Abstract
Rationale: Asthma is characterized by disease within the small airways. Several studies have suggested that forced oscillation technique–derived resistance at 5 Hz (R5) − resistance at 20 Hz (R20) is a measure of small airway disease; however, there has been limited validation of this measurement to date.
Objectives: To validate the use of forced oscillation R5 − R20 as a measure of small airway narrowing in asthma, and to investigate the role that small airway narrowing plays in asthma.
Methods: Patient-based complete conducting airway models were generated from computed tomography scans to simulate the impact of different degrees of airway narrowing at different levels of the airway tree on forced oscillation R5 − R20 (n = 31). The computational models were coupled with regression models in an asthmatic cohort (n = 177) to simulate the impact of small airway narrowing on asthma control and quality of life. The computational models were used to predict the impact on small airway narrowing of type-2 targeting biologics using pooled data from two similarly design randomized, placebo-controlled biologic trials (n = 137).
Measurements and Main Results: Simulations demonstrated that narrowing of the small airways had a greater impact on R5 − R20 than narrowing of the larger airways and was associated (above a threshold of approximately 40% narrowing) with marked deterioration in both asthma control and asthma quality of life, above the minimal clinical important difference. The observed treatment effect on R5 − R20 in the pooled trials equated to a predicted small airway narrowing reversal of approximately 40%.
Conclusions: We have demonstrated, using computational modeling, that forced oscillation R5 − R20 is a direct measure of anatomical narrowing in the small airways and that small airway narrowing has a marked impact on both asthma control and quality of life and may be modified by biologics.
Keywords: asthma, forced oscillation technique, small airways, imaging, integrative modelling
At a Glance Commentary
Scientific Knowledge on the Subject
Several studies have suggested that the forced oscillation measurement resistance at 5 Hz (R5) − resistance at 20 Hz (R20) is a marker of small airway disease. However, there has been limited validation of this measurement to date
What This Study Adds to the Field
Using computer models of the lungs, we show that R5 − R20 was found to be an anatomically sensitive measure of small airway disease. The models predicted that small airway narrowing would have a significant impact on both asthma control and quality of life, and that narrowing could, in part, be reversed by antiinflammatory biologics.
Asthma is a complex chronic inflammatory disease that involves both central and small airways (1, 2). Multiple lines of evidence suggest that, within subjects with asthma, the small airways are dysfunctional (3), inflamed (4), and damaged (5). Several in vivo imaging approaches have been used to study the small airways, including hyperpolarized gas, magnetic resonance imaging, and computed tomography (CT) imaging (6, 7). These studies have demonstrated that spatial disease in the small airways captured by both magnetic resonance imaging and CT of the lungs may be adequately reflected in more simple measures of small airway disease using the forced oscillation technique (FOT).
FOT is a simple technique that perturbs the respiratory system during tidal breathing, by using a series of pressure oscillations over a range of frequencies (typically, 5–35 Hz), applied at the mouth. Due to the lack of a required breathing maneuver, the FOT can be easily deployed across age groups, from young children to the elderly. This suggests that it may be a suitable and clinically applicable tool for the measurement of small airway disease in adults and children with asthma (8). The change in resistance from low- to high-frequency ranges (e.g., resistance at 5 Hz [R5] − resistance at 20 Hz [R20]) is often suggested as a putative marker of small airway obstruction and has been shown to predict loss of asthma control in children (9). This measure has also been shown to correlate with measures of small airway inflammation (10), exacerbations (11), and response to inhaled corticosteroids, including small particle formulations in adult asthma (12–14). Recently, a multinational study of small airway dysfunction markers in adult asthma identified that impulse oscillometry–measured R5 − R20 was the most strongly correlated marker of small airway disease of several small airway physiological markers. Furthermore, 42% of the adult asthma population demonstrated an abnormal R5 − R20 measurement. These observations provide strong evidence that FOT-derived R5 − R20 is a useful clinical tool to identify small airway disease (15).
However, despite these observational studies, the precise association between small airway anatomical narrowing and R5 − R20 is poorly understood. Furthermore, the impact of small airway narrowing on asthma control and quality of life has yet to be established.
Within this study, we hypothesized that: 1) anatomical disease within small airways (≤2 mm in diameter) is a critical determinant of forced oscillation–derived R5 − R20, accounting for relevant confounders, and that; 2) anatomical narrowing of the small airways would be predicted to have a significant impact on both asthma control and quality of life; and 3) we also attempted to predict the impact of antiinflammatory biologics targeting type-2 inflammation on small airway narrowing.
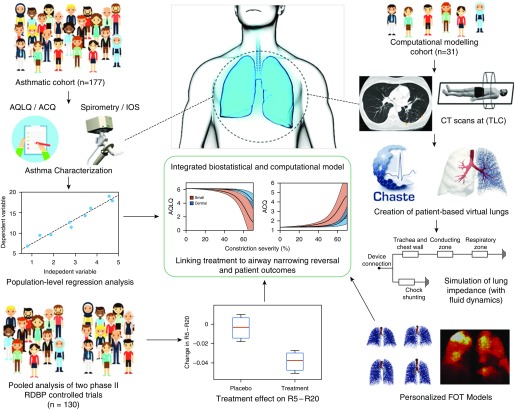
To investigate these hypotheses, we collected and analyzed data from a variety of different sources. Our approach uses patient-based computational modeling of the FOT (16) and statistical regression models that link R5 − R20 to asthma control and quality of life. Finally, post hoc evaluation of existing placebo-controlled clinical trial data was used to estimate the likely impact of antiinflammatory biologics on small airway narrowing. The overarching concept of the study is outlined visually in Figure 1.
Figure 1.
Diagram of the integrated modeling approach. The diagram shows many of the different clinical, statistical, and computational components that are used together in this integrated study. This includes the patient subset that underwent computed tomography (CT) scans, leading to the creation of patient-based lung structures, and personalized forced oscillation technique (FOT) modeling, as well as the larger asthmatic cohort used to create regressive links between FOT outcomes and more standardized asthmatic assessments. This integrative approach leads to a deeper understanding of the links between underlying physiology and patient outcomes. ACQ = asthma control questionnaire; AQLQ = asthma quality-of-life questionnaire; IOS = impulse oscillometry; R5 = resistance at 5 Hz; R20 = resistance at 20 Hz; RDBP = randomized, double-blind, placebo-controlled. Some components of Figure 1 were created (with permission) using stock photos from freepik.com.
Some of the results of these studies have been previously reported in the form of an abstract (17).
Methods
Results of this study were created through combined analysis of three separate data sources: 1) a large asthmatic cohort; 2) a smaller computational modeling cohort; and 3) pooled clinical trial cohorts.
Biostatistical Modeling Cohort
An adult asthmatic cohort (n = 177) was recruited from Glenfield Hospital in Leicester, United Kingdom. Current smokers and patients with a history of 10 pack-years or more were excluded. Asthma was diagnosed by a physician, according to current British Thoracic Society guidelines (18), with severity defined according to the GINA (Global Initiative for Asthma) treatment intensity steps (19). Details of the clinical study protocol are outlined in the online supplement, but in brief, participants attended for up to two visits, and underwent evaluation with asthma control questionnaire (ACQ)-6 and asthma quality-of-life questionnaire (AQLQ), and exacerbation frequency (20, 21); post-bronchodilator impulse oscillometry using a Jaeger MasterScreen (22); and spirometry, according to European Respiratory Society standards (23).
The study protocol for the recruitment and assessments in the two studies above was approved by the National Research Ethics Committee–East Midlands Leicester (approval no. 08/H0406/189), and all subjects gave their written informed consent.
The data collected from this cohort was used to create a statistical regression linking R5 − R20 to ACQ and AQLQ. The linear regression was calculated using a stepwise algorithm, and incorporated GINA treatment intensity, age, sex, smoking exposure in pack-years, and spirometry-measured FVC and FEV1/FVC as potential confounding variables. Discriminatory variables were retained by the stepwise regression model and include as independent predictors as a means of adjustment.
Computational Modeling Cohort
A subset of 20 of the 177 adults with asthma (9 female, 11 male), who consented to participate in a CT imaging substudy, were selected at random from the cohort of 177 patients, and 11 healthy control subjects were recruited for imaging. Full details of the CT protocol are outlined in the original study (15). All imaging was performed post-bronchodilator. Clinical characteristics of this cohort were well matched to the control subjects within the cohort and similar to patients with preserved spirometry in the overall cohort (Table E1 in the online supplement) and well matched to the 11 control subjects. GINA treatment step 3 (3–4) and R5 − R20 0.03 (0.01–0.11) kPa ⋅ s ⋅ L−1, aged 59 (47–65) years alongside 11 age-matched healthy volunteers (6 female, 5 male), R5 − R20 of 0.03 (0.02–0.06) kPa ⋅ s ⋅ L−1, and aged 59 (43–66) years. Healthy subjects had no prior history of respiratory disease, normal spirometry, and less than 10 pack-years smoking history.
Creation of patient-based airway models
From each of the 31 inspiratory CT scans, a patient-based virtual airway structure was derived by extracting center lines of the central airways (to generations 6–10) and using a recursive algorithm (24) to grow the remainder of the conducting zone (to an average of generation 16) within the identified lobar boundaries. The branch radii were scaled from TLC down to FRC using Lambert’s data (25). Each patient-based virtual lung consisted of 30,000–100,000 branches, with the entire set being originally presented by Bordas and colleagues (16). Within the original study, details of the structures were compared with histological data, with key features (radii, branch-lengths, etc.) lying within physiologically reasonable bounds (16).
Computational model of the FOT
The FOT was simulated using an electrical circuit–analogous model (26), with full details presented in the online supplement. In short, impedance of each airway branch was approximated using the wave equation (27). Total lung impedance was calculated by summing branch impedances in series and parallel, with each terminal bronchiole being subtended by a viscoelastic acinar model. This impedance was then added in series to contributions from the chest wall, trachea, and glottis, and in parallel to contributions from cheek and upper airway shunting, all of which were parameterized using experimental data from the literature (28, 29). All simulations were performed in MATLAB (Mathworks).
Alongside simulating resistance in all patient-based structures, simulations were also performed in one of the structures derived from a healthy control after application of artificial airway constrictions. Constrictions were applied by reducing branch radii in the structure by a fixed percentage (homogeneous) or drawing the reduction percentage from a normal distribution with a fixed mean (heterogeneous). Constrictions were either applied to all branches of a given Strahler order (marker of depth, 1 = terminal bronchiole, 12 = trachea), or to the small airways (orders 1–6) or large airways (7–10).
Clinical Trial Populations
To provide information on therapeutic intervention, we identified two similarly designed 3-month duration, antiinflammatory, randomized, double-blind, placebo-controlled, phase II trials, in moderate–severe uncontrolled asthma. Both trials have been previously reported (30, 31), and evaluated known targets relevant to asthma pathology in the small airways (IL-13 [n = 76] and the CRTH2 (chemoattractant receptor-homologous molecule expressed on Th2 cells) receptor DP2 (prostaglandin D2 receptor) [n = 61]) with systemic therapies that would be expected to engage with their target in the small airways.
Both trials used FOT-measured R5 − R20 as an exploratory outcome, alongside ACQ as an asthma control measure. Total cohort size (n = 137) was chosen to provide 90% power for identification of a clinically meaningful change in R5 − R20 of 0.03 kPa ⋅ s ⋅ L−1 (12–14, 32–34), with details given in the online supplement.
Combined analysis procedure for model integration
The procedure for systematically and jointly analyzing the various data sources is as follows. Resistance was simulated on each patient-based structure and compared against clinical measurements for validation. Then, model simulations were used to analyze the response of R5 − R20 to small and large airway constrictions. These results were compared with clinical stratifications seen in the larger asthmatic cohort. A regression model, derived from the larger cohort, was used to link R5 − R20 to ACQ and AQLQ, allowing for improved result interpretability. The combined model was then used to link biologic therapy results to changes in small airway narrowing and asthma control.
Statistical Analysis
Statistical analysis was performed using SAS 9.4 (SAS Institute), Prism 7 (GraphPad Software Inc.), and MATLAB. A P value of less than 0.05 was taken as the threshold for statistical significance. Comparisons across groups were performed using one-way ANOVA for parametric data or Kruskal-Wallis test for nonparametric data, and Fisher’s exact test or the chi-square test for proportions. Bonferroni/Dunn corrections for multiple comparisons were used, as appropriate. Correlations between continuous variables were calculated using Pearson’s correlation coefficient (R). We considered that FOT parameters were abnormally elevated when above 100% predicted of the 95th percentile predicted value from the KORA (Cooperative Health Research in the Augsburg Region) cohort (35).
Results
Identification of Small Airways within the Patient-based Lung Structures
Airway depth within the patient-based lung structures was annotated using Strahler order (SO = the number of branches from a given location to the nearest terminal bronchiole), a standardized method for describing branching networks (16). SO 1 refers to the terminal bronchioles, and, on average, SO 12 to the trachea. Across the set of 31 structures, the mean (SD) airway diameter at SO 7 was 2.12 (0.28) mm, and at SO 6 was 1.39 (0.21) mm, meaning orders 1–6 are treated as the small airways, and orders 7 and up as the large airways.
Correlation of Simulated and Clinical Resistance Measurements
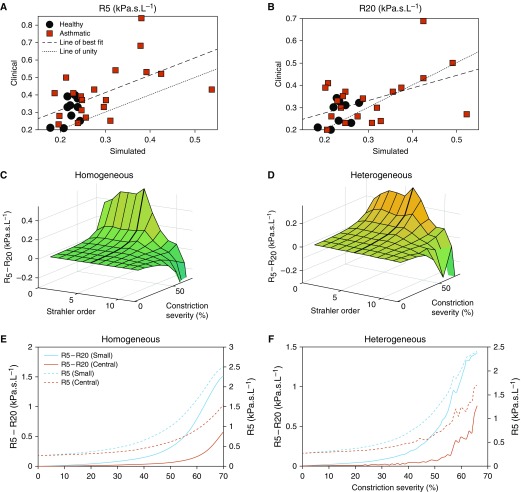
Within Figures 2A and 2B, we compare simulated (without artificial airway constriction) and clinically measured R5 and R20, across the 31 subjects who underwent CT imaging. As the figure shows, there is a moderate correlation (R2 [R5 = 0.35, P < 0.05]; R2 [R20 = 0.27, P < 0.05]) between simulated and clinical data, with only one significant outlier. The correlation of simulated and clinically measured R5 − R20 was modest, and individual R5 and R20 correlation errors were additive (data now shown).
Figure 2.
Analysis of the response of simulated resistance at 5 Hz (R5) − resistance at 20 Hz (R20) to airway constriction. (A and B) The ability of the model to simulate healthy subjects (black circles) and subjects with asthma (red squares) patient values is first shown, with R2 values for (A) R5: 0.35 and (B) R20: 0.27, and strong statistical significance (P < 0.05) in both cases. (C and D) The response of R5 − R20 to homogeneous (C) and heterogeneous (D) constrictions at different depths (denoted by Strahler order) is given. In both cases, R5 − R20 is seen to peak when constricting smaller airways (orders 1–6), and then decrease under upper airway constriction. (E and F) The response of R5 − R20 to small airway constriction is further illustrated by comparing homogeneous (E) and heterogeneous (F) constriction of all small airways (orders 1–6) and central airways (orders 7–12). A consistently stronger response is seen from the small airways. (E and F) R5 (right axis) and R5 − R20 (left axis) are both given, with R5 showing a larger response but a smaller distinction (particularly comparative to baseline values) between small and central airway constriction.
Sensitivity of R5 − R20 to Small Airway Constriction
In Figures 2C–2F, we illustrate the simulated response of R5 − R20 to various types of artificial airway constrictions applied to a structure based on a healthy control. Figures 2C and 2D illustrate how R5 − R20 responds to constriction, in both the presence and absence of heterogeneity (as defined in Methods). In both cases, R5 − R20 responds sensitively to mild constriction, with the response being amplified as constriction increases. Significantly, a larger response is seen when constricting small airways (SO 1–6) than large airways. Additional simulations confirmed that 5 Hz–20 Hz was the optimal difference for detection of small airway disease, though 5 Hz–25 Hz was similarly effective (Figure E3).
The response to small airway constriction is also illustrated in Figures 2E and 2F. As shown, constriction of the small airways consistently produced larger responses than constriction of the large airways, regardless of whether constrictions were homogeneous or heterogeneous. Taking care to note the different axis scales used for R5 and R5 − R20, the results clearly illustrate a larger relative increase in R5 − R20 when comparing large and small airway constriction, than in R5. These conclusions were the same when using other types of constriction distributions (see the online supplement).
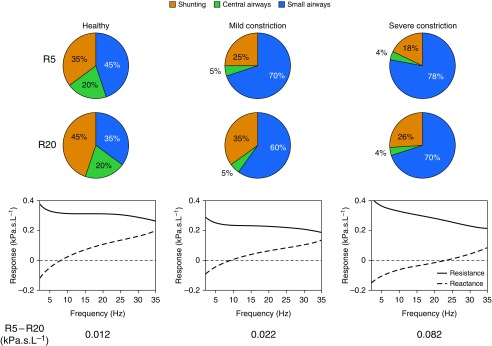
To further illustrate this sensitivity, in Figure 3 we show the relative contribution of the small airways, large airways, and cheek shunting, to R5 and R20, at baseline and under mild (20%) and severe (50%) constriction, imposed uniformly on the small airways. In all three cases, R5 was dominated by small airway contributions more than R20. Furthermore, in the presence of severe airway constriction, the model-derived R5 − R20 (0.08 kPa ⋅ s ⋅ L−1) was similar to the median reported R5 − R20 for subjects with asthma in our cross-sectional cohort (0.09 kPa ⋅ s ⋅ L−1; Table E1), suggesting that the models are generating physiologically relevant values.
Figure 3.
Relative contributions of small airways, central airways, and upper airway/cheek shunting to resistance at 5 Hz (R5) and resistance at 20 Hz (R20). The simulated relative contributions are shown for a healthy subject under mild constriction (20%) and severe constriction (50%) of all small airways, alongside the resistance and reactance curves from 2 to 35 Hz. In all three cases, small airways contribute more significantly to R5 than R20, with the magnitude of this effect increasing with disease severity. This suggests that R5 − R20 responds most strongly to small airway constriction.
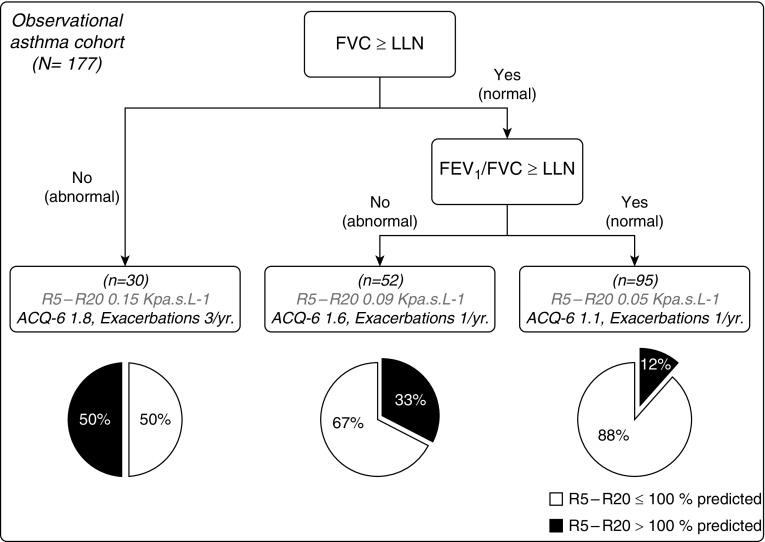
Large Asthmatic Cohort Stratification Based on R5 − R20
Within Figure 4, we evaluate the prevalence of small airway dysfunction in the large asthmatic cohort, measured using R5 − R20, and compared with spirometric values. The cohort was broken into three subgroups, based upon: 1) a low FVC less than the lower limit of normal (LLN) (36); 2) an abnormal FEV1/FVC less than LLN; and 3) normal spirometry (both FEV1 ≥ LLN and FVC ≥ LLN) outputs (clinical interpretation and statistical summaries of the data underlying Figure 4 are provided in the online supplement).
Figure 4.
Stratification of the clinical study population according to FVC z-score and FEV1/FVC lower limit of normal (LLN). Stratification according to spirometry culminated in three different groups: gas trapping, asthma/chronic obstructive pulmonary disease overlap, and early small airway disease. Pie charts show the percentage of patients in each group with resistance at 5 Hz (R5) − resistance at 20 Hz (R20) greater than 100% predicted, using the 95th percentile KORA (Cooperative Health Research in the Augsburg Region) cohort quantile–quantile regression equations (pie charts: black part corresponds to R5 − R20 > 100% predicted and white part corresponds to R5 − R20 ≤ 100% predicted). Further clinical details for each group can be found in Table E1. ACQ = asthma control questionnaire.
Associating R5 − R20 and Small Airway Narrowing with Patient Outcomes
To allow for contextualization of R5 − R20 in terms of reported patient outcomes, a linear regression model was built from the large asthmatic cohort data to allow for prediction of ACQ-6 and AQLQ, based on patient R5 − R20. This was done using a series of potential confounding independent variables (age, sex, GINA treatment intensity, FVC, FEV1/FVC, and smoking exposure in pack-years). Details of the regression model are summarized in Table 1.
Table 1.
Regression Models for ACQ and AQLQ
| Parameter Estimate | SE | P Value | 95% CI Lower Bound | 95% CI Upper Bound | |
|---|---|---|---|---|---|
| AQLQ model |
|
|
|
|
|
| Intercept | 5.832 | 0.445 | <0.001 | 4.953 | 6.712 |
| R5 − R20, kPa ⋅ s ⋅ L−1 | −3.292 | 0.967 | 0.001 | −5.202 | −1.383 |
| GINA (1–5) | −0.258 | 0.069 | <0.001 | −0.394 | −0.122 |
| FVC, L | 0.199 | 0.090 | 0.028 | 0.021 | 0.377 |
| ACQ-6 model | |||||
| Intercept | 1.911 | 0.715 | 0.008 | 0.499 | 3.322 |
| R5 − R20, kPa ⋅ s ⋅ L−1 | 2.195 | 0.787 | 0.006 | 0.640 | 3.749 |
| GINA (1–5) | 0.314 | 0.058 | <0.001 | 0.199 | 0.429 |
| Age, yr | −0.016 | 0.005 | 0.003 | −0.027 | −0.006 |
| Pack-years | 0.018 | 0.008 | 0.033 | 0.001 | 0.034 |
| Sex, M | 0.378 | 0.145 | 0.01 | 0.091 | 0.665 |
| FEV1/FVC | −0.016 | 0.007 | 0.026 | −0.030 | −0.002 |
Definition of abbreviations: ACQ = asthma control questionnaire; AQLQ = asthma quality-of-life questionnaire; CI = confidence interval; GINA = Global Initiative for Asthma; R5 = resistance at 5 Hz; R20 = resistance at 20 Hz.
Results of stepwise (forward selection) linear regression models. Dependent variables are AQLQ and ACQ. For AQLQ, independent variables are R5 − R20, GINA treatment step, and FVC. For the ACQ model, independent variables are R5 − R20, GINA treatment step, age, pack-years, sex, and FEV1/FVC.
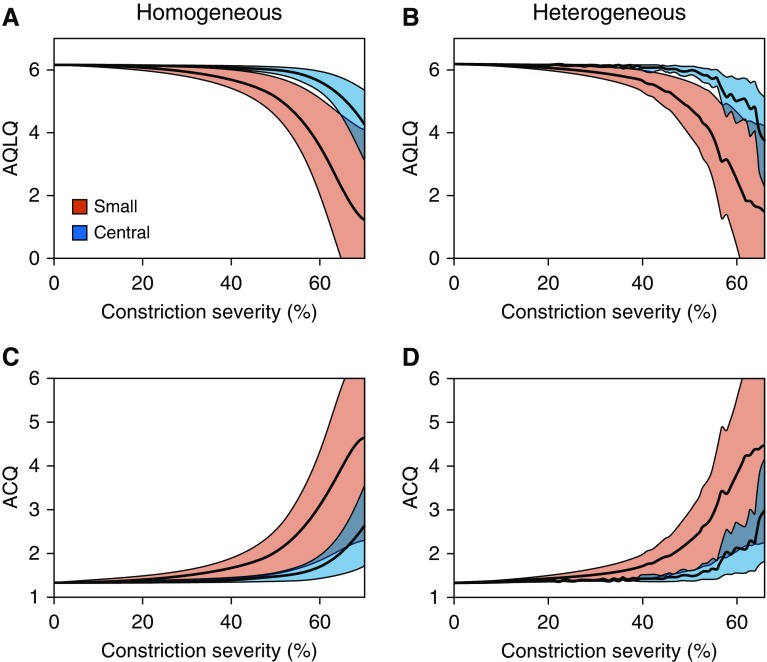
In Figure 5, we illustrate the potential value of the regression model, by transforming the R5 − R20 values in Figures 2E–2F to ACQ and AQLQ values, using a 95% confidence interval (CI) on the regression coefficients for R5 − R20. These results illustrate that deterioration in asthma control and quality of life occurs much more rapidly under constriction of the small airways than the large airways. Starting from a mild constricted state (20% constriction), small increases to constriction (moving from 20% to 40% constriction) would be expected to yield clinically relevant changes (>0.5 units) in both ACQ and AQLQ.
Figure 5.
Simulated response of asthma control questionnaire (ACQ) and asthma quality-of-life questionnaire (AQLQ) to airway constriction. The translation of the response of resistance at 5 Hz − resistance at 20 Hz to small and central airway constriction into ACQ and AQLQ uses regressive models from Table 1. Results were calculated using the mean regression parameter value for resistance at 5 Hz − resistance at 20 Hz (black line), as well as the 95% confidence interval (colored bands). For both ACQ and AQLQ, a much more severe response is seen under small airway constriction than central airway constriction, even after accounting for the uncertainty of the regression.
Linking Small Airway Changes to Treatment Outcomes
Having established that R5 − R20 is sensitive to small airway disease, and contextualizing this in terms of patient-related outcome measures, we seek to further evaluate these results in the context of clinical trial measured treatment outcomes. As shown in Table 2, the pooled treatment effect (95% CI) of two antiinflammatory therapies (30, 31) on R5 − R20 was −0.038 kPa ⋅ s ⋅ L−1 (−0.05 to −0.03; P < 0.0001), comparative to placebo. Based on the results in Figures 2 and 3, we estimate that this treatment effect would be equivalent to a 35% (20–50%) reduction in small airway constriction in the presence of moderate small airway dysfunction.
Table 2.
Pooled Clinical Trial Data Using R5 − R20 and Asthma Control Questionnaire as Markers
| Change from Baseline to Week 12 [Mean (SD)] | |||
|---|---|---|---|
| Tralokinumab (n = 36) | Placebo (n = 40) | Treatment Effect [Difference in Means (SE)] | |
| Change in ACQ-6 | −0.96 (0.14) | −0.87 (0.14) | −0.08 (0.20); P = 0.67 |
| Change in R5 − R20, kPa ⋅ s ⋅ L−1 | −0.04 (0.02) | −0.01 (0.01) | −0.03 (0.02); P = 0.19 |
| Change from Baseline to Week 12 [Mean (SD)] | |||
|---|---|---|---|
| Fevipiprant (n = 29) | Placebo (n = 32) | Treatment Effect [Difference in Means (SE)] | |
| Change in ACQ-7 | −0.18 (0.92) | 0.14 (0.96) | −0.32 (0.24); P = 0.19 |
| Change in R5 − R20, kPa ⋅ s ⋅ L−1 | −0.03 (0.11) | 0.02 (0.11) | −0.05 (0.03); P = 0.11 |
| Change from Baseline to Week 12 [Mean (SD)] | |||
|---|---|---|---|
| Drug (n = 65) | Placebo (n = 72) | Treatment Effect [Difference in Means (SE)] | |
| Pooled estimate change in ACQ | −0.612 (0.62) | −0.42 (0.64) | −0.19 (0.21); P < 0.0001; 95% CI = −0.26 to −0.11 |
| Pooled estimate change in R5 − R20, kPa ⋅ s ⋅ L−1 | −0.034 (0.07) | 0.002 (0.07) | −0.038 (0.024); P < 0.0001; 95% CI = −0.05 to −0.03 |
Definition of abbreviations: ACQ = asthma control questionnaire; CI = confidence interval; R5 = resistance at 5 Hz; R20 = resistance at 20 Hz.
Change and treatment effect on R5 − R20, and ACQ of two 3-month duration, antiinflammatory, randomized, double-blind, placebo-controlled phase-2 trials in moderate–severe uncontrolled asthma, for both intervention and placebo (20–21). Pooled estimated treatment effect and 95% CI of the two antiinflammatory therapies is shown. Note that, for ACQ, the two treatments used different validated variants of the questionnaire (ACQ6 and ACQ7). Given this, the pooled estimate treatment effect for ACQ is intended as approximate only.
In Table 2, we present the pooled estimate of treatment effect on ACQ (approximate only, as the two studies used different ACQ variants) as −0.19 (−0.26 to −0.11; P < 0.0001). We note that the predicted change in ACQ (from our model), based on the illustrated change in R5 − R20, would be −0.08 (−0.03 to −0.19), which is consistent with the actual change. This is particularly noteworthy, given the regression model was derived from a cohort independent of the clinical trials.
Discussion
In this study, we have used a combination of computational modeling on patient-based lung structures to identify that R5 − R20 is a clear marker of small airway anatomical constriction and is strongly associated with patient outcome measures, ACQ and AQLQ. Furthermore, using our models, we have demonstrated that narrowing above a threshold of 40% in the small airways generates clinical important changes in asthma control and quality of life, and that this effect is equivalent to the predicted narrowing reversal that would be expected with antiinflammatory biologics based upon the observed treatment effects on R5 − R20.
The validation of the computational lung impedance model, presented in Figures 2A and 2B, provides the largest (to our knowledge) patient-based validation of this model seen to date within the literature. The model that we have used has been used for a great variety of investigations in the literature (26, 28, 37, 38). Given this, this study helps provide validation to many prior results, built upon the theoretical foundations of the model.
Our results add further credence to the mounting evidence supporting R5 − R20 as a robust small airway detection tool. The recent multinational ATLANTIS (Assessment of Small Airways Involvement in Asthma) trial identified that R5 − R20 was one of the key physiological determinants of small airway disease in asthma (15). Furthermore, a recent study in chronic obstructive pulmonary disease has linked anatomical narrowing of airways generations 7–9 measured with bronchoscopic optical coherence tomography with R5 − R20 (39). Taken together, our observations provide further evidence supporting the validity of the claim that R5 − R20 is a physiological marker of small airway disease.
The data presented here used 5 Hz and 20 Hz as the low- and high-frequency points for calculating resistive difference. This choice was made primarily due to the prevalence of R5 − R20 in the literature compared to other index choices. However, for completeness, in the online supplement we also present a brief analysis of a variety of choices of low and high points within the frequency range, 5–35 Hz. Through this analysis it is shown that R5 − R20 is significantly more sensitive to small airway constriction than most other frequency choices.
The results in Figures 2 and 3 clearly illustrate the value of low–high frequency resistive difference as a marker of small airway dysfunction. This conclusion is furthered by the results in Figure 4, which suggest that R5 − R20 may stratify small airway disease, even in the presence of normal spirometric values. This compares well to other results in the literature (3, 15, 40), which have suggested the value of R5 − R20 as a predictor of small airway dysfunction.
The analysis in Figures 2–4 becomes more meaningful when interpreted in the context of the underlying statistical relationship between R5 − R20, ACQ, and AQLQ, as in Figure 5. This figure illustrates that small airway constriction leads to significantly larger reductions in asthma control and quality of life than large airway constrictions, suggesting that a major determinant of asthma control and quality of life may be disease within the small airways in keeping with the results of the multinational ATLANTIS study (15). The results in Figure 5 suggest that the initial effect of small airway constriction on ACQ and AQLQ is quite minimal, with an approximate 40% reduction in small airway radii needed to create the minimal clinically important change (0.5 units). However, once disease is already present, smaller increases in disease severity appear to lead to much larger degradations in patient quality of life (e.g., moving from 50% to 60% constriction decreases AQLQ from approximately 5 to 3.5).
The ability to more precisely connect morphological changes in lung structure with clinical pulmonary measurements and patient quality-of-life assessments is a truly valuable and novel contribution. This is further illustrated by connecting these results to the clinical trial results in Table 2. The pooled estimate effect on R5 − R20 had a 95% CI of −0.05 to −0.03 kPa ⋅ s ⋅ L−1. Interpreting this in terms of Figures 2E and 2F allows for an approximate interpretation of this change in the context of the underlying small airway morphology. Reductions in R5 − R20 of this magnitude could be interpreted as being driven by a 35% (20–50%) reduction in mean constriction severity of the small airways (a transition from mild/severe constriction toward a healthy state). Importantly, the statistical regression relationship was also able to accurately predict the observed treatment effect on ACQ, from the observed effect on R5 − R20, giving further confidence to the results.
Study Limitations
The concordance between simulated and clinically measured R5 − R20 in our study was modest but illustrates the ability of the model to distinguish between high and low responses. Part of the error between simulated and clinical values may be due to the use of mean population data for the shunting and tracheal impedances, and the use of a simple constant-phase model (26), for respiratory zone contributions.
In recent work by this group (17), CT voxel deformation data was used to improve structural accuracy of the virtual lungs. For a subset of the virtual lungs in this study, this process improved the R2 between simulated and clinical R5 from 0.61 to 0.78. This suggests that a significant degree of noise in the model may simply be due to limitations in imaging accuracy.
It should be noted that the computational modelling cohort was comprised of a randomly selected subset of the larger clinical cohort. This subset had R5 − R20 values that spanned the range of the larger cohort, and due to the randomized selection process, we do not believe that this subset biases the results.
Finally, the simulated results presented in this article relied on enforcing constricting patterns, either homogeneously or through a normal distribution. For completeness, in the online supplement, we investigate the effect of skew–normally distributed airway constrictions on R5 − R20, showing qualitatively similar results to those contained within the main article.
Conclusions
These findings illustrate a clear and consistent relationship between anatomical small airway disease and lung impedance measurement R5 − R20. Using a combination of patient-based computational modeling, large-scale clinical assessment, and clinical trial data, this relationship can be linked to patient outcome measures and be used to predict therapeutic intervention responses. Furthermore, this study stands as a prototype for the value of integrated computational–clinical approaches to understand the role of small airway disease in respiratory medicine.
Supplementary Material
Footnotes
Supported by grants from and National Institute for Health Research (NIHR) Leicester Biomedical Research Centre: Respiratory Theme grant RM65G0113, the East Midlands Comprehensive Clinical Research Network and EU FP7 AirPROM project grant 270194, an unrestricted grant from the Chiesi Onlus Foundation (Validation of Particle in Exhaled Air [PEx] as a Novel Matrix for Non-Invasive Detection of Small Airways Disease in Asthma), and by the Rhodes Trust (B.H.F.). The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health, United Kingdom.
Author Contributions: B.H.F. developed and implemented the computational modeling pipeline, analyzed model outputs, cowrote the manuscript, and provided scientific critique of the data; M.S. performed and analyzed impulse oscillometry and clinical data in the clinical study population, cowrote the manuscript with B.H.F. and S.S., and provided a scientific critique of the data; R.B. helped design and develop the computational modeling pipeline, reviewed the manuscript, and provided scientific critique of the data; M.R. performed the pooled clinical trial statistical analyses; A.B. constructed Figure 1, reviewed the manuscript, and provided a scientific critique of the data; A.S. and B.H. supported clinical patient recruitment in the observational cohorts and the trials; C.B. coordinated the AirPROM-FP7 consortium, was the chief investigator of the observational cohort and the clinical trials, and reviewed the manuscript and provided a scientific critique of the data; K.B. helped develop and implement the computational modeling pipeline, cowrote the manuscript with M.S. and S.S., and provided scientific critique of the data; D.K. helped design and conceive the overall study, supervised the development of the computational modeling pipeline, reviewed the manuscript, and provided a scientific critique of the data; J.O.-B. helped design and conceive the study, reviewed the manuscript, and provided a scientific critique of the data; and S.S. conceived the study, helped secure funding for the study with C.B., supervised the development of the modeling (computational and statistical) and key modeling questions, cowrote the manuscript, and provided a scientific critique of the data.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201812-2322OC on May 18, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Contoli M, Kraft M, Hamid Q, Bousquet J, Rabe KF, Fabbri LM, et al. Do small airway abnormalities characterize asthma phenotypes? In search of proof. Clin Exp Allergy. 2012;42:1150–1160. doi: 10.1111/j.1365-2222.2012.03963.x. [DOI] [PubMed] [Google Scholar]
- 2.Lipworth B, Manoharan A, Anderson W. Unlocking the quiet zone: the small airway asthma phenotype. Lancet Respir Med. 2014;2:497–506. doi: 10.1016/S2213-2600(14)70103-1. [DOI] [PubMed] [Google Scholar]
- 3.Usmani OS, Singh D, Spinola M, Bizzi A, Barnes PJ. The prevalence of small airways disease in adult asthma: a systematic literature review. Respir Med. 2016;116:19–27. doi: 10.1016/j.rmed.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Hamid Q, Song Y, Kotsimbos TC, Minshall E, Bai TR, Hegele RG, et al. Inflammation of small airways in asthma. J Allergy Clin Immunol. 1997;100:44–51. doi: 10.1016/s0091-6749(97)70193-3. [DOI] [PubMed] [Google Scholar]
- 5.Hyde DM, Hamid Q, Irvin CG. Anatomy, pathology, and physiology of the tracheobronchial tree: emphasis on the distal airways. J Allergy Clin Immunol. 2009;124(Suppl):S72–S77. doi: 10.1016/j.jaci.2009.08.048. [DOI] [PubMed] [Google Scholar]
- 6.Eddy RL, Westcott A, Maksym GN, Parraga G, Dandurand RJ. Oscillometry and pulmonary magnetic resonance imaging in asthma and COPD. Physiol Rep. 2019;7:e13955. doi: 10.14814/phy2.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell AJ, Foy BH, Richardson M, Singapuri A, Mirkes E, van den Berge M, et al. Functional CT imaging for identification of the spatial determinants of small-airways disease in adults with asthma. J Allergy Clin Immunol. 2019;144:83–93. doi: 10.1016/j.jaci.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Galant SP, Komarow HD, Shin HW, Siddiqui S, Lipworth BJ. The case for impulse oscillometry in the management of asthma in children and adults. Ann Allergy Asthma Immunol. 2017;118:664–671. doi: 10.1016/j.anai.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi Y, Aledia AS, Galant SP, George SC. Peripheral airway impairment measured by oscillometry predicts loss of asthma control in children. J Allergy Clin Immunol. 2013;131:718–723. doi: 10.1016/j.jaci.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 10.Williamson PA, Clearie K, Menzies D, Vaidyanathan S, Lipworth BJ. Assessment of small-airways disease using alveolar nitric oxide and impulse oscillometry in asthma and COPD. Hai. 2011;189:121–129. doi: 10.1007/s00408-010-9275-y. [DOI] [PubMed] [Google Scholar]
- 11.Gonem S, Umar I, Burke D, Desai D, Corkill S, Owers-Bradley J, et al. Airway impedance entropy and exacerbations in severe asthma. Eur Respir J. 2012;40:1156–1163. doi: 10.1183/09031936.00228611. [DOI] [PubMed] [Google Scholar]
- 12.Hozawa S, Terada M, Hozawa M. Comparison of budesonide/formoterol Turbuhaler with fluticasone/salmeterol Diskus for treatment effects on small airway impairment and airway inflammation in patients with asthma. Pulm Pharmacol Ther. 2011;24:571–576. doi: 10.1016/j.pupt.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Hozawa S, Terada M, Hozawa M. Comparison of the effects of budesonide/formoterol maintenance and reliever therapy with fluticasone/salmeterol fixed-dose treatment on airway inflammation and small airway impairment in patients who need to step-up from inhaled corticosteroid monotherapy. Pulm Pharmacol Ther. 2014;27:190–196. doi: 10.1016/j.pupt.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Hozawa S, Terada M, Haruta Y, Hozawa M. Comparison of early effects of budesonide/formoterol maintenance and reliever therapy with fluticasone furoate/vilanterol for asthma patients requiring step-up from inhaled corticosteroid monotherapy. Pulm Pharmacol Ther. 2016;37:15–23. doi: 10.1016/j.pupt.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Postma DS, Brightling C, Baldi S, Van den Berge M, Fabbri LM, Gagnatelli A, et al. ATLANTIS study group. Exploring the relevance and extent of small airways dysfunction in asthma (ATLANTIS): baseline data from a prospective cohort study. Lancet Respir Med. 2019;7:402–416. doi: 10.1016/S2213-2600(19)30049-9. [DOI] [PubMed] [Google Scholar]
- 16.Bordas R, Lefevre C, Veeckmans B, Pitt-Francis J, Fetita C, Brightling CE, et al. Development and analysis of patient-based complete conducting airways models. PLoS One. 2015;10:e0144105. doi: 10.1371/journal.pone.0144105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foy B, Bell A, Siddiqui SH, Kay D. Low frequency lung resistance is a global bronchoconstriction detection measure but is still sensitive to small airways disease [abstract]. Presented at the American Thoracic Society Meeting. May 22–25, 2018; San Diego, CA. [Google Scholar]
- 18.British Thoracic Society/Scottish Intercollegiate Guidelines Network. BTS/SIGN British guideline on the management of asthma. 2016 https://www.brit-thoracic.org.uk/standards-of-care/guidelines/btssign-british-guideline-on-the-management-of-asthma/ [accessed 2018 Jul 1]. Available from.
- 19.Global Initiative for Asthma (GINA) The global strategy for asthma management and prevention. 2017 http://www.ginasthma.org [accessed 2018 Jul 1]. Available from.
- 20.Juniper EF, Svensson K, Mörk AC, Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99:553–558. doi: 10.1016/j.rmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Juniper EF, Buist AS, Cox FM, Ferrie PJ, King DR. Validation of a standardized version of the asthma quality of life questionnaire. Chest. 1999;115:1265–1270. doi: 10.1378/chest.115.5.1265. [DOI] [PubMed] [Google Scholar]
- 22.Oostveen E, MacLeod D, Lorino H, Farré R, Hantos Z, Desager K, et al. ERS Task Force on Respiratory Impedance Measurements. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003;22:1026–1041. doi: 10.1183/09031936.03.00089403. [DOI] [PubMed] [Google Scholar]
- 23.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 24.Tawhai MH, Hunter P, Tschirren J, Reinhardt J, McLennan G, Hoffman EA. CT-based geometry analysis and finite element models of the human and ovine bronchial tree. J Appl Physiol (1985) 2004;97:2310–2321. doi: 10.1152/japplphysiol.00520.2004. [DOI] [PubMed] [Google Scholar]
- 25.Lambert RK, Wilson TA, Hyatt RE, Rodarte JR. A computational model for expiratory flow. J Appl Physiol. 1982;52:44–56. doi: 10.1152/jappl.1982.52.1.44. [DOI] [PubMed] [Google Scholar]
- 26.Lutchen KR, Gillis H. Relationship between heterogeneous changes in airway morphometry and lung resistance and elastance. J Appl Physiol (1985) 1997;83:1192–1201. doi: 10.1152/jappl.1997.83.4.1192. [DOI] [PubMed] [Google Scholar]
- 27.Benade AH. On the propagation of sound waves in a cylindrical conduit. J Acoust Soc Am. 1968;44:616–623. [Google Scholar]
- 28.Bhatawadekar SA, Leary D, Maksym GN. Modelling resistance and reactance with heterogeneous airway narrowing in mild to severe asthma. Can J Physiol Pharmacol. 2015;93:207–214. doi: 10.1139/cjpp-2014-0436. [DOI] [PubMed] [Google Scholar]
- 29.Cauberghs M, Van de Woestijne KP. Mechanical properties of the upper airway. J Appl Physiol. 1983;55:335–342. doi: 10.1152/jappl.1983.55.2.335. [DOI] [PubMed] [Google Scholar]
- 30.Gonem S, Berair R, Singapuri A, Hartley R, Laurencin MFM, Bacher G, et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med. 2016;4:699–707. doi: 10.1016/S2213-2600(16)30179-5. [DOI] [PubMed] [Google Scholar]
- 31.Russell RJ, Chachi L, FitzGerald JM, Backer V, Olivenstein R, Titlestad IL, et al. MESOS study investigators. Effect of tralokinumab, an interleukin-13 neutralising monoclonal antibody, on eosinophilic airway inflammation in uncontrolled moderate-to-severe asthma (MESOS): a multicentre, double-blind, randomised, placebo-controlled phase 2 trial. Lancet Respir Med. 2018;6:499–510. doi: 10.1016/S2213-2600(18)30201-7. [DOI] [PubMed] [Google Scholar]
- 32.Hoshino M. Comparison of effectiveness in ciclesonide and fluticasone propionate on small airway function in mild asthma. Allergol Int. 2010;59:59–66. doi: 10.2332/allergolint.09-OA-0122. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi M, Niimi A, Ueda T, Takemura M, Matsuoka H, Jinnai M, et al. Effect of inhaled corticosteroids on small airways in asthma: investigation using impulse oscillometry. Pulm Pharmacol Ther. 2009;22:326–332. doi: 10.1016/j.pupt.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Gonem S, Natarajan S, Desai D, Corkill S, Singapuri A, Bradding P, et al. Clinical significance of small airway obstruction markers in patients with asthma. Clin Exp Allergy. 2014;44:499–507. doi: 10.1111/cea.12257. [DOI] [PubMed] [Google Scholar]
- 35.Schulz H, Flexeder C, Behr J, Heier M, Holle R, Huber RM, et al. KORA Study Group. Reference values of impulse oscillometric lung function indices in adults of advanced age. PLoS One. 2013;8:e63366. doi: 10.1371/journal.pone.0063366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sorkness RL, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Chung KF, et al. National Heart, Lung, and Blood Institute Severe Asthma Research Program. Lung function in adults with stable but severe asthma: air trapping and incomplete reversal of obstruction with bronchodilation. J Appl Physiol (1985) 2008;104:394–403. doi: 10.1152/japplphysiol.00329.2007. [DOI] [PubMed] [Google Scholar]
- 37.Foy BH, Kay D. A computational comparison of the multiple-breath washout and forced oscillation technique as markers of bronchoconstriction. Respir Physiol Neurobiol. 2017;240:61–69. doi: 10.1016/j.resp.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 38.Kaczka DW, Ingenito EP, Suki B, Lutchen KR. Partitioning airway and lung tissue resistances in humans: effects of bronchoconstriction. J Appl Physiol (1985) 1997;82:1531–1541. doi: 10.1152/jappl.1997.82.5.1531. [DOI] [PubMed] [Google Scholar]
- 39.Su ZQ, Guan WJ, Li SY, Ding M, Chen Y, Jiang M, et al. Significances of spirometry and impulse oscillometry for detecting small airway disorders assessed with endobronchial optical coherence tomography in COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3031–3044. doi: 10.2147/COPD.S172639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Wiel E, ten Hacken NH, Postma DS, van den Berge M. Small-airways dysfunction associates with respiratory symptoms and clinical features of asthma: a systematic review. J Allergy Clin Immunol. 2013;131:646–657. doi: 10.1016/j.jaci.2012.12.1567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.