Abstract
Rationale: Many studies have linked short-term exposure to ozone (O3) with morbidity and mortality, but epidemiologic evidence of associations between long-term O3 exposure and mortality is more limited.
Objectives: To investigate associations of long-term (annual or warm season average of daily 8-h maximum concentrations) O3 exposure with all-cause and cause-specific mortality in the NIH-AARP Diet and Health Study, a large prospective cohort of U.S. adults with 17 years of follow-up from 1995 to 2011.
Methods: The cohort (n = 548,780) was linked to census tract–level estimates for O3. Associations between long-term O3 exposure (averaged values from 2002 to 2010) and multiple causes of death were evaluated using multivariate Cox proportional hazards models, adjusted for individual- and census tract–level covariates, and potentially confounding copollutants and temperature.
Measurements and Main Results: Long-term annual average exposure to O3 was significantly associated with deaths caused by cardiovascular disease (per 10 ppb; hazard ratio [HR], 1.03; 95% confidence interval [CI], 1.01–1.06), ischemic heart disease (HR, 1.06; 95% CI, 1.02–1.09), respiratory disease (HR, 1.04; 95% CI, 1.00–1.09), and chronic obstructive pulmonary disease (HR, 1.09; 95% CI, 1.03–1.15) in single-pollutant models. The results were robust to alternative models and adjustment for copollutants (fine particulate matter and nitrogen dioxide), although some evidence of confounding by temperature was observed. Significantly elevated respiratory disease mortality risk associated with long-term O3 exposure was found among those living in locations with high temperature (Pinteraction < 0.05).
Conclusions: This study found that long-term exposure to O3 is associated with increased risk for multiple causes of mortality, suggesting that establishment of annual and/or seasonal federal O3 standards is needed to more adequately protect public health from ambient O3 exposures.
Keywords: ozone, air pollution, mortality
At a Glance Commentary
Scientific Knowledge on the Subject
Effects of long-term exposure to ozone on mortality risk remain unclear. Past epidemiologic studies have reported mixed findings or have used exposure estimates for ozone with relatively coarse spatial resolutions.
What This Study Adds to the Field
This large U.S. prospective cohort study examined the association between long-term ambient ozone exposure and cause-specific mortality using spatiotemporally detailed exposure data from recent prediction models for ozone, particulate matter ≤2.5 μm in aerodynamic diameter, and nitrogen dioxide. The findings suggest that long-term exposures to ambient ozone may be associated with an increased risk of cardiovascular and respiratory disease mortality.
Ozone (O3) is a known respiratory irritant that causes bronchial inflammation and hyperresponsiveness (1). The dosimetry of O3 in the lung and its potential to cause oxidative injury and inflammation has been well established in mechanistic and human exposure studies (2). Among epidemiologic investigations, short-term exposures (a day or a few days) to O3 have been linked with increases in cardiopulmonary mortality and morbidity in many different regions of the world, and among both adults and children (3–6). For example, short-term increases in O3 increased risk of acute care visits and hospitalization for asthma (7) and chronic obstructive pulmonary disease (COPD) (8), as well as deterioration in asthma control, resulting in increased medication use (9).
In contrast to the robust body of evidence from short-term exposure studies, effects of long-term exposure to O3 on health are less well documented (2), with a recent meta-analysis finding no evidence of an association between long-term annual O3 concentration and mortality (10). Estimates of the global burden of mortality attributable to O3 (11–14) have relied on a single study population, analyses of effects of long-term O3 in the American Cancer Society CPS-II cohort by Jerrett and colleagues (15) and the updated CPS-II cohort estimates of mortality risks with longer follow-up and improved exposure estimates by Turner and colleagues (16). Thus, additional large cohort studies are required to independently confirm this association between long-term exposure to O3 on mortality risk and to develop improved concentration-response functions.
In this study, we evaluated the associations between long-term (annual or warm season averages of daily 8-hour maximum concentrations) exposures to O3 and cause-specific mortality risk in the NIH-AARP Diet and Health Study, a prospective cohort of more than a half million participants across multiple U.S. states with 17 years of follow-up from 1995 to 2011, with a detailed consideration of effect modification by individual risk factors and potential confounding by copollutants and temperature. We used recent spatiotemporally resolved national-level estimates for O3 from the U.S. Environmental Protection Agency (EPA) to assign exposure at the census-tract level.
Methods
Study Population
Briefly, the NIH-AARP Diet and Health Study was initiated when members of the AARP, 50–71 years of age from six U.S. states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) and two metropolitan areas (Atlanta, GA and Detroit, MI), responded to a mailed questionnaire in 1995–1996. Study participants completed a baseline questionnaire including demographic and lifestyle information. All participants provided written informed consent. The study was approved by the institutional review boards of the National Cancer Institute and the New York University School of Medicine.
Cohort Follow-up and Mortality Ascertainment
Each participant was followed from enrollment to the end of follow-up (December 31, 2011), the date of death, or the date a participant moved out of the study state or city where she or he lived at enrollment (movers included 42,786 participants), whichever occurred first. Vital status was ascertained through a periodic linkage of the cohort to the Social Security Administration Death Master File and follow-up searches of the National Death Index Plus for participants who matched to the Social Security Administration Death Master, cancer registry linkage, questionnaire responses, and responses to other mailings. The International Classification of Diseases, ninth Revision (ICD-9) and the International Statistical Classification of Diseases, 10th Revision (ICD-10) were used to define underlying cause of mortality due to cardiovascular diseases (ICD-9: 390–459; ICD-10: I00–I99); ischemic heart disease (ICD-9: 410–414; ICD-10: I20–I25); cerebrovascular disease (ICD-9: 430–438; ICD-10: I60–I69); cardiac arrests (ICD-9: 420–429; ICD-10: I30–I51); respiratory diseases (ICD-9: 460–519; ICD 10: J00–J98); COPD (ICD-9: 491–492, 494, 496; ICD-10: J40–J44); pneumonia and influenza (ICD-9: 480–48; ICD-10: J00–J98); and lung cancer (ICD-9: 162; ICD-10: C33–C34).
Out of 566,398 participants enrolled in the NIH-AARP cohort and available for analysis, after excluding those who responded via a proxy (n = 15,760), exited the study on the study entry date (n = 49), and those with missing particulate matter ≤2.5 μm in aerodynamic diameter (PM2.5) (n = 740), nitrogen dioxide (NO2) (n = 1,796), or temperature (n = 858) exposure data, the analytic cohort for this work includes 548,780 participants (96.9% of total cohort).
Exposure Assessment
Annual O3 concentrations were assigned to each participant’s residence census tract using the EPA Fused Air Quality Surface Using Downscaling data (17), which is available for the years after 2002. A bayesian space-time downscaler model is used to “fuse” daily 8-hour maximum O3 concentrations at the census tract centroid. The downscaler model develops a relationship between observed and modeled concentrations, and uses that relationship to spatially predict measurements at new locations in the spatial domain based on input data. Estimates are based on National Air Monitoring Stations/State and Local Air Monitoring Stations and Model-3/Community Multiscale Air Quality model data in 12 × 12-km grids.
Annual census-tract level PM2.5 exposure estimates were obtained from a published spatiotemporal prediction model (18) for the continental United States, available for 1980–2010. In brief, geographic predictors and annual average PM2.5 data from 1999 through 2010 were derived from the EPA Federal Reference Method network and the Interagency Monitoring of Protected Visual Environments network. Temporal trends before 1999 were estimated using 1) extrapolation based on PM2.5 data in Federal Reference Method/Interagency Monitoring of Protected Visual Environments, 2) PM2.5 sulfate data in the Clean Air Status and Trends Network, and 3) visibility data across the Weather-Bureau-Army-Navy network. The modeling approach was validated using PM2.5 data collected before 1999 from other data sources.
Annual NO2 data were derived at the census-tract level from a recent model (19) available for years 1990–2012, which applied kriging models combining land use regression methods with satellite data to improve model performance. The satellite data consist of total tropospheric NO2 measured via satellite images from the Ozone Monitoring Instrument on the Aurora satellite, whereas the estimation regression covariates were dimension-reduced components of 418 geographic variables.
To assess temperature exposure at the census-tract level, climate datasets were obtained from the PRISM Climate Group (20). The dataset uses the Parameter-elevation Relationships on Independent Slopes Model interpolation method to develop gridded data sets with 4-km resolution that reflect spatial climate patterns in the United States; surface stations used in the analysis numbered nearly 13,000 for precipitation and 10,000 for temperature. Annual averages calculated from daily maximum temperature (Tmax) data were linked to study participants from temperature grid value nearest to the census tract centroid.
Statistical Methods
Person-years were calculated from enrollment date to the date of death, the end of follow-up (December 31, 2011), or the date the participant moved out of the study state or city where she or he lived at enrollment, whichever occurred first. Cox proportional hazards models were used to examine the associations of mean O3, PM2.5, and NO2 concentration levels (average of annual levels for the years 2002–2010, to match more limited O3 data availability) with all-cause and cause-specific mortality. Warm season O3 exposure was also assigned by calculating averages for April to September. Hazard ratios (HR) were presented in relation to increments of 10 ppb for O3 and NO2 and 10 μg/m3 for PM2.5.
Fully adjusted multivariable models included the following individual-level variables: age (grouped into 3-yr categories), sex, and region (six U.S. states and two cities) as strata; race or ethnic group (non-Hispanic white; non-Hispanic black; Hispanic; Asian, Pacific Islander, or American Indian/Alaskan Native; and unknown); level of education (less than high school, some high school, high school completed, post high school or some college, college and postgraduate, and unknown); marital status (married, never-married, other, and unknown); body mass index (<18.5 kg/m2, 18.5 to <25.0, 25.0 to <30.0, 30 to <35, 35+, and unknown); alcohol (none, <1, 1 to <2, 2 to <3, 3 to <5, and 5+ drinks per d); smoking status (never smoker, former smoker of ≤1 pack/d, former smoker of >1 pack/d, current smoker of ≤1 pack/d, current smoker of >1 pack/d, and unknown), and diet (by the alternate Mediterranean diet index, which measures adherence to a Mediterranean diet pattern [21]), in addition to two contextual characteristics (median census-tract household income and percent of census-tract population with less than a high school education, based on the 2000 decennial census for the residence at study entry). The proportional hazards assumption was checked by examining the Schoenfeld residuals.
Potential effect modification by risk factors was assessed by including multiplicative interaction terms between the exposure term and covariates of interest in the models, and likelihood ratio tests comparing model fit with and without interaction terms were conducted to test their statistical significance. We similarly assessed potential effect modification and confounding by annual mean Tmax. For additional sensitivity analyses other models were tested, including: adjusting for other comorbid conditions (heart disease, diabetes, and stroke history) or limiting analysis to participants without these comorbid conditions, adding metropolitan statistical area–level random effects, adjusting for other pollutants (PM2.5 and NO2) in multipollutant models, and further adjusting for annual mean Tmax in multiexposure models. We also evaluated potential differences in O3-mortality associations by location, grouping the study locations into three regions according to geography: 1) California, 2) North (New Jersey, Pennsylvania, and Detroit), and 3) South (Florida, Louisiana, North Carolina, and Atlanta); and also tested whether timing of the exposures influenced the effect estimates by restricting the follow-up period to 2003–2011 (to match limited availability of O3 data) and conducting time-dependent (with 1-yr lagged exposures) and time-independent (for comparison purposes) analyses.
Packages “survival,” “coxme,” and “pspline” in the statistical package R version 3.4.0 were used for analysis.
Results
During the follow-up period considered, 126,806 of all study participants (23.1%) died of nonaccidental causes, of which 39,529 were caused by all cardiovascular diseases, 22,327 were caused by ischemic heart disease, 5,592 were caused by cerebrovascular disease, 6,811 were caused by cardiac arrests, 12,459 were caused by respiratory diseases, 7,748 were caused by COPD, 1,889 were caused by pneumonia, and 13,529 were caused by lung cancer.
The overall average annual O3 concentration during the study period was 39.0 with a SD of 4.6 (range, 26.8–56.3) ppb; average warm season O3 concentration was 46.2 with a SD of 7.6 (range, 29.5–70.4) ppb; average PM2.5 was 11.0 μg/m3, with a SD of 2.7 (range, 2.8–21.2) μg/m3; and for NO2 the average was 11.1 ppb with SD of 5.6 (range, 1.5–31.7) ppb. The annual O3 concentration did not exhibit temporal trends over the study period, whereas PM2.5 and NO2 levels decreased over that time (see Figure E1 in the online supplement). The mean correlation between annual and warm season O3 during the study was r = 0.71. The correlations between annual O3 with PM2.5 and NO2 were r = 0.12 and r = −0.17, respectively, whereas correlations between warm season O3 with PM2.5 and NO2 were r = 0.47 and r = 0.16, respectively; these values are lower (for annual) or similar (for warm season) than reported for the Turner and colleagues study (16). The correlation between annual O3 and annual Tmax was 0.35, and during warm season correlation between O3 and Tmax was 0.19. There were only small differences in pollutant concentrations across the range of cohort characteristics, suggesting that potential confounding by these factors is likely minimal (Table 1).
Table 1.
Descriptive Statistics for the NIH-AARP Cohort, by Quintile of Annual O3 Concentration Exposure
| Q1 (n = 109,756) | Q2 (n = 109,756) | Q3 (n = 109,756) | Q4 (n = 109,756) | Q5 (n = 109,756) | ||
|---|---|---|---|---|---|---|
| O3 | Range, ppb | 26.8–35.1 | 35.1–38.4 | 38.4–39.8 | 39.8–42.0 | 42.0–56.3 |
| Age, n (%) | ≥65 yr | 69,724 (63.5) | 68,821 (62.7) | 69,459 (63.3) | 69,687 (63.5) | 69,291 (63.1) |
| <65 yr | 40,032 (36.5) | 40,935 (37.3) | 40,297 (36.7) | 40,069 (36.5) | 40,465 (36.9) | |
| Sex, n (%) | M | 62,301 (56.8) | 66,240 (60.4) | 65,904 (60.0.) | 65,050 (59.3) | 64,589 (58.8) |
| F | 47,455 (43.2) | 43,516 (39.6) | 43,852 (40.0) | 44,706 (40.7) | 45,167 (41.2) | |
| Race, n (%) | White | 94,798 (86.4) | 99,944 (91.1) | 102,819 (93.7) | 101,350 (92.3) | 101,597 (92.6) |
| Black | 6,595 (6.0) | 5,062 (4.6) | 3,016 (2.7) | 4,086 (3.7) | 2,822 (2.6) | |
| Hispanic | 3,522 (3.2) | 1,559 (1.4) | 1,461 (1.3) | 1,492 (1.4) | 2,163 (2.0) | |
| Asian, Pacific | 3,009 (2.7) | 1,665 (1.5) | 1,134 (1.0) | 1,384 (1.3) | 1,687 (1.5) | |
| Education, n (%) | Less than high school | 6,243 (5.7) | 6,627 (6.0) | 7,834 (7.1) | 6,582 (6.0) | 5,969 (5.4) |
| Some high school | 19,252 (17.5) | 24,321 (22.2) | 25,660 (23.4) | 20,331 (18.5) | 17,808 (16.2) | |
| 12 yr or high school completed | 9,519 (8.7) | 10,928 (10.0) | 12,182 (11.1) | 11,145 (10.2) | 9,971 (9.1) | |
| Post high school or some college | 26,260 (23.9) | 22,978 (20.9) | 22,844 (20.8) | 25,982 (23.7) | 29,081 (26.5) | |
| College and postgraduate | 45,104 (41.1) | 41,669 (38.0) | 37,863 (34.5) | 42,412 (38.6) | 43,655 (39.8) | |
| BMI, n (%) | <18.5 kg/m2 | 897 (0.8) | 796 (0.7) | 885 (0.8) | 1,003 (0.9) | 1,070 (1.0) |
| 18.5–25 kg/m2 | 37,826 (34.5) | 35,616 (32.5) | 34,869 (31.8) | 37,303 (34) | 38,755 (35.3) | |
| 25–30 kg/m2 | 44,140 (40.2) | 46,314 (42.2) | 45,838 (41.8) | 45,415 (41.4) | 45,119 (41.1) | |
| 30–35 kg/m2 | 16,473 (15.0) | 17,237 (15.7) | 17,907 (16.3) | 16,705 (15.2) | 15,962 (14.5) | |
| >35 kg/m2 | 7,195 (6.6) | 6,877 (6.3) | 7,306 (6.7) | 6,444 (5.9) | 6,035 (5.5) | |
| Marriage, n (%) | Married | 68,687 (62.6) | 75,377 (68.7) | 76,575 (69.8) | 75,722 (69.0) | 77,176 (70.3) |
| Never married | 32,382 (29.5) | 27,964 (25.5) | 27,528 (25.1) | 28,464 (25.9) | 27,706 (25.2) | |
| Other | 7,582 (6.9) | 5,532 (5) | 4,778 (4.4) | 4,688 (4.3) | 3,986 (3.6) | |
| Smoking, n (%) | Never | 37,781 (34.4) | 38,550 (35.1) | 38,768 (35.3) | 37,498 (34.2) | 38,419 (35.0) |
| Less than a pack, 1+ yr quit | 29,847 (27.2) | 29,126 (26.5) | 28,598 (26.1) | 28,834 (26.3) | 29,475 (26.9) | |
| More than a pack, 1+ yr quit | 4,882 (4.4) | 5,015 (4.6) | 5,706 (5.2) | 5,769 (5.3) | 5,395 (4.9) | |
| Less than a pack, stopped less than a year/current | 10,181 (9.3) | 9,963 (9.1) | 9,601 (8.7) | 9,684 (8.8) | 9,410 (8.6) | |
| More than a pack, stopped less than a year or current | 4,882 (4.4) | 5,015 (4.6) | 5,706 (5.2) | 5,769 (5.3) | 5,395 (4.9) | |
| Alcohol, n (%) | 0 drinks/d | 23,684 (21.6) | 23,422 (21.3) | 28,616 (26.1) | 29,259 (26.7) | 30,950 (28.2) |
| <1 drinks/d | 59,235 (54.0) | 60,524 (55.1) | 57,627 (52.5) | 55,398 (50.5) | 53,958 (49.2) | |
| 1–2 drinks/d | 13,346 (12.2) | 12,644 (11.5) | 11,354 (10.3) | 12,372 (11.3) | 12,260 (11.2) | |
| 2–3 drinks/d | 4,771 (4.3) | 4,141 (3.8) | 3,617 (3.3) | 4,004 (3.6) | 4,164 (3.8) | |
| 3–5 drinks/d | 3,938 (3.6) | 4,134 (3.8) | 3,950 (3.6) | 4,036 (3.7) | 3,946 (3.6) | |
| 5+ drinks/d | 4,782 (4.4) | 4,891 (4.5) | 4,592 (4.2) | 4,687 (4.3) | 4,478 (4.1) | |
| aMED index, n (%) | 0–2 | 18,865 (17.2) | 22,532 (20.5) | 22,483 (20.5) | 20,198 (18.4) | 18,011 (16.4) |
| 3 | 18,445 (16.8) | 19,980 (18.2) | 20,696 (18.9) | 19,806 (18.0) | 18,629 (17.0) | |
| 4 | 22,240 (20.3) | 22,350 (20.4) | 22,818 (20.8) | 22,814 (20.8) | 22,344 (20.4) | |
| 5 | 21,397 (19.5) | 20,566 (18.7) | 20,340 (18.5) | 21,371 (19.5) | 22,076 (20.1) | |
| 6–9 | 28,809 (26.2) | 24,328 (22.2) | 23,419 (21.3) | 25,567 (23.3) | 28,696 (26.1) |
Definition of abbreviations: aMED = alternate Mediterranean diet; BMI = body mass index; O3 = ozone.
In single-pollutant models, long-term annual average exposure to O3 was significantly associated with cardiovascular disease mortality (HR, 1.03; 95% confidence interval [CI], 1.01–1.06), ischemic heart disease (HR, 1.06; 95% CI, 1.02–1.09), respiratory disease mortality (HR, 1.04; 95% CI, 1.00–1.09), and COPD (HR, 1.09; 95% CI, 1.03–1.15) after adjustment for covariates (Table 2). We did not observe a significant association between O3 and all-cause mortality (HR, 0.99; 95% CI, 0.98–1.00), cerebrovascular disease mortality (HR, 1.01; 95% CI, 0.95–1.08), cardiac arrests mortality (HR, 0.99; 95% CI, 0.93–1.06), pneumonia (HR, 1.00; 95% CI, 0.90–1.11), or lung cancer (HR, 0.96; 95% CI, 0.92–1.00). Using warm season averages for O3 changed the effect estimates marginally, but the associations remained statistically significant. Adjusting for PM2.5 and NO2 as copollutants in two-pollutant (Table E1) and multipollutant models (Table 2) also did not significantly change the results. Additional adjustment for annual Tmax average in multiexposure models significantly reduced cerebrovascular disease mortality risk, and cardiovascular and respiratory disease mortality risks were also attenuated. Effect estimates were robust to (i.e., not significantly changed by) consideration of alternative model specifications, including metropolitan-level random effects, adjusting for other preexisting comorbidities, and restricting analysis to those without preexisting comorbidities only (results not shown). Restricting the follow-up period to 2003–2011 and assigning O3 as time-varying exposures also did not significantly change the results (Table E2). Differences in cause-specific mortality risk associated with long-term O3 exposure were observed across the study regions (Table E3). Examination of the Schoenfeld residuals revealed that the proportional hazards assumption was satisfied.
Table 2.
All-Cause and Cause-Specific Air Pollutant Mortality HRs (per 10 mg/m3 for PM2.5; per 10 ppb for NO2 and O3) by Model Specifications
| Single-Pollutant Model |
Multipollutant Model |
Multiexposure Model |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annual O3 |
Warm Season O3 |
PM2.5 |
NO2 |
Annual O3 Adjusting for PM2.5 + NO2 |
Warm Season O3 Adjusting for PM2.5 + NO2 |
Annual O3 Adjusting for PM2.5 + NO2 + Tmax |
Warm Season O3 Adjusting for PM2.5 + NO2 + Tmax |
||||||||||
| Cause | n | HR* | 95% CI* | HR* | 95% CI* | HR* | 95% CI* | HR* | 95% CI* | HR* | 95% CI* | HR* | 95% CI* | HR* | 95% CI* | HR* | 95% CI* |
| All-cause | 126,806 | 0.99 | 0.98–1.00 | 1.00 | 0.99–1.01 | 1.03 | 1.01–1.06 | 1.04 | 1.02–1.05 | 1.00 | 0.96–1.01 | 1.00 | 0.99–1.01 | 0.99 | 0.97–1.00 | 1.00 | 0.99–1.01 |
| Cardiovascular disease | 39,529 | 1.03 | 1.01–1.06 | 1.02 | 1.01–1.04 | 1.11 | 1.06–1.16 | 1.07 | 1.04–1.09 | 1.05 | 1.02–1.08 | 1.04 | 1.02–1.06 | 1.02 | 0.99–1.05 | 1.02 | 0.99–1.03 |
| Ischemic heart disease | 22,327 | 1.06 | 1.02–1.09 | 1.03 | 1.02–1.05 | 1.15 | 1.08–1.22 | 1.09 | 1.05–1.12 | 1.08 | 1.04–1.12 | 1.05 | 1.03–1.08 | 1.05 | 1.01–1.10 | 1.03 | 1.00–1.06 |
| Cerebrovascular | 5,592 | 1.01 | 0.95–1.08 | 1.02 | 0.99–1.06 | 1.11 | 0.98–1.25 | 1.02 | 0.97–1.09 | 1.00 | 0.93–1.07 | 1.01 | 0.97–1.06 | 0.89 | 0.82–0.97 | 0.92 | 0.86–0.98 |
| Cardiac arrest, heart failure | 6,811 | 0.99 | 0.93–1.06 | 0.99 | 0.96–1.03 | 0.97 | 0.86–1.09 | 1.02 | 0.97–1.08 | 1.01 | 0.95–1.08 | 1.01 | 0.96–1.06 | 1.01 | 0.94–1.09 | 1.01 | 0.97–1.05 |
| Respiratory disease | 12,459 | 1.04 | 1.00–1.09 | 1.04 | 1.02–1.06 | 1.09 | 1.00–1.18 | 1.04 | 0.99–1.08 | 1.05 | 1.00–1.10 | 1.05 | 1.01–1.08 | 1.04 | 0.99–1.10 | 1.04 | 1.00–1.08 |
| COPD | 7,748 | 1.09 | 1.03–1.15 | 1.05 | 1.02–1.08 | 1.07 | 0.97–1.18 | 1.03 | 0.98–1.08 | 1.09 | 1.03–1.16 | 1.07 | 1.03–1.11 | 1.08 | 1.01–1.15 | 1.06 | 1.01–1.12 |
| Pneumonia and influenza | 1,889 | 1.00 | 0.90–1.11 | 1.05 | 0.99–1.10 | 1.53 | 1.26–1.86 | 1.22 | 1.11–1.35 | 0.99 | 0.89–1.11 | 1.02 | 0.95–1.10 | 0.99 | 0.86–1.13 | 1.06 | 0.99–1.14 |
| Lung cancer | 13,529 | 0.96 | 0.92–1.00 | 0.98 | 0.96–1.00 | 0.96 | 0.88–1.04 | 1.00 | 0.97–1.04 | 0.97 | 0.93–1.02 | 0.98 | 0.96–1.01 | 0.96 | 0.91–1.01 | 0.98 | 0.95–1.00 |
Definition of abbreviations: CI = confidence interval; COPD = chronic obstructive pulmonary disease; HR = hazard ratio; NO2 = nitrogen dioxide; O3 = ozone; PM2.5 = particulate matter ≤2.5 μm in aerodynamic diameter; Tmax = daily maximum temperature.
Adjusted for age, sex, and location, as strata; for race, body mass index, education, smoking, diet, and marital status, at individual level; and for median income and percentage with high school education at census-tract level.
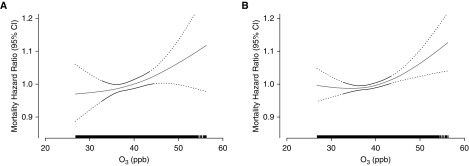
The overall exposure–response relationship using splines was plotted, with the best df (df = 2) selected via Akaike information criterion and bayesian information criterion values. We observed the significant associations between O3 with cardiovascular disease and respiratory disease mortality to be monotonic, and positively linear across the range of concentration levels in the cohort (Figure 1).
Figure 1.
Concentration–response curves (solid lines) and 95% confidence intervals (dashed lines) relative to the effect at mean concentration, based on natural spline models with 2 df, for standard Cox models adjusted for individual-level and contextual covariates; (A and B) respiratory disease mortality (A) and cardiovascular disease mortality (B). The tick marks on the x-axis identify the distribution of observations according to annual average ozone concentrations. CI = confidence interval; O3 = ozone.
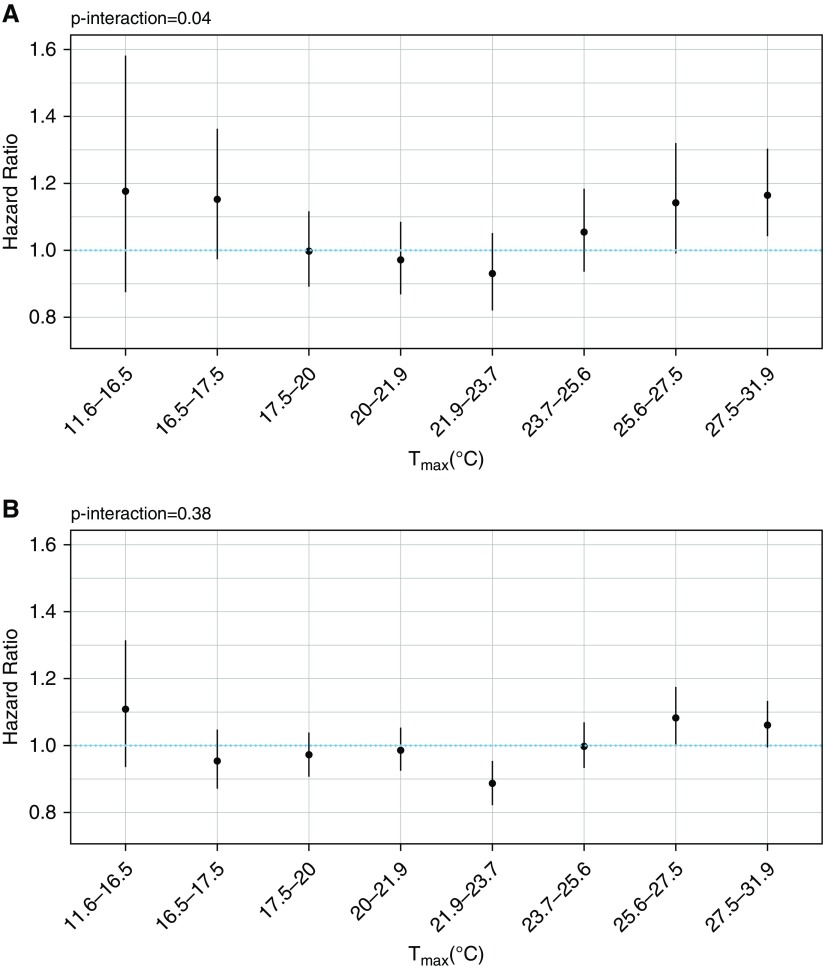
As seen in Table 3, we did not observe effect modification by covariates. We did observe significant effect modification by temperature (Figure 2), with increased respiratory disease mortality risk and higher annual mean maximum temperature in a U-shaped dose–response relationship (Pinteraction = 0.04); we did not find such modification for cardiovascular disease.
Table 3.
Annual Average Ozone Mortality HRs (per 10 ppb), by Category of Selected Risk Factors
| Cardiovascular Disease |
Ischemic Heart Disease |
Respiratory Disease |
COPD |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR* | 95% CI* | Pinteraction | HR* | 95% CI* | Pinteraction | HR* | 95% CI* | Pinteraction | HR* | 95% CI* | Pinteraction | ||
| Sex | M | 1.01 | 0.97–1.05 | 0.92 | 1.03 | 0.97–1.10 | 0.64 | 1.09 | 1.02–1.17 | 0.39 | 1.13 | 1.05–1.23 | 0.66 |
| F | 1.05 | 1.02–1.08 | 1.06 | 1.02–1.10 | 1.02 | 0.96–1.13 | 1.06 | 0.98–1.13 | |||||
| Age | ≥65 yr | 1.04 | 1.01–1.07 | 0.60 | 1.05 | 1.01–1.09 | 0.49 | 1.05 | 0.99–1.10 | 0.50 | 1.09 | 1.03–1.17 | 0.48 |
| <65 yr | 1.01 | 0.97–1.05 | 1.07 | 1.02–1.12 | 1.06 | 0.99–1.21 | 1.11 | 1.02–1.21 | |||||
| Race | White | 1.03 | 1.00–1.06 | 0.36 | 1.05 | 1.02–1.09 | 0.29 | 1.03 | 0.99–1.07 | 0.08 | 1.07 | 1.02–1.13 | 0.15 |
| Black | 1.02 | 0.89–1.17 | 0.97 | 0.81–1.16 | 1.28 | 0.97–1.68 | 1.16 | 0.76–1.55 | |||||
| Other | 1.08 | 0.92–1.27 | 1.08 | 0.87–1.32 | 1.11 | 0.77–1.60 | 1.29 | 0.77–2.17 | |||||
| BMI | <25 kg/m2 | 1.00 | 0.96–1.04 | 0.54 | 1.02 | 0.96–1.08 | 0.24 | 1.06 | 0.99–1.13 | 0.29 | 1.09 | 1.01–1.18 | 0.50 |
| 25–35 kg/m2 | 1.07 | 1.03–1.11 | 1.08 | 1.03–1.14 | 1.04 | 0.97–1.12 | 1.10 | 1.00–1.21 | |||||
| >35 kg/m2 | 1.02 | 0.97–1.07 | 1.06 | 1.00–1.13 | 1.02 | 0.93–1.11 | 1.06 | 0.94–1.20 | |||||
| Education | ≤High school | 1.04 | 1.00–1.09 | 0.58 | 1.06 | 1.00–1.12 | 0.99 | 1.02 | 0.96–1.09 | 0.21 | 1.08 | 0.99–1.17 | 0.27 |
| Some college | 0.99 | 0.95–1.04 | 1.01 | 0.95–1.08 | 1.05 | 0.97–1.14 | 1.12 | 1.02–1.24 | |||||
| College or post | 1.05 | 1.01–1.10 | 1.09 | 1.04–1.15 | 1.05 | 0.97–1.14 | 1.04 | 0.94–1.16 | |||||
| Smoking | Never | 1.03 | 0.99–1.09 | 0.26 | 1.03 | 0.97–1.11 | 0.08 | 1.13 | 1.00–1.27 | 0.69 | 1.35 | 1.07–1.69 | 0.54 |
| Former | 1.03 | 0.99–1.06 | 1.05 | 1.01–1.10 | 1.02 | 0.96–1.07 | 1.05 | 0.98–1.13 | |||||
| Current | 1.05 | 0.99–1.11 | 1.06 | 0.98–1.15 | 1.08 | 0.99–1.17 | 1.13 | 1.03–1.23 | |||||
| aMED index | Quintile 1 | 1.06 | 1.01–1.12 | 0.43 | 1.11 | 1.03–1.19 | 0.18 | 1.07 | 0.99–1.17 | 0.65 | 1.14 | 1.03–1.26 | 0.55 |
| Quintile 2 | 1.04 | 0.98–1.10 | 1.07 | 0.99–1.16 | 1.03 | 0.93–1.12 | 1.07 | 0.95–1.19 | |||||
| Quintile 3 | 1.01 | 0.96–1.06 | 1.05 | 0.98–1.13 | 1.01 | 0.92–1.11 | 1.03 | 0.92–1.15 | |||||
| Quintile 4 | 1.00 | 0.95–1.06 | 1.00 | 0.93–1.07 | 1.08 | 0.97–1.19 | 1.07 | 0.94–1.22 | |||||
| Quintile 5 | 1.05 | 0.99–1.10 | 1.05 | 0.99–1.12 | 1.03 | 0.93–1.15 | 1.13 | 0.99–1.30 | |||||
Definition of abbreviations: aMED = alternate Mediterranean diet index; BMI = body mass index; CI = confidence interval; COPD = chronic obstructive pulmonary disease; HR = hazard ratio.
Adjusted for age, sex, and location, as strata; for race, BMI, education, smoking, diet, and marital status, at individual level; and for median income and percentage with high school education at census-tract level.
Figure 2.
Hazard ratios and 95% confidence intervals for the association between annual average ozone (per 10 ppb) and respiratory disease (A) and cardiovascular disease mortality (B) as a function of annual average daily maximum temperature (Tmax).
Discussion
In this extensive analysis of a large, well-characterized U.S. prospective cohort, long-term exposure to O3 was significantly associated with multiple causes of mortality, including cardiovascular disease, ischemic heart disease, respiratory disease, and COPD. The associations here were generally robust to alternative model specifications and additional adjustment for PM2.5, NO2, and temperature. This study leverages a recent national-level O3 exposure model that estimates annual and warm season O3 concentration levels at the census-tract level, providing improved spatial resolution for the pollutant compared with similar past studies.
To date, studies examining the effects of long-term exposure to ambient O3 on mortality have reported mixed results. A recent analysis of the association between long-term O3 exposure and total mortality in the U.S. Medicare population of more than 60 million reported positive findings; the daily O3 was estimated using a neural network approach that incorporated monitoring data, satellite-based measurements, a chemical transport model, land use terms, and other data at a 1-km resolution (22). The Turner and colleagues study (16) linked EPA gridded estimates for daily 8-hour maximum O3 concentrations in 36-km resolutions in the analysis of multiple mortality causes in the American Cancer Society CPS-II Cohort of 669,046 and found that chronic O3 exposure was significantly associated with multiple causes of death, including all-cause, cardiovascular, and respiratory disease mortality, but not ischemic heart disease mortality. The Turner and colleagues study (16) also similarly evaluated the 12-km resolution downscaler data used in the present study and found similar results between the two exposure estimates, although analysis was limited to a subset of the cohort because an earlier version of the downscaler dataset limited geographically to the Eastern United States was applied.
Analysis of the CanCHEC (Canadian Census Health and Environment Cohort) linked 2.5 million adults to estimates of average daily 8-hour maximum concentrations in the warm seasons with 21-km resolution, and reported significant associations of long-term exposure to O3 with cardiovascular disease and ischemic heart disease mortality, but not with cerebrovascular disease, respiratory disease, or COPD mortality (23). A study linked a national English cohort of 835,607 with 1-km gridded estimates from air dispersion models and reported negative associations between long-term O3 and mortality (24), whereas another study of a French cohort of 20,327 observed nonsignificant associations in relation to O3 estimated from a 2-km resolution chemical transport model (25).
Evidence for a causal association between long-term O3 exposure and cardiovascular disease is more limited, with the U.S. EPA (2) concluding that the evidence is only “suggestive of a causal relationship between long-term exposure to O3 and cardiovascular effects.” Recent estimates of the global burden of disease of O3 did not include cardiovascular diseases in their analyses (13, 14). In this study, we report significant associations between long-term O3 exposure and both cardiovascular disease and ischemic heart disease mortality but not cerebrovascular mortality in single-pollutant models, providing additional evidence that is consistent with recent results from the aforementioned CPS-II and CanCHEC cohorts. We also report a positive and significant association with COPD mortality, which adds to the limited evidence from previous studies, including an ecologic analysis of the relationship between exposure to O3 and chronic lower respiratory disease mortality across continental U.S. counties (26). We observed smaller respiratory disease mortality and pneumonia mortality risks associated with long-term O3 exposure than the CPS-II cohort.
Possible biologic mechanisms by which O3 contributes to development of respiratory diseases include airway hyperreactivity and lung inflammation with release of inflammatory mediators and neutrophil recruitment (27). Long-term O3 exposure resulted in narrowing of the respiratory bronchiole among monkeys, primarily by peribronchiolar inflammation (28). Chronic O3 exposure in mice resulted in epithelial injury and significant increase in airspace density and airspace diameter along with decrease of the number of airspaces, thereby inducing small airway remodeling and emphysema (29). In a longitudinal panel study of 43 young adults that measured personal-level exposure in Shanghai, China, short-term O3 resulted in acute respiratory inflammation, with increase in fractional exhaled nitric oxide and inducible nitric oxide synthase, and decreased average methylation of NOS2A, which encodes inducible nitric oxide synthase (30). Low levels of O3 also adversely affects respiratory health; in a randomized, controlled exposure study of 87 healthy adults (MOSES study), exposure to near ambient levels of O3 induced statistically significant lung function decrease, airway injury, and airway inflammation (31).
The evidence for the relationship between O3 exposure and cardiovascular outcomes is more mixed, although several studies have observed appreciable changes in circulating biomarkers of inflammation, oxidative stress, coagulation, vasoreactivity, and glucose metabolism (32). Increases in systemic proinflammatory markers and adverse changes in cardiac autonomic effects were observed in young, healthy human participants in controlled exposure studies (33, 34). A panel study of air pollution in a population with extant coronary heart disease (n = 13) observed alternations in several pathways associated with cardiovascular morbidity and mortality, including fibrinolysis, systemic inflammation, and vascular reactivity at levels below the EPA standards (35). A study of 89 healthy adults in China found that short-term O3 exposure was associated with platelet activation and increased blood pressure (36). However, in the aforementioned MOSES study no significant effects of O3 exposure on any of the primary or secondary measures of autonomic function, repolarization, ST-segment elevation change, arrhythmia, or vascular function were observed (37).
We also observed significant effect modification by long-term temperature, with elevated respiratory disease mortality risks associated with long-term O3 exposure at high annual mean maximum temperature. Higher temperatures directly influence O3 levels and occurrences of episodes (38), but evidence also points to a potentially interactive effect between O3 and temperature to influence health. A controlled exposure study found that O3-induced systemic effects varied according to temperature, with O3 impairing the fibrinolytic pathway at elevated (32.5°C) temperature, whereas O3 activated the pathway at moderate (22°C) temperature (39). Examination of the long-term effect of O3 exposure on cardiovascular mortality in the CanCHEC cohort found that the association was modified by spatial synoptic classification zone, which is a daily weather classification scheme (40).
Our findings are consistent with findings from the CPS-II studies (15, 16) that observed effect modification by annual average temperature with higher O3-related mortality risks observed with both high and low annual temperatures, and also with multiple short-term O3 exposure studies to date that similarly have observed effect modification by temperature extremes for total and cardiovascular mortality risks (41). Temperature strongly predicts O3 levels and is also a potential confounder (42) of the O3-mortality association. Unlike the CPS-II cohort, the associations between long-term O3 exposure and several causes of death were confounded by temperature, especially for cerebrovascular disease, suggesting that temperature is an important risk factor for this cause of death. The observed disparity between the two cohorts may be caused by differences in cohort characteristics and the spatial resolution and correlation structure of O3 and temperature, especially as daily exposure estimates were averaged and applied in this current study.
This study offers many strengths, including: the large size of the cohort; long follow-up period of 17 years; and the availability of detailed individual-level information, especially for behavioral risk factors, such as smoking and diet. However, limitations are also present in this study; residence census tract and personal covariates were recorded at baseline and prospective changes in these factors could not be accounted for. Another limitation is that the NIH-AARP cohort has a smaller number of participants in races other than white and black non-Hispanic, limiting power to study other races/ethnicities. We were also unable to control for smoking duration, although it is unlikely that this would be a major source of residual confounding because O3-related mortality estimates did not vary significantly across major smoking groups, and observed O3-related mortality effects were generally larger among the never-smokers.
Given the ubiquitous nature of outdoor air pollution exposures, the global burden of disease from O3 is substantial, with estimates that 1.04 to 1.23 million respiratory deaths in adults were attributable to O3 exposure in 2010 (14). The results from this study suggest that long-term exposure to ambient O3 increases cardiovascular disease and respiratory disease mortality risk; taken together in consideration with recent evidence consistent with our results (15, 22), these findings suggest that an annual and/or seasonal standard for ambient O3 concentrations, in addition to the present daily standard, is needed to adequately protect public health. The correlations between daily O3 values with annual and warm season averages are 0.41 and 0.68, respectively, suggesting that these metrics (and potential standards) are moderately distinct, especially for annual exposure.
In summary, this analysis of a large U.S. cohort found that long-term exposure to ambient O3 is associated with elevated risk of death from cardiovascular disease, ischemic heart disease, respiratory disease, and COPD. The results were robust to alternative models and adjustment for copollutants, although some evidence of confounding by temperature was observed. We also observed significantly elevated respiratory disease mortality risk associated with long-term O3 exposure in areas with higher annual average daily maximum temperatures. This study provides additional evidence linking excess mortality to long-term O3 exposure, suggesting that policies aimed at lowering the long-term O3 concentration level are important in alleviating the public health burden associated with ambient O3 exposures.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Joel Kaufman, Michael T. Young, and Sun-Young Kim from the University of Washington for providing the air pollution data used in this study.
Footnotes
Supported by the NIH National Institute of Environmental Health Sciences (1R01 ES019584-01A1, R21 ES021194, and 5T32ES007324-18), U.S. Environmental Protection Agency (RD835871), American Lung Association (Lung Dissertation Grant), and New York University National Institute of Environmental Health Sciences Center of Excellence (ES00260). Air pollution data used in this study were developed under Environmental Protection Agency award Nos. RD831697 (MESA Air) and RD-83830001 (MESA Air Next Stage) and NIH awards K24ES013195 and P30ES07033.
Author Contributions: C.C.L. designed the study, analyzed the data, and wrote the manuscript. J.A., Y.S., D.T.S., R.R.J., and M.L.B. edited the manuscript and contributed to discussion. C.G. aided with developing the exposure datasets and edited the manuscript. R.B.H. and G.D.T. are the main investigators for the study, contributed to interpretation of findings, and edited the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201806-1161OC on May 3, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Song H, Tan W, Zhang X. Ozone induces inflammation in bronchial epithelial cells. J Asthma. 2011;48:79–83. doi: 10.3109/02770903.2010.529224. [DOI] [PubMed] [Google Scholar]
- 2.US Environmental Protection Agency. Washington, DC: US Environmental Protection Agency; 2013. 2013 Final report: integrated science assessment of ozone and related photochemical oxidants. [Google Scholar]
- 3.Gent JF, Triche EW, Holford TR, Belanger K, Bracken MB, Beckett WS, et al. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA. 2003;290:1859–1867. doi: 10.1001/jama.290.14.1859. [DOI] [PubMed] [Google Scholar]
- 4.Bell ML, Dominici F, Samet JM. A meta-analysis of time-series studies of ozone and mortality with comparison to the national morbidity, mortality, and air pollution study. Epidemiology. 2005;16:436–445. doi: 10.1097/01.ede.0000165817.40152.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji M, Cohan DS, Bell ML. Meta-analysis of the association between short-term exposure to ambient ozone and respiratory hospital admissions. Environ Res Lett. 2011;6:02400. doi: 10.1088/1748-9326/6/2/024006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin P, Chen R, Wang L, Meng X, Liu C, Niu Y, et al. Ambient ozone pollution and daily mortality: a nationwide study in 272 Chinese cities. Environ Health Perspect. 2017;125:117006. doi: 10.1289/EHP1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glad JA, Brink LL, Talbott EO, Lee PC, Xu X, Saul M, et al. The relationship of ambient ozone and PM(2.5) levels and asthma emergency department visits: possible influence of gender and ethnicity. Arch Environ Occup Health. 2012;67:103–108. doi: 10.1080/19338244.2011.598888. [DOI] [PubMed] [Google Scholar]
- 8.Ko FW, Tam W, Wong TW, Chan DP, Tung AH, Lai CK, et al. Temporal relationship between air pollutants and hospital admissions for chronic obstructive pulmonary disease in Hong Kong. Thorax. 2007;62:780–785. doi: 10.1136/thx.2006.076166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thurston GD, Lippmann M, Scott MB, Fine JM. Summertime haze air pollution and children with asthma. Am J Respir Crit Care Med. 1997;155:654–660. doi: 10.1164/ajrccm.155.2.9032209. [DOI] [PubMed] [Google Scholar]
- 10.Atkinson RW, Carey IM, Kent AJ, van Staa TP, Anderson HR, Cook DG. Long-term exposure to outdoor air pollution and the incidence of chronic obstructive pulmonary disease in a national English cohort. Occup Environ Med. 2015;72:42–48. doi: 10.1136/oemed-2014-102266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anenberg SC, Horowitz LW, Tong DQ, West JJ. An estimate of the global burden of anthropogenic ozone and fine particulate matter on premature human mortality using atmospheric modeling. Environ Health Perspect. 2010;118:1189–1195. doi: 10.1289/ehp.0901220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [Published erratum appears in Lancet 381:1276.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389:1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malley CS, Henze DK, Kuylenstierna JCI, Vallack HW, Davila Y, Anenberg SC, et al. Updated global estimates of respiratory mortality in adults ≥30 years of age attributable to long-term ozone exposure. Environ Health Perspect. 2017;125:087021. doi: 10.1289/EHP1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jerrett M, Burnett RT, Pope CA, III, Ito K, Thurston G, Krewski D, et al. Long-term ozone exposure and mortality. N Engl J Med. 2009;360:1085–1095. doi: 10.1056/NEJMoa0803894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner MC, Jerrett M, Pope CA, III, Krewski D, Gapstur SM, Diver RW, et al. Long-term ozone exposure and mortality in a large prospective study. Am J Respir Crit Care Med. 2016;193:1134–1142. doi: 10.1164/rccm.201508-1633OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berrocal VJ, Gelfand AE, Holland DM. Space-time data fusion under error in computer model output: an application to modeling air quality. Biometrics. 2012;68:837–848. doi: 10.1111/j.1541-0420.2011.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SY, Olives C, Sheppard L, Sampson PD, Larson TV, Keller JP, et al. Historical prediction modeling approach for estimating long-term concentrations of PM2.5 in cohort studies before the 1999 implementation of widespread monitoring. Environ Health Perspect. 2017;125:38–46. doi: 10.1289/EHP131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young MT, Bechle MJ, Sampson PD, Szpiro AA, Marshall JD, Sheppard L, et al. Satellite-based NO2 and model validation in a national prediction model based on universal kriging and land-use regression. Environ Sci Technol. 2016;50:3686–3694. doi: 10.1021/acs.est.5b05099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daly C, Halbleib M, Smith JI, Gibson WP, Doggett MK, Taylor GH, et al. Physiographically sensitive mapping of climatological temperature and precipitation across the conterminous United States. Int J Climatol. 2008;28:2031–2064. [Google Scholar]
- 21.Lim CC, Hayes RB, Ahn J, Shao Y, Silverman DT, Jones RR, et al. Mediterranean diet and the association between air pollution and cardiovascular disease mortality risk. Circulation. 2019;139:1766–1775. doi: 10.1161/CIRCULATIONAHA.118.035742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, et al. Air pollution and mortality in the Medicare population. N Engl J Med. 2017;376:2513–2522. doi: 10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crouse DL, Peters PA, Hystad P, Brook JR, van Donkelaar A, Martin RV, et al. Ambient PM2.5, O3, and NO2 exposures and associations with mortality over 16 years of follow-up in the Canadian Census Health and Environment Cohort (CanCHEC) Environ Health Perspect. 2015;123:1180–1186. doi: 10.1289/ehp.1409276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carey IM, Atkinson RW, Kent AJ, van Staa T, Cook DG, Anderson HR. Mortality associations with long-term exposure to outdoor air pollution in a national English cohort. Am J Respir Crit Care Med. 2013;187:1226–1233. doi: 10.1164/rccm.201210-1758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bentayeb M, Wagner V, Stempfelet M, Zins M, Goldberg M, Pascal M, et al. Association between long-term exposure to air pollution and mortality in France: a 25-year follow-up study. Environ Int. 2015;85:5–14. doi: 10.1016/j.envint.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Hao Y, Balluz L, Strosnider H, Wen XJ, Li C, Qualters JR. Ozone, fine particulate matter, and chronic lower respiratory disease mortality in the United States. Am J Respir Crit Care Med. 2015;192:337–341. doi: 10.1164/rccm.201410-1852OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michaudel C, Couturier-Maillard A, Chenuet P, Maillet I, Mura C, Couillin I, et al. Inflammasome, IL-1 and inflammation in ozone-induced lung injury. Am J Clin Exp Immunol. 2016;5:33–40. [PMC free article] [PubMed] [Google Scholar]
- 28.Fujinaka LE, Hyde DM, Plopper CG, Tyler WS, Dungworth DL, Lollini LO. Respiratory bronchiolitis following long-term ozone exposure in bonnet monkeys: a morphometric study. Exp Lung Res. 1985;8:167–190. doi: 10.3109/01902148509057520. [DOI] [PubMed] [Google Scholar]
- 29.Michaudel C, Fauconnier L, Julé Y, Ryffel B. Functional and morphological differences of the lung upon acute and chronic ozone exposure in mice. Sci Rep. 2018;8:10611. doi: 10.1038/s41598-018-28261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niu Y, Chen R, Xia Y, Cai J, Lin Z, Liu C, et al. Personal ozone exposure and respiratory inflammatory response: the role of DNA methylation in the arginase-nitric oxide synthase pathway. Environ Sci Technol. 2018;52:8785–8791. doi: 10.1021/acs.est.8b01295. [DOI] [PubMed] [Google Scholar]
- 31.Arjomandi M, Balmes JR, Frampton MW, Bromberg P, Rich DQ, Stark P, et al. MOSES (The Multicenter Ozone Study in Older Subjects) Respiratory responses to ozone exposure: MOSES (the multicenter ozone study in older subjects) Am J Respir Crit Care Med. 2018;197:1319–1327. doi: 10.1164/rccm.201708-1613OC. [DOI] [PubMed] [Google Scholar]
- 32.Goodman JE, Prueitt RL, Sax SN, Pizzurro DM, Lynch HN, Zu K, et al. Ozone exposure and systemic biomarkers: evaluation of evidence for adverse cardiovascular health impacts. Crit Rev Toxicol. 2015;45:412–452. doi: 10.3109/10408444.2015.1031371. [DOI] [PubMed] [Google Scholar]
- 33.Devlin RB, Duncan KE, Jardim M, Schmitt MT, Rappold AG, Diaz-Sanchez D. Controlled exposure of healthy young volunteers to ozone causes cardiovascular effects. Circulation. 2012;126:104–111. doi: 10.1161/CIRCULATIONAHA.112.094359. [DOI] [PubMed] [Google Scholar]
- 34.Arjomandi M, Wong H, Donde A, Frelinger J, Dalton S, Ching W, et al. Exposure to medium and high ambient levels of ozone causes adverse systemic inflammatory and cardiac autonomic effects. Am J Physiol Heart Circ Physiol. 2015;308:H1499–H1509. doi: 10.1152/ajpheart.00849.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mirowsky JE, Carraway MS, Dhingra R, Tong H, Neas L, Diaz-Sanchez D, et al. Ozone exposure is associated with acute changes in inflammation, fibrinolysis, and endothelial cell function in coronary artery disease patients. Environ Health. 2017;16:126. doi: 10.1186/s12940-017-0335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Day DB, Xiang J, Mo J, Li F, Chung M, Gong J, et al. Association of ozone exposure with cardiorespiratory pathophysiologic mechanisms in healthy adults. JAMA Intern Med. 2017;177:1344–1353. doi: 10.1001/jamainternmed.2017.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rich DQ, Balmes JR, Frampton MW, Zareba W, Stark P, Arjomandi M, et al. Cardiovascular function and ozone exposure: the Multicenter Ozone Study in oldEr Subjects (MOSES) Environ Int. 2018;119:193–202. doi: 10.1016/j.envint.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 38.Likhvar VN, Pascal M, Markakis K, Colette A, Hauglustaine D, Valari M, et al. A multi-scale health impact assessment of air pollution over the 21st century. Sci Total Environ. 2015;514:439–449. doi: 10.1016/j.scitotenv.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Kahle JJ, Neas LM, Devlin RB, Case MW, Schmitt MT, Madden MC, et al. Interaction effects of temperature and ozone on lung function and markers of systemic inflammation, coagulation, and fibrinolysis: a crossover study of healthy young volunteers. Environ Health Perspect. 2015;123:310–316. doi: 10.1289/ehp.1307986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cakmak S, Hebbern C, Vanos J, Crouse DL, Burnett R. Ozone exposure and cardiovascular-related mortality in the Canadian Census Health and Environment Cohort (CANCHEC) by spatial synoptic classification zone. Environ Pollut. 2016;214:589–599. doi: 10.1016/j.envpol.2016.04.067. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Woodward A, Hou XY, Zhu T, Zhang J, Brown H, et al. Modification of the effects of air pollutants on mortality by temperature: a systematic review and meta-analysis. Sci Total Environ. 2017;575:1556–1570. doi: 10.1016/j.scitotenv.2016.10.070. [DOI] [PubMed] [Google Scholar]
- 42.Chen K, Wolf K, Hampel R, Stafoggia M, Breitner S, Cyrys J, et al. OP VII – 2 does temperature confounding control influence the modifying effect of air temperature in ozone–mortality associations? Occup Environ Med. 2018;75:A14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.