | 863, 866, 812 | ||
| Gamma | se 5′-ATTTGGATTCCAGACACYWTCTT | 392 bp | 427, 430, 376 | |
| as 5′-AAGTAGCCCATTCTTCKRCTCAG | 796, 799, 745 | |||
| γ1 (nested) | se 5′-CGCCTGCTGCGGATTTG | 180 bp | 496 | NM_010252 |
| as 5′-CACAGAGGGCTTTTTCCACTTGT | 653 | |||
| γ2 (nested) | se 5′-AAAAMRGCTGAGGCTCACTGGAT | 211 bp | 463 | NM_177408 |
| as 5′-AACTGCGCTTCCATTGATAAACA | 651 | |||
| γ3 (nested) | se 5′-AAAAMRGCTGAGGCTCACTGGAT | 272 bp | 409 | NM_008074 |
| as 5′-CTGAGGCCCATGAAGTCAAACTGA | 657 | |||
| PDGFα-receptor | se 5′-TCAAAGGGAGGACGTTCAAGACC | 303 bp | 578 | NM_011058 |
| as 5′-GACGGGCAGCACATTCATACTC | 859 | |||
| PDGFα-receptor | se 5′-TGTGTATAAGGCAGGAGAAACGAT | 167 bp | 669 | |
| (nested) | as 5′-TGGGGACGGTCAAAGTGTA | 817 |
“Se” and “as” indicate sense and antisense primers, respectively. Position 1 is the first nucleotide of the initiation codon. The primer pairs for Alpha, Beta, and Gamma recognize all subunits of the respective receptor family. For the subunits α2, α3, and α4 a common antisense primer was used. For the subunits γ2 and γ3 a common sense primer was used. All sense and antisense primers are located on different exons, respectively.
Data analysis
To describe modulation of GABAA receptor responses a ratio R was defined as follows:
Data are given as mean ± SD. Differences between data were tested for significance using Student's t test (paired Student's t test as indicated) or Pearson's χ2 test (see Fig. 6D). The level of significance was set at p < 0.05.
Figure 6.
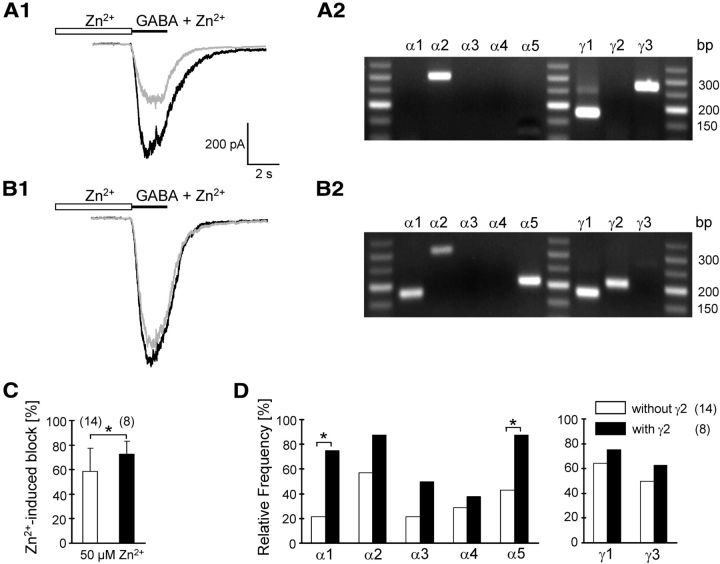
Zn2+ sensitivity and subunit composition of NG2 cell GABAA receptors in situ. A1, B1, Pressure pre-application of Zn2+ (50 μm, 30 s) was followed by coapplication of Zn2+ with GABA (50 μm, 2 s, gray traces). Black traces represent control GABA responses before Zn2+ application. Zn2+ substantially reduced the GABA-evoked current in one NG2 cell (A1) while in another cell the block was much smaller (B1). A2, B2, Agarose gels show the α- and γ-subunit composition of the cells given in A1 and B1. A low molecular weight marker (New England BioLabs) was used as a base pair ladder. C, Summary of Zn2+-mediated inhibition of GABA responses (white bars, cells lacking the γ2-subunit; black bars, cells expressing γ2). Cell numbers are given in parentheses. D, Comparison of α- and γ-subunit expression of cells lacking (white bars) and expressing γ2 (black bars). Experiments were performed at –70 mV, [Cl−]i = 135 mm. Bath solution was supplemented with 25 μm CNQX. Asterisks indicate significant differences.
Results
Pharmacological properties of GABAA receptors in NG2 cells
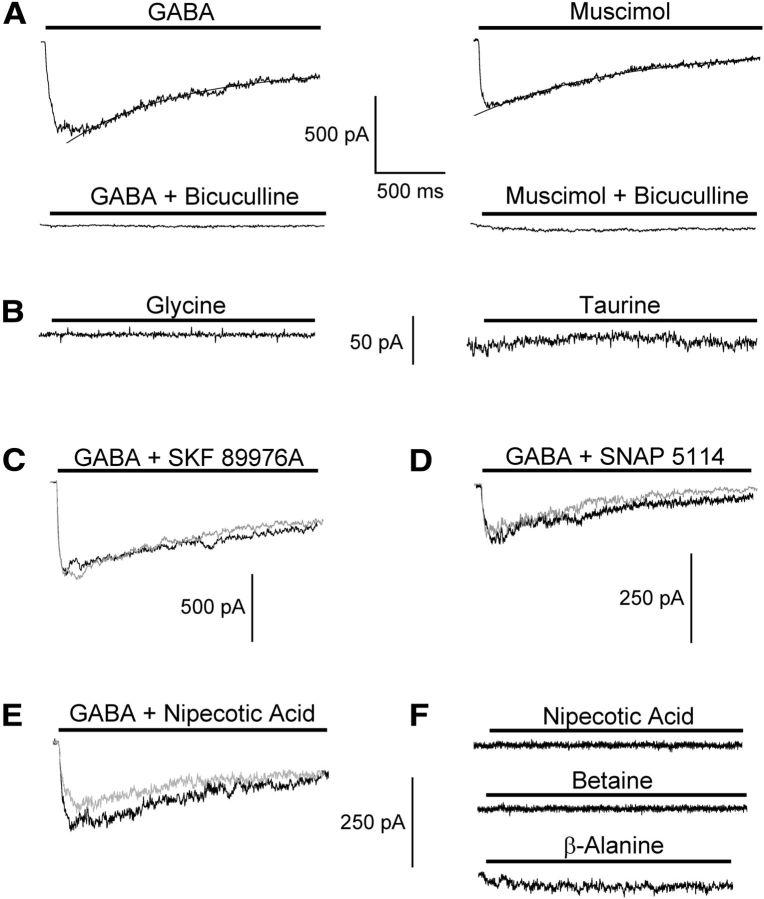
To quantify receptor current kinetics, freshly isolated cells were used. Fast application of GABA (100 μm) to isolated NG2 cells activated slowly desensitizing inward currents (corresponding to Cl− outward currents) at holding potential (Fig. 1A). Receptor responses amounted to –413 ± 260 pA (n = 70), corresponding to a current density of 133 ± 112 pA/pF. Current desensitization could be fit by a mono-exponential function yielding a time constant τ = 1.25 ± 0.65 s (n = 41). In another 22 cells, two decay time constants were found with τfast = 218 ± 207 ms (amplitude factor 0.22 ± 0.16) and τslow = 2.48 ± 1.95 s. Increasing GABA concentration to 1 mm produced significantly larger amplitudes (−631 ± 489 pA, corresponding to 298 ± 276 pA/pF, n = 10) while the desensitization time constants remained unchanged (τfast = 95.1 ± 60.0 ms, amplitude factor 0.19 ± 0.03; τslow = 1.63 ± 1.32 s, n = 7). Bicuculline (20 μm), a competitive receptor antagonist, almost completely blocked the responses (100 μm GABA: decrease to 3.5 ± 2.4% of the control, n = 12; 1 mm GABA: decrease to 7.2 ± 5.7%, n = 8; Fig. 1A, bottom). Responses evoked by muscimol, a GABAA receptor agonist, mimicked those elicited by GABA (100 μm muscimol: current amplitudes −523 ± 213 pA, corresponding to 163 ± 122 pA/pF, mono-exponential current decay with τ = 1.40 ± 0.93 s, n = 5; Fig. 1A, right). Muscimol-evoked currents were sensitive to bicuculline (20 μm bicuculline: block to 10.0 ± 0.8%, n = 3; Fig. 1A, bottom). Fast application of glycine (100 μm, n = 3; 500 μm, n = 6) and taurine, an agonist at glycine and GABAA receptors, at concentrations of up to 10 mm (n = 3) failed to induce receptor currents at isolated cells (Fig. 1B).
Figure 1.
GABA-induced currents in freshly isolated NG2 cells are mediated by GABAA receptors and not by GABA transporters. A, Fast application of GABA and muscimol (100 μm each; holding potential –70 mV; [Cl−]i = 135 mm) induced slowly desensitizing responses (top). Current desensitization could be described by a single exponential function (smooth lines, τGABA = 767 ms; τMuscimol = 965 ms). In the same cell, coapplication of bicuculline (20 μm) blocked the responses (bottom). B, Fast application of glycine (100 μm) and taurine (5 mm) did not evoke receptor responses in acutely isolated cells. C–E, Coapplication of GABA (100 μm) with the GABA uptake inhibitors SKF 89976A, SNAP 5114, and nipecotic acid (100 μm each, gray traces) did not affect the responses. F, Neither betaine (1 mm), a substrate of the betaine/GABA transporters mGAT2, nor nipecotic acid (500 μm), a transportable antagonist of mGAT1 and mGAT4, evoked membrane currents in NG2 cells. Only β-alanine (1 mm) activated tiny responses in some cells. Timescale bar in A also applies to B–F. Amplitude scaling in F also applies to E.
GABA-evoked currents in NG2 cells are not mediated by GABA transporters
To test for a potential contribution of transport currents to the GABA-induced responses, we rapidly applied GABA (100 μm) together with transport inhibitors to freshly isolated NG2 cells. Four GABA transporters have been cloned. Among them, mouse GAT-1 (mGAT-1) is predominantly expressed by neurons and particularly found on GABAergic terminals in the hippocampus although it has also been localized to glial processes (Minelli et al., 1995; Ribak et al., 1996). Coapplication of the mGAT-1-specific, nontransportable inhibitor SKF 89976A (100 μm) had no effect on GABA-evoked responses (93 ± 18%, n = 5; Fig. 1C). The glia-specific transporter mGAT-4 is found in astrocytic processes enwrapping GABAergic synapses (Minelli et al., 1996; Ribak et al., 1996). To probe NG2 cells for mGAT-4 we applied the nontransportable inhibitor SNAP 5114 (100 μm), which did not reduce GABA responses (94 ± 12%; n = 10; Fig. 1D). The nonspecific, transportable inhibitor nipecotic acid (100 μm) also had no significant effect compared with GABA controls (91 ± 17%; n = 6; Fig. 1E). Neither nipecotic acid (500 μm, n = 9) nor betaine (1 mm, n = 6), an mGAT-2 agonist, induced any currents on its own (Fig. 1F). Fast application of β-alanine (1 mm), which might potentially activate glial mGAT-3 and mGAT-4 transporters, produced tiny inward currents in 5/12 cells tested (24 ± 7 pA). The corresponding current densities were 6.2 ± 1.6 pA/pF, i.e., much smaller than the responses evoked by GABA.
Together, these findings suggest that responses of NG2 cells to GABA were mainly mediated by GABAA receptors. Only in a few cases GABA transport (possibly mGAT-4) might have marginally added to the responses.
Modulation of GABAA receptor currents in situ
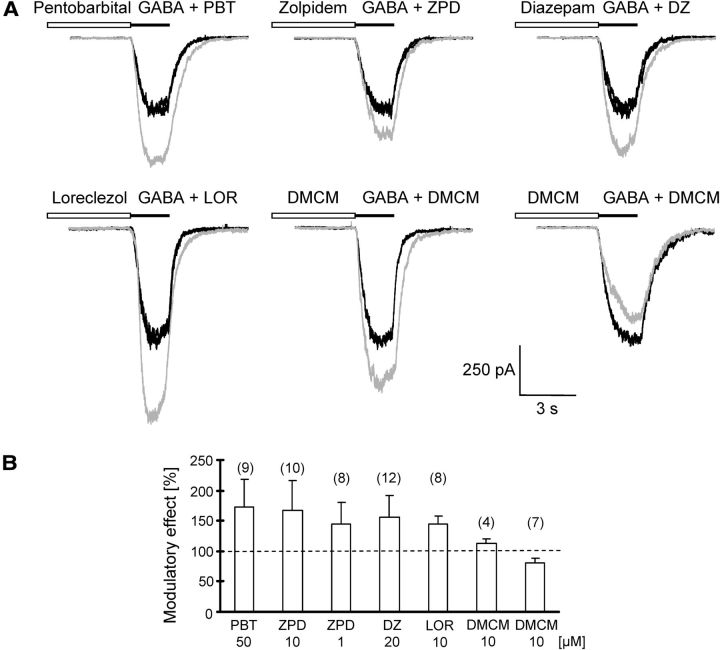
GABAA receptors contain binding sites for barbiturates and benzodiazepines. To test for their presence in the glial receptors, local pressure application in situ was used. Application of GABA (50 μm) with pentobarbital (50 μm) reversibly potentiated the control GABA responses in all cells tested (to 172 ± 46%, n = 9; Fig. 2). Zolpidem modulates GABA receptor function through the benzodiazepine site, with high efficiency if receptors contain the α1- and the γ2-subunit (Olsen and Sieghart, 2009). At a concentration of 10 μm, zolpidem increased the GABA (50 μm) responses to 167 ± 49% (n = 10). To enhance specificity for the α1-subunit, zolpidem was applied at a lower concentration. At a concentration of 1 μm, zolpidem still increased the GABA (50 μm)-evoked responses to 145 ± 35% (n = 8/11 cells tested; Fig. 2). Diazepam (20 μm) and clonazepam (10 μm), which bind at the α/γ-interface of the receptor (Olsen and Sieghart, 2009), enhanced the GABA (50 and 80 μm, respectively) responses to 156 ± 35% (n = 12; Fig. 2) and 187 ± 22% (n = 3; data not shown), respectively. The β-carboline, methyl-6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate (DMCM), is an inverse agonist acting at the benzodiazepine site of GABAA receptors. It usually mediates current decreases, except at receptors containing the γ1- and/or β2/3-subunits where positive modulatory effects have been observed (Fraser et al., 1995). In 7 of 14 NG2 cells tested, DMCM (10 μm) led to an inhibition of GABA (50 μm)-induced control currents (to 81 ± 6%). In 4/14 cells currents increased upon DMCM coapplication (to 113 ± 8%; Fig. 2), while in another three cells DMCM had no effect (data not shown). In contrast to the other modulators, the effect of DMCM was irreversible in most cells. Loreclezole (10 μm), a specific modulator of GABAA receptors containing the β2/3-subunits, enhanced GABA-evoked currents in all cells (to 145 ± 13%, n = 8; Fig. 2). All the modulatory effects were statistically significant. Coapplication of GABA (50 μm) together with antagonists of GABAB receptors in TTX (1 μm)-containing solution did not significantly change the responses activated by GABA alone (200 μm phaclofen, to 101 ± 10%, n = 7; 10 μm CGP 55845, to 94 ± 17%, n = 10; data not shown). Thus, it is unlikely that GABAB receptors were involved in the GABA-induced responses of NG2 cells.
Figure 2.
Modulation of GABAA receptor currents in NG2 cells in situ. A, Pre-application of receptor modulators (30 s) was followed by coapplication of GABA (50 μm) with the modulators (pressure application, 2 s). Gray traces represent responses upon coapplication, black traces give GABA responses before and after application of the modulator. The following concentrations were used: pentobarbital (PBT), 50 μm; diazepam (DZ), 20 μm; DMCM, 10 μm; loreclezole (LOR), 10 μm; and zolpidem (ZPD), 1 μm. Traces showing PBT, ZPD, DZ and LOR, DMCM (middle, bottom) were obtained from the same cell, respectively. B, Summary of receptor modulation. Data were normalized to the first GABA control response (Eq. 1), cell numbers are given in parentheses. All modulators significantly (paired t test, p < 0.05) affected the GABA responses. Experiments were performed at –70 mV, [Cl−]i = 135 mm. Bath solution was supplemented with 10 mm BaCl2, 4 mm 4-AP, 30 μm CdCl2, and 1 μm TTX.
GABAA receptor activation produces depolarization of NG2 cells
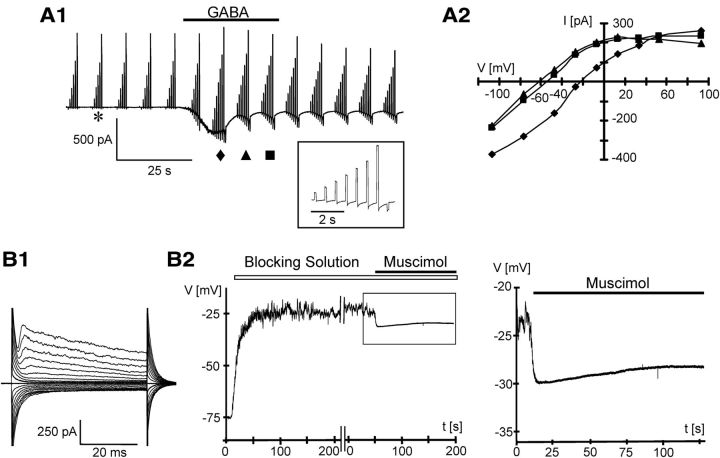
GABAA receptors may depolarize or hyperpolarize cells dependent on the intracellular Cl− concentration [Cl−]i. In neurons, developmental upregulation of the KCl cotransporter, KCC2, results in a reduction of [Cl−]i and hyperpolarization upon receptor activation. In immature neurons and astrocytes, KCC2 is not yet active or absent, resulting in a depolarizing effect of receptor activation. KCC2 immunoreactivity has also been found in oligodendrocytes of the optic nerve (Malek et al., 2003). To determine the reversal potential of GABAA receptor-mediated currents in NG2 cells, perforated patches were used using gramicidin A, which leaves the intrinsic [Cl−]i unaffected (Kyrozis and Reichling, 1995). GABA- or muscimol-mediated currents (Fig. 3A1) were isolated in K+ channel blocking solution (cf. Materials and Methods) and current–voltage relationships were determined by subtracting currents at corresponding potentials before and during application of the agonist. In these voltage-clamp experiments, we noted an increasing negative shift in the reversal potential, which outlasted GABA application, reaching −44.5 ± 8.6 mV (n = 4) at 24 s after peak inward currents (Fig. 3A2). This shift might have been due to a depletion in [Cl−]i due to the permanent Cl− outflow while clamping the cell at –70 mV (DeFazio and Hablitz, 2001; Karlsson et al., 2011). To prevent this presumed Cl− shift, we switched to the current-clamp mode. Wash-in of K+ channel blockers, which is necessary to avoid GABA-mediated K+ channel inhibition (Bekar et al., 1999; Bekar and Walz, 2002), led to a significant depolarization of the resting potential (from −78.5 ± 4.9 mV to values between –45.0 and −20.2 mV, n = 6; Fig. 3B2). In this blocking solution, the muscimol (400 μm, bath application)-activated Cl− conductance shifted the membrane potential to –27.3 ± 5.8 mV (n = 6) (Fig. 3B2). This membrane potential corresponds to a [Cl−]i in NG2 cells of ∼50 mm. Washout of the GABAA receptor agonist led to a slow recovery of the membrane potential, which was profoundly accelerated by bicuculline (50 μm) or picrotoxin (100 μm; data not shown).
Figure 3.
Reversal potential analysis of GABAA receptor responses in situ. A1, An NG2 cell was analyzed in the perforated patch mode (gramicidin A; bath solution supplemented with 10 mm BaCl2, 4 mm 4-AP, 30 μm CdCl2, and 1 μm TTX). The membrane was repeatedly clamped between −100 and +100 mV; the inset shows the current family marked by an asterisk at higher time resolution. At −70 mV, 0.5 mm GABA induced a peak inward current of 400 pA. A2, I/V curves of the GABA response shown in A1 were determined by subtracting current families evoked before (asterisk) from those at different time points during (♦, ▴, and ■) GABA application, after off-line correction for liquid junction potential. Note the time-dependent shift of the I/V curve (right). B1, Membrane currents of an NG2 cell in the perforated patch mode after depolarization and hyperpolarization of the membrane between −160 and +20 mV (left). B2, To determine the reversal potential after activation of the Cl− conductance, cells were analyzed in the current-clamp mode. After wash-in of K+ channel blocking solution, a profound depolarization of the membrane potential to approximately −25 mV was observed. Application of muscimol (400 μm) provoked a hyperpolarization to –30 mV and led to a stabilization of the resting potential (left). Right side shows the boxed response at higher magnification.
Modulation of phasic and tonic GABAA receptor currents in NG2 cells
To decide which GABAA receptor subunits are preferentially located at postsynaptic sites in NG2 cells, GABAergic interneurons were stimulated (Jabs et al., 2005) and the glial responses were recorded in the presence of diazepam and zolpidem. In diazepam (10 μm), ePSCs reached 126 ± 9% of the control (n = 5) (Fig. 4A, top left) while in another three NG2 cells, diazepam had no effect on ePSCs (Fig. 4A, bottom). Zolpidem (1 μm) in a majority of cells potentiated ePSCs (to 128 ± 5%, n = 6; Fig. 4A, top right), while in another three cells ePSCs were not affected (Fig. 4A, bottom). These results indicate that most of the NG2 cells contained postsynaptic GABAA receptors carrying the γ2-subunit.
Figure 4.
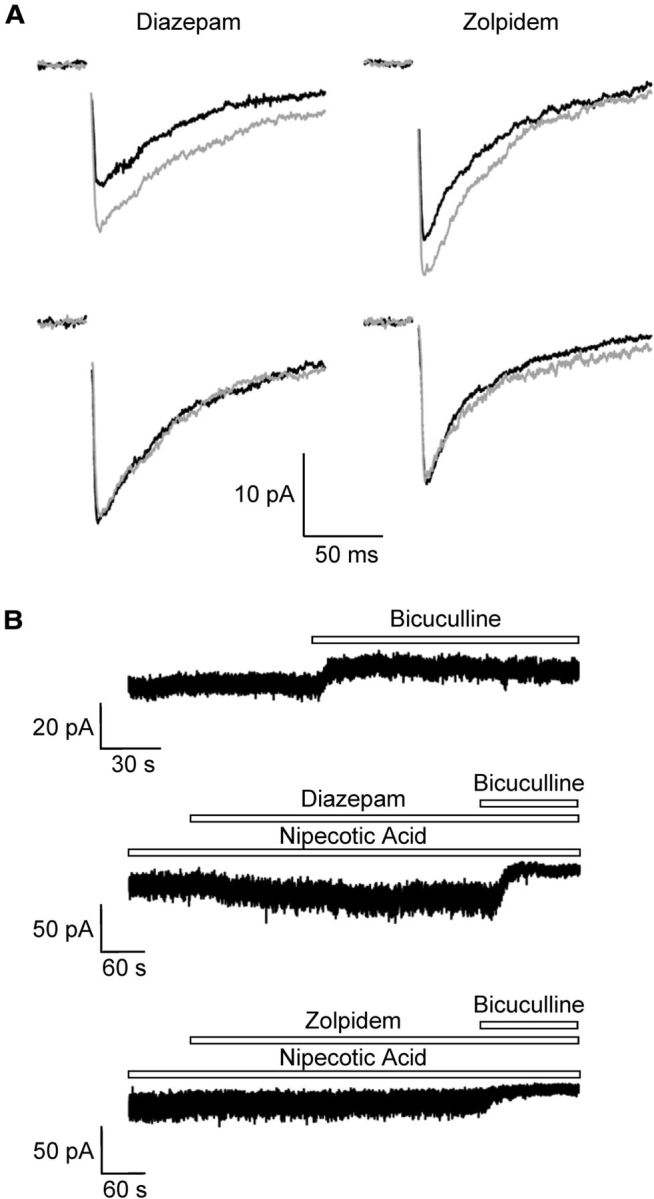
Modulation of synaptic and tonic GABAA receptor currents in NG2 cells in situ. A, Near-field stimulation in the stratum radiatum of the CA1 region elicited ePSCs in NG2 cells (holding potential −70 mV; [Cl−]i = 135 mm). Bath solution contained NBQX (5 μm) to avoid AMPA receptor-mediated ePSCs. Bath application of diazepam (10 μm) and zolpidem (1 μm) increased the ePSCs (gray traces, top). Some of the cells were insensitive to diazepam or zolpidem (bottom). B, Bath application of bicuculline (10 μm) unmasked tonic currents (−70 mV) (top). Coapplication of diazepam (10 μm) with nipecotic acid (1 mm) enhanced the bicuculline (20 μm)-sensitive tonic currents (middle). In contrast, zolpidem did not further increase bicuculline (20 μm)-sensitive tonic currents (bottom). K+ currents were blocked by using CsCl-based pipette solution.
Apart from fast synaptic neurotransmission, tonic activation of extrasynaptic GABAA receptors has been observed in neurons (Farrant and Nusser, 2005; Belelli et al., 2009). To test NG2 cells for tonic GABAA receptor currents, we used a Cs+-based pipette solution to decrease K+ currents. In all cells tested (n = 7), application of bicuculline (10 μm) led to a positive shift of the holding current (by 5.3 ± 3.3 pA, n = 7; Fig. 4B, top). To investigate pharmacological properties of the tonic currents, the ambient GABA concentration in the tissue was enhanced by wash-in of nipecotic acid (1 mm), an inhibitor of the GABA transporters mGAT-1 and mGAT-4. Under these conditions, tonic current amounted to 18.7 ± 7.2 pA (n = 6). Coapplication of nipecotic acid together with diazepam (10 μm) significantly increased tonic currents in 4/6 NG2 cells tested (to 140 ± 32%; Fig. 4B, middle) while in two other cells these currents remained unaffected by diazepam. Interestingly, zolpidem (1 μm) never did enhance tonic currents of NG2 cells recorded in the presence of nipecotic acid (control: 18.3 ± 8.5 pA; zolpidem: 19.0 ± 9.3 pA; n = 5; Fig. 4B, bottom). These results indicated the existence of tonic currents in NG2 cells, presumably mediated by extrasynaptic GABAA receptors devoid of the γ2-subunit.
Analysis of GABAA receptor subunits by single cell RT-PCR
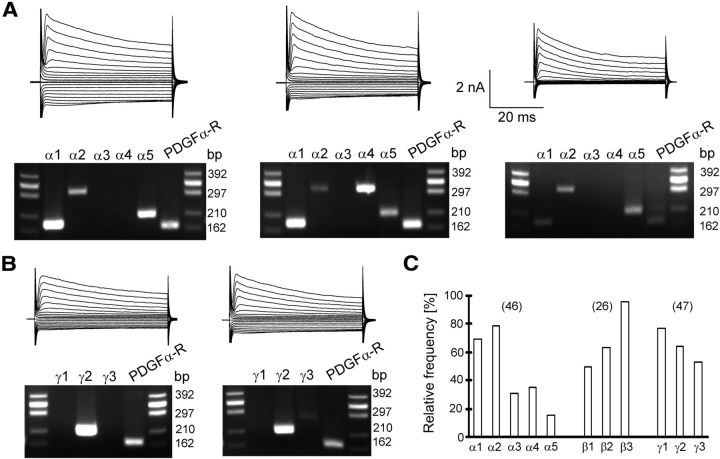
To identify the receptor subunits expressed by NG2 cells, transcript analysis was performed after acute isolation and in situ, subsequent to functional characterization of membrane currents with single-cell RT-PCR. After RT we performed a two-round PCR using primer sets for α-subunits, β-subunits, or γ-subunits in the first round, respectively. For the second PCR round subunit-specific primers were used. Restriction analysis was used to test specificity of the primers and identify β2- versus β3- subunits. The subunits α1 to α5 were found in 70, 78, 30, 35, and 15% of the NG2 cells tested (n = 46). β1 to β3 were expressed at a frequency of 50, 64, and 95% (n = 22). All three β-subunits were found in 7/22 cells. Among the γ-subunits γ1 (77%) and γ2 (64%) were most frequently detected while 53% of the NG2 cells contained γ3 mRNA (n = 47; Fig. 5). The subunit combination γ1/γ2 was found in 21 cells, and among them 13 cells coexpressed all three γ-subunits. Since subunit expression did not correlate with membrane resistance or capacitance, the variability of the expression pattern probably does not reflect different stages of differentiation of the cells investigated.
Figure 5.
Single-cell RT-PCR analysis of GABAA receptor subunits in NG2 cells. A, B, Current patterns of different NG2 cells in situ (depolarization and hyperpolarization between –160 and +20 mV) are shown together with the respective agarose gels of PCR products for the α-subunit (A) and γ-subunit (B). PDGFα-receptor transcripts served as positive controls. The PCR products corresponded to the predicted lengths (Table 1). Phi X174 HincII digest (Eurogentec) was used as a molecular weight marker. C, Summary of the relative frequency of subunit expression by individual cells. Cell numbers are given in parentheses.
In another set of experiments we investigated the functional impact of γ2 expression by determining effects of Zn2+, an endogenous inhibitor of GABAA receptors. The sensitivity of the receptors to this cation is strongly influenced by the particular α- and γ-subunits forming the pore (Draguhn et al., 1990; Smart et al., 1991). To directly correlate Zn2+ sensitivity with subunit expression in individual NG2 cells, Zn2+ (50 μm) was coapplied with GABA (50 μm) while AMPA receptors were blocked by CNQX (25 μm). Subsequently, single-cell transcript analysis was performed with RT-PCR (Fig. 6A,B). We found that cells lacking the γ2-subunit display a significantly higher sensitivity to Zn2+ block than those cells expressing γ2 (50 μm Zn2+; block to 58.6 ± 18.8, n = 14 vs 72.5 ± 10.6%, n = 8; Fig. 6C). Notably, in cells with higher Zn2+ sensitivity lacking γ2, α1 and α5 were also significantly less prevalent (Fig. 6D).
Discussion
GABAergic interneurons in the hippocampus synapse onto NG2 cells through monosynaptic innervation (Bergles et al., 2010). To better understand the physiological impact of these intriguing neuron–glia synapses here we investigated properties of GABAA receptors in NG2 cells combining pharmacological and molecular analyses. With regard to their modulatory properties and subunit composition, the glial receptors resembled many properties of neuronal GABAA receptors. However, slower receptor desensitization was found in NG2 cells compared with neuronal receptors. Importantly, our data indicate that postsynaptic GABAA receptors in NG2 cells carry the γ2-subunit while extrasynaptic receptors mostly lack γ2.
Functional properties of GABAA receptors in NG2 glial cells
Fast application of GABA or muscimol to isolated NG2 cells in most cases activated slowly desensitizing currents. This finding is in line with the relatively slow decay constants of mIPSCs and stimulus-evoked IPSCs in NG2 glia/GluR cells previously reported (Lin and Bergles, 2004; Jabs et al., 2005). In hippocampal and cortical neurons, GABAA responses decline bi-exponentially with a rapid time constant (Galarreta and Hestrin, 1997; Berger et al., 1998; τfast < 10 ms). IPSCs and GABAA receptor currents with slower desensitization time constants were, however, also observed in dentate gyrus granule cells (Celentano and Wong, 1994; Draguhn and Heinemann, 1996).
Receptor desensitization is determined by the subunit composition (Puia et al., 1994; Tia et al., 1996). The α5-subunit causes slowly desensitizing GABAA receptors, although our transcript analysis identified many NG2 cells lacking this subunit. The rapid concentration-clamp technique makes it unlikely that slow agonist application accounted for this particular property, although our application techniques might have been too slow to detect very rapidly desensitizing receptor currents (<3 ms). Rather, the long-lasting application of GABA onto NG2 cells might play a role because of potential reopening of the receptors from the closed state in the presence of the agonist (Jones and Westbrook, 1995). Slow receptor desensitization might also have been caused by the high intracellular Cl− concentration used in our experiments (Houston et al., 2009).
Correlation of pharmacological properties with transcript analysis
The benzodiazepine binding site of GABAA receptors is located at the interface of α- and γ-subunits of the receptor complex. Diazepam increased GABA-evoked responses, indicating expression of γ1- and γ2-subunits by NG2 cells (Khom et al., 2006; Olsen and Sieghart, 2009). Moreover, the modulator enhanced ePSCs in most NG2 cells tested. To further distinguish GABAA receptor subunits by pharmacological analysis we used zolpidem, a selective modulator of receptors containing α1/γ2-subunits. Zolpidem was highly efficient at the glial GABAA receptors and, importantly, also increased ePSCs in the majority of NG2 cells. A third of the investigated cells displayed diazepam- and zolpidem-insensitive ePSCs, which have been described in a previous study (Lin and Bergles, 2004). Zolpidem shows cross-reactivity with α2/α3-containing receptors (Puia et al., 1991; Wafford et al., 1993). Our RT-PCR analysis revealed frequent expression of α1- and α2-subunits in hippocampal NG2 cells. Together, the sensitivity of the receptors to diazepam and zolpidem suggested expression of α1-, α2-, and γ2-containing receptors at postsynaptic sites of hippocampal NG2 cells, resembling properties of neuronal receptors in this brain region (Olsen and Sieghart, 2008). To further test for the presence of γ-subunits we applied DMCM, an inverse agonist acting at the benzodiazepine site, except for receptors containing γ1 (Ymer et al., 1990; Puia et al., 1991; Wafford et al., 1993; Hevers and Lüddens, 1998). The modulatory effect of DMCM was inconsistent, suggesting variable expression of γ1. All NG2 cells were sensitive to loreclezole, which indicated abundant expression of β2/3-subunits, which was confirmed by our single-cell RT-PCR. Heterogeneity in mRNA expression patterns has also been observed in cultured oligodendrocyte progenitors, although these cells lacked α1 and showed more abundant expression of α5-subunits (Williamson et al., 1998).
The Zn2+ sensitivity of GABAA receptors is determined by γ- and α-subunits (Draguhn et al., 1990; Smart et al., 1991; White and Gurley, 1995; Hosie et al., 2003). In hippocampal neurons, GABAA receptors are strongly inhibited by Zn2+ (Westbrook and Mayer, 1987; Berger et al., 1998) with Zn2+ sensitivity decreasing during development (Martina et al., 1996). Our data show that hippocampal NG2 cells are heterogeneous with respect to their Zn2+ sensitivity. To directly correlate receptor function with subunit expression, single-cell RT-PCR was performed after determining the Zn2+ sensitivity of individual NG2 cells. Despite some variability, we report that NG2 cells lacking the γ2-subunit display a significantly higher Zn2+ sensitivity. Moreover, α1 and α5 were much less abundant in the Zn2+-sensitive cells compared with those showing low Zn2+ sensitivity. γ2, together with β3, also determines the Zn2+ sensitivity of cortical interneurons (Alsbo et al., 2001) and, together with the scaffolding protein gephyrin, the γ2-subunit is required for postsynaptic clustering of GABAA receptors. It has been reported that the turnover rate of GABAA receptor transcripts is much faster than downregulation of the corresponding proteins (Lyons et al., 2000), which might have contributed to the variability in Zn2+ sensitivity among the γ2 mRNA-lacking NG2 cells.
The pharmacological properties of GABAA receptors in NG2 cells differ from those in hippocampal astrocytes, Bergmann glia and Müller cells. α2, a main constituent of GABAA receptors, was also observed in Bergmann glia (Wisden et al., 1989; Müller et al., 1994; Riquelme et al., 2002) and hippocampal astrocytes (Fraser et al., 1995), but the insensitivity of the responses to diazepam and its potentiation by DMCM and Zn2+ (Müller et al., 1994; Fraser et al., 1995; Biedermann et al., 2004) indicated prominent expression of γ1-subunits by these glial cell types (Wisden et al., 1989; Riquelme et al., 2002).
Evidence for tonic GABA receptor currents in glial cells
In neurons, GABA may induce phasic and tonic receptor responses (Semyanov et al., 2004). To probe NG2 cells for tonic currents bicuculline was applied, which consistently revealed small resting Cl− outward currents. Thus, in addition to activating synaptic receptors, ambient GABA stimulates NG2 cells through extrasynaptic receptors. To get further insight into the molecular composition of these receptors, tonic currents were enhanced by applying the GABA uptake inhibitor nipecotic acid. While the extrasynaptic receptors were sensitive to diazepam, zolpidem failed to increase tonic currents. These findings suggest that γ1- or γ3-containing receptors may be located extrasynaptically while γ2 is located postsynaptically in NG2 cells. In hippocampal neurons, tonic GABA currents are mediated by extrasynaptic receptors comprising the α5-subunit (Kneussel and Loebrich, 2007; Glykys et al., 2008). In cortical NG2 cells, synaptic activation of GABAA receptors was only observed during the first two postnatal weeks and subsequently disappeared. This change might be due to altered subunit composition and/or impaired postsynaptic receptor clustering (Vélez-Fort et al., 2010). Tonic activation of GABAA receptors in glial cells was recently shown to represent a chemotactic cue that is crucial for NG2 cell migration (Tong et al., 2009).
Functional implications
Reversal potential analysis in the voltage-clamp mode revealed GABA current reversal at −44 mV in NG2 cells, very similar to the data previously reported (Lin and Bergles, 2004). However, we noted a significant shift in the reversal potential during receptor activation, presumably indicating depletion in [Cl−]i due to holding the cells at −70 mV (Karlsson et al., 2011). To avoid this bias, we switched to the current-clamp mode, which yielded a reversal potential of approximately −30 mV, corresponding to a [Cl−]i of 50 mm. Thus, the [Cl−]i of NG2 cells is probably higher than estimated previously. Due to this high [Cl−]i, opening of GABAA receptors leads to depolarization of the cells, which might activate voltage-gated Ca2+ channels (Akopian et al., 1996) and Ca2+-induced Ca2+ release (Haberlandt et al., 2011). Indeed, Ca2+ signaling upon GABAA receptor activation has been demonstrated in cultured glial precursor cells (Kirchhoff and Kettenmann, 1992) and in NG2 cells (Tanaka et al., 2009; Haberlandt et al., 2011). Depolarization of NG2 glia through presynaptic stimulation also induced [Ca2+]i increases in these cells (Haberlandt et al., 2011). GABA-dependent Ca2+ transients might stimulate BDNF secretion as has been demonstrated in cultured NG2+/nestin+ cells (Tanaka et al., 2009) and regulate process motility (Haberlandt et al., 2011). While GABA-mediated excitation is crucial for proper neuronal morphology (Cancedda et al., 2007) and neural progenitor cell proliferation (Young et al., 2012), its physiological impact on NG2 cells is not yet understood. Future research has to unravel whether GABA signaling is also needed for glial process maturation, secretion of neurotransmitters, and possibly formation of neuron–glia synapses.
Footnotes
Supported by Deutsche Forschungsgemeinschaft (SFB/TR3, TP C1; SPP1172, SE774/3-2) and EU (FP7-202167 NeuroGLIA; ESF EuroEPINOMICS). We thank T. Erdmann and I. Fiedler for excellent technical assistance.
The authors declare no competing financial interests.
References
- Akopian G, Kressin K, Derouiche A, Steinhäuser C. Identified glial cells in the early postnatal mouse hippocampus display different types of Ca2+ currents. Glia. 1996;17:181–194. doi: 10.1002/(SICI)1098-1136(199607)17:3<181::AID-GLIA1>3.3.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Alldred MJ, Mulder-Rosi J, Lingenfelter SE, Chen G, Lüscher B. Distinct gamma2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin. J Neurosci. 2005;25:594–603. doi: 10.1523/JNEUROSCI.4011-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsbo CW, Kristiansen U, Møller F, Hansen SL, Johansen FF. GABAA receptor subunit interactions important for benzodiazepine and zinc modulation: a patch-clamp and single cell RT-PCR study. Eur J Neurosci. 2001;13:1673–1682. doi: 10.1046/j.0953-816x.2001.01539.x. [DOI] [PubMed] [Google Scholar]
- Bekar LK, Walz W. Intracellular chloride modulates A-type potassium currents in astrocytes. Glia. 2002;39:207–216. doi: 10.1002/glia.10096. [DOI] [PubMed] [Google Scholar]
- Bekar LK, Jabs R, Walz W. GABAA receptor agonists modulate K+ currents in adult hippocampal glial cells in situ. Glia. 1999;26:129–138. doi: 10.1002/(SICI)1098-1136(199904)26:2<129::AID-GLIA4>3.3.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger T, Schwarz C, Kraushaar U, Monyer H. Dentate gyrus basket cell GABAA receptors are blocked by Zn2+ via changes of their desensitization kinetics: an in situ patch-clamp and single-cell PCR study. J Neurosci. 1998;18:2437–2448. doi: 10.1523/JNEUROSCI.18-07-02437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Jabs R, Steinhäuser C. Neuron-glia synapses in the brain. Brain Res Rev. 2010;63:130–137. doi: 10.1016/j.brainresrev.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedermann B, Bringmann A, Franze K, Faude F, Wiedemann P, Reichenbach A. GABA(A) receptors in Muller glial cells of the human retina. Glia. 2004;46:302–310. doi: 10.1002/glia.20004. [DOI] [PubMed] [Google Scholar]
- Cancedda L, Fiumelli H, Chen K, Poo MM. Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo. J Neurosci. 2007;27:5224–5235. doi: 10.1523/JNEUROSCI.5169-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celentano JJ, Wong RK. Multiphasic desensitization of the GABAA receptor in outside-out patches. Biophys J. 1994;66:1039–1050. doi: 10.1016/S0006-3495(94)80885-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFazio RA, Hablitz JJ. Chloride accumulation and depletion during GABA(A) receptor activation in neocortex. Neuroreport. 2001;12:2537–2541. doi: 10.1097/00001756-200108080-00049. [DOI] [PubMed] [Google Scholar]
- Dimou L, Simon C, Kirchhoff F, Takebayashi H, Götz M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci. 2008;28:10434–10442. doi: 10.1523/JNEUROSCI.2831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draguhn A, Heinemann U. Different mechanisms regulate IPSC kinetics in early postnatal and juvenile hippocampal granule cells. J Neurophysiol. 1996;76:3983–3993. doi: 10.1152/jn.1996.76.6.3983. [DOI] [PubMed] [Google Scholar]
- Draguhn A, Verdorn TA, Ewert M, Seeburg PH, Sakmann B. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+ Neuron. 1990;5:781–788. doi: 10.1016/0896-6273(90)90337-F. [DOI] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Lüscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fraser DD, Duffy S, Angelides KJ, Perez-Velazquez JL, Kettenmann H, MacVicar BA. GABAA/benzodiazepine receptors in acutely isolated hippocampal astrocytes. J Neurosci. 1995;15:2720–2732. doi: 10.1523/JNEUROSCI.15-04-02720.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Properties of GABAA receptors underlying inhibitory synaptic currents in neocortical pyramidal neurons. J Neurosci. 1997;17:7220–7227. doi: 10.1523/JNEUROSCI.17-19-07220.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I. Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberlandt C, Derouiche A, Wyczynski A, Haseleu J, Pohle J, Karram K, Trotter J, Seifert G, Frotscher M, Steinhäuser C, Jabs R. Gray Matter NG2 cells display multiple Ca-signaling pathways and highly motile processes. PLoS One. 2011;6:e17575. doi: 10.1371/journal.pone.0017575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevers W, Lüddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Dunne EL, Harvey RJ, Smart TG. Zinc-mediated inhibition of GABA(A) receptors: discrete binding sites underlie subtype specificity. Nat Neurosci. 2003;6:362–369. doi: 10.1038/nn1030. [DOI] [PubMed] [Google Scholar]
- Houston CM, Bright DP, Sivilotti LG, Beato M, Smart TG. Intracellular chloride ions regulate the time course of GABA-mediated inhibitory synaptic transmission. J Neurosci. 2009;29:10416–10423. doi: 10.1523/JNEUROSCI.1670-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs R, Pivneva T, Hüttmann K, Wyczynski A, Nolte C, Kettenmann H, Steinhäuser C. Synaptic transmission onto hippocampal glial cells with hGFAP promoter activity. J Cell Sci. 2005;118:3791–3803. doi: 10.1242/jcs.02515. [DOI] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Karlsson U, Druzin M, Johansson S. Cl(-) concentration changes and desensitization of GABA(A) and glycine receptors. J Gen Physiol. 2011;138:609–626. doi: 10.1085/jgp.201110674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khom S, Baburin I, Timin EN, Hohaus A, Sieghart W, Hering S. Pharmacological properties of GABAA receptors containing gamma1 subunits. Mol Pharmacol. 2006;69:640–649. doi: 10.1124/mol.105.017236. [DOI] [PubMed] [Google Scholar]
- Kirchhoff F, Kettenmann H. GABA triggers a [Ca2+]i increase in murine precursor cells of the oligodendrocyte lineage. Eur J Neurosci. 1992;4:1049–1058. doi: 10.1111/j.1460-9568.1992.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Betz H. Clustering of inhibitory neurotransmitter receptors at developing postsynaptic sites: the membrane activation model. Trends Neurosci. 2000;23:429–435. doi: 10.1016/S0166-2236(00)01627-1. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Loebrich S. Trafficking and synaptic anchoring of ionotropic inhibitory neurotransmitter receptors. Biol Cell. 2007;99:297–309. doi: 10.1042/BC20060120. [DOI] [PubMed] [Google Scholar]
- Kyrozis A, Reichling DB. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. J Neurosci Methods. 1995;57:27–35. doi: 10.1016/0165-0270(94)00116-X. [DOI] [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat Neurosci. 2004;7:24–32. doi: 10.1038/nn1162. [DOI] [PubMed] [Google Scholar]
- Lyons HR, Gibbs TT, Farb DH. Turnover and down-regulation of GABA(A) receptor alpha1, beta2S, and gamma1 subunit mRNAs by neurons in culture. J Neurochem. 2000;74:1041–1048. doi: 10.1046/j.1471-4159.2000.0741041.x. [DOI] [PubMed] [Google Scholar]
- Malek SA, Coderre E, Stys PK. Aberrant chloride transport contributes to anoxic/ischemic white matter injury. J Neurosci. 2003;23:3826–3836. doi: 10.1523/JNEUROSCI.23-09-03826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina M, Mozrzymas JW, Strata F, Cherubini E. Zinc modulation of bicuculline-sensitive and -insensitive GABA receptors in the developing rat hippocampus. Eur J Neurosci. 1996;8:2168–2176. doi: 10.1111/j.1460-9568.1996.tb00738.x. [DOI] [PubMed] [Google Scholar]
- Matthias K, Kirchhoff F, Seifert G, Hüttmann K, Matyash M, Kettenmann H, Steinhäuser C. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003;23:1750–1758. doi: 10.1523/JNEUROSCI.23-05-01750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. An update on GABAA receptors. Brain Res Rev. 1999;29:196–217. doi: 10.1016/S0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- Minelli A, Brecha NC, Karschin C, DeBiasi S, Conti F. GAT-1, a high-affinity GABA plasma membrane transporter, is localized to neurons and astroglia in the cerebral cortex. J Neurosci. 1995;15:7734–7746. doi: 10.1523/JNEUROSCI.15-11-07734.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minelli A, DeBiasi S, Brecha NC, Zuccarello LV, Conti F. GAT-3, a high-affinity GABA plasma membrane transporter, is localized to astrocytic processes, and it is not confined to the vicinity of GABAergic synapses in the cerebral cortex. J Neurosci. 1996;16:6255–6264. doi: 10.1523/JNEUROSCI.16-19-06255.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T, Fritschy JM, Grosche J, Pratt GD, Möhler H, Kettenmann H. Developmental regulation of voltage-gated K+ channel and GABAA receptor expression in Bergmann glial cells. J Neurosci. 1994;14:2503–2514. doi: 10.1523/JNEUROSCI.14-05-02503.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Nolte C, Matyash M, Pivneva T, Schipke CG, Ohlemeyer C, Hanisch UK, Kirchhoff F, Kettenmann H. GFAP promoter-controlled EGFP-expressing transgenic mice: a tool to visualize astrocytes and astrogliosis in living brain tissue. Glia. 2001;33:72–86. doi: 10.1002/1098-1136(20010101)33:1<72::AID-GLIA1007>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puia G, Vicini S, Seeburg PH, Costa E. Influence of recombinant gamma-aminobutyric acid-A receptor subunit composition on the action of allosteric modulators of gamma-aminobutyric acid-gated Cl- currents. Mol Pharmacol. 1991;39:691–696. [PubMed] [Google Scholar]
- Puia G, Costa E, Vicini S. Functional diversity of GABA-activated Cl− currents in Purkinje versus granule neurons in rat cerebellar slices. Neuron. 1994;12:117–126. doi: 10.1016/0896-6273(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Tong WM, Brecha NC. GABA plasma membrane transporters, GAT-1 and GAT-3, display different distributions in the rat hippocampus. J Comp Neurol. 1996;367:595–606. doi: 10.1002/(SICI)1096-9861(19960415)367:4<595::AID-CNE9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Riquelme R, Miralles CP, De Blas AL. Bergmann glia GABA(A) receptors concentrate on the glial processes that wrap inhibitory synapses. J Neurosci. 2002;22:10720–10730. doi: 10.1523/JNEUROSCI.22-24-10720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Seifert G, Steinhäuser C. Glial cells in the mouse hippocampus express AMPA receptors with an intermediate Ca2+ permeability. Eur J Neurosci. 1995;7:1872–1881. doi: 10.1111/j.1460-9568.1995.tb00708.x. [DOI] [PubMed] [Google Scholar]
- Seifert G, Weber M, Schramm J, Steinhäuser C. Changes in splice variant expression and subunit assembly of AMPA receptors during maturation of hippocampal astrocytes. Mol Cell Neurosci. 2003;22:248–258. doi: 10.1016/S1044-7431(03)00039-3. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure, pharmacology, and function of GABAA receptor subtypes. Adv Pharmacol. 2006;54:231–263. doi: 10.1016/S1054-3589(06)54010-4. [DOI] [PubMed] [Google Scholar]
- Smart TG, Moss SJ, Xie X, Huganir RL. GABAA receptors are differentially sensitive to zinc: dependence on subunit composition. Br J Pharmacol. 1991;103:1837–1839. doi: 10.1111/j.1476-5381.1991.tb12337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein V, Hermans-Borgmeyer I, Jentsch TJ, Hübner CA. Expression of the KCl cotransporter KCC2 parallels neuronal maturation and the emergence of low intracellular chloride. J Comp Neurol. 2004;468:57–64. doi: 10.1002/cne.10983. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Tozuka Y, Takata T, Shimazu N, Matsumura N, Ohta A, Hisatsune T. Excitatory GABAergic activation of cortical dividing glial cells. Cereb Cortex. 2009;19:2181–2195. doi: 10.1093/cercor/bhn238. [DOI] [PubMed] [Google Scholar]
- Tia S, Wang JF, Kotchabhakdi N, Vicini S. Distinct deactivation and desensitization kinetics of recombinant GABAA receptors. Neuropharmacology. 1996;35:1375–1382. doi: 10.1016/S0028-3908(96)00018-4. [DOI] [PubMed] [Google Scholar]
- Tong XP, Li XY, Zhou B, Shen W, Zhang ZJ, Xu TL, Duan S. Ca(2+) signaling evoked by activation of Na(+) channels and Na(+)/Ca(2+) exchangers is required for GABA-induced NG2 cell migration. J Cell Biol. 2009;186:113–128. doi: 10.1083/jcb.200811071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vélez-Fort M, Maldonado PP, Butt AM, Audinat E, Angulo MC. Postnatal switch from synaptic to extrasynaptic transmission between interneurons and NG2 cells. J Neurosci. 2010;30:6921–6929. doi: 10.1523/JNEUROSCI.0238-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Steinhäuser C. Ion channels in glial cells. Brain Res Rev. 2000;32:380–412. doi: 10.1016/S0165-0173(99)00093-4. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Bain CJ, Whiting PJ, Kemp JA. Functional comparison of the role of gamma subunits in recombinant human gamma-aminobutyric acidA/benzodiazepine receptors. Mol Pharmacol. 1993;44:437–442. [PubMed] [Google Scholar]
- Westbrook GL, Mayer ML. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987;328:640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- White G, Gurley DA. Alpha subunits influence Zn block of gamma2 containing GABAA receptor currents. Neuroreport. 1995;6:461–464. doi: 10.1097/00001756-199502000-00014. [DOI] [PubMed] [Google Scholar]
- Williamson AV, Mellor JR, Grant AL, Randall AD. Properties of GABAA receptors in cultured rat oligodendrocyte progenitor cells. Neuropharmacology. 1998;37:859–873. doi: 10.1016/S0028-3908(98)00016-1. [DOI] [PubMed] [Google Scholar]
- Wisden W, McNaughton LA, Darlison MG, Hunt SP, Barnard EA. Differential distribution of GABAA receptor mRNAs in bovine cerebellum–localization of alpha 2 mRNA in Bergmann glia layer. Neurosci Lett. 1989;106:7–12. doi: 10.1016/0304-3940(89)90193-6. [DOI] [PubMed] [Google Scholar]
- Ymer S, Draguhn A, Wisden W, Werner P, Keinänen K, Schofield PR, Sprengel R, Pritchett DB, Seeburg PH. Structural and functional characterization of the gamma 1 subunit of GABAA/benzodiazepine receptors. EMBO J. 1990;9:3261–3267. doi: 10.1002/j.1460-2075.1990.tb07525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SZ, Taylor MM, Wu S, Ikeda-Matsuo Y, Kubera C, Bordey A. NKCC1 knockdown decreases neuron production through GABA(A)-regulated neural progenitor proliferation and delays dendrite development. J Neurosci. 2012;32:13630–13638. doi: 10.1523/JNEUROSCI.2864-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]