Abstract
Rationale: The impact of lung insult on the bone marrow (BM) and subsequent disease is unknown.
Objectives: To study alterations in the BM in response to lung injury/fibrosis and examine their impact on subsequent lung insult.
Methods: BM cells from control or bleomycin-treated donor mice were transplanted into naive mice, which were subsequently evaluated for bleomycin-induced pulmonary fibrosis. In addition, the effect of prior bleomycin treatment on subsequent fibrosis was examined in wild-type and B7H3-knockout mice. Samples from patients with idiopathic pulmonary fibrosis were analyzed for potential clinical relevance of the findings.
Measurements and Main Results: Recipient mice transplanted with BM from bleomycin-pretreated donors showed significant exacerbation of subsequent fibrosis with increased B7H3+ cell numbers and a T-helper cell type 2–skewed phenotype. Pretreatment with a minimally fibrogenic/nonfibrogenic dose of bleomycin also caused exacerbation, but not in B7H3-deficient mice. Exacerbation was not observed if the mice received naive BM cell transplant after the initial bleomycin pretreatment. Soluble B7H3 stimulated BM Ly6Chi monocytic cell expansion in vitro and caused similar expansion in the lung in vivo. Notably, soluble B7H3 was elevated in plasma of patients with idiopathic pulmonary fibrosis and in BAL fluid in those with acute exacerbation. Finally, ST2 deficiency diminished the bleomycin-induced B7H3 and IL-13 upregulation, suggesting a role for type 2 innate lymphoid cells.
Conclusions: Pulmonary fibrosis caused significant alterations in BM with expansion and activation of monocytic cells, which enhanced fibrosis when transplanted to naive recipients with potential mediation by a novel role for B7H3 in the pathophysiology of pulmonary fibrosis in both mice and humans.
Keywords: bleomycin, bone marrow transplantation, group 2 innate lymphoid cells, idiopathic pulmonary fibrosis, monocytes
At a Glance Commentary
Scientific Knowledge on the Subject
Disease in a distal organ may impact the bone marrow, which could in turn influence subsequent disease in the originally affected distal organ. This has not been studied in the context of bone marrow alterations in response to distal lung injury and fibrosis. To clarify the impact of pulmonary fibrosis on the bone marrow, bone marrow cells from control or bleomycin-treated donor mice were transplanted into naive recipient mice. Mice that received bone marrow transplanted from bleomycin-pretreated donors showed significant exacerbation of fibrosis upon subsequent bleomycin treatment. This was accompanied by increased numbers of lung B7H3+ cells and a T-helper cell type 2–skewed cytokine phenotype. Similar enhancement of fibrosis was noted in mice pretreated 9 or 24 weeks before with low-dose bleomycin. Interestingly, soluble B7H3 was significantly higher in plasma samples from patients with idiopathic pulmonary fibrosis than in control samples. Moreover, the concentrations of soluble B7H3 were significantly higher in BAL fluid samples from patients with progressive pulmonary fibrosis than in samples from those with stable disease.
What This Study Adds to the Field
These results suggested that pulmonary fibrosis caused significant alterations in bone marrow, which could promote fibrosis when bone marrow cells are transplanted to naive recipients with potential mediation by a novel role for B7H3 in the pathophysiology of pulmonary fibrosis in both mice and humans.
The potential importance of bone marrow (BM)-derived cells in fibrotic interstitial lung diseases, such as idiopathic pulmonary fibrosis (IPF), is suggested by animal model studies (1–4). This is not unique to the lung, because myocardial infarction alters the BM cells, resulting in impaired efficacy when used in BM cell therapy after myocardial infarction (5). In patients with refractory autoimmune diseases, improvement in pulmonary fibrosis occurs after bone marrow transplant (BMT) (6). This use of BMT is based on the idea of “resetting of dysregulated immune systems” (7). Although distal organ injury can cause alterations in BM cells (8, 9), the altered BM in turn can also affect disease in distal organs (10–12). Thus, distal organ insult may alter the BM cells and perhaps prime them for an enhanced response to subsequent stimuli in the same organ. Potential alterations in BM cells in response to distal lung insult and their impact on subsequent lung insult and fibrosis require further elucidation.
Members of the B7 family, identified as T-cell costimulatory molecules, are believed to be important regulators of the immune system (13). To date, several B7 homologs (B7Hs) and their receptors have been identified. Recently, B7H1/CD274/programmed cell death ligand 1 and programmed cell death protein 1 pathways have been spotlighted as an important regulator of tumor immunity (14, 15). However, the role of the B7 family in nonmalignant diseases is not fully understood. Among them, B7H3/CD276/B7RP2 has been implicated in regulation of the immune system (16), including in allergic disease (17–19) and graft-versus-host disease (20). The existence of a soluble form of B7H3 (sB7H3) has been reported and is purportedly released via matrix metalloproteinase–dependent cleavage of the membrane-bound form (21), although a more recent study suggests derivation by alternative splicing (22). Immunoregulation by B7H3 is in part mediated by an enhancing effect on T-helper cell type 2 (Th2) immune response with suppression of Th1 response, thus resulting in a net Th2 polarization (17, 18, 23). Although the Th2 cytokine IL-13 has been implicated in fibrosis (24, 25), the potential importance of B7H3 in pulmonary fibrosis is unknown, but it is suggested by the noted elevated expression of B7H3 in lung and blood samples from patients with IPF with progressive disease relative to samples from subjects with relatively stable disease (26).
On the basis of these previous observations, we hypothesize that the lung response to an initial insult, such as bleomycin (BLM) treatment, alters the phenotype of BM cells such that they will contribute to exacerbation of pulmonary fibrosis upon subsequent lung insult. The objectives of this study were to clarify the importance of these altered BM cells and B7H3 expression with Th2 skewing using the murine model of BLM-induced pulmonary fibrosis and to assess the potential relevance of B7H3 upregulation to human IPF.
Methods
Animals
All mice were housed in the university laboratory animal facility under animal protocols approved by the institutional animal care and use committee at the University of Michigan. Pulmonary fibrosis was induced by endotracheal injection of BLM as previously described (27–29). BMT was performed as reported elsewhere (1, 3). Additional details are provided in the online supplement. In vivo treatment with sB7H3 (R&D Systems) was undertaken by intravenous injection via the tail vein with a loading dose of 150 μg/kg followed by half the loading dose every other day until mice were killed on Day 4 after initial injection. These doses were selected on the basis of previous in vivo studies using comparable doses (125–165 μg/kg, assuming 20-g body weight) (30, 31). The lungs were removed for enumeration of CD11b+ and Ly6Chi cells by flow cytometry. For analysis of fibrosis, the lungs were analyzed for Col1a2 (collagen type I, α2 chain), Acta2, and Tgfb1 (transforming growth factor-β1 [TGF-β1]) mRNA by quantitative PCR at 3 weeks after the last dose of sB7H3.
Flow Cytometry and Cell Sorting
Flow cytometric analyses of lung cells obtained by enzymatic dissociation of lung tissue were performed as previously described (1). Additional details are provided in the online supplement.
Real-Time Quantitative PCR
RNA extraction, reverse transcription to cDNA, and real-time quantitative PCR were performed as described previously (1). Additional details are provided in the online supplement.
Histological Analysis and Immunofluorescence Staining
Harvested murine lungs were inflated and fixed with 4% paraformaldehyde, then embedded in paraffin. After cutting the lungs into 5-μm sections, we stained them with hematoxylin and eosin or Masson’s trichrome. Human lung tissue sections were antigen retrieved in 10 mmol/L sodium citrate (pH 6.0) using microwaves for 20 minutes, followed by staining with mouse antihuman B7H3 (DCN.70; BioLegend) as primary antibody and with antimouse IgG-NL577 (NL007; R&D Systems) as secondary antibody. Mounting medium containing DAPI (Vector Laboratories) was applied, and the sections were examined with a Nikon Eclipse E600 fluorescence microscope (Nikon) using the Nuance 3.0.2 multispectral imaging system (PerkinElmer, Inc.).
Hydroxyproline Assay
Lung hydroxyproline content was measured using left lung homogenates as described previously (27, 28).
Subjects
BAL fluid (BALF) samples were obtained from 20 consecutive newly diagnosed patients with IPF (19 males; aged 66.5 ± 7.0 yr) who underwent BAL at Hiroshima University Hospital between January 2009 and December 2013. The diagnosis and acute exacerbation of IPF were based on published guidelines or criteria (32, 33). The study protocol was approved by the institutional review board of Hiroshima University (IRB no. 326). Written informed consent was obtained from each subject for the use of clinical samples. Deidentified plasma samples and lung tissue sections from patients with IPF and control subjects were obtained from the NIH Lung Tissue Research Consortium. The control sample donors ranged in age from 43 to 72 years (three males and nine females), and the IPF sample donors ranged in age from 56 to 79 years (six males and four females).
Statistical Analysis
All experiments were performed two or three times, and representative data or pooled data from repeat experiments were recorded. Data were shown as median ± interquartile range, with inclusion of 90th and 10th percentiles in some graphs. Differences between groups were analyzed using the Kruskal-Wallis test for median values. Correlation coefficients for parameters were calculated using the Spearman’s rank correlation coefficient analysis. To evaluate exacerbation-free days, Kaplan-Meier analysis and the log-rank test were used. P values less than 0.05 were considered significant. All analyses were performed using the JMP software package (version 12; SAS Institute Inc.).
Results
Transplant of BM from BLM-pretreated Donors Enhanced BLM-induced Pulmonary Fibrosis in Recipients
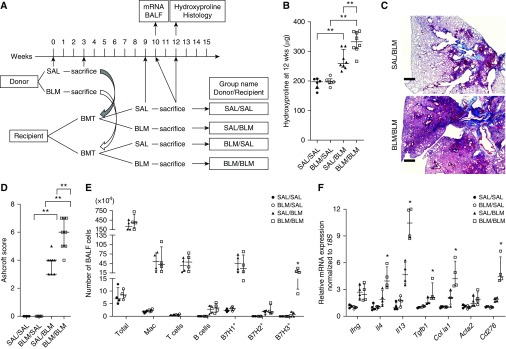
To evaluate if BLM insult to the lung alters the BM, we performed BMT using BLM-pretreated donors (Figure 1A). BMT of BM from BLM-pretreated donors plus subsequent saline (SAL) treatment did not induce pulmonary fibrosis. However, mice that received BM transplant from BLM-pretreated donors showed significant worsening of BLM-induced pulmonary fibrosis relative to mice that received BM transplant from SAL-pretreated donors (Figure 1B). Histopathology with Ashcroft scoring confirmed enhanced pulmonary fibrosis (Figures 1C and 1D). BAL cell analyses revealed significant increases in B7H3-expressing cells, but not B7H1- or B7H2-expressing cells, in the BLM/BLM group compared with the SAL/BLM group (Figure 1E). Quantitative PCR analysis revealed significantly increased lung Th2 cytokines (IL4 and IL13), TGF-β1 (Tgfb1), type I collagen (Col1a1), and B7H3 (Cd276) mRNAs in the BLM/BLM group compared with the SAL/BLM group (Figure 1F). Thus, lung BLM pretreatment in donors altered their BM cells in a manner that could exacerbate BLM-induced pulmonary fibrosis in the naive recipients in association with upregulation of B7H3 expression and B7H3+ cell recruitment as well as Th2 immune response.
Figure 1.
Bleomycin (BLM)-induced pulmonary fibrosis in recipients transplanted with bone marrow (BM) cells from BLM- or saline (SAL)-pretreated donors. (A) Experimental scheme and group naming. Recipients received BM cell transplants from BLM- or SAL-pretreated donors at the indicated time points and evaluated as indicated at the specified time points. (B) Lung hydroxyproline content in each group (n = 6–8/group). **P < 0.01. (C) Masson’s trichrome–stained lung tissue obtained 3 weeks after BLM treatment (or 9 wk after bone marrow transplant) in recipient mice. (D) Ashcroft scores in each group (n = 5–8/group) were obtained from analysis of histological sections as described in C. **P < 0.01. (E) Flow cytometric analysis of cell surface markers in BAL fluid (BALF) cells obtained 1 week after SAL or BLM treatment in recipients (n = 4/group). *P < 0.05 versus SAL/BLM group. (F) Real-time PCR analysis for the indicated lung mRNAs in recipients at 1 week after treatment (n = 4/group). Measured mRNA concentrations were expressed relative to internal control 18S mRNA concentration and normalized to the lowest value in the analyzed groups (which equaled 1). Scale bars, 400 μm. *P < 0.05 versus SAL/BLM group. BMT = bone marrow transplant; Mac = macrophages.
Monocyte Lineage, but Not Hematopoietic Stem Cells, in Donor BM Mediated the Exacerbation of Fibrosis in Recipient Mice
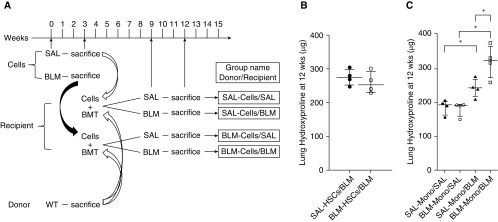
To determine which cell population in donor BM contributed to the enhanced BLM-induced fibrosis in recipients, we sorted BM cell populations from SAL- or BLM-pretreated mice into hematopoietic stem cells (HSCs) (cKit+/Sca1+/Lin−) and monocytic fractions (Figure 2A). After depletion of differentiated blood cells positive for CD3ε, CD45R/B220, NK1.1, Ly6G, and CD49b using a monocyte isolation kit, the monocytic fraction consisted primarily (>90%) of Ly6C+ monocytic cells (Figure E1 in the online supplement; see Methods section of the online supplement for details). The monocytic fraction but not HSCs obtained from BLM-pretreated donor BM significantly exacerbated the BLM-induced pulmonary fibrosis in recipients (Figures 2B and 2C). Thus, exacerbation of fibrosis by donor BM from BLM-pretreated mice was mediated by the monocytic population.
Figure 2.
Bone marrow (BM) cell transplant (BMT) from bleomycin (BLM)- or saline (SAL)-pretreated mice affects pulmonary fibrosis. (A) Experimental scheme and group naming. Recipients received transplants of normal BM cells (from naive donors) together with the indicated sorted cells obtained from BLM- or SAL-pretreated mice and then analyzed as described at the indicated time points. (B) Lung hydroxyproline content in recipients of transplanted normal BM cells together with sorted hematopoietic stem cells (HSCs) from SAL- or BLM-pretreated donors (n = 4/group). (C) Lung hydroxyproline content in recipients of transplanted normal BM cells together with sorted monocytic cells (Mono) from SAL- or BLM-pretreated donors (n = 4/group). *P < 0.05. WT = wild type.
BLM Pretreatment Caused Exacerbation of Fibrosis and Induction of B7H3
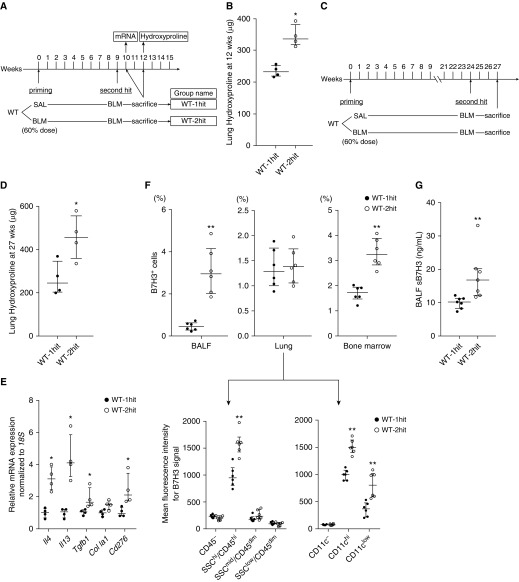
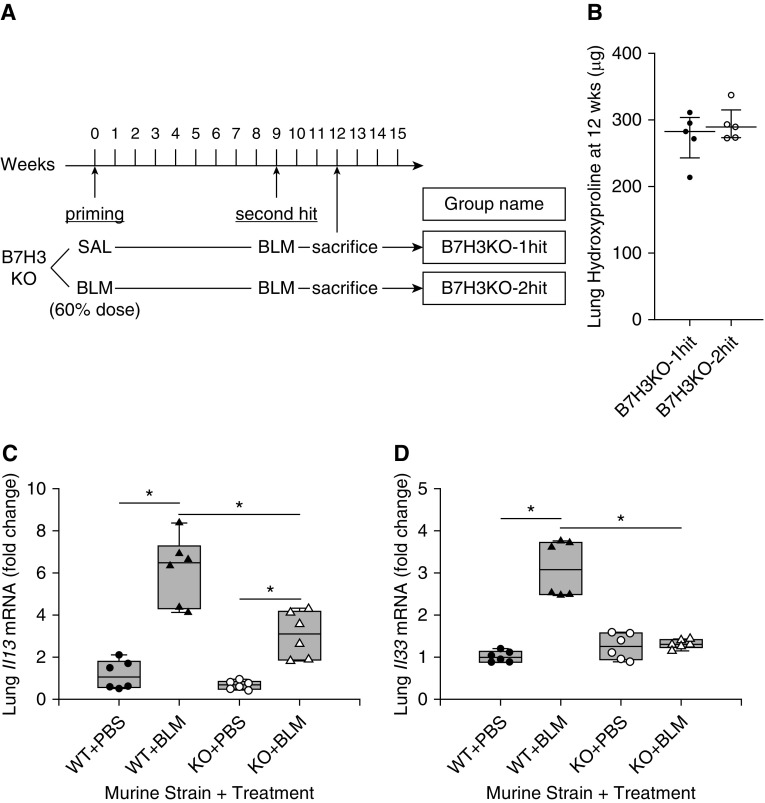
To rule out irradiation or BMT as the cause of exacerbation, the effects of BLM pretreatment on subsequent BLM challenge were evaluated in the same mouse (Figure 3A). In this model, initial low-dose BLM treatment (60% of normal dose) caused insignificant fibrosis 3 or 6 weeks later (Figure E2A), although the numbers of Ly6Chi cells and other inflammatory cells, including CD11+F4/80+ macrophages and CD3+ T cells, were increased in both BALF and lung tissue at 1 week after treatment (Figures E2B–E2D). However, this low-dose BLM treatment did not cause a detectable change in the BM Ly6Chi population at this time point (Figure E2E). Upon subsequent treatment with a full dose of BLM at 9 weeks after pretreatment, hydroxyproline measurement (Figure 3B) revealed enhanced fibrosis relative to the group receiving SAL pretreatment. The exacerbation effect caused by low-dose BLM pretreatment persisted for at least 6 months after pretreatment (Figures 3C and 3D). The BLM-pretreated group demonstrated significantly upregulated mRNA expression for Th2 cytokines (IL4 and IL13), TGF-β1 (Tgfb1), and Cd276 upon subsequent treatment with BLM (Figure 3E). The percentage of B7H3+ cells was also significantly increased in BALF and BM but not in lung tissue of BLM-pretreated mice (Figure 3F). However, when lung cells were further analyzed on the basis of side scatter and CD45 or CD11c expression, the mean fluorescence intensity for B7H3 was significantly upregulated on SSChi/CD45hi cells (Figure 3F, lower left panel) and CD11c+ cells (Figure 3F, lower right panel). Mean fluorescence intensity for B7H3 did not differ when gated on B cells or T cells (data not shown). BALF from the two-hit group had significantly higher concentrations of sB7H3 protein than that from the one-hit group (Figure 3G). Thus, the exacerbation of fibrosis noted with BLM pretreatment was also associated with enhanced B7H3+ cell recruitment and Th2 immune response.
Figure 3.
Effect of bleomycin (BLM) pretreatment on subsequent BLM-induced pulmonary fibrosis in wild-type (WT) mice. (A) Experimental scheme and group naming. Mice were pretreated with a reduced dose (60% of normal dose) of BLM at Time 0. After the indicated time intervals, mice were then treated with the normal dose of BLM. (B) Lung hydroxyproline content at 3 weeks after the subsequent full-dose BLM treatment (n = 4/group). *P < 0.05 versus WT one-hit group. (C) Modified experimental scheme with extended time interval after BLM pretreatment. (D) Lung hydroxyproline content at 3 weeks after the subsequent full-dose BLM treatment (n = 4/group). *P < 0.05 versus WT one-hit group. (E) Real-time PCR analysis for indicated lung mRNAs at 1 week after the subsequent full-dose BLM treatment in WT one-hit and WT two-hit groups (n = 4/group). Measured mRNA concentrations were expressed relative to internal control 18S mRNA concentration and normalized to the value in WT one-hit group (which equaled 1). *P < 0.05 versus WT one-hit group. (F) Flow cytometric analysis for B7H3 expression on cells obtained from BAL fluid (BALF), lung tissue, and bone marrow (upper panel) at 3 weeks after the full-dose BLM treatment in WT one-hit and WT two-hit groups (n = 6/group). The percentages of total cells are shown. For lung tissue, total lung cells were divided according to side scatter (SSC) and CD45 or CD11c expression, then further analyzed for each mean fluorescence intensity for B7H3 signal (lower panel). **P < 0.01 versus WT one-hit group. (G) BALF concentrations of soluble B7H3 protein at 3 weeks after the subsequent full-dose BLM treatment in WT one-hit and WT two-hit groups (n = 7/group). **P < 0.01 versus WT one-hit group. SAL = saline.
Exacerbation Effect of BLM Pretreatment Was Abolished by BMT Using BM from Naive Donors
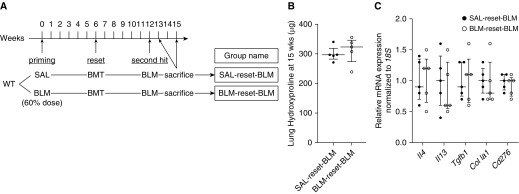
In the preceding experiments, BLM pretreatment caused exacerbation of fibrosis, but it is unclear if the effect was mediated by alterations in the lung or BM caused by the pretreatment. To help distinguish between these two possibilities, we performed BMT using BM from naive mice at 6 weeks after the BLM pretreatment (i.e., to “reset” the BM) (Figure 4A). The results showed that resetting the BM using naive donor BM abolished the exacerbation due to BLM pretreatment (Figure 4B). Exacerbation effects with respect to Th2 immune response and B7H3 expression were also abolished (Figure 4C).
Figure 4.
Bone marrow transplant (BMT) using bone marrow (BM) from naive donors abolished exacerbation of fibrosis by bleomycin (BLM) pretreatment. (A) Experimental scheme and group naming. Mice received transplanted BM cells from naive donors (“reset”) after the initial priming BLM treatment (60% of normal dose; control mice received saline [SAL] alone). The recipient mice were subsequently treated with full-dose BLM at the indicated time point. (B) Lung hydroxyproline content at 3 weeks after the full-dose BLM treatment (n = 5/group). (C) Real-time PCR analysis for the indicated lung mRNAs at 1 week after the full-dose BLM treatment in SAL reset BLM and BLM reset BLM groups (n = 5/group). Measured mRNA concentrations were expressed relative to internal control 18S mRNA concentration and normalized to the value in the SAL reset BLM group (which equaled 1). WT = wild type.
B7H3 as a Key Molecule for Exacerbation of Pulmonary Fibrosis in BLM-pretreated Mice
To determine if upregulated B7H3 was essential for exacerbation of pulmonary fibrosis in BLM-pretreated mice, the effects of B7H3 deficiency were evaluated (Figure 5A). The results showed that exacerbation of BLM-induced pulmonary fibrosis in BLM-pretreated wild-type (WT) mice was absent in B7H3-knockout (B7H3-KO) mice (Figure 5B). Interestingly, in a separate experiment, single full-dose BLM-induced lung IL-13 expression was significantly diminished in B7H3-deficient mice (Figure 5C). Because type 2 innate lymphoid cells (ILC2) are a significant source of IL-13 in BLM-induced pulmonary fibrosis (34, 35), the effect of B7H3 deficiency on expression of IL-33, a key activator of ILC2, was also examined. The results showed similar profound diminution of IL-33 induction by BLM in B7H3-deficient mice (Figure 5D). Thus, B7H3 induction played a significant role in mediating the Th2 skewing associated with BLM-induced pulmonary fibrosis.
Figure 5.
Effect of bleomycin (BLM) pretreatment on subsequent BLM-induced pulmonary fibrosis in B7H3-knockout (B7H3 KO) mice. (A) Experimental scheme and group naming. The experimental protocol is identical to that in Figure 3, except that B7H3 KO mice were used instead of wild-type (WT) mice. (B) Lung hydroxyproline content at 3 weeks after the subsequent full-dose BLM treatment (n = 5/group). (C) Lung Il13 and (D) Il33 mRNA analysis by real-time PCR at 1 week after saline (SAL) or full-dose BLM treatment in WT or B7H3 KO mice. Results were expressed as fold change relative to the SAL-treated WT group mean value (n = 6/group). Box plots show the median and interquartile range, and the lower and upper bars denote the 10th and 90th percentiles, respectively. PBS = phosphate-buffered saline. *P < 0.05 between the two indicated groups.
Effect of sB7H3 on Induction of Monocytic Cells in BM
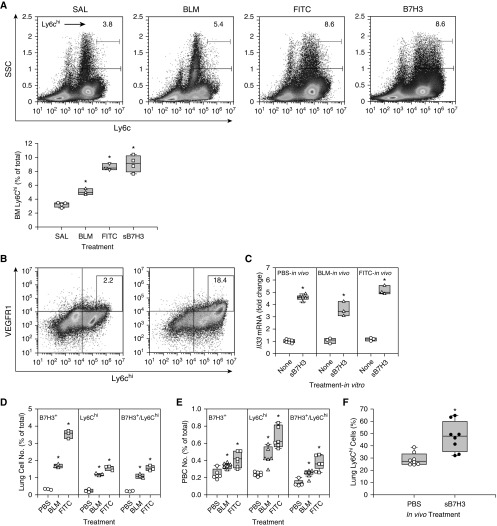
Elevated BALF sB7H3 (Figure 3G) concentrations were associated with the significantly increased numbers of B7H3+ cells in the lung and BM (Figures 1E and 3F), which suggested a potential role in mediating the alteration in the BM in response to prior lung insult. To evaluate this possibility, the effects of sB7H3 on BM monocytic cells (Ly6C+) were compared with alterations in BM cells from Day 7 SAL-, BLM-, or fluorescein isothiocyanate (FITC)-treated mice. The FITC model was included to see if any effect observed was unique to the BLM model. The results revealed that sB7H3 caused a significant increase in Ly6Chi cells comparable to that caused by in vivo BLM or FITC treatment in the lung (Figure 6A), although the lower priming dose of BLM alone failed to have a significant effect (Figure E2E). In addition, vascular endothelial growth factor receptor 1 (VEGFR1)-expressing Ly6Chi cell number was increased almost ninefold after sB7H3 treatment (Figure 6B), indicating the potential activation of BM monocytic cells recruited in the BLM model (1, 3, 4). Moreover, sB7H3 stimulated expression of IL-33, a key inducer of ILC2 differentiation in differentiated CD11c+-enriched BM cells (Figure 6C). IL-33 was also induced in BM cells by in vivo BLM or FITC treatment, which was further enhanced by in vitro treatment with sB7H3. These BM alterations were accompanied by increased numbers of lung B7H3+ or Ly6Chi cells, as well as B7H3+/Ly6Chi cells (Figure 6D) and peripheral blood (Figure 6E) of BLM- or FITC-treated animals. Moreover, in vivo treatment with sB7H3 alone caused a significant increase in lung Ly6Chi cells (Figure 6F) but not fibrosis (data not shown). These findings suggested that sB7H3, perhaps derived from injured lung, could signal to the BM to cause alterations in the BM monocytic cells, leading to the noted enhancement of fibrosis upon subsequent lung insult. The comparable results in the FITC model indicated that the noted changes were not unique to the BLM model.
Figure 6.
Effect of soluble B7H3 (sB7H3) on bone marrow (BM) monocytic cells. (A) BM cells isolated from saline (SAL)-treated (control), bleomycin (BLM)-treated, or fluorescein isothiocyanate (FITC)-treated mice and naive BM cells (from untreated mice) treated with 5 μg/ml sB7H3 for 24 hours were analyzed for Ly6C expression by flow cytometry. The quantitative data are shown on the right panel. All box plots show the median and interquartile range, and the lower and upper bars denote the 10th and 90th percentiles, respectively. *P < 0.05 from the respective SAL or PBS controls. (B) sB7H3-treated control BM cells described in A were analyzed for VEGFR1 and Ly6Chi double-positive cells. Representative plots from three separate analyses are shown in A (left panel) and B. (C) SAL BM cells obtained from SAL-, BLM-, or FITC-treated mice were treated with 10 ng/ml granulocyte–macrophage colony–stimulating factor for 5 days and then further treated with or without sB7H3 for another 24 hours. IL-33 mRNA expression was analyzed by real-time PCR (n = 3–6). (D) Whole-lung cell suspension or (E) peripheral blood cells (PBC) obtained from SAL-, BLM-, or FITC-treated mice were analyzed for B7H3+, Ly6Chi, and double-positive cells by flow cytometry (n = 3–6). (F) Mice were treated with sB7H3 by intravenous injection and analyzed for lung Ly6Chi cell population by flow cytometry. Data were expressed as percentage of CD11b+ cells (n = 8). PBS = phosphate-buffered saline; SSC = side scatter. *P < 0.05 compared with respective untreated or PBS controls in C–F.
sB7H3 in Human IPF
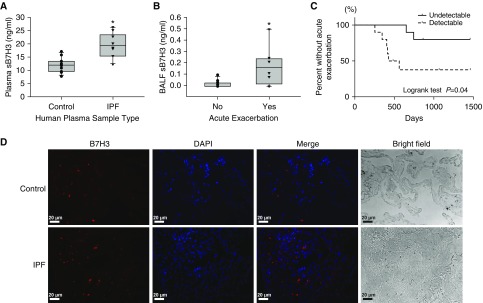
To relate the potential significance of the B7H3 findings in murine models to human IPF, we measured concentrations of plasma or BALF sB7H3 in healthy human subjects or patients with IPF. The plasma concentrations of sB7H3 in IPF were significantly induced compared with in healthy subjects (Figure 7A). Interestingly, patients with IPF who developed acute exacerbation in their clinical course showed significantly higher concentrations of sB7H3 in their BALF at diagnosis than concentrations patients without acute exacerbation (Figure 7B). Similarly, compared with patients with IPF with undetectable sB7H3 in BALF, patients with IPF who showed detectable concentrations of sB7H3 developed acute exacerbation more frequently in their clinical course (Figure 7C). Thus, the detection and concentrations of sB7H3 in BALF were associated with acute exacerbation in IPF. Furthermore, immunostaining of control or IPF lung tissue sections for B7H3 protein expression revealed that B7H3-positive signals were more frequently observed in IPF than in control lungs (Figure 7D), suggesting that B7H3-expressing cells may be induced in human IPF lung.
Figure 7.
B7H3 was induced in human plasma, BAL fluid (BALF), and lung tissue. (A) Plasma concentrations of soluble B7H3 (sB7H3) in healthy control subjects (n = 12) and patients with idiopathic pulmonary fibrosis (IPF) (n = 10). *P < 0.05. (B) BALF concentrations of sB7H3 in patients with IPF who did (n = 8) or did not (n = 12) develop acute exacerbation in their clinical course. *P < 0.05. Box plots show the median and interquartile range, and the lower and upper bars denote the 10th and 90th percentiles, respectively, in A and B. (C) Kaplan-Meier analysis of frequency of acute exacerbation in patients with IPF. The proportion of patients with IPF without acute exacerbations were compared between patients whose BALF concentrations of sB7H3 were detectable (n = 10; dashed line) or undetectable (n = 10; solid line). (D) The immunofluorescence staining of paraffin-embedded human lung tissue sections obtained from control subjects or patients with IPF is shown. B7H3 signals are shown in red and nuclei in purple-blue with DAPI. Representative single and merged images are shown. Scale bars, 20 μm.
IL-33/ST2 Signaling Regulation of B7H3 Effect on BM Cells
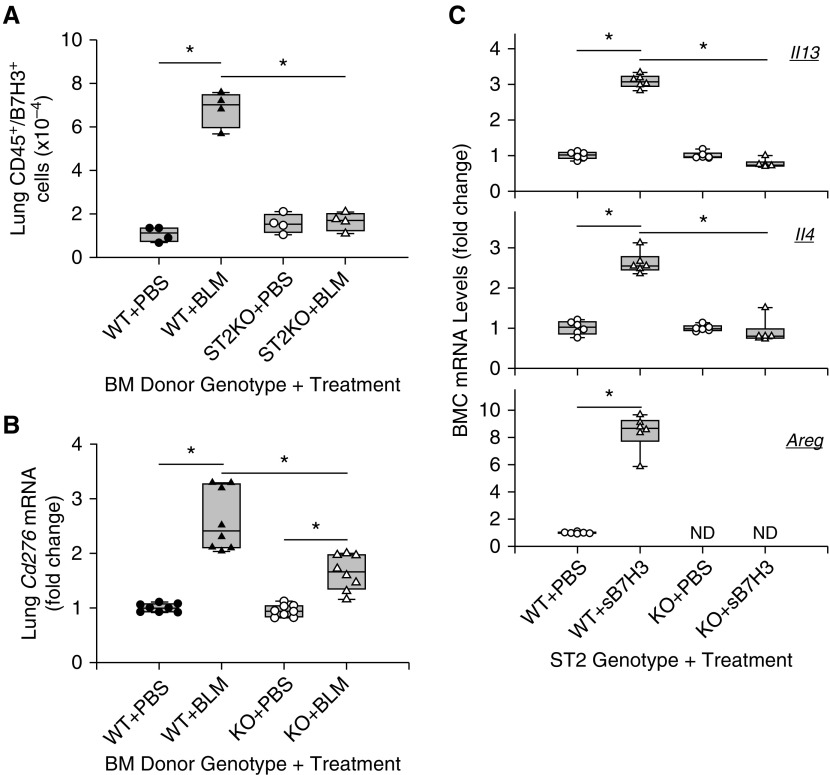
IL-33/ST2 signaling is activated in the WT fibrotic lung and was markedly diminished in B7H3-KO lung (Figure 5D), whereas sB7H3 enhanced IL-33 expression in BM cells (Figure 6C). To see if IL-33/ST2 signaling mediated the sB7H3 effects on the BM compartment, we assessed the effects of BM ST2 deficiency using BM ST2-KO chimera mice. The results revealed that the number of lung CD45+/B7H3+ cells was significantly increased in mice that received WT BM transplant at 7 days after BLM treatment (Figure 8A). In contrast, this increase was completely abrogated in mice that received ST2-KO BM transplant (BM ST2-KO chimera) (Figure 8A), which was accompanied by a significant reduction of BLM-induced increase in lung B7H3 mRNA concentration compared with that in mice that received WT BM (Figure 8B). Thus, deficiency of ST2 in the BM had a significant impact on the induction of lung B7H3 expression and the expansion of the B7H3+ myeloid (CD45+) cell population in BLM-induced pulmonary fibrosis. Because ST2 is essential for ILC2 differentiation and activation, we investigated the impact of ST2 deficiency on sB7H3-induced alterations in lineage-negative BM cells. The results showed that sB7H3 caused significant induction of IL-13, IL-4, and amphiregulin mRNA in WT lineage-negative BM cells, which was essentially abolished (for IL-13 and IL-4) or was not detectable (for amphiregulin) in ST2-KO BM cells (Figure 8C). These observations suggested that IL-33/ST2 signaling activation might participate in B7H3 mediation of fibrosis enhancement/exacerbation by increasing elaboration of Th2 and fibrogenic cytokines in the BM, as well as in induction of lung B7H3 expression with expansion of the lung B7H3+ myeloid cell population.
Figure 8.
ST2 effect on B7H3 induction and function. Mice received transplants with wild-type (WT) or ST-knockout (ST2KO) bone marrow (BM), and, after stable engraftment, they were treated with phosphate-buffered saline (PBS) or bleomycin (BLM). Box plots show the median and interquartile range, and the lower and upper bars denote the 10th and 90th percentiles, respectively. (A) The number of lung CD45+/B7H3+ cells was assessed by flow cytometry (n = 4). (B) Lung tissue B7H3 (Cd276) mRNA was analyzed by real-time PCR, and the results are expressed as fold change from the SAL-treated group with WT BM (n = 8/group). (C) Lineage-negative bone marrow cells (BMC) from SAL WT or ST2KO mice were treated with or without 5 μg/ml sB7H3 for 48 hours, and they were then analyzed for the indicated mRNA by real-time PCR. Results are shown as fold changes from their respective untreated controls (n = 6/group). *P < 0.05 between the two indicated groups.
Discussion
The present study showed that the BLM lung insult caused significant and stable alterations in BM cells to enhance fibrogenic responses to a subsequent lung insult. The exacerbation effect was mediated by the monocytic population, but not HSCs, in the BMT model. With use of the two-hit model, an exacerbation effect on BLM-induced pulmonary fibrosis was also noted when WT mice, but not B7H3-KO mice, were pretreated with BLM up to as long as 6 months earlier. In both experimental situations, the BLM pretreatment altered BM cells to potentially heighten their responsiveness in response to the forthcoming BLM treatment, in association with enhancement of both BLM-induced B7H3 expression and Th2-skewed phenotype. Notably, exacerbation was mediated by the monocytic population in the BMT model. The exacerbation of pulmonary fibrosis by BLM pretreatment was abolished by BMT using BM from naive donor mice before the second BLM insult. Taken together, these findings suggested that injury/fibrosis in a distal organ, such as the lung, could impact the BM compartment, inducing alterations in the monocytic population that enabled monocytes to exacerbate injury/fibrosis upon reinjury of the same animal or in recipient naive mice that received transplants of this altered population of BM cells in a B7H3-dependent manner and associated with Th2 skewing. Potential clinical relevance was suggested by the detection of B7H3 in plasma, BALF, and lung sections from patients with IPF. BALF sB7H3 may be of prognostic significance and/or value as a biomarker for disease progression in IPF.
BM alterations have also been reported in response to myocardial injury (5). Healthy donor BM cells improved myocardial infarction in recipient mice, but those from donors after myocardial infarction had diminished therapeutic effect. Although our findings confirmed BM alterations in response to distal organ injury, the nature of the alterations and the mechanism of enhanced response to repeat insult are unclear. The upregulation of B7H3 expression and Th2-skewed phenotype offer some clues. Because B7H3 can downregulate Th1-mediated immune response (23), it is reasonable to infer that B7H3 contributes to the Th2 skewing. Furthermore, anti-B7H3 antibody treatment reduces Th2 cytokine production in a murine asthma model (17). Induction of lung IL-13 in BLM-induced pulmonary fibrosis was in part dependent on BM ST2 expression, which is implicated in the expansion of lung ILC2 (36). ST2-expressing ILC2 is implicated in tissue repair and fibrosis (37–39), in part by producing Th2 cytokines IL-13, IL-4, and amphiregulin (35, 40). Moreover, ILC2 has recently been reported to have memory-like properties (41), making it a suitable candidate to mediate the persistence/stability of the BM alterations induced by distal lung insult. Furthermore, sB7H3-induced Th2 cytokine expression was ST2 dependent, thus implicating ILC2. Because sB7H3 also induced the ST2 ligand, IL-33, a product of monocytic cells, including dendritic cells and macrophages (37, 42), the induction/exacerbation of pulmonary fibrosis could be initiated by sB7H3-mediated induction of IL-33 expression by monocytic cells with consequent ILC2 differentiation/activation manifested by induction of IL-13. Interestingly BLM-induced lung B7H3 expression and expansion of B7H3+ myeloid cells also depend on intact ST2 expression in the BM, thus suggesting a potential positive feedback loop by which B7H3 induces IL-13 in an ST2-dependent manner, which in turn promotes lung B7H3 expression, and so forth. Further studies are warranted to elucidate the detailed mechanism. Thus, at least one potential effect of the upregulated B7H3 in altered BM-induced exacerbation of fibrosis is activation of a Th2 response, which is implicated in pulmonary fibrosis (43, 44).
The association of B7H3 with exacerbation of BLM-induced pulmonary fibrosis was also observed in BLM-pretreated mice, an approach that was independent of the irradiation or BMT effects intrinsic to transplanting BM from BLM-treated donors. In the model using repeated BLM treatments, more sustained fibrosis was observed (45) owing to the accumulated cytotoxicity of BLM on epithelial cells (46). Our findings suggest that the repetitive model could also be due to the potential effects of prior BLM treatment(s) on the BM compartment. Enhanced pulmonary fibrosis induced by repetitive BLM exposure is associated with both reduced endothelial CXCR7 expression and recruitment of VEGFR1-expressing macrophages (47). Indeed, the present study showed sB7H3-induced expansion of BM Ly6Chi monocytic cells in vitro and in the lung in vivo, which is reported to promote the progression of pulmonary fibrosis (48), and their VEGFR1 expression is consistent with this previous observation regarding the importance of these recruited VEGFR1+ macrophages in pulmonary fibrosis. Priming with low-dose BLM also caused inflammatory changes in the lung, including influx of Ly6Chi cells but no detectable change in BM Ly6Chi cell number, suggesting that cell activation and/or Ly6C− populations might be involved in BM cell priming. Indeed, previous studies suggest the importance of BM-derived Ly6C− lung hematopoietic progenitor cells and segregated nucleus–containing atypical monocytes in lung fibrosis (1, 4). This may represent an underlying mechanism by which B7H3 facilitates the progression of pulmonary fibrosis via mediating BM monocytic cell alteration/activation and recruitment (Figure 9). The alterations in B7H3, Th2 skewing, and Ly6Chi cells were also seen in the FITC model, indicating that these effects were not unique to the BLM model. In the absence of information on sB7H3 pharmacokinetics, a relatively high dose of sB7H3 was used to document its in vivo effects, which might not be in the physiological range. Despite this high dose, sB7H3 alone was insufficient to cause fibrosis. However, its association with and ability to cause an expansion in BM Ly6Chi monocytic cells (Figures 6A and 6B), together with increased profibrogenic cytokine expression (Figures 6C, 6F, and C8C), could result in enhanced fibrosis when combined with a lung insult (i.e., second hit). Furthermore, the expression and effects of sB7H3 might be enhanced upon BLM treatment (Figures 5C, 5D, 6A, 6D, and E6E), resembling a two-hit model of pathogenesis.
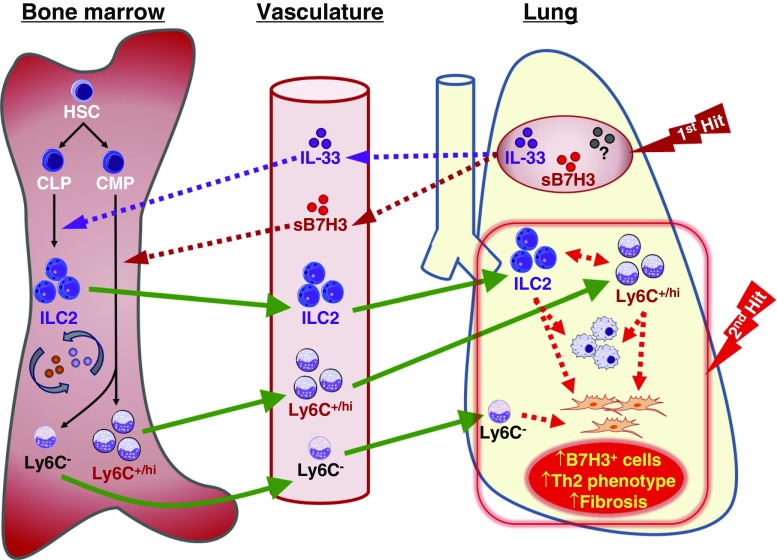
Figure 9.
Schematic summary of the alteration in bone marrow cells and B7H3/IL-33 signaling in pulmonary fibrosis. Initial injury or first hit in the lung causes elevated concentrations of soluble B7H3 (sB7H3) and other mediators, such as IL-33, etc. (?), to be detected in the lung and plasma. These factors (sB7H3 and IL-33 are shown) are hypothesized to stably influence/alter bone marrow (BM) progenitor cell (e.g., hematopoietic stem cells [HSC], common lymphoid progenitors [CLP], and common myeloid progenitors [CMP]) differentiation (BM priming) (e.g., IL-33–mediated activation of type 2 innate lymphoid cell [ILC2] development from CLP) and sB7H3 promotion of myeloid cell differentiation (e.g., to Ly6C+/hi population and/or other Ly6C− cells or progenitors as shown) from CMP (1, 4). These “primed” or preactivated BM cells exhibit enhanced responsiveness for recruitment to the lung upon a second hit or insult, where they may play a paracrine role to promote myeloid differentiation/activation with expression of B7H3, T-helper cell type 2 (Th2) skewing, and fibroblast proliferation and activation, resulting in exacerbation of pulmonary fibrosis. Solid arrows indicate cell differentiation (black) or migration (green), and dotted arrows indicate regulatory effects of mediators (e.g., sB7H3 or IL-33 from the indicated sources as shown). Parts of images were adapted from Motifolio drawing toolkits (www.motifolio.com).
The potential relevance of B7H3 in human IPF is suggested by elevated concentrations of sB7H3 in certain patient clinical samples. Although the role of B7H3 in human IPF has not been clarified, a gene expression array profile in one study revealed that B7H3 is induced in patients with IPF and is associated with progression of disease (26) (Gene Expression Omnibus accession no. GDS4279). Indeed, in our study, the BALF concentrations of sB7H3 were found to reflect the exacerbation of disease. Currently, it is unclear what the pathogenetic significance of sB7H3 in BALF is, if any, but the association with increased numbers of B7H3+ BAL cells and increased BALF concentrations of sB7H3 in the murine exacerbation experiments suggested a significant role in fibrosis and disease progression/exacerbation. In addition, or alternatively, BALF sB7H3 might be useful as a possible biomarker for patients with IPF with increased likelihood of developing exacerbation and/or rapid disease progression.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Professor Tak W. Mak (University of Toronto) for providing B7H3-KO mice and Dr. Stefan Wirtz (Friedrich Alexander University Erlangen, Erlangen, Germany) for ST2-KO mice. The authors also thank Andrew E. Rinke, Lisa R. Riggs, Ronald Craig, and Yukari Iyanaga for excellent technical assistance.
Footnotes
Supported by NIH grants HL052285, HL112880, and HL138417 and Japan Society for the Promotion of Science KAKENHI (Grand-in-Aid for Scientific Research) grants JP25870463 and JP15K21190. B7H3-knockout mice on C57BL/6 background were kindly provided by Professor Tak W. Mak (University of Toronto). ST2-knockout mice on C57BL/6 background were kindly provided by Dr. Stefan Wirtz (Friedrich Alexander University Erlangen, Erlangen, Germany).
Author Contributions: T.N.: Conception and design, collection of data, financial support, data analysis and interpretation, and manuscript writing. T.L.: Conception and design, collection of data, data analysis and interpretation, and manuscript writing. B.H. and L.D.: Data analysis and interpretation. Z.W., M.U., and K.O.: Collection of data, provision of study material, and technical help. N.H.: Financial support, data analysis and interpretation, and provision of clinical material. S.H.P.: Conception and design, financial support, data analysis and interpretation, and manuscript writing.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201808-1560OC on May 18, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Nakashima T, Liu T, Yu H, Ding L, Ullenbruch M, Hu B, et al. Lung bone marrow-derived hematopoietic progenitor cells enhance pulmonary fibrosis. Am J Respir Crit Care Med. 2013;188:976–984. doi: 10.1164/rccm.201303-0479OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu T, Yu H, Ullenbruch M, Jin H, Ito T, Wu Z, et al. The in vivo fibrotic role of FIZZ1 in pulmonary fibrosis. PLoS One. 2014;9:e88362. doi: 10.1371/journal.pone.0088362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding L, Liu T, Wu Z, Hu B, Nakashima T, Ullenbruch M, et al. Bone marrow CD11c+ cell-derived amphiregulin promotes pulmonary fibrosis. J Immunol. 2016;197:303–312. doi: 10.4049/jimmunol.1502479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Satoh T, Nakagawa K, Sugihara F, Kuwahara R, Ashihara M, Yamane F, et al. Identification of an atypical monocyte and committed progenitor involved in fibrosis. Nature. 2017;541:96–101. doi: 10.1038/nature20611. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Takagawa J, Lam VC, Haddad DJ, Tobler DL, Mok PY, et al. Donor myocardial infarction impairs the therapeutic potential of bone marrow cells by an interleukin-1-mediated inflammatory response. Sci Transl Med. 2011;3:100ra90. doi: 10.1126/scitranslmed.3002814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsukamoto H, Nagafuji K, Horiuchi T, Miyamoto T, Aoki K, Takase K, et al. A phase I-II trial of autologous peripheral blood stem cell transplantation in the treatment of refractory autoimmune disease. Ann Rheum Dis. 2006;65:508–514. doi: 10.1136/ard.2005.037879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyndall A. Successes and failures of stem cell transplantation in autoimmune diseases. Hematology (Am Soc Hematol Educ Program) 2011;2011:280–284. doi: 10.1182/asheducation-2011.1.280. [DOI] [PubMed] [Google Scholar]
- 8.Cogle CR, Wise E, Meacham AM, Zierold C, Traverse JH, Henry TD, et al. Cardiovascular Cell Therapy Research Network (CCTRN) Detailed analysis of bone marrow from patients with ischemic heart disease and left ventricular dysfunction: BM CD34, CD11b, and clonogenic capacity as biomarkers for clinical outcomes. Circ Res. 2014;115:867–874. doi: 10.1161/CIRCRESAHA.115.304353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamirault G, Susen S, Forest V, Hemont C, Parini A, Le Corvoisier P, et al. Difference in mobilization of progenitor cells after myocardial infarction in smoking versus non-smoking patients: insights from the BONAMI trial. Stem Cell Res Ther. 2013;4:152. doi: 10.1186/scrt382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu PY, Mariani J, Finch S, McMullen JR, Sadoshima J, Marshall T, et al. Bone marrow-derived cells contribute to fibrosis in the chronically failing heart. Am J Pathol. 2010;176:1735–1742. doi: 10.2353/ajpath.2010.090574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verda L, Kim DA, Ikehara S, Statkute L, Bronesky D, Petrenko Y, et al. Hematopoietic mixed chimerism derived from allogeneic embryonic stem cells prevents autoimmune diabetes mellitus in NOD mice. Stem Cells. 2008;26:381–386. doi: 10.1634/stemcells.2006-0262. [DOI] [PubMed] [Google Scholar]
- 12.Fujita Y, Abe R, Inokuma D, Sasaki M, Hoshina D, Natsuga K, et al. Bone marrow transplantation restores epidermal basement membrane protein expression and rescues epidermolysis bullosa model mice. Proc Natl Acad Sci USA. 2010;107:14345–14350. doi: 10.1073/pnas.1000044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schildberg FA, Klein SR, Freeman GJ, Sharpe AH. Coinhibitory pathways in the B7-CD28 ligand-receptor family. Immunity. 2016;44:955–972. doi: 10.1016/j.immuni.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greaves P, Gribben JG. The role of B7 family molecules in hematologic malignancy. Blood. 2013;121:734–744. doi: 10.1182/blood-2012-10-385591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yi KH, Chen L. Fine tuning the immune response through B7-H3 and B7-H4. Immunol Rev. 2009;229:145–151. doi: 10.1111/j.1600-065X.2009.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagashima O, Harada N, Usui Y, Yamazaki T, Yagita H, Okumura K, et al. B7-H3 contributes to the development of pathogenic Th2 cells in a murine model of asthma. J Immunol. 2008;181:4062–4071. doi: 10.4049/jimmunol.181.6.4062. [DOI] [PubMed] [Google Scholar]
- 18.Lombardi V, Singh AK, Akbari O. The role of costimulatory molecules in allergic disease and asthma. Int Arch Allergy Immunol. 2010;151:179–189. doi: 10.1159/000242355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen ZR, Zhang GB, Wang YQ, Yan YD, Zhou WF, Zhu C, et al. Therapeutic effects of anti-B7-H3 antibody in an ovalbumin-induced mouse asthma model. Ann Allergy Asthma Immunol. 2013;111:276–281. doi: 10.1016/j.anai.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 20.Veenstra RG, Flynn R, Kreymborg K, McDonald-Hyman C, Saha A, Taylor PA, et al. B7-H3 expression in donor T cells and host cells negatively regulates acute graft-versus-host disease lethality. Blood. 2015;125:3335–3346. doi: 10.1182/blood-2014-09-603357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang G, Hou J, Shi J, Yu G, Lu B, Zhang X. Soluble CD276 (B7-H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology. 2008;123:538–546. doi: 10.1111/j.1365-2567.2007.02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen W, Liu P, Wang Y, Nie W, Li Z, Xu W, et al. Characterization of a soluble B7-H3 (sB7-H3) spliced from the intron and analysis of sB7-H3 in the sera of patients with hepatocellular carcinoma. PLoS One. 2013;8:e76965. doi: 10.1371/journal.pone.0076965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4:899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- 24.McHedlidze T, Waldner M, Zopf S, Walker J, Rankin AL, Schuchmann M, et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357–371. doi: 10.1016/j.immuni.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doucet C, Brouty-Boyé D, Pottin-Clémenceau C, Canonica GW, Jasmin C, Azzarone B. Interleukin (IL) 4 and IL-13 act on human lung fibroblasts: implication in asthma. J Clin Invest. 1998;101:2129–2139. doi: 10.1172/JCI741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meltzer EB, Barry WT, D’Amico TA, Davis RD, Lin SS, Onaitis MW, et al. Bayesian probit regression model for the diagnosis of pulmonary fibrosis: proof-of-principle. BMC Med Genomics. 2011;4:70. doi: 10.1186/1755-8794-4-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu T, Yu H, Ding L, Wu Z, Gonzalez De Los Santos F, Liu J, et al. Conditional knockout of telomerase reverse transcriptase in mesenchymal cells impairs mouse pulmonary fibrosis. PLoS One. 2015;10:e0142547. doi: 10.1371/journal.pone.0142547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu B, Wu Z, Bai D, Liu T, Ullenbruch MR, Phan SH. Mesenchymal deficiency of Notch1 attenuates bleomycin-induced pulmonary fibrosis. Am J Pathol. 2015;185:3066–3075. doi: 10.1016/j.ajpath.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu T, Chung MJ, Ullenbruch M, Yu H, Jin H, Hu B, et al. Telomerase activity is required for bleomycin-induced pulmonary fibrosis in mice. J Clin Invest. 2007;117:3800–3809. doi: 10.1172/JCI32369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, Li Y, Blankson S, Liu M, Huang D, Redmond HP, et al. B7-H3 augments inflammatory responses and exacerbates brain damage via amplifying NF-κB p65 and MAPK p38 activation during experimental pneumococcal meningitis. PLoS One. 2017;12:e0171146. doi: 10.1371/journal.pone.0171146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X, Quinn EM, Ni H, Wang J, Blankson S, Redmond HP, et al. B7-H3 participates in the development of experimental pneumococcal meningitis by augmentation of the inflammatory response via a TLR2-dependent mechanism. J Immunol. 2012;189:347–355. doi: 10.4049/jimmunol.1103715. [DOI] [PubMed] [Google Scholar]
- 32.Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society. Diagnosis of idiopathic pulmonary fibrosis: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 33.Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis: an international working group report. Am J Respir Crit Care Med. 2016;194:265–275. doi: 10.1164/rccm.201604-0801CI. [DOI] [PubMed] [Google Scholar]
- 34.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CGK, Doering TA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Y, De Los Santos FG, Wu Z, Liu T, Phan SH. An ST2-dependent role of bone marrow-derived group 2 innate lymphoid cells in pulmonary fibrosis. J Pathol. 2018;245:399–409. doi: 10.1002/path.5092. [DOI] [PubMed] [Google Scholar]
- 37.Li D, Guabiraba R, Besnard AG, Komai-Koma M, Jabir MS, Zhang L, et al. IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J Allergy Clin Immunol. 2014;134:1422–1432, e11. doi: 10.1016/j.jaci.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luzina IG, Kopach P, Lockatell V, Kang PH, Nagarsekar A, Burke AP, et al. Interleukin-33 potentiates bleomycin-induced lung injury. Am J Respir Cell Mol Biol. 2013;49:999–1008. doi: 10.1165/rcmb.2013-0093OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu J, Zheng J, Song P, Zhou Y, Guan S. IL-33/ST2 pathway in a bleomycin-induced pulmonary fibrosis model. Mol Med Rep. 2016;14:1704–1708. doi: 10.3892/mmr.2016.5446. [DOI] [PubMed] [Google Scholar]
- 40.Halim TYF, Steer CA, Mathä L, Gold MJ, Martinez-Gonzalez I, McNagny KM, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez-Gonzalez I, Ghaedi M, Steer CA, Mathä L, Vivier E, Takei F. ILC2 memory: recollection of previous activation. Immunol Rev. 2018;283:41–53. doi: 10.1111/imr.12643. [DOI] [PubMed] [Google Scholar]
- 42.Tada H, Suzuki R, Nemoto E, Shimauchi H, Matsushita K, Takada H. Increases in IL-33 production by fimbriae and lipopeptide from Porphyromonas gingivalis in mouse bone marrow-derived dendritic cells via Toll-like receptor 2. Biomed Res (Aligarh) 2017;38:189–195. doi: 10.2220/biomedres.38.189. [DOI] [PubMed] [Google Scholar]
- 43.Bagnato G, Harari S. Cellular interactions in the pathogenesis of interstitial lung diseases. Eur Respir Rev. 2015;24:102–114. doi: 10.1183/09059180.00003214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Degryse AL, Tanjore H, Xu XC, Polosukhin VV, Jones BR, McMahon FB, et al. Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2010;299:L442–L452. doi: 10.1152/ajplung.00026.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Degryse AL, Lawson WE. Progress toward improving animal models for idiopathic pulmonary fibrosis. Am J Med Sci. 2011;341:444–449. doi: 10.1097/MAJ.0b013e31821aa000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao Z, Lis R, Ginsberg M, Chavez D, Shido K, Rabbany SY, et al. Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nat Med. 2016;22:154–162. doi: 10.1038/nm.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gibbons MA, MacKinnon AC, Ramachandran P, Dhaliwal K, Duffin R, Phythian-Adams AT, et al. Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am J Respir Crit Care Med. 2011;184:569–581. doi: 10.1164/rccm.201010-1719OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.