Abstract
Introduction
Continuous EEG recordings (cEEGs) are increasingly used in evaluation of acutely ill adults. Pre-screening using compressed data formats, such as compressed spectral array (CSA), may accelerate EEG review. We tested whether screening with CSA can enable detection of seizures and other relevant patterns.
Methods
Two individuals reviewed the CSA displays of 113 cEEGs. While blinded to the raw EEG data, they marked each visually homogeneous CSA segment. An independent experienced electroencephalographer reviewed the raw EEG within 60 seconds on either side of each mark, and recorded any seizures (and isolated epileptifom discharges, periodic epileptiform discharges (PEDs), rhythmic delta activity (RDA), and focal or generalized slowing). Seizures were considered to have been detected if the CSA mark was within 60 seconds of the seizure. The electroencephalographer then determined the total number of seizures (and other critical findings) for each record by exhaustive, page-by-page review of the entire raw EEG.
Results
Within each of the 39 cEEG recordings containing seizures, one CSA reviewer identified at least one seizure, while the second CSA reviewer identified 38/39 patients with seizures. The overall detection rate was 89.0% of 1,190 total seizures. When present, an average of 87.9% of seizures were detected per individual patient. Detection rates for other critical findings were: epileptiform discharges, 94.0%; PEDs, 100%; RDA, 97.9%; focal slowing, 100%; and generalized slowing, 100%.
Conclusions
CSA-guided review can support sensitive screening of critical pathological information in cEEG recordings. However, some patients with seizures may not be identified.
Keywords: Continuous EEG monitoring, quantitative EEG, compressed spectral array, seizures
1. Introduction
Continuous EEG monitoring (cEEG) is an increasingly integral part of caring for acutely-ill hospitalized patients. The expansion of cEEG has been driven by the growing recognition that subclinical seizures are common in many acute neurological conditions, particularly in intensive care units1-11. While direct review by expert encephalographers remains the gold standard for interpretation, the increased use of cEEG has resulted in dramatic increases in data volume, leading to the need for compressed data formats to enable efficient pre-screening of data 12-16.
Spectrograms, or compressed spectral arrays (CSA) 17,18, are the most widely used compressed data format, consisting of 3-dimensional plots with time on the x-axis, frequency on the y-axis, and EEG power on the z-axis (Figure 1). Whereas standard EEG displays no more than 10-15 seconds of data per screen and requires simultaneous inspection of numerous channels, CSA displays may show several hours of data on a single page. This enables the electroencephalographer to identify ‘suspicious’ regions of the EEG from their gross features, then selectively ‘zoom in’ on these regions for more detailed review. However, the sensitivity of CSA to detect clinically significant patterns, as compared with standard exhaustive visual review, has never been quantified.
Figure 1. Seizures and Artifact in CSA Displays.
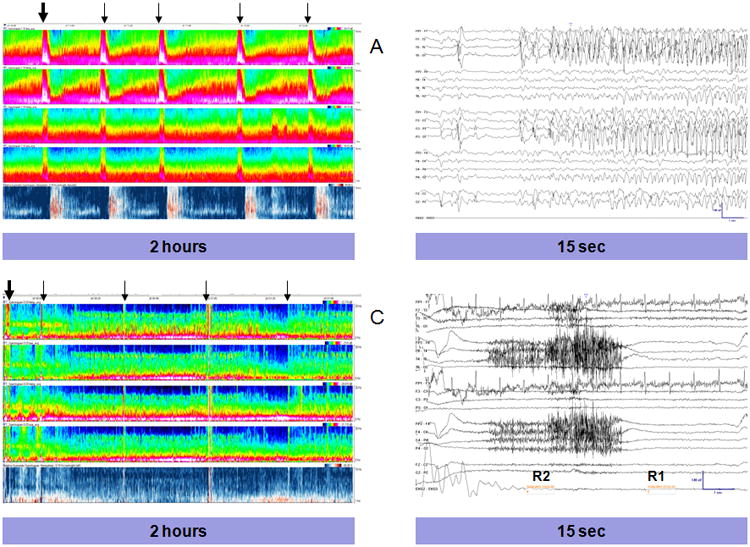
Compressed Spectral Array (CSA) displays, demonstrating a seizure (A) and muscle artifact (C). Each CSA displays 2 hours of EEG data. X-axis:time, Y-Axis:frequency (0-20Hz), Z axis: power with black representing lowest and white highest power. From top-to-bottom, the individual segments represent: left lateral power (Fp1-F7, F7-T3, T3-T5, T5-O1), left parasaggital power (Fp1-F3, F3-C3, C3-P3, P3-O1), right lateral power (Fp2-F8, F8-T4, T4-T6, T6-O2), right parasaggital power (Fp1-F4, F3-C4, C4-P4, P4-O2) and the relative asymmetry index. For the relative asymmetry index, red represents increased right-sided power and blue increased left-sided power. (A) Five seizures are present, marked by arrows (B) Section of the EEG corresponding to the EEG segment marked by the thick arrow, demonstrating seizure onset. (C) CSA display with several segments with muscle artifact, each marked by an arrow corresponding to where a CSA reviewer placed a mark. (D) Section of the EEG corresponding to the CSA segment marked by the thick arrow, displaying muscle artifact.
We hypothesized that CSA could be used to screen cEEG recordings for seizures and other clinically relevant pathological patterns. This hypothesis was tested on a collection of 113 cEEG studies, using a CSA review strategy designed to assess the sensitivity with which CSA screening can be used to identify seizures, compared against gold-standard exhaustive visual review.
2. Methods
Study population
We identified a convenience sample of 118 adult patients (age > 18) who underwent cEEG at Massachusetts General Hospital between September 2011 and February 2012. All patients were admitted for an acute illness and were hospitalized in an ICU, neurological or medical/surgical ward. Patients admitted for elective long-term EEG monitoring were not included. All cEEGs had at least 10 hours of recorded data. In patients who had greater than 24 hours of data per cEEG, only the first 24 hours were reviewed. For patients with multiple cEEGs, only one was reviewed. Clinical information was obtained from review of the hospital electronic medical record, as well as the Epilepsy Service EEG report database. This retrospective review of EEG recordings and associated medical records was carried out with approval of the local institutional review board.
EEG recording and CSA Display
Continuous EEG data was recorded using 19 silver / silver chloride electrodes, affixed to the scalp according to the international 10-20 system. CSA displays were computed using the standard Fast Fourier Transform (FFT), implemented in Magic Marker clinical software (Persyst 11, San Diego, CA). Epoch sizes for FFT calculations were set to 2 seconds. The sampling rate was 512Hz. CSA displays were configured to show the average spectrogram over each region on a logarithmic colormap scale. During review, a 24-inch monitor was used to display two hours of data from the following spectral arrays from top-to-bottom: left lateral power (Fp1-F7, F7-T3, T3-T5, T5-O1), left parasaggital power (Fp1-F3, F3-C3, C3-P3, P3-O1), right lateral power (Fp2-F8, F8-T4, T4-T6, T6-O2), right parasaggital power (Fp1-F4, F3-C4, C4-P4, P4-O2) and the relative asymmetry index. For all CSA displays, the y-axis represented frequency (0-20 Hz) and the x-axis represented time. For all displays other than the relative asymmetry index, the z-axis represented frequency-specific power depicted as a range of colors, with black representing low power and blue, green, orange, pink, and white representing successively higher spectral power. The relative asymmetry index's z-axis colormap displayed the power difference between left and right hemispheres at each frequency, with darker red corresponding to increased right-sided power and darker blue corresponding to increased left-sided power.
Review of CSA and EEG data
Two neurology residents (“CSA reviewers” CAW and SW) without prior experience using CSA displays received a two-hour tutorial in which the senior authors reviewed the basic theory of spectral EEG analysis, and reviewed several examples (not included among the cases subsequently analyzed) of CSA displays of seizures, background slowing, artifacts and periodic epileptiform discharges, along with the corresponding raw EEG tracings. The two CSA reviewers were then instructed to mark each visually homogenous segment, rather than only marking segments that they believed were most likely to represent seizure activity, ensuring an exhaustive CSA-based review of each EEG. Marking was accomplished by placing a cursor on the review screen and making an annotation wherever a significant change in the spectral array occurred. The CSA reviewers were blinded to the presence or absence of seizures and not allowed to view the primary cEEG data. Training of the CSA reviewers for the study was completed by performing a trial review of 5 cEEGs jointly to establish consistent methodology. Subsequently, the two CSA reviewers independently reviewed and exhaustively marked each visually homogenous segment of the remaining 113 cEEGs, making note of the total time required to review each cEEG.
Ground truth for the number of seizures and presence vs. absence of key pathological patterns in each EEG was determined by one of two experienced clinical electroencephalographers (MMS or MBW). One of these two electroencephalographers reviewed every page of each EEG recording to identify and count electrographic seizures. Electrographic seizures were defined according to the criteria of Table 2 in Chong and Hirsch19 (adapted from Young et al.20). In situations where the presence, onset or offset of a seizure was questionable, the two electroencephalographers jointly reviewed the EEG and arrived at a consensus. For each seizure identified, the reviewing electroencephalographer determined the precise beginning and end time of the seizure, and then looked at the CSA marks placed by the blinded CSA reviewers to see if there were any marks within 60 seconds of either side of the seizure. If there were no CSA marks within 60 seconds of the beginning or ending time of the seizure, the seizure was counted as missed; otherwise the seizure was considered to have been detected. In the course of reviewing the raw EEG data for seizures, the electroencephalographers also noted whether sporadic epileptiform discharges (EDs), PEDs, RDA, focal slowing (FS) or generalized slowing (GS) were present at any time, then determined whether these patterns were present within any of the 120 second windows (60 seconds to either side) centered on any CSA mark. If these patterns were present within one or more of these windows, they were counted as “detected” by the CSA reviewers.
Table 2. Percentage of seizures and other patterns of interest identified and mean and median CSA review timesa.
| Reviewer 1 | Reviewer 2 | Combined | |
|---|---|---|---|
| Sz pts identified | 38/39 (97.4%) | 39/39 (100%) | 98.7% |
| Total szs identified | 1039/1190 (87.3%) | 1080/1190 (90.8%) | 89.0% |
| Szs identified per pt, mean % (SD) | 85.8 (20.8) | 89.8 (15.8) | 87.9 (18.4) |
| Szs identified per pt, median % | 92.9 | 97.0 | 94.2 |
| PEDs identified | 41/41 (100%) | 41/41 (100%) | 100% |
| EDs identified | 64/67 (95.5%) | 62/67 (92.5%) | 94.0% |
| RDA identified | 31/32 (96.9%) | 31/32 (96.9%) | 96.9% |
| FS identified | 72/72 (100%) | 72/72 (100%) | 100% |
| GS identified | 96/96 (100%) | 96/96 (100%) | 100% |
| CSA review time, mean min (SD) | 10.4 (5.0) | 10.2 (5.8) | 10.3 (5.4) |
| CSA review time, median min (range) | 9.7 (1.5-25.0) | 9.1 (1.6-42.2) | 9.1 (1.5-42.2) |
Abbreviations and symbols: Sz = seizure; pt = patient; % = percent, SD = standard deviation, PEDs = periodic epileptiform discharges, EDs = epileptiform discharges, RDA = rhythmic delta activity, FS = focal slowing, GS = generalized slowing, CSA = compressed spectral array, min = minutes
Data are number identified/total number (percent identified) unless otherwise specified
Analysis
The primary outcome measure was the percentage of seizures detected (sensitivity) overall and per individual reviewer. Secondary outcomes included the sensitivity with which PEDs, EDs, FS, GS and RDA were identified, the number of false-positive segments identified, and the overall time for cEEG review. The false-positive rate for seizure detection was calculated by determining the total number of segments marked divided by the number of seizure-containing segments, and was used to determine the false-positive rate per hour of cEEG. Because each discrete cEEG segment was marked there were no “true negatives,” so a specificity could not be calculated; however, the false negative rate was determined by calculating the rate of missed seizures. Excel (Version 14.2, Microsoft, Redmond, WA) was used for data storage and determination of sensitivities, false-positive rates, means, medians and standard deviations.
3. Results
Of the 113 total cEEG recordings that were reviewed individually, 39 contained from 1 to 151 seizures (median 20, mean 30.5). As would be expected in a population of acutely ill neurological and medical patients, the vast majority of the seizures (87%) were partial. Three patients with hypoxic-ischemic injury had myoclonic status, one patient had a partial seizure with secondary generalization and one patient had generalized status epilepticus. Diagnoses and demographic data for the entire cohort and subdivided by seizure presence are listed in Table 1. The average patient age was 59.6, and approximately half were men. Fifty-eight percent of the continuous EEGs were recorded in an ICU and the remainder on an acute neurological, medical, or surgical ward.
Table 1. Patient Demographic Data.
| All patients (n=113) | Patients without seizures (n=74) | Seizure patients (n=39) | |
|---|---|---|---|
| Age, mean ± SD (range) | 59.6 ± 18.5 (19-95) | 59.6 ± 18.6 (19-95) | 59.6 ± 18.6 (23-88) |
| Male | 58 (51.3%) | 38 (51.4%) | 20 (51.3%) |
| ICU | 66 (58.4%) | 47 (63.5%) | 19 (48.7%) |
| Diagnosis | |||
| ICH | 21 (18.6%) | 16 (21.6%) | 5 (12.8%) |
| Ischemic stroke | 7 (6.2%) | 6 (8.1%) | 1 (2.6%) |
| TBI | 9 (8.0%) | 7 (9.5%) | 2 (5.1%) |
| CNS tumor | 11 (9.7%) | 5 (6.8%) | 6 (15.4%) |
| CNS infection/autoimmunity | 11 (9.7%) | 7 (9.5%) | 4 (10.3%) |
| Hypoxic-ischemic injury | 8 (7.1%) | 4 (5.4%) | 4 (10.3%) |
| Seizure disorder or spells | 29 (25.7%) | 20 (27.0%) | 9 (23.1%) |
| General medical disease | 17 (15.0%) | 9 (12.2%) | 8 (20.5%) |
Abbreviations: ICU = intensive care unit; ICH = intracranial hemorrhage; TBI = traumatic brain injury; CNS = central nervous system
Values are n (percentage) unless otherwise indicated
Data for the rates of seizure detection and the presence of PEDs, EDs, RDA, FS and GS are displayed in table 2. Of the 39 patients who had seizures, reviewer 1 identified at least 1 seizure in 38, while reviewer 2 identified at least one seizure in all 39. The patient who was not identified by reviewer 1 had a single, brief, right centrotemporal seizure lasting 16 seconds.
EEG and corresponding CSA for this seizure are displayed in Figure 2. Reviewer 1 marked1,039 of 1,190 total seizures (87.3%, false negative rate 12.7%), and reviewer 2 marked 1,080 of 1,190 (90.8%, false negative rate 9.2%). Of the 39 patients with seizures, reviewer 1 identified an average of 85.8% [median 92.9%, standard deviation (SD) 20.8] of each patient's seizures, while reviewer 2's CSA markings identified on average 89.8% (median 97.0%, SD 15.8) of the seizures in each recording. The seizure detection rate for each patient with seizures is displayed in Figure 3. Combined, a median of 94.2% and an average of 87.9% of seizures were identified per patient by CSA. The time expenditure to review each CSA was low, with the reviewers spending, on average, 10.3 minutes per recording (median 9.1, SD 5.0).
Figure 2. Examples of Seizures Missed By CSA Screening.
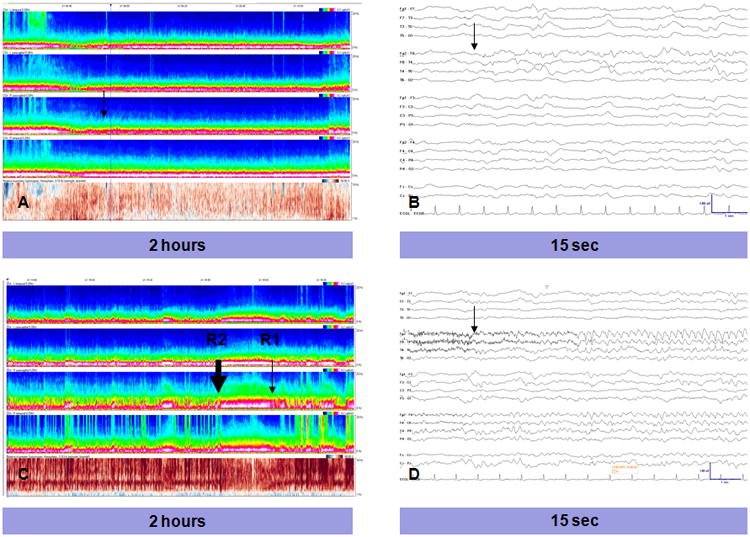
Case 1 (A, B): A very focal right temporal seizure (onset marked by black arrows), lasting 20 sec, with no significant change in the CSA background, missed by both reviewers. Case 2 (C,D): A right frontotemporal seizure lasting 83 sec. This seizure was marked by reviewer 2 near the seizure onset (thick black arrow), but was ‘missed’ by reviewer 2 (thin black arrow) whose nearest CSA mark occurred 90 sec after the end of the seizure.
Figure 3. Sensitivity of CSA Screening for Seizures.
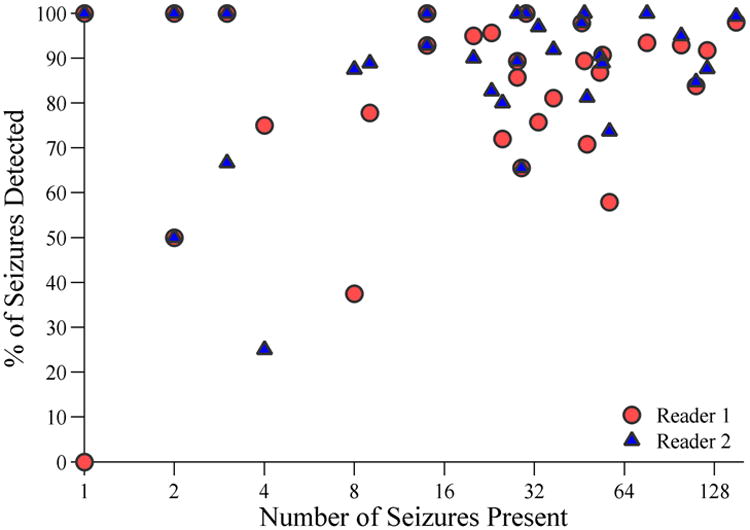
The sensitivity (% of seizures present which were detected) for each of the two CSA reviewers is displayed on the y-axis vs. the number of seizures present in each cEEG recording (x-axis). The number of seizures is displayed on a logarithmic scale in order to provide better visualization of each data point.
By design, the number of marked segments that did not contain seizures was high. The current data does not permit calculation of specificity, but the number of false-positives (i.e. number of marked segments that did not contain seizures) was determined. Reviewer 1 identified fewer seizures but had a lower false-positive rate, marking for review a median of 5.4 and an average of 6.1 (SD 3.4) segments per hour of cEEG, while correctly detecting an average of 0.52 seizures per hour (SD 1.39). Consequently, there was 1 seizure identified for every 11.7 segments marked. Reviewer 2 marked a median of 7.5 and an average of 8.6 (SD 5.8) segments per hour, while correctly identifying 0.54 seizures per hour (SD 1.42), giving a false positive rate of 15.7 segments marked per each seizure identified. The combined average number of segments marked per hour was 7.3 (SD 4.9). Across all subjects, there was an average of 0.53 (SD 1.4) seizures that were successfully identified per hour. Thus, for every one seizure identified there were 13.8 segments that did not contain seizures.
Discussion
The present study provides novel evidence that compressed spectral array can be used as a screening tool in adult cEEGs for detecting seizures and other clinically significant pathological patterns. This method has a high sensitivity, while requiring direct inspection of a smaller fraction of the total cEEG record. To our knowledge, this is the first study to rigorously evaluate the performance of CSA displays for seizure detection in adult patients. A unique feature of the study design is that we evaluate CSA in a manner that simulates the way it is typically used in clinical practice, that is, as a screening tool to select portions of the raw EEG for closer inspection21.
The most similar study for comparison, by Stewart et al., evaluated the sensitivity with which seizures were identified by two quantitative EEG techniques, compressed spectral array (specifically color density spectral array as was used in the present study) and amplitude-integrated EEG (aEEG), in pediatric ICU patients ranging in age from 1.5 months to 12 years 22. In that study, three reviewers identified a median of 83.3% (range 73.3%–86.7%) of seizures per recording using compressed spectral array and a median of 81.5% (range 80.6%–83.9%) of seizures per recording using aEEG. All 3 reviewers failed to identify seizures in 2 of 17 patients using CSA, whereas at least one reviewer identified some seizures in all 17 patients using aEEG. In the present study, a median of 94.2% of seizures were identified per recording, while 38 of 39 patients with seizures were identified by one reviewer and all 39 by the other. The two reviewers identified 89.0% of all 1,190 seizures that were present (overall false negative rate of 11.0%). Earlier studies of the sensitivity of quantitative EEG for seizure detection used aEEG or related techniques, were confined to neonatal ICU patients, and showed widely varying sensitivities 23-29.
The present study achieved high seizure detection rates by deliberately accepting a higher rate of false positives, i.e. by framing the goal of CSA review as that of screening, deferring the final determination of whether or not a suspicious CSA segment contained a seizure to a second stage of raw-data review. In some cEEGs with a large amount of artifact resulting in frequent changes in the frequency spectra, this approach necessarily led to a higher rate of false-positives, but was more likely to ensure that seizures were not missed.
While designed to simulate the use of CSA in practice as a screening tool rather than a substitute for direct data review, the use of CSA in the present study differed from its use in routine cEEG review in one important respect. In order to investigate the sensitivity of review with CSA alone, the reviewers were blinded to the raw EEG data when selecting which segments to mark. If CSA information could be combined with review of EEG data, as occurs in clinical practice, it is possible that more seizures would have been identified. It is also likely that the false positive rate would have been reduced since review of EEG data would allow the reviewer to identify which CSA patterns are due to artifact, and once these patterns are recognized they could subsequently be ignored. This process of adaptation to the individual patient's pattern by a continual suspect-and-verify process of feedback likely affords increased time-efficiency.
There are several limitations to the current study which suggest directions for future research. CSA is only one of several methods that can be used to graphically display compressed EEG data, and was used in the current study because of its intuitive nature and ability to represent subtle changes in EEG pattern. However, in future studies it may be useful to compare its efficacy with other quantitative techniques, such as amplitude-integrated EEG. Additionally, instead of experienced electroencephalographers, CSA review was performed by neurology residents without prior quantitative EEG exposure and limited overall EEG experience. The approach to simply mark visually homogeneous segments is a simple and easily learned technique, which can be taught to novices in EEG interpretation. Therefore, our findings suggest that it may be possible to train bedside nurses or EEG technicians to perform an initial screen to identify areas for closer review, thereby allowing less intermittent seizure screening. However, how best to implement such an approach without placing undue burden on physician responders due to false positives requires further investigation. Finally, by enabling electroencephalographers to review a smaller portion of the raw EEG, it is probable that this method will reduce overall EEG review time. However, further investigation is needed to determine if CSA indeed results in clinically meaningful time-savings.
Overall, this study suggests that the use of a CSA display as a screening tool is a reasonable alternative to reviewing the entirety of raw cEEG data in adult patients, resulting in a minimal reduction in seizure detection rates as compared with exhaustive direct visual review. Nevertheless, not all seizures were identified, and one patient with a single brief focal seizure was missed by one of the reviewers. Therefore, if this approach is to be implemented on a larger scale, it should be done with awareness of its benefits and limitations.
Acknowledgments
This study was supported in part by the following grants: American Brain Foundation (MBW), NIH/NINDS NS062092 (MBW); NIH/NCATS 8KL2TR000168-05 (MS).
Footnotes
Authorship details: All four authors played equal part in conceptualization and design of the study. CAW analyzed data and drafted the initial manuscript. SW drafted the initial manuscript. All authors reviewed and revised the manuscript. All authors contributed equally to the overall work.
Drs. Williamson, Wahlster, Shafi and Westover declare that they have no conflicts of interest.
Contributor Information
Craig A. Williamson, Email: craigaw@med.umich.edu.
Sarah Wahlster, Email: swahlster@partners.org.
M. Brandon Westover, Email: mwestover@partners.org.
References
- 1.Jordan KG. Continuous EEG and evoked potential monitoring in the neuroscience intensive care unit. J Clin Neurophysiol. 1993;10:445–75. doi: 10.1097/00004691-199310000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Towne AR, Waterhouse EJ, Boggs JG, et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000;54:340–340. doi: 10.1212/wnl.54.2.340. [DOI] [PubMed] [Google Scholar]
- 3.Pandian J, Cascino G, Elson L, Manno E, Fulgham JR. Digital video-electroencephalographic monitoring in the neurological-neurosurgical intensive care unit. Arch Neurol. 2004;61:1090–1094. doi: 10.1001/archneur.61.7.1090. [DOI] [PubMed] [Google Scholar]
- 4.Oddo M, Carrera E, Claassen J, Mayer SA, Hirsch LJ. Continuous electroencephalography in the medical intensive care unit. Crit Care Med. 2009;37:2051–2056. doi: 10.1097/CCM.0b013e3181a00604. [DOI] [PubMed] [Google Scholar]
- 5.Jette N, Claassen J, Emerson RG, Hirsch LJ. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol. 2006;63:1750–1755. doi: 10.1001/archneur.63.12.1750. [DOI] [PubMed] [Google Scholar]
- 6.Vespa PM, Nuwer MR, Nenov V, et al. Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J Neurosurg. 1999;91:750–760. doi: 10.3171/jns.1999.91.5.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young GB, Doig GS. Continuous EEG monitoring in comatose intensive care patients: epileptiform activity in etiologically distinct groups. Neurocrit Care. 2005;2:5–10. doi: 10.1385/NCC:2:1:005. [DOI] [PubMed] [Google Scholar]
- 8.Hosain SA, Solomon GE, Kobylarz EJ. Electroencephalographic patterns in unresponsive pediatric patients. Pediatr Neurol. 2005;32:162–165. doi: 10.1016/j.pediatrneurol.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–1748. doi: 10.1212/01.wnl.0000125184.88797.62. [DOI] [PubMed] [Google Scholar]
- 10.Jordan KG. Nonconvulsive status epilepticus in acute brain injury. J Clin Neurophysiol. 1999;16:332–340. doi: 10.1097/00004691-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Wittman JJ, Hirsch LJ. Continuous electroencephalogram monitoring in the critically ill. Neurocrit Care. 2005:330–341. doi: 10.1385/ncc:2:3:330. [DOI] [PubMed] [Google Scholar]
- 12.Liu A, Hahn JS, Heldt GP, Coen RW. Detection of neonatal seizures through computerized EEG analysis. Electroencephalogr Clin Neurophysiol. 1992;82:30–37. doi: 10.1016/0013-4694(92)90179-l. [DOI] [PubMed] [Google Scholar]
- 13.Nuwer MR. Quantitative EEG analysis in clinical settings. Brain Topogr. 1996;8:201–208. doi: 10.1007/BF01184770. [DOI] [PubMed] [Google Scholar]
- 14.Nuwer M. Assessment of digital EEG, quantitative EEG, and EEG brain mapping: Report of the American Academy of Neurology and the American Clinical Neurophysiology Society. Neurology. 1997;49:277–292. doi: 10.1212/wnl.49.1.277. [DOI] [PubMed] [Google Scholar]
- 15.Talwar D, Torres F. Continuous electrophysiologic monitoring of cerebral function in the pediatric intensive care unit. Pediatr Neurol. 1988;4:137–147. doi: 10.1016/0887-8994(88)90001-x. [DOI] [PubMed] [Google Scholar]
- 16.Scheuer ML, Wilson SB. Data analysis for continuous EEG monitoring in the ICU: seeing the forest and the trees. J Clin Neurophysiol. 2004;21:353–378. [PubMed] [Google Scholar]
- 17.Bickford RG, Billinger TW, Fleming N, Steward L. The compressed spectral array - A pictorial EEG. Proc San Diego Biomed Symp. 1972;11:365–370. [Google Scholar]
- 18.Bricolo A, Turazzi S, Faccioli F, Odorizzi F, Sciaretta G, Erculiani P. Clinical application of compressed spectral array in long-term EEG monitoring of comatose patients. Electroencephalogr Clin Neurophysiol. 1978;45:211–225. doi: 10.1016/0013-4694(78)90005-6. [DOI] [PubMed] [Google Scholar]
- 19.Chong DJ, Hirsch LJ. Which EEG Patterns Warrant Treatment in the Critically Ill? Reviewing the Evidence for Treatment of Periodic Epileptiform Discharges and Related Patterns. J Clin Neurophysiol. 2005;22:79–91. doi: 10.1097/01.wnp.0000158699.78529.af. [DOI] [PubMed] [Google Scholar]
- 20.Young GB, Wang JT, Connolly JF. Prognostic determination in anoxic-ischemic and traumatic encephalopathies. J Clin Neurophysiol. 2004;21:379–90. [PubMed] [Google Scholar]
- 21.Bleck TP. Status epilepticus and the use of continuous EEG monitoring in the intensive care unit. Continuum Lifelong Learning Neurol. 2012;18:560–578. doi: 10.1212/01.CON.0000415428.61277.90. [DOI] [PubMed] [Google Scholar]
- 22.Stewart CP, Otsubo H, Ochi A, Sharma R, Hutchison JS, Hahn CD. Seizure identification in the ICU using quantitative EEG displays. Neurology. 2010;75:1501–1508. doi: 10.1212/WNL.0b013e3181f9619e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abend NS, Dlugos D, Herman S. Neonatal seizure detection using multichannel display of envelope trend. Epilepsia. 2008;49:349–352. doi: 10.1111/j.1528-1167.2007.01425.x. [DOI] [PubMed] [Google Scholar]
- 24.Shah DK, Mackay MT, Lavery S, et al. Accuracy of bedside electroencephalographic monitoring in comparison with simultaneous continuous conventional electroencephalography for seizure detection in term infants. Pediatrics. 121:1146–1154. doi: 10.1542/peds.2007-1839. [DOI] [PubMed] [Google Scholar]
- 25.Shellhaas RA, Soaita AI, Clancy RR. Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics. 2007;120:770–777. doi: 10.1542/peds.2007-0514. [DOI] [PubMed] [Google Scholar]
- 26.Rennie J, Chorley G, Boylan G, Pressler R, Nguyen Y, Hooper R. Non-expert use of the cerebral function monitor for neonatal seizure detection. Arch Dis Child Fetal Neonatal Ed. 2004;89:F37–F40. doi: 10.1136/fn.89.1.F37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toet MC, van der Meij W, de Vries LS, Uiterwaal CSPM, van Huffelen KC. Comparison between simultaneously recorded amplitude integrated electroencephalogram (cerebral function monitor) and standard electroencephalogram in neonates. Pediatrics. 2002;109:772–779. doi: 10.1542/peds.109.5.772. [DOI] [PubMed] [Google Scholar]
- 28.Bourez-Swart MD, van Rooij L, Rizzo C, et al. Detection of subclinical electroencephalographic seizure patterns with multichannel amplitude-integrated EEG in full-term neonates. Clin Neurophysiol. 2009;120:1916–1922. doi: 10.1016/j.clinph.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 29.El-Dib M, Chang T, Tsuchida TN, Clancy RR. Amplitude-integrated electroencephalography in neonates. Pediatr Neurol. 2009;4:315–326. doi: 10.1016/j.pediatrneurol.2009.05.002. [DOI] [PubMed] [Google Scholar]


