Abstract
An estimated 14.1 million patients survive sepsis each year. Many survivors experience poor long-term outcomes, including new or worsened neuropsychological impairment; physical disability; and vulnerability to further health deterioration, including recurrent infection, cardiovascular events, and acute renal failure. However, clinical trials and guidelines have focused on shorter-term survival, so there are few data on promoting longer-term recovery. To address this unmet need, the International Sepsis Forum convened a colloquium in February 2018 titled “Understanding and Enhancing Sepsis Survivorship.” The goals were to identify gaps and limitations of current research and shorter- and longer-term priorities for understanding and enhancing sepsis survivorship. Twenty-six experts from eight countries participated. The top short-term priorities identified by nominal group technique culminating in formal voting were to better leverage existing databases for research, develop and disseminate educational resources on postsepsis morbidity, and partner with sepsis survivors to define and achieve research priorities. The top longer-term priorities were to study mechanisms of long-term morbidity through large cohort studies with deep phenotyping, build a harmonized global sepsis registry to facilitate enrollment in cohorts and trials, and complete detailed longitudinal follow-up to characterize the diversity of recovery experiences. This perspective reviews colloquium discussions, the identified priorities, and current initiatives to address them.
Keywords: survivorship, critical illness, rehabilitation
Sepsis—life-threatening organ dysfunction caused by a dysregulated host response to infection (1)—is a leading cause of global morbidity and mortality. An estimated 14.1 million adults and 2.5 million children survive sepsis each year (2, 3), and many survivors experience poor long-term outcomes (4). Patients develop an average one or two new functional limitations after sepsis (5), and 10–40% experience new cognitive impairment (5–8). Anxiety (9), depression (10), and post-traumatic stress disorder (11) symptoms exceed population-level norms. Furthermore, sepsis survivors are vulnerable to further health problems (4). Up to 40% are rehospitalized within 90 days (12), and rates of recurrent infection, sepsis, cardiovascular events, acute renal failure, and aspiration are increased relative to age- and comorbidity-matched control subjects (4, 13–15). As a result, sepsis survivors are often unable to live independently after sepsis (16), cannot return to work (17), and have increased risk of dying for up to 2 years (18). Thus, sepsis should be viewed as a life-changing and disability-inducing event.
A 2017 World Health Organization resolution on sepsis called on member states to address the needs of survivors, recognizing the burden of longer-term sepsis-related morbidity (19). However, guidelines have traditionally focused on early recognition and management, not on mitigation of longer-term sequelae (20). Likewise, clinical trials typically use shorter-term mortality endpoints and only rarely collect data on functional outcomes or quality of life (21, 22). Perhaps not surprisingly, given the lack of attention to sepsis survivorship, many patients report dissatisfaction with follow-up care after hospitalization (23).
To address this unmet need, a colloquium titled “Understanding and Enhancing Sepsis Survivorship,” sponsored by the International Sepsis Forum, was held in February 2018. The colloquium brought together a diverse group of healthcare professionals, researchers, and patient representatives to distill essential findings on sepsis survivorship and articulate how to improve longer-term recovery. This perspective reports on gaps and limitations of current knowledge on sepsis survivorship, research priorities and their rationales, and current initiatives to address these priorities.
Methods
The colloquium chairs (H.C.P., K.M.R., and D.C.A.) identified participants on the basis of expertise and through snowball sampling by recommendation. Participants outside critical care and infectious disease were intentionally invited to provide experiences and examples of successes in analogous areas. Collectively, the group had expertise in sepsis, critical care, infectious diseases, aging, physical medicine and rehabilitation, psychology, and physiotherapy.
During the colloquium, we used “nominal group technique” to rapidly gain consensus on research priorities. This process involves problem identification, solution generation, and decision making by group vote (24). Before the colloquium, participants were asked to consider gaps and limitations of current research (based on literature review and their expert opinion), then generate potential next steps to move the field forward. Participants identified recent systematic reviews pertinent to sepsis survivorship that informed their thinking for inclusion in a “review of reviews” included in this article. During the colloquium, ideas were shared through presentations and group discussion (see Appendix E1 in the online supplement for colloquium agenda). Our discussion was informed by recent comprehensive reviews on adult sepsis survivorship (4) and pediatric critical illness survivorship (25), as well as by systematic reviews identified by participants.
At the end of the colloquium, participants listed potential next steps over 2-year and 10-year horizons to “do more with what we have” in the shorter term and “develop and deliver more” in the longer term, respectively. Ideas were prioritized by group vote. Each participant could cast 12 votes, 6 for shorter-term and 6 for longer-term priorities. Votes could be allocated in any way—all six for a single idea or split among several ideas. After the colloquium, the organizing chairs drafted the manuscript of this article, which was circulated to participants for critical appraisal, revision, and final approval.
Results
Summary of Evidence
Participants also identified 30 recent systematic reviews pertinent to sepsis survivorship (Table E1). The main findings, as well as the gaps and limitations identified by our review of systematic reviews, are summarized in Table E2.
Limitations of Existing Research
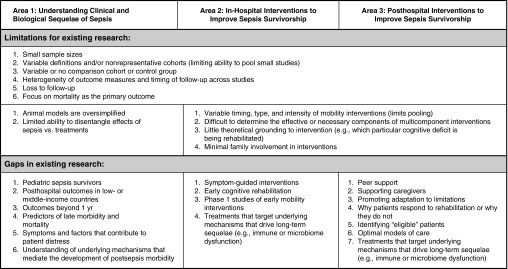
Participants identified the following limitations of existing research as most important: 1) variable inclusion/exclusion criteria, outcomes measures, and timing of outcome assessments, making it difficult to pool studies to yield larger and more generalizable study populations; and 2) small or nonrepresentative patient populations (Figure 1 and Tables E2 and E3).
Figure 1.
Key limitations and gaps of existing research.
Gaps in Research
Participants identified the following gaps in research as most important: 1) limited data on longer-term outcomes of specific patient populations, such as pediatric sepsis survivors and the majority of sepsis survivors who reside in low- or middle-income countries; 2) limited data on outcomes beyond 1 year; 3) few studies of in-hospital or posthospital interventions to enhance longer-term survival and quality of life; and 4) limited data on how to identify patients most likely to benefit from interventions (Figure and Tables E2 and E4). Additional research is needed to guide clinical management during and after sepsis to best promote long-term recovery (Table 1). In particular, studies are needed to define the benefit and optimal delivery of early mobilization, physical rehabilitation, early cognitive rehabilitation, peer support, supportive interventions for caregivers, and interventions to assist survivors in adapting to new limitations. In the meantime, we refer readers to a recent review (4) for an evidence summary and pragmatic recommendations for clinical practice.
Table 1.
Key Questions for Clinical Management of Sepsis Survivors
| In-hospital treatments |
| • How do common in-hospital treatments for sepsis (e.g., antibiotics, fluid resuscitation, vasopressors, and organ support) impact physical function, healthcare use, and quality of life 6–12 mo after sepsis? |
| • Can in-hospital sepsis treatments be refined to optimize physical function, quality of life, and days spent at home in the 6–12 mo after sepsis? |
| Early physical and cognitive rehabilitation |
| • What are the optimal characteristics of early rehabilitation (e.g., timing, dosage, intensity, and duration)? |
| • Are there specific subsets of patients for whom early rehabilitation may be harmful? |
| • How should rehabilitation be tailored to specific subsets of patients? |
| • Does early rehabilitation result in improved long-term physical and cognitive function at 6–12 mo after sepsis? |
| Transitions of care |
| • What is the optimal mechanism to transition patients from ICU, to ward, to post–acute care facilities, and ultimately to primary care management? |
| Follow-up care |
| • Does earlier outpatient follow-up (e.g., within 7–14 d) result in improved patient and caregiver satisfaction and in a greater number of days alive and out of hospital at 6 mo? |
| • Do specialized postsepsis follow-up programs lead to reduced healthcare use, improved physical function, and/or improved patient and caregiver satisfaction at 6–12 mo? |
| • Which patients are most likely to benefit from specialized postsepsis follow-up care? |
| • What are the necessary components of sepsis aftercare, and how can they be scaled for delivery outside of specialized follow-up programs? |
| • Does referral to peer support programs result in improved patient satisfaction, caregiver satisfaction, or patient health-related quality of life? |
Successes in Related Fields
Relevant expert participants presented models of success in the fields of cancer, dementia, stroke, and traumatic brain injury that each have research programs promoting recovery from and/or adaptation to new disease-related physical, cognitive, or psychological impairment (Table 2). Dedicated follow-up clinics, which serve both to support patients and to generate and test research hypotheses, exist for each of these conditions in at least some countries. In addition, these fields, particularly cancer, have large-scale public awareness campaigns, philanthropy-funded research programs, successful integration of patients into the prioritization of research questions, and large-scale longitudinal registries. These solutions should be adapted and applied to sepsis survivorship.
Table 2.
Examples and Models of Success from Other Fields
| Analogous Condition | Similarities | Differences | Successful Programs That May Be Applied to Sepsis |
|---|---|---|---|
| Cancer | Like sepsis, cancer and its treatment commonly result in new morbidity, increased risk for certain medical complications, and post–acute mortality | The duration of cancer treatment is longer, such that patients are more likely to self-identify as cancer survivors. A defined specialty group provides both the acute and longer-term care | Large-scale registries and International Association of Cancer Registries (59) |
| Registry–RCT linkages | |||
| Public awareness campaigns | |||
| Philanthropy-funded research | |||
| Long-term follow-up clinics | |||
| Peer support groups | |||
| Dementia | Like sepsis, dementia typically occurs in older patients with multimorbidity and often requires family members to take on caregiving roles. Like sepsis, dementia has suffered from a lack of targeted therapies entering the market despite improved understanding of its pathophysiology | Unlike sepsis, many dementias are slowly progressive diseases. A defined specialty group provides both the acute and longer-term care | Regional registries of patients with dementia (60) |
| Industry–academia research collaboration with multinational register, standardized follow-up, and intentional invitations to participate in early-stage “adaptive” clinical trials (e.g., European Prevention of Alzheimer’s Dementia Consortium [75, 76]) | |||
| Stroke/TBI | Like sepsis, stroke and TBI may be followed by profound new functional and cognitive limitations | More focal injuries with discrete lesions and associated functional and cognitive limitations. A defined specialty group provides both the acute and longer-term care | Structured acute rehabilitation and long-term follow-up programs |
| Regional registries of patients with TBI (61, 62) |
Definition of abbreviations: RCT = randomized controlled trial; TBI = traumatic brain injury.
Is Sepsis Survivorship Unique?
During the colloquium, we considered the extent to which sepsis survivorship is a unique problem. Many challenges are shared with broader populations of patients surviving an acute illness (26, 27) as described by “posthospital syndrome” (28) (an acquired, transient period of generalized risk for a range of adverse health events), “post–intensive care syndrome” (29) (new or worsened physical, cognitive, or mental health impairment after critical illness), and “persistent inflammation, immunosuppression, and catabolism syndrome” (30) (a collection of persistent physiological derangements after sepsis, trauma, or major surgery). Moreover, patients’ experiences after sepsis are often influenced by multimorbidity, frailty, and progressively declining health before sepsis (31, 32). For this reason, the magnitude and type of postsepsis problems measured in studies depend heavily on the comparison—whether sepsis survivors are compared with age- and sex-matched population control subjects, patients hospitalized for infection, or other ICU patients. It is important to consider the control population when interpreting and designing matched cohort studies measuring the impact of sepsis (Table E5).
Despite the overlap with other populations, there are some benefits to focusing research and treatment on sepsis survivors rather than on general ICU survivors. Serious sequelae of sepsis are not limited to patients treated in an ICU. Furthermore, organizing educational information around sepsis may be more accessible to patients, who rarely self-identify as ICU survivors. Indeed, a growing number of websites provide information on “life after sepsis” (33, 34), “postsepsis symptoms” (35), or “postsepsis syndrome” (36, 37).
Discussion Themes
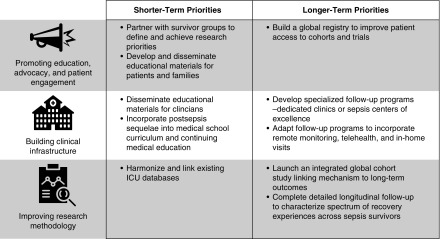
The following themes emerged as central foci (Figure 2):
-
1.
Promoting education, advocacy, and patient engagement, including more robust involvement of patients and caregivers in setting research priorities;
-
2.
Building clinical infrastructure, such as critical illness follow-up clinics or sepsis centers of excellence to address the multifaceted needs of sepsis survivors, concentrate learning by doing, and provide a setting to pilot and test novel rehabilitation strategies more efficiently (e.g., as in the Society of Critical Care Medicine THRIVE International Peer Support Collaborative [38] and ICU follow-up clinics [39]); and
-
3.
Improving research methods.
Particular areas of methodological need include robust and proximal surrogate outcome measures that distinguish underlying mechanism of injury, measures that precisely characterize patient outcomes while minimizing response burden, theory-guided interventions (i.e., tailoring interventions to the mechanism/type of impairment), longer duration of longitudinal follow-up, and translational studies leveraging multimodal assessment (from gene expression through patient-reported outcomes).
Figure 2.
Shorter and longer-term research priorities, by theme. Building clinical infrastructure was viewed as critically important not only to meet the multifaceted needs of sepsis survivors but also to provide a concentrated venue to learn about sepsis survivors and pilot novel interventions to promote adaptation and/or recovery more efficiently.
Prioritization of Shorter- and Longer-Term Goals
The top shorter-term priorities were 1) merging ICU databases across countries and developing consensus-harmonized data elements for such databases (15.2% of votes); 2) developing and disseminating educational materials for patients, families, and clinicians (15.2%); and 3) making deep connections with survivor groups to define and achieve research priorities (14.5%) (Figure 2). The full list of potential next steps is presented in Table E6. Voting on shorter-term priorities is presented in Table E7.
The top longer-term priorities were 1) building an integrated global cohort study linking mechanism to long-term outcomes (17.4% of votes), 2) building a global sepsis cohort to feed into observational research and therapy trials (15.9%), and 3) incorporating detailed long-term longitudinal follow-up to characterize trajectories of recovery/survivorship across patients (14.5%) (Figure 3). Voting on longer-term priorities is presented in Table E8.
Figure 3.
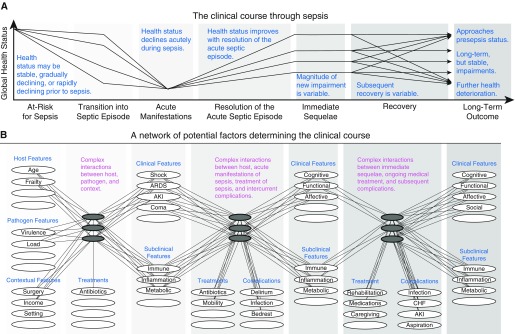
Conceptual diagram of patients’ clinical course through sepsis and underlying factors that influence an individual patient’s trajectory. There are many potential clinical courses that a patient may experience after a hospitalization for sepsis, from rapid complete recovery to recurrent complications and death. (A and B) This figure (adapted by permission from a conceptual diagram first promoted by Prescott and Angus [4]) depicts common clinical trajectories (A) and presents factors important to shaping a patient’s clinical course and long-term outcome (B). This figure draws from the Wilson-Cleary model (90), which links underlying biological factors to physical function and quality of life but extends the representation of the biological factors to demonstrate their complex and unmeasurable interactions. Observable factors, such as presenting features and clinical manifestations of disease, are presented as white ellipses, and the unmeasurable biological interactions are presented as dark gray ellipses. Not all ellipses are labeled, representing the incomplete knowledge of the factors determining clinical course. This diagram is intended to convey that innumerable factors interact in complex ways to determine a patient’s long-term outcome and that the measurable manifestations of disease cannot fully predict the evolution of a patient’s recovery, owing to the unmeasurable biological interactions at play. AKI = acute kidney injury; ARDS = acute respiratory distress syndrome; CHF = congestive heart failure.
Shorter-term priority 1: harmonizing and linking existing ICU databases
A number of high-quality ICU databases (40–42), although not primarily developed for research, have proven valuable to answering research questions. Moreover, ICU databases have recently been developed in several lower- and middle-income countries (43–45). However, these databases are rarely linked to each other or to other data sources. As such, research questions are often limited to those that can be answered within the single database, constraining generalizability to select regions or hospital systems and to the shorter-term outcomes collected.
Investing in data linkages would realize the full potential of existing data, facilitate longer-term follow-up, and enable cross-system comparisons. For example, Brazil’s ORCHESTRA (Organizational Characteristics in Critical Care) study database was recently linked to the United Kingdom’s Intensive Care National Audits and Research Centre Case Mix Program database to compare prevalence and outcomes of ICU-treated sepsis between Brazil and England (46).
Importantly, it is not necessary to share patient-level data. Rather, analyses can be completed in secure data enclaves (47) or by pooling aggregate results, as was done for the Sepsis-3 validation (48) and evaluation of U.S. sepsis incidence (49).
Beyond linking existing databases, ICU dataset specifications should be harmonized, such that basic demographic, illness severity, and treatment data are collected in a consistent manner on consistent scales to facilitate comparison. Similarly, core outcome sets for sepsis research are needed to facilitate robust meta-analyses with harmonized study definitions (e.g., PRISM [Protocolized Resuscitation in Sepsis Meta-Analysis] study [50]). Several initiatives are underway to achieve this goal (51–53).
Shorter-term priority 2: developing and disseminating educational materials to raise awareness of the consequences of sepsis
Public awareness of sepsis has increased in recent years, but recognition still lags behind other acute medical conditions (54), and awareness of long-term sequelae (e.g., physical and neuropsychological impairment and increased risk for recurrent infection) remains particularly low. The challenges of sepsis survivorship are not covered in current sepsis guidelines (20) and are rarely discussed during hospitalization (23, 55).
Several educational resources on sepsis survivorship have been developed (33, 34, 37, 56, 57). However, these materials must be disseminated more broadly. Panelists made the following recommendations:
-
1.
Educate patients and families about life after sepsis in the peridischarge period;
-
2.
Develop and disseminate educational materials to clinicians working in post–acute care facilities and the outpatient setting, such as a recent perspective on sepsis survivorship geared toward physical therapists (58); and
-
3.
Incorporate education on sepsis sequelae into medical school curricula, professional society conferences, and continuing medical education opportunities.
Shorter-term priority 3: building deep connections with survivor groups to define and achieve research priorities
Patient advocacy groups play an important role in defining research priorities and funding research for many diseases. For example, the Cystic Fibrosis Foundation funds drug development and randomized clinical trials. Their website reports that “nearly every CF drug was made possible by the Foundation and because of funds raised from Great Strides [walks]” (59).
Sepsis advocacy groups—such as the Global Sepsis Alliance (60), the UK Sepsis Trust (61), the Latin American Sepsis Institute (62), the Sepsis Alliance (63), and the Rory Staunton Foundation (64)—have spurred large-scale awareness and quality improvement initiatives, such as World Sepsis Day (65, 66), nationwide quality improvement programs in Brazil (67), and “Rory’s Regulations” in New York State (68, 69). These efforts have saved lives. Moreover, in 2017, the World Health Organization passed a resolution recognizing sepsis as a global health priority (19).
Despite these successes, patients have historically been absent from defining sepsis research priorities. One challenge is that patients surviving sepsis may be less inclined to define themselves as disease survivors than patients surviving cancer or a lifelong disease such as cystic fibrosis. Going forward, however, researchers must better engage with sepsis survivors to advance sepsis research. Recent examples of increased public involvement include 1) collaboration of patients, caregivers, and clinicians to create the James Lind Alliance’s top 10 research questions for intensive care (70); 2) inclusion of multiple public members on the current Surviving Sepsis Campaign guidelines panel; 3) inclusion of patient and caregiver representatives on the Delphi panel for developing core outcome measures for acute respiratory distress syndrome survivors (71, 72); and 4) codesign of critical illness follow-up clinics by patients and clinicians (73, 74).
Longer-term priorities
The following were the top longer-term priorities:
-
1.
A global cohort study linking mechanism to long-term outcomes;
-
2.
A global sepsis registry from which patients could be enrolled in observational and interventional studies;
-
3.
Detailed long-term longitudinal follow-up to characterize the heterogeneity of recovery across sepsis survivors.
We believe the longer-term priorities are best tackled jointly through a systematic research program on sepsis survivorship (Figure 4). The European Prevention of Alzheimer’s Dementia (EPAD) Consortium—a multinational industry–academia initiative to “create a novel environment for testing numerous interventions targeted at the prevention of Alzheimer’s dementia”—could serve as a model (75). EPAD aims to advance antidementia research and treatment by 1) improving patients’ access to existing cohorts and registries, 2) developing a master registry of patients at increased risk of Alzheimer’s dementia, 3) establishing a longitudinal cohort study of 6,000 patients, and 4) deploying a proof-of-concept adaptive trial enrolling patients from the master registry of at-risk patients (76). In essence, EPAD connects existing registries and serves as a unified entry point for early-phase clinical trials.
Figure 4.
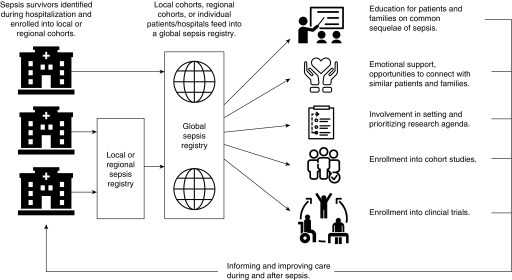
Conceptual diagram of a global sepsis registry. Although some services (education and emotional support) are ideally provided locally or regionally, they are not universally available. A global sepsis registry could provide universal opportunities for enrollment in cohorts and interventional trials, as well as safety net services for patients without local sepsis survivorship resources.
Similar infrastructure could dramatically accelerate sepsis research. Akin to EPAD, a sepsis consortium could start as a harmonized international registry, bringing together existing sepsis registries such as the Mid-German Sepsis Cohort (77) and patients with sepsis from ICU registries. From the harmonized registry, patients could be invited to join longitudinal cohort studies. The consortium could provide a venue for education and peer support, potentially providing immediate benefits to participants and encouraging retention. Over the longer term, it would improve understanding of postsepsis sequelae. Ultimately, adaptive platform trials could be incorporated to test promising interventions after sepsis.
The major limitation of a freestanding sepsis consortium, however, is that data on presepsis health status could be collected only retrospectively. To overcome this limitation, the consortium could be embedded within ongoing population cohort studies (e.g., UK Biobank [78], Norway’s HUNT Study [Nord-Trøndelag Health Study] [79], the NIH’s All of Us Research Program [80], the Department of Veterans Affairs Million Veterans Program [81], and the New South Wales 45 and Up Study [82]) that already collect genomic and health data on millions of individuals.
Cohort studies (e.g., Health and Retirement Study [83] and Cardiovascular Health Study [84]) have already been leveraged to measure the impact of presepsis health status/trajectory on sepsis outcomes (31, 85). However, in these studies, sepsis cases were identified by diagnosis codes in linked claims data, and there is limited data on patient outcomes in the months immediately after sepsis hospitalization.
Prospectively embedding a sepsis consortium within ongoing cohort studies would provide several distinct benefits over existing research, including 1) accurate identification of sepsis cases by prospectively collecting data on all potential sepsis hospitalizations, 2) better characterization of sepsis, and 3) increased intensity of data collection to characterize recovery after sepsis (e.g., serial collection of biospecimens and patient-reported outcomes).
A global, harmonized sepsis consortium would be a natural arena in which to advance standardized core baseline variables and core outcome sets for sepsis (51); to refine their measurement across the continuum of sepsis; and to develop platforms to collect such information across participating sites, drawing directly from electronic health records and existing databases when possible. The consortium could also promote nonmortality outcomes, following the U.S. Food and Drug Administration’s Critical Path Initiative process for proxy outcome development (86), and select from among existing (or develop new) item-response theory-based instruments to measure core outcomes in a way that maximizes information and minimizes participant burden.
Sepsis survivorship research has often focused on a particular outcome (e.g., cognitive function, physical function, or healthcare use) or a particular aspect of the underlying mechanistic pathways driving morbidity and mortality (e.g., genomics, transcriptomics, or proteomics in isolation). However, a sepsis consortium could support broad translational studies, simultaneously examining genomic and transcriptomic host response together with behavioral adaptations and multifaceted patient outcomes, to understand the mechanisms driving the long-term morbidity and mortality (Figure 3).
Finally, although prior cohort studies have defined the average experience of patients in the year after sepsis, there is evidence of wide heterogeneity of experiences across individual patients. It is hypothesized that there are characteristic trajectories of recovery, adaptation, and ongoing/progressive disability after sepsis (87). A sepsis consortium could support large, population-based cohorts with detailed longitudinal follow-up necessary to 1) objectively identify and define the characteristic pathways of recovery versus disability after sepsis, 2) predict a patient’s likely postsepsis trajectory, and 3) identify modifiable factors influencing a patient’s recovery that could be targeted in future interventional studies.
Additional Areas of Focus
Beyond the top priorities, there was considerable interest in developing 1) an item-response theory computerized adaptive testing question bank for long-term sepsis outcomes and 2) better animal models of sepsis. Current instruments used to assess postsepsis outcomes (e.g., neuropsychological status and quality of life) may not detect subtle declines, but detailed assessments impose expense and respondent burden. Computerized adaptive testing characterizes a person’s ability more precisely and efficiently than standard surveys, is used widely in other settings (e.g., intelligence testing), and was recently used to study functional recovery after pediatric critical illness (88). Computerized adaptive testing would be useful for characterizing heterogeneous outcomes of sepsis survivors, but questions must first be selected and calibrated for use.
For preclinical studies, animal sepsis models can reproduce many of the acute immune defects seen in patients with sepsis, but they would need to be adapted considerably to be useful for studying longer-term recovery. In most instances, the animals used are young, have no comorbid disease, and are not treated with typical sepsis therapies (e.g., antibiotics, fluids, or supplemental oxygen). Furthermore, because the goal is often to study short-term survival, animal models have been designed such that only a minority of animals survive the acute insult. These limitations have been addressed in a recent expert consensus initiative for improving animal modeling in sepsis that aims to improve the translation of preclinical findings (89).
Conclusions
Sepsis is a common cause of hospitalization that frequently results in new morbidity. Shorter-term priorities to improve outcomes for survivors include leveraging existing databases, improving awareness of postsepsis morbidity, and connecting with sepsis survivors to define and achieve research priorities. Longer-term priorities are to understand the mechanisms driving long-term sequelae and to characterize heterogeneity of recovery experiences, both of which will inform future interventions. These longer-term priorities may be best accomplished through a global, harmonized sepsis research consortium embedded within existing large prospective cohorts.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the Canadian Critical Care Conference, Zena Davidson, and Elaine Rinicker for coordinating meeting space for the colloquium.
Footnotes
The colloquium was sponsored by the International Sepsis Forum and InFACT (International Forum for Acute Care Trials). Supported by NIH grant K08 GM115859 (H.C.P.), Academic Health Science Centre Alternative Funding Plan Innovation Fund no. 3576.3014214 (K.C.), Australian National Health and Medical Research Council Practitioner Fellowship Grant 1117230 (S.F.), NIH grant R01 HL135144 (T.D.G.), a Heart Foundation Future Leader Fellowship from Australia (C.H.), NIH grant R01 GM097471 (S.Y.), and the Intramural Research Program at the National Institute on Aging (L.F.). The views in this article do not reflect the position or policy of the U.S. government or the Department of Veterans Affairs.
Author Contributions: Drafting of the manuscript: H.C.P., K.M.R., and D.C.A. Contribution to the conception and design, critical revision for important intellectual content, final approval, and agreement to be accountable for all aspects of the work: all authors.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201812-2383CP on June 4, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: on behalf of the International Sepsis Forum
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischmann C, Scherag A, Adhikari NKJ, Hartog CS, Tsaganos T, Schlattmann P, et al. International Forum of Acute Care Trialists. Assessment of global incidence and mortality of hospital-treated sepsis: current estimates and limitations. Am J Respir Crit Care Med. 2016;193:259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 3.Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. 2018;6:223–230. doi: 10.1016/S2213-2600(18)30063-8. [DOI] [PubMed] [Google Scholar]
- 4.Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA. 2018;319:62–75. doi: 10.1001/jama.2017.17687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Needham DM, Colantuoni E, Dinglas VD, Hough CL, Wozniak AW, Jackson JC, et al. Rosuvastatin versus placebo for delirium in intensive care and subsequent cognitive impairment in patients with sepsis-associated acute respiratory distress syndrome: an ancillary study to a randomised controlled trial. Lancet Respir Med. 2016;4:203–212. doi: 10.1016/S2213-2600(16)00005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al. BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girard TD, Thompson JL, Pandharipande PP, Brummel NE, Jackson JC, Patel MB, et al. Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. Lancet Respir Med. 2018;6:213–222. doi: 10.1016/S2213-2600(18)30062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikayin S, Rabiee A, Hashem MD, Huang M, Bienvenu OJ, Turnbull AE, et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2016;43:23–29. doi: 10.1016/j.genhosppsych.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabiee A, Nikayin S, Hashem MD, Huang M, Dinglas VD, Bienvenu OJ, et al. Depressive symptoms after critical illness: a systematic review and meta-analysis. Crit Care Med. 2016;44:1744–1753. doi: 10.1097/CCM.0000000000001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker AM, Sricharoenchai T, Raparla S, Schneck KW, Bienvenu OJ, Needham DM. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med. 2015;43:1121–1129. doi: 10.1097/CCM.0000000000000882. [DOI] [PubMed] [Google Scholar]
- 12.Prescott HC, Langa KM, Iwashyna TJ. Readmission diagnoses after hospitalization for severe sepsis and other acute medical conditions. JAMA. 2015;313:1055–1057. doi: 10.1001/jama.2015.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yende S, Linde-Zwirble W, Mayr F, Weissfeld LA, Reis S, Angus DC. Risk of cardiovascular events in survivors of severe sepsis. Am J Respir Crit Care Med. 2014;189:1065–1074. doi: 10.1164/rccm.201307-1321OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen HN, Lu CL, Yang HH. Risk of recurrence after surviving severe sepsis: a matched cohort study. Crit Care Med. 2016;44:1833–1841. doi: 10.1097/CCM.0000000000001824. [DOI] [PubMed] [Google Scholar]
- 15.Ou SM, Chu H, Chao PW, Lee YJ, Kuo SC, Chen TJ, et al. Long-term mortality and major adverse cardiovascular events in sepsis survivors: a nationwide population-based study. Am J Respir Crit Care Med. 2016;194:209–217. doi: 10.1164/rccm.201510-2023OC. [DOI] [PubMed] [Google Scholar]
- 16.Yende S, Austin S, Rhodes A, Finfer S, Opal S, Thompson T, et al. Long-term quality of life among survivors of severe sepsis: analyses of two international trials. Crit Care Med. 2016;44:1461–1467. doi: 10.1097/CCM.0000000000001658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poulsen JB, Møller K, Kehlet H, Perner A. Long-term physical outcome in patients with septic shock. Acta Anaesthesiol Scand. 2009;53:724–730. doi: 10.1111/j.1399-6576.2009.01921.x. [DOI] [PubMed] [Google Scholar]
- 18.Prescott HC, Osterholzer JJ, Langa KM, Angus DC, Iwashyna TJ. Late mortality after sepsis: propensity matched cohort study. BMJ. 2016;353:i2375. doi: 10.1136/bmj.i2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing sepsis as a global health priority: a WHO resolution. N Engl J Med. 2017;377:414–417. doi: 10.1056/NEJMp1707170. [DOI] [PubMed] [Google Scholar]
- 20.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 21.Harhay MO, Wagner J, Ratcliffe SJ, Bronheim RS, Gopal A, Green S, et al. Outcomes and statistical power in adult critical care randomized trials. Am J Respir Crit Care Med. 2014;189:1469–1478. doi: 10.1164/rccm.201401-0056CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaudry S, Messika J, Ricard JD, Guillo S, Pasquet B, Dubief E, et al. Patient-important outcomes in randomized controlled trials in critically ill patients: a systematic review. Ann Intensive Care. 2017;7:28. doi: 10.1186/s13613-017-0243-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang CY, Daniels R, Lembo A, Hartog C, O’Brien J, Heymann T, et al. Life after sepsis: an international survey of survivors to understand the post-sepsis syndrome. Int J Qual Health Care. 2019;31:191–198. doi: 10.1093/intqhc/mzy137. [DOI] [PubMed] [Google Scholar]
- 24.Delbecq AL, Van de Ven AH. A group process model for problem identification and program planning. J Appl Behav Sci. 1971;7:466–492. [Google Scholar]
- 25.Watson RS, Choong K, Colville G, Crow S, Dervan LA, Hopkins RO, et al. Life after critical illness in children—toward an understanding of pediatric post-intensive care syndrome. J Pediatr. 2018;198:16–24. doi: 10.1016/j.jpeds.2017.12.084. [DOI] [PubMed] [Google Scholar]
- 26.Thompson K, Taylor C, Jan S, Li Q, Hammond N, Myburgh J, et al. Health-related outcomes of critically ill patients with and without sepsis. Intensive Care Med. 2018;44:1249–1257. doi: 10.1007/s00134-018-5274-x. [DOI] [PubMed] [Google Scholar]
- 27.Hodgson CL, Walsh TS, Lone N. The long road home: are outcomes different for patients with sepsis? Intensive Care Med. 2018;44:1556–1557. doi: 10.1007/s00134-018-5301-y. [DOI] [PubMed] [Google Scholar]
- 28.Krumholz HM. Post-hospital syndrome: an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100–102. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 30.Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72:1491–1501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prescott HC, Carmichael AG, Langa KM, Gonzalez R, Iwashyna TJ. Paths into sepsis: trajectories of presepsis healthcare use. Ann Am Thorac Soc. 2019;16:116–123. doi: 10.1513/AnnalsATS.201806-391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. The association of frailty with post-ICU disability, nursing home admission, and mortality: a longitudinal study. Chest. 2018;153:1378–1386. doi: 10.1016/j.chest.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Australian Sepsis Network. Life after sepsis [accessed 2019 Apr 8]. Available from: https://www.australiansepsisnetwork.net.au/community-awareness/life-sepsis.
- 34.Centers for Disease Control and Prevention. Life after sepsis fact sheet: what sepsis survivors need to know [accessed 2019 Apr 8]. Available from: https://www.cdc.gov/sepsis/pdfs/life-after-sepsis-fact-sheet.pdf.
- 35.Global Sepsis Alliance. Post-sepsis symptoms (PSS) [accessed 2019 Apr 8]. Available from: https://www.global-sepsis-alliance.org/sepsis.
- 36.UK Sepsis Trust. Post sepsis syndrome [accessed 2019 Apr 8]. Available from: https://sepsistrust.org/get-support/support-for-survivors/post-sepsis-syndrome/
- 37.Sepsis Alliance. Post-sepsis syndrome [accessed 2018 Nov 4]. Available from: https://www.sepsis.org/sepsis-basics/post-sepsis-syndrome/
- 38.McPeake J, Hirshberg EL, Christie LM, Drumright K, Haines K, Hough CL, et al. Models of peer support to remediate post-intensive care syndrome: a report developed by the Society of Critical Care Medicine THRIVE International Peer Support Collaborative. Crit Care Med. 2019;47:e21–e27. doi: 10.1097/CCM.0000000000003497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Society of Critical Care Medicine. THRIVE Post ICU Clinic Collaborative [accessed 2019 Apr 8]. Available from: https://www.sccm.org/Research/Quality/THRIVE/THRIVE-Post-ICU-Clinic-Collaborative.
- 40.Harrison DA, Brady AR, Rowan K. Case mix, outcome and length of stay for admissions to adult, general critical care units in England, Wales and Northern Ireland: the Intensive Care National Audit & Research Centre Case Mix Programme Database. Crit Care. 2004;8:R99–R111. doi: 10.1186/cc2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van de Klundert N, Holman R, Dongelmans DA, de Keizer NF. Data resource profile: the Dutch National Intensive Care Evaluation (NICE) registry of admissions to adult intensive care units. Int J Epidemiol. 2015;44:1850–1850h. doi: 10.1093/ije/dyv291. [DOI] [PubMed] [Google Scholar]
- 42.Australian and New Zealand Intensive Care Society (ANZICS) Adult patient database (APD) [accessed 2018 Dec 21]. Available from: https://www.anzics.com.au/adult-patient-database-apd/
- 43.Hashmi M, Beane A, Taqi A, Memon MI, Athapattu P, Khan Z, et al. Pakistan Registry of Intensive CarE (PRICE): expanding a lower middle-income, clinician-designed critical care registry in South Asia [editorial] J Intensive Care Soc. 2019;20:190–195. doi: 10.1177/1751143718814126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zampieri FG, Soares M, Borges LP, Salluh JIF, Ranzani OT. The Epimed Monitor ICU Database®: a cloud-based national registry for adult intensive care unit patients in Brazil. Rev Bras Ter Intensiva. 2017;29:418–426. doi: 10.5935/0103-507X.20170062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Intensive Care Surveillance. A critical care clinical registry and bed availability system for Sri Lanka [accessed 2019 Apr 8]. Available from: https://nicslk.wordpress.com.
- 46.Ranzani OT, Shankar-Hari M, Harrison DA, Rabello LS, Salluh JIF, Rowan KM, et al. A comparison of mortality from sepsis in Brazil and England: the impact of heterogeneity in general and sepsis-specific patient characteristics. Crit Care Med. 2019;47:76–84. doi: 10.1097/CCM.0000000000003438. [DOI] [PubMed] [Google Scholar]
- 47.Platt R, Lieu T. Data enclaves for sharing information derived from clinical and administrative data. JAMA. 2018;320:753–754. doi: 10.1001/jama.2018.9342. [DOI] [PubMed] [Google Scholar]
- 48.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, et al. CDC Prevention Epicenter Program. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009–2014. JAMA. 2017;318:1241–1249. doi: 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rowan KM, Angus DC, Bailey M, Barnato AE, Bellomo R, Canter RR, et al. PRISM Investigators. Early, goal-directed therapy for septic shock: a patient-level meta-analysis. N Engl J Med. 2017;376:2223–2234. doi: 10.1056/NEJMoa1701380. [DOI] [PubMed] [Google Scholar]
- 51.Blackwood B, Marshall J, Rose L. Progress on core outcome sets for critical care research. Curr Opin Crit Care. 2015;21:439–444. doi: 10.1097/MCC.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 52.COMET Initiative. Core outcomes set (COS) for pediatric critical care medicine (PCCM) research [accessed 2019 Apr 27]. Available from: http://www.comet-initiative.org/studies/details/1131.
- 53.Blackwood B, Ringrow S, Clarke M, Marshall J, Rose L, Williamson P, et al. Core Outcomes in Ventilation Trials (COVenT): protocol for a core outcome set using a Delphi survey with a nested randomised trial and observational cohort study. Trials. 2015;16:368. doi: 10.1186/s13063-015-0905-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kerrigan SW, Martin-Loeches I. Public awareness of sepsis is still poor: we need to do more. Intensive Care Med. 2018;44:1771–1773. doi: 10.1007/s00134-018-5307-5. [DOI] [PubMed] [Google Scholar]
- 55.Govindan S, Iwashyna TJ, Watson SR, Hyzy RC, Miller MA. Issues of survivorship are rarely addressed during intensive care unit stays: baseline results from a statewide quality improvement collaborative. Ann Am Thorac Soc. 2014;11:587–591. doi: 10.1513/AnnalsATS.201401-007BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Recovery after a hospitalization for sepsis [patient education video] [accessed 2019 Apr 8]. Available from: https://www.youtube.com/watch?v=LmTMrdrKMjU.
- 57.Prescott HC, Angus DC. Postsepsis morbidity. JAMA. 2018;319:91. doi: 10.1001/jama.2017.19809. [DOI] [PubMed] [Google Scholar]
- 58.Jette AM. Enhancing recovery after sepsis. Phys Ther. 2018;98:459–460. doi: 10.1093/ptj/pzy043. [DOI] [PubMed] [Google Scholar]
- 59.Cystic Fibrosis Foundation. Why we stride [accessed 2018 Aug 3]. Available from: http://fightcf.cff.org/site/PageServer?pagename=gs_why_we_stride.
- 60.Global Sepsis Alliance. [accessed 2018 Nov 4]. Available from: https://www.global-sepsis-alliance.org/
- 61.UK Sepsis Trust. [accessed 2018 Nov 4]. Available from: https://sepsistrust.org/
- 62.Instituto Latino Americano de Sepse (ILAS) 2018 [accessed 2018 Nov 4]. Available from: http://www.ilas.org.br/
- 63. Sepsis Alliance [accessed 2018 Nov 4]. Available from: https://www.sepsis.org/
- 64.Rory Staunton Foundation. Awareness and prevention [accessed 2018 Nov 4]. Available from: https://rorystauntonfoundationforsepsis.org/
- 65.Reinhart K, Daniels R, Kissoon N, O’Brien J, Machado FR, Jimenez E GSA Executive Board and WSD Executive Board. The burden of sepsis—a call to action in support of World Sepsis Day 2013. J Crit Care. 2013;28:526–528. doi: 10.1016/j.jcrc.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 66.Savelkoel J, Claushuis TAM, van Engelen TSR, Scheres LJJ, Wiersinga WJ. Global impact of World Sepsis Day on digital awareness of sepsis: an evaluation using Google Trends. Crit Care. 2018;22:61. doi: 10.1186/s13054-018-1981-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noritomi DT, Ranzani OT, Monteiro MB, Ferreira EM, Santos SR, Leibel F, et al. Implementation of a multifaceted sepsis education program in an emerging country setting: clinical outcomes and cost-effectiveness in a long-term follow-up study. Intensive Care Med. 2014;40:182–191. doi: 10.1007/s00134-013-3131-5. [DOI] [PubMed] [Google Scholar]
- 68.Staunton O, Staunton C. The urgency of now: attacking the sepsis crisis. Crit Care Med. 2018;46:809–810. doi: 10.1097/CCM.0000000000003047. [DOI] [PubMed] [Google Scholar]
- 69.Levy MM, Gesten FC, Phillips GS, Terry KM, Seymour CW, Prescott HC, et al. Mortality changes associated with mandated public reporting for sepsis: the results of the New York State initiative. Am J Respir Crit Care Med. 2018;198:1406–1412. doi: 10.1164/rccm.201712-2545OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.James Lind Alliance. Intensive care top 10 [accessed 2019 Apr 8]. Available from: http://www.jla.nihr.ac.uk/priority-setting-partnerships/intensive-care/top-10-priorities/
- 71.Turnbull AE, Sepulveda KA, Dinglas VD, Chessare CM, Bingham CO, III, Needham DM. Core domains for clinical research in acute respiratory failure survivors: an international modified Delphi consensus study. Crit Care Med. 2017;45:1001–1010. doi: 10.1097/CCM.0000000000002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Needham DM, Sepulveda KA, Dinglas VD, Chessare CM, Friedman LA, Bingham CO, III, et al. Core outcome measures for clinical research in acute respiratory failure survivors: an international modified Delphi consensus study. Am J Respir Crit Care Med. 2017;196:1122–1130. doi: 10.1164/rccm.201702-0372OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McPeake J, Iwashyna TJ, Devine H, MacTavish P, Quasim T. Peer support to improve recovery following critical care discharge: a case-based discussion. Thorax. 2017;72:856–858. doi: 10.1136/thoraxjnl-2016-209661. [DOI] [PubMed] [Google Scholar]
- 74.Haines KJ, Holdsworth C, Cranwell K, Skinner EH, Holton S, MacLeod-Smith B, et al. Development of a peer support model using experience-based co-design to improve critical care recovery. Crit Care Explor. 2019;1:e0006. doi: 10.1097/CCE.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.European Prevention of Alzheimer’s Dementia Consortium (EPAD) [accessed 2018 Jun 21]. Available from: at http://ep-ad.org/
- 76.Ritchie CW, Molinuevo JL, Truyen L, Satlin A, Van der Geyten S, Lovestone S European Prevention of Alzheimer’s Dementia (EPAD) Consortium. Development of interventions for the secondary prevention of Alzheimer’s dementia: the European Prevention of Alzheimer’s Dementia (EPAD) project. Lancet Psychiatry. 2016;3:179–186. doi: 10.1016/S2215-0366(15)00454-X. [DOI] [PubMed] [Google Scholar]
- 77.Scherag A, Hartog CS, Fleischmann C, Ouart D, Hoffmann F, König C, et al. Mid-German Sepsis Cohort investigators. A patient cohort on long-term sequelae of sepsis survivors: study protocol of the Mid-German Sepsis Cohort. BMJ Open. 2017;7:e016827. doi: 10.1136/bmjopen-2017-016827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krokstad S, Langhammer A, Hveem K, Holmen TL, Midthjell K, Stene TR, et al. Cohort profile: the HUNT study, Norway. Int J Epidemiol. 2013;42:968–977. doi: 10.1093/ije/dys095. [DOI] [PubMed] [Google Scholar]
- 80.National Institutes of Health. All of Us Research Program [accessed 2019 Apr 23]. Available from: https://allofus.nih.gov.
- 81.Gaziano JM, Concato J, Brophy M, Fiore L, Pyarajan S, Breeling J, et al. Million Veteran Program: a mega-biobank to study genetic influences on health and disease. J Clin Epidemiol. 2016;70:214–223. doi: 10.1016/j.jclinepi.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 82.45 and Up Study Collaborators. Cohort profile: the 45 and Up Study. Int J Epidemiol. 2008;37:941–947. doi: 10.1093/ije/dym184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JWR, Weir DR. Cohort profile: the Health and Retirement Study (HRS) Int J Epidemiol. 2014;43:576–585. doi: 10.1093/ije/dyu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 85.Shah FA, Pike F, Alvarez K, Angus D, Newman AB, Lopez O, et al. Bidirectional relationship between cognitive function and pneumonia. Am J Respir Crit Care Med. 2013;188:586–592. doi: 10.1164/rccm.201212-2154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.U.S. Food and Drug Administration. Critical Path Initiative [accessed 2019 Apr 8]. Available from: https://www.fda.gov/scienceresearch/specialtopics/criticalpathinitiative/default.htm.
- 87.Iwashyna TJ. Trajectories of recovery and dysfunction after acute illness, with implications for clinical trial design. Am J Respir Crit Care Med. 2012;186:302–304. doi: 10.1164/rccm.201206-1138ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choong K, Fraser D, Al-Harbi S, Borham A, Cameron J, Cameron S, et al. Functional recovery in critically ill children, the “WeeCover” multicenter study. Pediatr Crit Care Med. 2018;19:145–154. doi: 10.1097/PCC.0000000000001421. [DOI] [PubMed] [Google Scholar]
- 89.Osuchowski MF, Ayala A, Bahrami S, Bauer M, Boros M, Cavaillon JM, et al. Minimum Quality Threshold in Pre-Clinical Sepsis Studies (MQTiPSS): an international expert consensus initiative for improvement of animal modeling in sepsis. Shock. 2018;50:377–380. doi: 10.1097/SHK.0000000000001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life: a conceptual model of patient outcomes. JAMA. 1995;273:59–65. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.