In theory, the application of extracorporeal membrane oxygenation (ECMO) in severe respiratory failure allows lung treatments varying from a lung at rest (continuous positive airway pressure) to all different levels of ventilatory support or even pure, spontaneous breathing. Although ECMO is increasingly used worldwide, very little is known about the respiratory settings applied during the course of ECMO, and even less is known about the optimal “balance” of ventilatory and extracorporeal support to minimize ventilator- or ventilation-induced lung injury, and the optimal conditions for lung healing and repair. In this issue of the Journal, Schmidt and coauthors (pp. 1002–1012) present an international, multicenter, prospective cohort study (LIFEGARDS [Ventilation Management of Patients with Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome]) in which data from 350 patients with ECMO in 23 international ICUs were collected during a 1-year period (1). In addition to demographics, the authors carefully compiled data regarding the ventilator settings applied before and during ECMO, the use of adjunctive therapies, and ICU and 6-month outcomes. The authors and their participating centers should be congratulated for providing the community with such sound data from different countries and ICUs, as well as the preferential ventilator settings used before and during the application of ECMO. The primary outcome measured was 6-month mortality, but the study also provides data on the type and use of adjunctive therapies, as well as the changes in driving pressure and mechanical power before and during the ECMO run. Some of these observational data are in part confirmatory and quite striking (2, 3). This study included only ICUs with an annual ECMO volume of more than 15 cases, and all of the participating centers treated a median of 30 patients with ECMO in the year before the study. Therefore, they could be clearly classified as “experienced.” In this context, it is more than striking that the prone position was not used in more than 26% of the patients, especially when a plateau pressure of 32 cm H2O was applied. Instead, the fact that a reported 15% of patients were turned to prone even during the ECMO course gives reason to hope that proning will be more regularly applied also in patients without ECMO. In contrast, with a Vt of 6.4 ± 2.0 ml/kg, patients were ventilated close to the magic “protective” value. However, the ventilatory setup as a whole led to a plateau pressure of 32 ± 7 cm H2O, a ventilatory rate of 26 ± 8, a driving pressure (ΔP) of 20 ± 7 cm H2O, and a mechanical power of 26 ± 12.7 J/min. It is interesting to note that after the ECMO initiation, while the reduction in DELTAP was only 30%, the reduction in mechanical power was as great as 75%, reflecting the importance of the frequency for energy transmission. With an overall 6-month survival of 61%, the study presents impressive outcome findings. The changes in respiratory settings after ECMO initiation resulted in both ΔP and power values below the thresholds that have been considered “critical” in both experimental and clinical studies (4–7). It is thus not surprising that the ventilator settings applied during the first 2 days after ECMO onset had no impact on survival, whereas age, immunocompromised state, extrapulmonary sepsis, and lactate and fluid balance—all of which could be considered indicators for the general severity of disease—were positively correlated. Given the ΔP and power values observed before ECMO was initiated, it is not unexpected that each day of delaying intubation to ECMO was also positively correlated with a higher 6-month mortality. Moreover, higher spontaneous respiratory rates during the first 2 days of ECMO were associated with higher 6-month mortality.
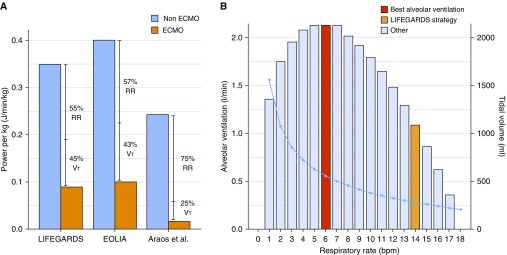
The strength of this study, which used data from different ICUs in 10 different countries, lies in the amount and quality of the data and the homogeneity of the treatment, including the use or nonuse of adjunctive measures. At the same time, this is also a limitation, as these data certainly do not reflect the real world of patients with ECMO treated in non-university hospitals or in hospitals with lower ECMO volumes and less experience in treating patients with severe respiratory failure and/or acute respiratory distress syndrome. In addition, the study describes how the patients were ventilated after the onset of ECMO, but it does not provide the reasons for the chosen partitioning between gas exchange across the native lungs and the artificial lung, or the rationale behind each specific ventilatory pattern. It is also unclear why a Vt of 3.7 ± 2.0 ml/kg ideal body weight and a respiratory rate of 14 ± 6, including 8 ± 11 spontaneous breaths at a positive end-expiratory pressure of 11 ± 3 cm H2O, was chosen. This study clearly identifies crucial questions for further research: how much unloading of the lungs is most beneficial for healing and repair, and what is the best composition (i.e., ventilatory pattern) of the chosen load? It seems reasonable to choose a ventilator setting that enables the greatest alveolar ventilation (i.e., the highest amount of CO2 removal) with the lowest price to pay (resulting power). A simplified mathematical approach makes it possible to determine for any given power the combination of Vt and frequency that will result in the highest alveolar ventilation (see Figure 1A). The ECMO settings applied will determine how low the power could theoretically become to reach equivalent CO2 removal. Figure 1B demonstrates the reduction in power achieved in LIFEGARDS, the EOLIA (Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome) trial (8), and the animal experiment by Araos and colleagues (9), with the goal of near-apneic ventilation. Ultimately, the question remains as to what creates the best conditions for an organ accustomed to rhythmically expanding and relaxing: more rest or more movement?
Figure 1.
(A) Mechanical power (MP) normalized per kilogram of body weight delivered during mechanical ventilation before and after onset of extracorporeal membrane oxygenation (ECMO) in the LIFEGARDS (Ventilation Management of Patients with Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome) and EOLIA (Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome) studies, as well as in the experimental study by Araos and colleagues (9), indicating a reduction (in percent) of MP attributed to the respiratory rate (RR) or the Vt. (B) We built a model for an MP (here we use the one delivered during ECMO in the LIFEGARDS study, 6.6 J/min) and a given dead space (200 ml) to establish the best combination of Vt and RR, with the aim of maximizing alveolar ventilation. Each column represents the alveolar ventilation at each different RR (left y-axis), and the light blue line represents the associated Vt (right y-axis). Positive end-expiratory pressure was kept constant (11 cm H2O) in this model, as were the airway resistances. bpm = breaths/min.
Schmidt and coauthors did a great job of letting us know where—at least in experienced centers—we actually are on this issue. The LIFEGARDS study provides a more than solid basis for us to move forward.
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.201906-1164ED on June 19, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Schmidt M, Pham T, Arcadipane A, Agerstrand C, Ohshimo S, Pellegrino V, et al. International ECMO Network (ECMONet), and the LIFEGARDS Study Group. Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome: an international multicenter prospective cohort. Am J Respir Crit Care Med. 2019;200:1002–1012. doi: 10.1164/rccm.201806-1094OC. [DOI] [PubMed] [Google Scholar]
- 2.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [Published erratum appears in JAMA 316:350.] [DOI] [PubMed] [Google Scholar]
- 3.Li X, Scales DC, Kavanagh BP. Unproven and expensive before proven and cheap: extracorporeal membrane oxygenation versus prone position in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2018;197:991–993. doi: 10.1164/rccm.201711-2216CP. [DOI] [PubMed] [Google Scholar]
- 4.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 5.Cressoni M, Gotti M, Chiurazzi C, Massari D, Algieri I, Amini M, et al. Mechanical power and development of ventilator-induced lung injury. Anesthesiology. 2016;124:1100–1108. doi: 10.1097/ALN.0000000000001056. [DOI] [PubMed] [Google Scholar]
- 6.Serpa Neto A, Deliberato RO, Johnson AEW, Bos LD, Amorim P, Pereira SM, et al. PROVE Network Investigators. Mechanical power of ventilation is associated with mortality in critically ill patients: an analysis of patients in two observational cohorts. Intensive Care Med. 2018;44:1914–1922. doi: 10.1007/s00134-018-5375-6. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z, Zheng B, Liu N, Ge H, Hong Y. Mechanical power normalized to predicted body weight as a predictor of mortality in patients with acute respiratory distress syndrome. Intensive Care Med. 2019;45:856–864. doi: 10.1007/s00134-019-05627-9. [DOI] [PubMed] [Google Scholar]
- 8.Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. EOLIA Trial Group, REVA, and ECMONet. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 9.Araos J, Alegria L, Garcia P, Cruces P, Soto D, Erranz B, et al. Near-apneic ventilation decreases lung injury and fibroproliferation in an acute respiratory distress syndrome model with extracorporeal membrane oxygenation. Am J Respir Crit Care Med. 2019;199:603–612. doi: 10.1164/rccm.201805-0869OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.