Introduction
Since the discovery of inhibitory post-synaptic potentials (IPSPs) by Sir John Eccles in 1952, and the identification of GABA (gamma-Aminobutyric acid) as the major inhibitory neurotransmitter in the brain by Dr. Krešimir Krnjević in the early 1970s (see for review, Avoli and Krnjević, 2016), it has largely been assumed that synaptic inhibition functionsto arrest or restrain focal seizures (Levy and O’Leary, 1965). Dr. David Prince demonstrated in 1967 that following cortical application of penicillin, neurons exhibited depolarizing shifts during epileptiform discharges at the focus, while neurons surrounding the focus exhibited prominent IPSPs (Prince and Wilder, 1966). This finding lent further credence that synaptic inhibition served to restrain focal seizures. The advent of advanced microelectrode recording techniques in patients (Babb et al., 1987)(Stead et al., 2010)(Truccolo et al., 2011)(Schevon et al., 2012)(Lambrecq et al., 2017)(Misra et al., 2018)(Elahian et al., 2018) has only recently provided a means to test the validity of this assumption with respect to spontaneous seizures in epileptic patients. Microelectrode recordings are extracellular and cannot be used to distinguish excitatory from inhibitory post-synaptic activity. However, single unit analysis of action potentials in the local field potentials (LFPs) recorded by microelectrodes can be used to distinguish excitatory principal neuron firing from interneuron firing on the basis of morphology (Elahian et al., 2018). Unfortunately, consensus has not been reached by investigators with respect to the role of synaptic inhibition in human seizure genesis and spread. While some teams of investigators have promoted the classical interpretation that synaptic inhibition functions to restrict and perhaps stop the spread of focal seizures (Schevon et al., 2012), others have suggested that inhibition may actually promote the initiation and the spread of seizure activity (Elahian et al., 2018).
The role of synaptic inhibition in seizure genesis and spread is undoubtedly complex, and the mechanisms responsible are unlikely monolithic. For instance, the morphology of seizure onset patterns are not homogeneous (Perucca et al., 2014), and each pattern may reflect distinct cellular and network events. In the human neocortex, the most common seizure onset pattern is called low-voltage fast (LVF) and consists of beta-(15–30 Hz) or gamma (30–80 Hz) EEG activity with an amplitude typically less than 80μV (Gnatkovsky et al., 2014; Perucca et al., 2014; de Curtis and Gnatkovsky., 2009). In contrast, in patients with mesial temporal lobe epilepsy exclusively, the hypersynchronous (HYP) pattern is seen most frequently and consists of evolving repetitive sharply contoured high-amplitudeictal discharges at a frequency of 0.5–2 Hz (Velasco et al., 2000; Devinsky et al., 2018). Adding to the complexity is the fact that one seizure onset pattern can sometimes evolve into another pattern (Weiss et al., 2016). Also, in restricted cortical microcolumns, microseizures in the local field potential with HYP-like morphology can precede the EEG seizure onset pattern recorded from the clinical macroelectrode (Stead et al., 2010; Weiss et al., 2016). Thus, to properly dissect out the role of synaptic inhibition in seizure genesis and spread it is important to implement, at the very least, a taxonomic approach based on seizure onset morphologies (Perucca et al., 2014).
With respect to the role of synaptic inhibition in LVF onset seizures, several unique and complementary approaches have yielded important clues for investigators. This review focuses on how the role of synaptic inhibition in the LVF onset pattern contrasts with its role in the HYP onset pattern in humans. We will examine results from human studies performed with microelectrode recordings of LVF onset seizures, by doing so, we will consider this evidence within the context of several studies that have been obtained from animal models of mesial-temporal lobe and neocortical epilepsy. Few human microelectrode studies have differentiated LVF onset seizures from other EEG seizure onset types (Elahian et al., 2018) (Lambrecq et al., 2017)(Weiss et al., 2016). In contrast, most studies utilizing animal models have carefully distinguished LVF from other seizure onset types (see: Lévesque et al., 2012; Bragin et al., 2005; Bragin et al., 1999).
An important early clue that inhibition may promote seizure genesis was found in acute brain slices treated with 4-amonopyridine (4-AP). In this preparation, bath application of glutamate receptor antagonists revealed a depolarizing GABAergic potential that occurred during seizure discharges that contained LVF-like features (Avoli et al., 1993; Avoli et al., 1996). Building on this unexpected finding, in vivo microelectrode recording (Karunakaran et al., 2016)(Grasse et al., 2013), and in vitro intra-(Uva et al., 2015)(Ziburkus et al., 2006)(Gnatkovsky et al., 2008)(Lopantsev and Avoli, 1998) and extra-(Lévesque et al., 2016)(Lévesque et al., 2012) cellular recordings confirmed that LVF activity begins with the increased firing of inhibitory interneurons. Most recently, optogenetic stdies have demonstrated that LVF seizures can even be elicited in acute slices by optogenetically stimulating different types of inhibitory interneurons(Chang et al., 2018)(Shiri et al., 2016) (Shiri et al., 2015)(Yekhlef et al., 2015). Finally, modeling studies have been used to further investigate how the increased firing rate of inhibitory interneurons could potentially trigger seizure genesis and/or spread (Ho and Truccolo, 2016).
LVF onset seizures progress through distinct stages that reflect seizure genesis mechanisms
LVF onset seizures exhibit a consistent morphologicalevolution but often are preceded by HYP-like discharges (Figure 1). The evolution of the seizure morphology, containing an LVF epoch, reflects distinct mechanistic processes that are indeed sufficient for seizure genesis and spread (Weiss et al., 2016) (Figure 1). Although, HYP onset seizures and LVF onset seizures can occur exclusively in a single subject, it has been postulated that some LVF onset seizures begin with a HYP discharges (Elahian et al., 2018)(Weiss et al., 2016), and that these discharges can take the form of a micro-seizure that is recognized only in the local field potential, and not the EEG (Weiss et al., 2016)(Stead et al., 2010). Because high-density LFP recordings of an entire mesial-temporal structure cannot ethically be performed in humans, and have not yet been performed in animal models of mesial temporal lobe epilepsy, this assumption has not been proven. In support of the alternative hypothesis that LVF onset seizures can occur independent of a HYP event, the inter-ictal iEEG of patients with HYP onset seizures inter-ictal epileptiform discharges exhibit a distinct morphology from patients with exclusively LVF onset seizures (Tanaka et al., 2018). If patients with mesial-temporal LVF onset seizures always had preceding HYP-onset events, this result would be less likely. Neuroimaging studies have demonstrated differences in brain structure and atrophy in patients with exclusively, or primarily, HYP or LVF onsets, but these findings do not exclude the possibility that in the group of patients with LVF onset, a HYP onset microseizure can precede the LVF onset (Memarian et al., 2015)(Ogren et al., 2009).
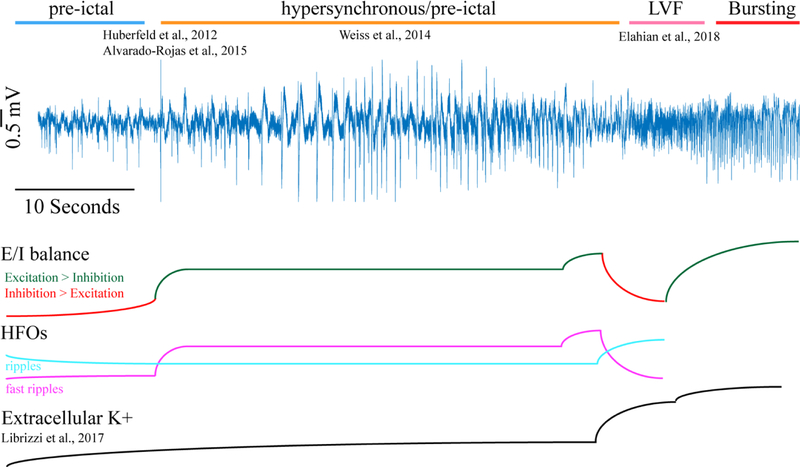
Figure 1: An illustration of the changes in excitatory-inhibitory balance, high-frequency oscillations, and extracellular potassium concentration during the progression through the different stages of a low-voltage fast (LVF) onset seizure as determined by human and animal investigations.
Prior to the seizure pre-ictal spikes associated with the firing of inhibitory interneuronsand ripples predominate. The transition to hypersynchronous/pre-ictal spikes is associated with spikes with fast ripples that incrementally increase in size, these spikes persist throughout the hypersynchronous epoch. The transition to LVF activity is also associated with fast ripples that incrementally increase in size, when the LVF activity begins the firing rate of inhibitory interneurons increase dramatically and ripple oscillations predominate over fast ripple oscillations as the extracellular potassium rises dramatically. The transition from LVF activity to the bursting stage is associated with a rebound of the firing rate of excitatory neurons.
Assuming that HYP-like micro-seizures always precede LVF events, it becomes essential to examine the transition from the inter-ictal state to the initiation of the HYP seizure or microseizure. Intra-and extracellular recordings from the epileptogenic subiculum of patients with mesial-temporal lobe epilepsy showed that inter-ictal discharges were distinct from the HYP (i.e. “pre-ictal” ) discharges. The inter-ictal discharges were associated with the increased firing of inhibitory interneurons, while the HYP discharges were associated with the increased firing of excitatory neurons (Huberfeld et al., 2011)(Köhling et al., 2016). Further studies using this preparation have demonstrated that the inter-ictal discharges were associated with ripple (80–200 Hz) oscillations, whereas the HYP/pre-ictal discharges were associated with fast ripple (250–600 Hz) oscillations (Alvarado-Rojas et al., 2015). The significance of these two observations toward understanding seizure genesis is strengthened by the independent observation that the “pre-ictal” discharges during the inter-ictal to HYP transition exhibit fast-ripples that incrementally increase in amplitude and power up until the HYP seizure begins in both the kainic acid model of MTLE (Bragin et al., 2005), and in patients with HYP seizures (Weiss et al., 2016)(Figure 1).
These data suggest that seizure genesis coincides with fast ripples that occur during the inter-ictal to HYP transition, and also during the HYP onset seizure. These fast ripples that incrementally grow in power represent the synchronized firing of pathologically interconnected neuronal (PIN) clusters that grow in size and amalgamate (Bragin et al., 2002). An alternative hypothesis has also been introduced that fast ripples are generated by the out-of-phase firing of pyramidal neurons (Ibarz et al., 2010; Foffani et al., 2007). In support of this proposed mechanism, optogenetic stimulation of principal neurons in the entorhinal cortex was found to elicit HYP onset events with high rates of fast ripples, but not LVF onset events (Shiri et al., 2016). Assuming again that LVF onset events are always preceded by HYP discharges, it is important to ask what precedes LVF events in the neocortex, and also what drives the transition from HYP or another EEG patterns, including the LVF pattern. Recordings from neocortex show little evidence of HYP onset seizures, but in contrast most LVF onset seizures there begin with one or several sentinel spikes (i.e. ictal discharges) prior to the onset of the LVF activity (Perucca et al., 2014), that can be associated with high-frequency oscillations (Weiss et al., 2013). One possibility is that during these sentinel spikes, pathologically interconnected neuron (PIN) cluster activation and synchronization also drives forward the transition from the pre-ictal to ictal state. Very little is known about the transition from HYP onset to LVF activity, but a single recording during this transition has shown that fast ripples of incrementally increasing power occur just prior to the onset of LVF activity (Weiss et al., 2016)(Figure 1).
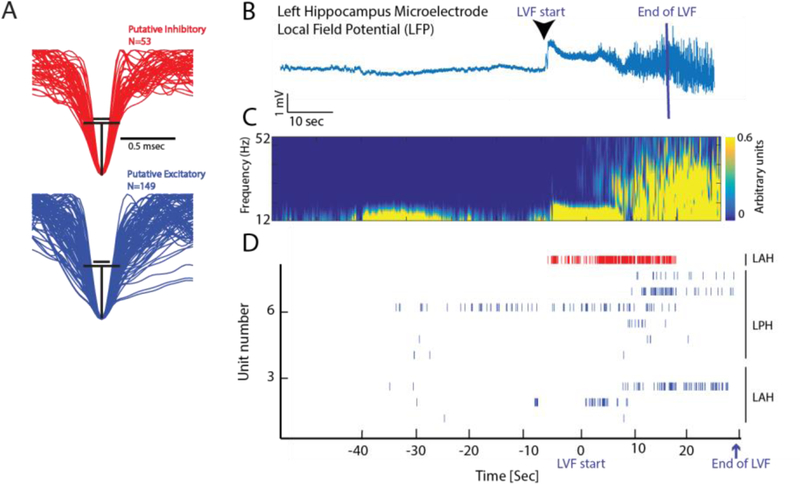
When LVF activity begins inhibitory interneurons increase their firing rate prior to a rebound in the firing rate of principal neurons (Figure 1). This observation was most recently shown during spontaneous mesial-temporal lobe LVF seizures in patients (Elahian et al., 2018)(Figure 2), but was first demonstrated using animal models over two decades ago (Lopantsev and Avoli, 1998)(Avoli et al., 1993). Extra-and intracellular recordings from mesial-temporal lobe brain slices, and the isolated Guinea pig brain preparation, after bath application of 4-AP (Lévesque et al., 2016)(Uva et al., 2015)(Ziburkus et al., 2006)(Lopantsev and Avoli, 1998)(Avoli et al., 1993) or bicuculine (Uva et al., 2015)(Gnatkovsky et al., 2008) demonstrated that increased firing of inhibitory neurons preceded a rebound of the principal neuron firing rates during LVF activity (Figure 1). In vivo extracellular recordings from pilocarpine treated animals (Karunakaran et al., 2016)(Grasse et al., 2013) utilizing single unit spike sorting offered further evidence that this pattern of neuronal spiking was relevant to clinical seizures.
Figure 2: In patients LVF onset seizures begin with an increase in the firing rate of inhibitory interneurons.
(A) Mean normalized waveforms of putative inhibitory and excitatory neurons isolated from microelectrodes during spontaneous LVF seizures in patients. Black lines illustrate an estimation of the halfwidth of the inhibitory neuron action potentials(top), projected on to the excitatory neuron action potentials (bottom). (B) Aligned LFP recorded from a microelectrode in the left hippocampus indicating the beginning and end of the LVF onset. (C) Corresponding spectrogram of the increase in the power in faster frequencies during LVF onset. (D) Raster plot of spiking activity of excitatory (blue) and inhibitory neurons (red) prior to and during LVF onset. Note that the raster plot is not aligned with panels A and B, and LVF onset ended at different times for each microelectrode recording. Abbreviations (LAH: left anterior hippocampus, LPH: left posterior hippocampus).
Why inhibitory interneuronsincrease their firing rate prior to excitatory neurons is unclear, and answering this question will likely require consensus on the significance of the events that precede LVF onset. During LVF activity, ripples predominate over fast ripples (Weiss et al., 2016)(Lévesque et al., 2012) presumably because the ripples are generated by synchronized IPSPs (Buzsáki, 2015). It is a distinct possibility that LVF activity may represent a maladaptive attempt to restrain the run-away excitation that takes place during preceding HYP, or “sentinel spike” events just prior to the LVF transition. One challenge to this interpretation is that the sentinel spike may by associated with IPSPs and not EPSPs (Lopantsev and Avoli 1998, Avoli et al, 1983).
One reason that LVF activity could signify a maladaptive response is that during the LVF activity extracellular potassium concentrations increase dramatically due to the rapid firing rates of the inhibitory interneurons (Librizzi et al., 2017; D’Antunono et al., 2004; Louvel et al., 2001; Avoli et al., 1997; Avoli et al., 1996;) (Figure 1). Thus, the neuronal activity associated with the LVF pattern may be an attempt to restrain the seizure that inadvertently only magnifies its severity. It remains unexplained however, why the cellular events associated with “sentinel spikes” rapidly engage inhibitory networks resulting in LVF activity, whereas the cellular events associated with the HYP onset pattern, that produce a prolonged increase in excitability, engage the inhibitory network after a pronounced delay. HYP activity is also associated with increased extracellular potassium concentration (Köhling et al., 2016), but LVF activity is associated with a more dramatic increase that may drive a state transition in to the bursting stage)(de Curtis et al., 2018)(Librizzi et al., 2017) (Figure 1). Also, simulations suggest that the inhibitory interneurons may reach a depolarization block, due to the rapid firing rates, that could also lead to run-away excitation (Ho and Truccolo, 2016). Possibly the combination of a loss of inhibitory tone from the depolarization block of inhibitory neurons, and the depolarization of principal neurons via the increase in the extracellular potassium concentration leads to the bursting stage of the seizure that follows the seizure onset pattern and persists until seizure termination. One critical recent observation is that during this bursting stage, increasing the inhibitory tone by optogenetic stimulation of dentate gyrus mossy fiber neurons results in a decrease in seizure duration and a failure of secondarily generalization (Bui et al., 2018).
Seizure spread, LVF activity, and the roles of inhibition
While LVF is largely regarded as a seizure onset pattern (Velasco et al., 2000)(Perucca et al., 2014) it may also representative of a seizure spread pattern (Schevon et al., 2012)(Weiss et al., 2013)(Grinenko et al., 2018). During the LVF epoch, microelectrode array recordings from the epileptogenic human neocortex in at least one subject-seizure exhibited seemingly directional propagation of multi-unitactivity firing rate increases and LFP amplitudes (see subject C7 in (Schevon et al., 2012)(Weiss et al., 2013). Also, quantitative iEEG studies have demonstrated that as the seizure evolves there is an increase in the number of sites where LVF activity can be recorded (Grinenko et al., 2018).
A concept related to the LVF spread pattern is the “ictal wavefront” (Schevon et al., 2012), which premises that after the “LVF” spread pattern, ictal discharges originate from the “recruited area” (i.e. where LVF spread to and neuron firing rates increased) and directionally propagate in to the “penumbra” (i.e. regions where multi-unit firing rates did not increase during the seizure despite a seizure appearing on the EEG there) (Smith et al., 2016)(Proix et al., 2018). Central to this theory is the concept that LVF spread signifies a slow (.2 mm/sec) spread of excitability across the neocortex, which contrasts with the known increased inhibitory neuron firing observed during LVF onset, but not the diffusion dynamics of potassium and the known role of KCC2 transporters in seizure genesis (Gonzalez et al., 2018; Di Cristo et al., 2018).
Unfortunately, very few studies utilizing pharmacological animal models of mesial-temporal or neocortical seizures have explicitly examined the role of excitatory-inhibitory balance during LVF spread, because experiments utilizing these models do not explicitly differentiate between LVF onset-and LVF spread-events (Uva et al., 2015)(Ziburkus et al., 2006)(Lopantsev and Avoli, 1998)(Uva et al., 2015)(Gnatkovsky et al., 2008). One unique investigation utilizing a 4-AP model of neocortical epilepsy showed that applying bicuculline to a second site, remote from the site of seizure initiation resulting from the 4-AP administration, resulted in a second seizure focus arguing that inhibition functions to restrain the spread of the primary ictal focus (Liou et al., 2018). One surprising observation made by this team of investigators, was that parvalbumin inhibitory interneurons at sites remote from the primary focus exhibited a near instantaneous increase in firing at the time of seizure onset at the primary focus, as revealed by calcium imaging (Liou et al., 2018). Inhibition of the penumbra territory had been conceptualized as a result of feed-forward inhibition, from the ictal core territory (Schevon et al., 2012), not local inhibition. It is also not yet clear if the firing rate increase of inhibitory neuronsat sites remote from the focus promotes or restrains seizure spread, since the firing rate of excitatory neurons at the remote site were not imaged as well (Liou et al., 2018).
Optogenetics may prove to be a very important tool to better understand excitatory-inhibitory balance during LVF spread because of its high spatial and temporalresolution. A recent breakthrough optogenetic study was successfully able to generate seizures using optogenetic stimulation of excitatory neurons in the neocortex and then to use genetically encoded calcium indicators to examine the role of different families of inhibitory and excitatory neurons contralateral to the site of stimulation (Khoshkhoo et al., 2017). These investigators were also able to optogentically inhibit the different types of inhibitory interneurons (parvalbumin, somatostatin, and VIP) in the hemisphere contralateral to the optogenetic kindling. One shortcoming of this study was that the seizure onset morphology was not properly characterized, and was unlikely LVF morphology (Sohal VS, personal communication). During these optogenetic seizures, inhibitory neuronsincreased firing 4–5 seconds prior to the increase in excitatory neuron firing at the site of spread (Khoshkhoo et al., 2017). Furthermore, inhibiting somatastatin or parvalbumin inhibitory interneurons at the site of spread, raised the seizure threshold (Khoshkhooet al., 2017). Although simultaneously inhibiting the somatastatin or parvalbumin inhibitory interneurons induced a decrease in the seizure threshold (Khoshkhoo et al., 2017). Thus, the results of this study support the premise that inhibition may paradoxically act to promote seizure spread, although the relevance to LVF spread in particular is speculative.
Human microelectrode studies have also made meaningful contributions to understanding excitatory-inhibitory balance during LVF spread. Elahian et al., found in a small cohort of patients that in mesial-temporal lobe contralateral to the site of seizure-onset the firing rate of inhibitory neuronsincreased during the appearance of LVF activity, and prior to an increase of excitatory neuron firing. Oddly, during some seizures an increase in excitatory neuron firing was not evident at all during the LVF spread (Elahain et al., 2018). Published human evidence to the contrary, and supporting a role of inhibition in restraining seizure spread, includes an observed decrease in inhibitory neuron firing rates in the mesial-temporal lobe during neocortical seizure spread (Misra et al., 2018). Also, microelectrode array recordings have demonstrated that at sites outside the region of seizure onset, LFPs are less sharply contoured and synchronized, while multi-unit activity is less phase-locked to the ictal LFP and EEG (Eissa et al., 2016)(Weiss et al., 2013)(Schevon et al., 2012). Together, these observations have been used as a foundation to infer that in regions outside of the site of seizure onset, inhibition functions to restrain the spread of the seizure. It is challenging to synthesize the conflicting results of these studies, because often the seizure-onset morphology was not used as a classifier and often the data sets included seizures with diverse morphologies (Misra et al., 2018) (Weiss et al., 2013)(Schevon et al., 2012).
Conclusion
Microelectrode investigations in patients with medically refractory epilepsy have succeeded in uncovering critical events in seizure genesis despite disagreement regarding interpretation. We believe that dramatic shifts in excitatory-inhibitory balance drive forward the transition between the inter-ictal to ictal state. In the case of mesial-temporal lobe seizures, excitation may come to dominate inhibition during the HYP onset pattern as a result of the synchronization of pathologically-interconnected neurons (Weiss et al., 2016) (Bragin et al., 2006). By contrast, synaptic inhibition dominatessynaptic excitation during the LVF onset pattern (Elahian et al., 2018). One important future avenue of research may be to combine human microelectrode recordings with ion sensitive electrodes, sensors, or microdialysis (Fried et al., 1999), to determine how changes in extracellular potassium concentrations contribute to seizure genesis (de Curtis et al., 2018; (Librizzi et al., 2017; D’Antunono et al., 2004; Louvel et al., 2001; Avoli et al., 1997; Avoli et al., 1996;). It is also important to design experimental approaches in which human data can be neatly integrated with animal models of mesial-temporal or neocortical epilepsy (Liou et al., 2018) (Schevon et al., 2012). Finally, it is essential that investigators agree on terminology for classifying seizures recorded in the local field potential, as an accurate understanding of the mechanisms of seizure genesis may require a “Galapagos Island” approach, in which seizure morphologies at different stages are carefully classified and sub-classified in to families prior to further analysis, since heterogeneity in morphology may reflect heterogeneity in underlying mechanisms.
Based on the results from these and other studies, we propose that synaptic inhibition promotes LVF-onset seizure onset and spread, rather than restraining seizure spread, during spontaneous human LVF-onset seizures and in animal models of mesial temporal lobe epilepsy. Resolving the mechanism responsible for LVF onset and spread is important because it can lead to new pharmacological and neuroengineering interventionalstrategies to treat epilepsy, as well as promote insights in to mechanisms of epileptogenesis (de Curtis and Avoli, 2016)(Trevelyan and Schevon, 2013).
Acknowledgements
This work was supported by R01 NS033310 to J.E., NS106957 to R.S., and a Commonwealth Universal Research Enhancement (CURE) Program grant #4100077067 to S.A.W and 5K23NS094633 – 02 to S.A.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Conflicts of Interest
S.A.W. is a founder of Fastwave, an EEG software manufacturer.
References
- Alvarado-Rojas C, Huberfeld G, Baulac M, Clemenceau S, Charpier S, Miles R, de la Prida LM, and Le Van Quyen M (2015). Different mechanisms of ripple-like oscillations in the human epileptic subiculum. Ann. Neurol 77, 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, and Krnjević K (2016). The Long and Winding Road to Gamma-Amino-Butyric Acid as Neurotransmitter. Can. J. Neurol. Sci. J. Can. Sci. Neurol 43, 219–226. [DOI] [PubMed] [Google Scholar]
- Avoli M, Psarropoulou C, Tancredi V, and Fueta Y (1993). On the synchronous activity induced by 4-aminopyridine in the CA3 subfield of juvenile rat hippocampus. J. Neurophysiol 70, 1018–1029. [DOI] [PubMed] [Google Scholar]
- Avoli M, Barbarosie M, Lücke A, Nagao T, Lopantsev V, Köhling R. (1996). Synchronous GABA-mediated potentials and epileptiform discharges in the rat limbic system in vitro. J Neurosci June 15;16(12):3912–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, Louvel J, Kurcewicz I, Pumain R, Barbarosie M. (1996). Extracellular free potassium and calcium during synchronous activity induced by 4-aminopyridine in the juvenile rat hippocampus. J Physiol June 15;493 ( Pt 3):707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb TL, Wilson CL, and Isokawa-Akesson M (1987). Firing patterns of human limbic neurons uring stereoencephalography (SEEG) and clinical temporal lobe seizures. Electroencephalogr. Clin. Neurophysiol 66, 467–482. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J Jr, Wilson CL, Vizentin E, Mathern GW. (1999). Electrophysiologic analysisof a chronic seizure model after unilateralhippocampal KA injection. Epilepsia September;40(9):1210–21. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Staba RJ, Reddick M, Fried I, and Engel J (2002). Interictal high-frequency oscillations (80–500 Hz) in the human epileptic brain: entorhinalcortex. Ann. Neurol 52, 407–415. [DOI] [PubMed] [Google Scholar]
- Bragin A, Azizyan A, Almajano J, Wilson CL, and Engel J (2005). Analysis of chronic seizure onsets after intrahippocampalkainic acid injection in freely moving rats. Epilepsia 46, 1592–1598. [DOI] [PubMed] [Google Scholar]
- Bui AD, Nguyen TM, Limouse C, Kim HK, Szabo GG, Felong S, Maroso M, and Soltesz I (2018). Dentate gyrus mossy cells control spontaneous convulsive seizures and spatial memory. Science 359, 787–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G (2015). Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus 25, 1073–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M, Dian JA, Dufour S, Wang L, Moradi Chameh H, Ramani M, Zhang L, Carlen PL, Womelsdorf T, Valiante TA. (2018). Brief activation of GABAergic interneurons initiatesthe transition to ictal events through post-inhibitory rebound excitation. Neurobiol Dis January;109(Pt A):102–116. doi: 10.1016/j.nbd.2017.10.007. [DOI] [PubMed] [Google Scholar]
- D’Antuono M, Louvel J, Köhling R, Mattia D, Bernasconi A, Olivier A, Turak B, Devaux A, Pumain R, Avoli M (2004). GABAA receptor-dependent synchronization leads to ictogenesis in the human dysplastic cortex. Brain July;127(Pt 7):1626–40. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Gnatkovsky V. (2009). Reevaluating the mechanisms of focal ictogenesis: The role of low-voltage fast activity. Epilepsia December;50(12):2514–25. doi: 10.1111/j.1528-1167.2009.02249.x. [DOI] [PubMed] [Google Scholar]
- de Curtis M, and Avoli M (2016). GABAergic networks jump-start focal seizures. Epilepsia 57, 679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis M, Uva L, Gnatkovsky V, and Librizzi L (2018). Potassium dynamics and seizures: Why is potassium ictogenic? Epilepsy Res 143, 50–59. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Vezzani A, O’Brien TJ, Jette N, Scheffer IE, de Curtis M, Perucca P. (2018). Epilepsy. Nat Rev Dis Primers 2018 May 3;4:18024. doi: 10.1038/nrdp.2018.24. [DOI] [PubMed] [Google Scholar]
- Di Cristo G, Awad PN, Hamidi S, Avoli M. (2018). KCC2, epileptiform synchronization, and epileptic disorders. Prog Neurobiol March;162:1–16. [DOI] [PubMed] [Google Scholar]
- Eissa TL, Tryba AK, Marcuccilli CJ, Ben-Mabrouk F, Smith EH, Lew SM, Goodman RR, McKhann GM, Frim DM, Pesce LL, et al. (2016). Multiscale Aspects of Generation of High-Gamma Activity during Seizures in Human Neocortex. ENeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahian B, Lado NE, Mankin E, Vangala S, Misra A, Moxon K, Fried I, Sharan A, Yeasin M, Staba R, Bragin A, Avoli M, Sperling MR, Engel J Jr, Weiss SA. (2018) Low-voltage fast seizures in humans begin with increased interneuron firing. Ann Neurol October;84(4):588–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried I, Wilson CL, Maidment NT, Engel J, Behnke E, Fields TA, MacDonald KA, Morrow JW, and Ackerson L (1999). Cerebral microdialysis combined with single-neuron and electroencephalographic recording in neurosurgical patients. Technical note. J. Neurosurg 91, 697–705. [DOI] [PubMed] [Google Scholar]
- Foffani G, Uzcategui YG, Gal B, Menendez de la Prida L (2007). Reduced spike-timing reliability correlates with the emergence of fast ripples in the rat epileptic hippocampus. Neuron September 20;55(6):930–41. [DOI] [PubMed] [Google Scholar]
- González OC, Shiri Z, Krishnan GP, Myers TL, Williams S, Avoli M, Bazhenov M. (2018) Role of KCC2-dependent potassium efflux in 4-Aminopyridine-induced Epileptiform synchronization. Neurobiol Dis January;109(Pt A):137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnatkovsky V, Librizzi L, Trombin F, and de Curtis M (2008). Fast activity at seizure onset is mediated by inhibitory circuits in the entorhinal cortex in vitro. Ann. Neurol 64, 674–686. [DOI] [PubMed] [Google Scholar]
- Gnatkovsky V, de Curtis M, Pastori C, Cardinale F, Lo Russo G, Mai R, Nobili L, Sartori I, Tassi L, Francione S. (2014). Biomarkers of epileptogenic zone defined by quantified stereo-EEG analysis. Epilepsia February;55(2):296–305. doi: 10.1111/epi.12507. Epub 2014 Jan 13. [DOI] [PubMed] [Google Scholar]
- Grasse DW, Karunakaran S, and Moxon KA (2013). Neuronal synchrony and the transition to spontaneous seizures. Exp. Neurol 248, 72–84. [DOI] [PubMed] [Google Scholar]
- Grinenko O, Li J, Mosher JC, Wang IZ, Bulacio JC, Gonzalez-Martinez J, Nair D, Najm I, Leahy RM, and Chauvel P (2018). A fingerprint of the epileptogenic zone in human epilepsies. Brain J. Neurol 141, 117–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberfeld G, Menendez de la Prida L, Pallud J, Cohen I, Le Van Quyen M, Adam C, Clemenceau S, Baulac M, and Miles R (2011). Glutamatergic pre-ictal discharges emerge at the transition to seizure in human epilepsy. Nat. Neurosci 14, 627–634. [DOI] [PubMed] [Google Scholar]
- Ibarz JM, Foffani G, Cid E, Inostroza M, Menendez de la Prida L (2010). Emergent dynamics of fast ripples in the epileptic hippocampus. J Neurosci 2010 December 1;30(48):16249–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunakaran S, Grasse DW, and Moxon KA (2016). Role of CA3 theta-modulated interneuronsduring the transition to spontaneousseizures. Exp. Neurol 283, 341–352. [DOI] [PubMed] [Google Scholar]
- Khoshkhoo S, Vogt D, and Sohal VS (2017). Dynamic, Cell-Type-Specific Roles for GABAergic Interneurons in a Mouse Model of Optogenetically Inducible Seizures. Neuron 93, 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhling R, D’Antuono M, Benini R, de Guzman P, and Avoli M (2016). Hypersynchronous ictal onset in the perirhinalcortex results from dynamic weakening in inhibition. Neurobiol. Dis 87, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecq V, Lehongre K, Adam C, Frazzini V, Mathon B, Clemenceau S, Hasboun D, Charpier S, Baulac M, Navarro V, et al. (2017). Single-unit activities during the transition to seizures in deep mesial structures. Ann. Neurol 82, 1022–1028. [DOI] [PubMed] [Google Scholar]
- Lévesque M, Salami P, Gotman J, and Avoli M (2012). Two seizure-onset types reveal specific patterns of high-frequency oscillations in a model of temporal lobe epilepsy. J. Neurosci. Off. J. Soc. Neurosci 32, 13264–13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque M, Herrington R, Hamidi S, and Avoli M (2016). Interneuronsspark seizure-like activity in the entorhinal cortex. Neurobiol. Dis 87, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Librizzi L, Losi G, Marcon I, Sessolo M, Scalmani P, Carmignoto G, and de Curtis M (2017). Interneuronal Network Activity at the Onset of Seizure-Like Events in Entorhinal Cortex Slices. J. Neurosci. Off. J. Soc. Neurosci 37, 10398–10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J-Y, Ma H, Wenzel M, Zhao M, Baird-Daniel E, Smith EH, Daniel A, Emerson R, Yuste R, Schwartz TH, et al. (2018). Role of inhibitory control in modulating focal seizure spread. Brain J. Neurol 141, 2083–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopantsev V, and Avoli M (1998). Participation of GABAA-mediated inhibition in ictallike discharges in the rat entorhinal cortex. J. Neurophysiol 79, 352–360. [DOI] [PubMed] [Google Scholar]
- Louvel J, Papatheodoropoulos C, Siniscalchi A, Kurcewicz I, Pumain R, Devaux B, Turak B, Esposito V, Villemeure JG, Avoli M. (2001). GABA-mediated synchronization in the human neocortex: elevations in extracellular potassium and presynaptic mechanisms. Neuroscience; 105(4):803–13. [DOI] [PubMed] [Google Scholar]
- Memarian N, Madsen SK, Macey PM, Fried I, Engel J, Thompson PM, and Staba RJ (2015). Ictal depth EEG and MR structural evidence for two different epileptogenic networks in mesial temporal lobe epilepsy. PloS One 10, e0123588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merricks EM, Smith EH, McKhann GM, Goodman RR, Bateman LM, Emerson RG, Schevon CA, Trevelyan AJ. (2015). Single unit action potentials in humans and the effect of seizure activity. Brain 2015 October;138(Pt 10):2891–906. doi: 10.1093/brain/awv208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra A, Long X, Sperling MR, Sharan AD, and Moxon KA (2018). Increased neuronal synchrony prepares mesial temporal networks for seizures of neocortical origin. Epilepsia 59, 636–649. [DOI] [PubMed] [Google Scholar]
- Ogren JA, Wilson CL, Bragin A, Lin JJ, Salamon N, Dutton RA, Luders E, Fields TA, Fried I, Toga AW, et al. (2009). Three-dimensional surface maps link local atrophy and fast ripples in human epileptic hippocampus. Ann. Neurol 66, 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucca P, Dubeau F, and Gotman J (2014). Intracranial electroencephalographic seizure-onset patterns: effect of underlying pathology. Brain J. Neurol 137, 183–196. [DOI] [PubMed] [Google Scholar]
- Prince DA, Wilder J (1967). Control mechanisms in cortical epileptogenic foci. Arch Neurol 1967;16(2):194–202. [DOI] [PubMed] [Google Scholar]
- Proix T, Jirsa VK, Bartolomei F, Guye M, and Truccolo W (2018). Predicting the spatiotemporal diversity of seizure propagation and termination in human focal epilepsy. Nat. Commun 9, 1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevon CA, Weiss SA, McKhann G, Goodman RR, Yuste R, Emerson RG, and Trevelyan AJ (2012). Evidence of an inhibitory restraintof seizure activity in humans. Nat. Commun 3, 1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessolo M, Marcon I, Bovetti S, Losi G, Cammarota M, Ratto GM, Fellin T, Carmignoto G. (2015). Parvalbumin-Positive Inhibitory Interneurons Oppose Propagation But Favor Generation of Focal Epileptiform Activity. J Neurosci July 1;35(26):9544–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiri Z, Manseau F, Lévesque M, Williams S, and Avoli M (2015). Interneuron activity leads to initiation of low-voltage fast-onset seizures. Ann. Neurol 77, 541–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiri Z, Manseau F, Lévesque M, Williams S, and Avoli M (2016). Activation of specific neuronal networks leads to different seizure onset types. Ann. Neurol 79, 354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EH, Liou J, Davis TS, Merricks EM, Kellis SS, Weiss SA, Greger B, House PA, McKhann GM, Goodman RR, et al. (2016). The ictal wavefront is the spatiotemporalsource of discharges during spontaneous human seizures. Nat. Commun 7, 11098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead M, Bower M, Brinkmann BH, Lee K, Marsh WR, Meyer FB, Litt B, Van Gompel J, and Worrell GA (2010). Microseizures and the spatiotemporal scales of human partial epilepsy. Brain J. Neurol 133, 2789–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Khoo HM, Dubeau F, and Gotman J (2018). Association between scalp and intracerebral electroencephalographic seizure-onsetpatterns: A study in different lesional pathological substrates. Epilepsia 59, 420–430. [DOI] [PubMed] [Google Scholar]
- Trevelyan AJ, and Schevon CA (2013). How inhibition influences seizure propagation. Neuropharmacology 69, 45–54. [DOI] [PubMed] [Google Scholar]
- Truccolo W, Donoghue JA, Hochberg LR, Eskandar EN, Madsen JR, Anderson WS, Brown EN, Halgren E, and Cash SS (2011). Single-neuron dynamics in human focal epilepsy. Nat. Neurosci 14, 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uva L, Breschi GL, Gnatkovsky V, Taverna S, and de Curtis M (2015). Synchronous inhibitory potentials precede seizure-like events in acute models of focal limbic seizures. J. Neurosci 35, 3048–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco AL, Wilson CL, Babb TL, and Engel J (2000). Functional and anatomic correlates of two frequently observed temporal lobe seizure-onset patterns. Neural Plast 7, 49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SA, Banks GP, McKhann GM, Goodman RR, Emerson RG, Trevelyan AJ, and Schevon CA (2013). Ictal high frequency oscillations distinguish two types of seizure territories in humans. Brain J. Neurol 136, 3796–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SA, Alvarado-Rojas C, Bragin A, Behnke E, Fields T, Fried I, Engel J, and Staba R (2016). Ictal onset patterns of local field potentials, high frequency oscillations, and unit activity in human mesial temporal lobe epilepsy. Epilepsia 57, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Y Ho EC, and Truccolo W (2016). Interaction between synaptic inhibition and glial-potassium dynamics leads to diverse seizure transition modes in biophysical models of human focal seizures. J. Comput. Neurosci 41, 225–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekhlef L, Breschi GL, Lagostena L, Russo G, Taverna S. (2015). Selective activation of parvalbumin-or somatostatin-expressing interneurons triggers epileptic seizurelike activity in mouse medial entorhinalcortex. J Neurophysiol March 1;113(5):1616–30. [DOI] [PubMed] [Google Scholar]
- Ziburkus J, Cressman JR, Barreto E, and Schiff SJ (2006). Interneuron and pyramidal cell interplay during in vitro seizure-like events. J. Neurophysiol 95, 3948–3954. [DOI] [PMC free article] [PubMed] [Google Scholar]