Abstract
Rationale: Alveolar capillary dysplasia with misalignment of pulmonary veins (ACDMPV) is a lethal congenital disorder causing respiratory failure and pulmonary hypertension shortly after birth. There are no effective treatments for ACDMPV other than lung transplant, and new therapeutic approaches are urgently needed. Although ACDMPV is linked to mutations in the FOXF1 gene, molecular mechanisms through which FOXF1 mutations cause ACDMPV are unknown.
Objectives: To identify molecular mechanisms by which S52F FOXF1 mutations cause ACDMPV.
Methods: We generated a clinically relevant mouse model of ACDMPV by introducing the S52F FOXF1 mutation into the mouse Foxf1 gene locus using CRISPR/Cas9 technology. Immunohistochemistry, whole-lung imaging, and biochemical methods were used to examine vasculature in Foxf1WT/S52F lungs and identify molecular mechanisms regulated by FOXF1.
Measurements and Main Results: FOXF1 mutations were identified in 28 subjects with ACDMPV. Foxf1WT/S52F knock-in mice recapitulated histopathologic findings in ACDMPV infants. The S52F FOXF1 mutation disrupted STAT3–FOXF1 protein–protein interactions and inhibited transcription of Stat3, a critical transcriptional regulator of angiogenesis. STAT3 signaling and endothelial proliferation were reduced in Foxf1WT/S52F mice and human ACDMPV lungs. S52F FOXF1 mutant protein did not bind chromatin and was transcriptionally inactive. Furthermore, we have developed a novel formulation of highly efficient nanoparticles and demonstrated that nanoparticle delivery of STAT3 cDNA into the neonatal circulation restored endothelial proliferation and stimulated lung angiogenesis in Foxf1WT/S52F mice.
Conclusions: FOXF1 acts through STAT3 to stimulate neonatal lung angiogenesis. Nanoparticle delivery of STAT3 is a promising strategy to treat ACDMPV associated with decreased STAT3 signaling.
Keywords: alveolar capillary dysplasia with misalignment of pulmonary veins, ACDMPV, FOXF1 transcription factor, STAT3, neonatal pulmonary angiogenesis
At a Glance Commentary
Scientific Knowledge on the Subject
Alveolar capillary dysplasia with misalignment of pulmonary veins (ACDMPV) is a lethal congenital disorder causing cyanosis and respiratory failure shortly after birth. There are no effective treatments for ACDMPV other than lung transplant, and new therapeutic approaches are urgently needed.
What This Study Adds to the Field
We generated the first clinically relevant animal model of ACDMPV by inserting the S52F FOXF1 mutation into the mouse Foxf1 gene locus. S52F FOXF1 mutation disrupted formation of alveolar capillaries and decreased STAT3, a key regulator of pulmonary endothelial cells. Nanoparticle delivery of STAT3 increased vascularity and improved lung structure in FOXF1 mutant mice, suggesting that the STAT3 gene therapy can be effective for human ACDMPV.
Alveolar capillary dysplasia with misalignment of pulmonary veins (ACDMPV) is a fatal congenital disorder, characterized by defects in morphogenesis of pulmonary capillaries, positioning of pulmonary veins, and impaired lobular development, resulting in cyanosis, respiratory failure, and pulmonary hypertension following birth (1). Structural abnormalities of the cardiovascular, gastrointestinal, and genitourinary systems are often associated with ACDMPV. Because of the severity of the developmental defects and respiratory insufficiency in infants with ACDMPV, mortality usually occurs within a month of birth despite intensive care (1). Lung transplantation is an effective treatment for patients with ACDMPV with delayed clinical presentation but these cases are rare (2). Although genetic factors associated with ACDMPV are not fully characterized, heterozygous copy-number variant deletions and point mutations involving the Forkhead Box F1 (FOXF1) gene locus account for most ACDMPV cases (3, 4). Thus far more than 50 distinct point mutations in FOXF1 have been linked to ACDMPV (4). In addition to ACDMPV, mutations in the FOXF1 gene were identified in prenatal cystic hygroma (5), pulmonary capillary hemangiomatosis (6), and VATER/VACTERL association (7). Diminished FOXF1 expression was found in lungs of patients with acute lung injury and acute respiratory distress syndrome (8), whereas FOXF1 expression was increased in human rhabdomyosarcoma tumors (9).
FOXF1 is an important transcriptional regulator of embryonic development in mice and humans (3, 10). Foxf1−/− mice exhibited an embryonic lethal phenotype caused by the lack of vasculature in the yolk sac and allantois (11). Foxf1 haploinsufficiency (Foxf1+/−) increased mortality after birth, associated with lung hypoplasia, fusion of the lung lobes, and reduced lung angiogenesis (12–14). Gallbladder morphogenesis was disrupted in Foxf1+/− mice, resulting in either malformations or loss of the organ (15). Absence of the gallbladder was reported in patients with ACDMPV (1). In the lung, FOXF1 is expressed in mesenchyme-derived cells, including capillary endothelial cells, fibroblasts, and peribronchial smooth muscle cells (8, 16, 17). Endothelium-specific inactivation of Foxf1 revealed that FOXF1 regulates transcription of genes critical for angiogenesis and maintenance of endothelial cell junctions (8, 16, 17). Although multiple FOXF1 point mutations have been found in human ACDMPV, these mutations have not been modeled in vivo. Molecular mechanisms whereby the FOXF1 point mutations cause ACDMPV are unknown.
In the present study, we have focused on the P49-Y53 region in FOXF1, harboring multiple point mutations linked to ACDMPV. In particular, the S52F Foxf1 mutation was further studied in vitro and in transgenic mice. Some of the results of these studies have been previously reported in the form of abstracts (18, 19).
Methods
The data, analytic methods, and study materials will be made available on request from the corresponding author of this manuscript to other researchers for purposes of reproducing the results or replicating the procedure. All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee at the Cincinnati Children’s Hospital Medical Center, and NIH guidelines for laboratory animal care and safety were strictly followed.
Mice
Foxf1+/− mouse line was described previously (14, 17). Foxf1WT/S52F line was generated using CRISPR/Cas9 editing approach as described (20). Specific details on generation of Foxf1WT/S52F mouse line are provided in Table E1 in the online supplement.
Mutation Analysis of the FOXF1 Gene
Patients with histopathologically verified ACDMPV were recruited after signing consent for the study. Institutional review board (IRB) protocol #H-8712 was approved by the IRB for Baylor College of Medicine and Affiliated Hospitals. DNA was isolated from peripheral blood. Overlapping amplicons covering the entire coding region of exons 1 and 2 of FOXF1 were amplified and sequenced by conventional Sanger method (Lone Star Labs; Molecular Core Lab) as described (3, 4, 21). Genomic analysis in the clinical laboratory was performed under IRB protocol #H-42680 (“Genomic sequencing and retrospective analysis of de-identified subjects from a clinical laboratory”) approved by the IRB for Baylor College of Medicine.
Cloning, Constructs, and Retroviruses
Cloning of murine Foxf1 cDNA, generation of retroviruses and stably transfected cell lines expressing HF-tagged FOXF1 and its mutants have been described previously (22) and in the online supplement.
Cell Fractionation, Gel Filtration, Immunoprecipitation Western Blot, and Luciferase Assay
Subcellular and cofractionation experiments were performed as described (22). Two-step affinity purification, immunoprecipitation, and Western blots were performed as described previously (22, 23) and in the online supplement. A dual-luciferase assay (Promega) was performed 48 hours after transfection as described (24–26).
qRT-PCR
qRT-PCR analysis was performed as described previously using a StepOnePlus Real-Time PCR system (Applied Biosystems) (27, 28). Samples were amplified using inventoried TaqMan primers (Table E2).
Immunohistochemistry and Immunofluorescence
Paraffin sections of the human lungs were obtained after informed consent according to IRB protocol #H-8712. Human and mouse lung sections were stained with hematoxylin-eosin or used for immunostaining as described (29, 30). Images were obtained using a Zeiss Axioplan 2 microscope as described (31, 32). Information about antibodies and isolectin-B4 labeling is provided in the online supplement.
siRNA Transfection
MFLM-91 U cell cultures and siRNA transfections were performed as described (17, 33). Specific siRNA sequences are provided in the online supplement.
Chip-Seq and Preparation of Nanoparticles
ChIP was performed using SX-8G IP-STAR robot (Diagenode) (34). PEI600 and PEG2k were conjugated with myristic acid (PEI600-MA5) and oleic acid (PEG2k-OA), respectively, using EDC/NHS coupling (35). Colloidal nanoparticles were formulated by addition of PEG2k-OA and cholesterol to PEI600-MA5 (see online supplement).
Fluorescence-activated Cell Sorter Analysis
Cell suspensions were prepared from mouse lungs (8, 17). Antibodies are listed in Table E3. Stained cells were analyzed using FACSAria II.
Statistical Analysis
One-way ANOVA and Student’s t test were used to determine statistical significance. The log-transformation was applied for skewed data to confirm with normality. P values less than 0.05 were considered significant. Values for all measurements were expressed as the mean ± SD. Survival data were analyzed using Kaplan-Meier method.
Results
FOXF1 Interacts with STAT3
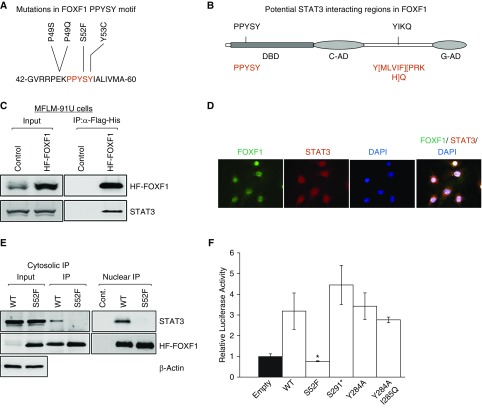
Using whole exome sequencing and targeted Sanger sequencing, we identified 27 FOXF1 mutations in 28 patients with ACDMPV (Table E4), including 19 novel FOXF1 mutations and 8 mutations reported previously (4). Cumulative analysis of all pathogenic FOXF1 mutations in patients with ACDMPV was performed (Figure E1) identifying a P49-Y53 aa sequence (PPYSY) in the FOXF1 DNA binding domain as a frequently mutated region (Figure 1A). The P49-Y53 aa region is evolutionary conserved among species and other FOX proteins (Figures E2A and E2B). Bioinformatic analysis suggested two potential SH2 domain-binding sites in the FOXF1 protein: the P49-Y53 and Y284-Q287 region; the latter is located between the transcriptional activation domains (Figure 1B).
Figure 1.
S52F mutation disrupts FOXF1–STAT3 protein–protein interaction and decreases FOXF1 transcriptional activity. (A) Protein sequence shows a frequently mutated region, PPYSY (P49-Y53), located in DNA-binding domain of FOXF1. (B) Schematic diagram shows two potential SH2 domain-binding regions in FOXF1. Consensus STAT3-binding sequences are shown in red. (C) Immunoblots show STAT3 and FOXF1 proteins in immunoprecipitation fractions of MFLM-91 U cells after HF-FOXF1 purification. Cells stably expressing HF-Foxf1 were subjected to two-step affinity purification using anti-Flag Ab followed by nickel affinity columns. Vector alone–transduced cells were used as a control. (D) Immunofluorescent images show colocalization of FOXF1 and STAT3 proteins in nuclei of MFLM-91 U cells. Magnification ×400. (E) Immunoblots show that transfected S52F-FOXF1 protein does not interact with STAT3. Exogenous HF-FOXF1 mutant proteins were detected by α-Flag antibody. (F) S52F FOXF1 mutation inhibits transcriptional activity. Dual-luciferase assay was performed using 6× FOXF1-LUC reporter plasmid (n = 5 in each group). *P < 0.05. IP = immunoprecipitation; WT = wild type.
To determine whether FOXF1 binds to SH2 domain-containing STAT3 protein, we used a retroviral-mediated gene transfer and generated a fetal lung endothelial MFLM-91 U cell line (22) stably expressing Foxf1 tagged with an N-terminal Flag and the C-terminal His-6 tags (HF-FOXF1). STAT3 physically bound to HF-FOXF1 as shown by immunoprecipitation of HF-FOXF1 followed by immunoblot with STAT3 antibody (Figure 1C). Immunoprecipitation of the protein lysate obtained from mouse lung tissue demonstrated that STAT3 binds to endogenous FOXF1 (Figure E2C). STAT3-FOXF1 interactions were stronger after lung injury caused by butylated hydroxytoluene (Figure E2C). Consistent with STAT3-FOXF1 binding, fractionation profiles of endogenous FOXF1 and STAT3 proteins partially overlapped during Superose 6 gel filtration chromatography (Figure E2D). FOXF1 colocalized with STAT3 in nuclei of endothelial cells in vitro (Figure 1D).
To determine whether the P49-Y53 or the Y284-Q287 aa regions of FOXF1 bind to SH2 domain of STAT3, as predicted by bioinformatic analysis (Figure 1B), we generated a series of Foxf1 variants corresponding to ACDMPV causative mutations, mapping into those two protein regions, and expressed them in MFLM-91 U cells. The selected mutations included S52F, Y284A, and I285Q, and S291* mapping 3′ to the Y284-Q287 FOXF1 region. FOXF1 mutants had similar molecular weights compared with endogenous FOXF1, except the S291* mutant, which was smaller because of premature termination causing the loss of the C-terminal transactivation domain (Figures E3A and E3B). S52F FOXF1 did not bind to STAT3 (Figure 1E), whereas the Y284A, I285Q, and S291* mutations maintained FOXF1-STAT3 interactions (Figures E3C and E3D). Thus, serine 52 of FOXF1 is essential for interaction with STAT3. Among the examined FOXF1 mutants only the S52F mutant was transcriptionally inactive as shown by LUC reporter assay (Figure 1F), suggesting that serine 52 of FOXF1 is required for FOXF1 transcriptional activity.
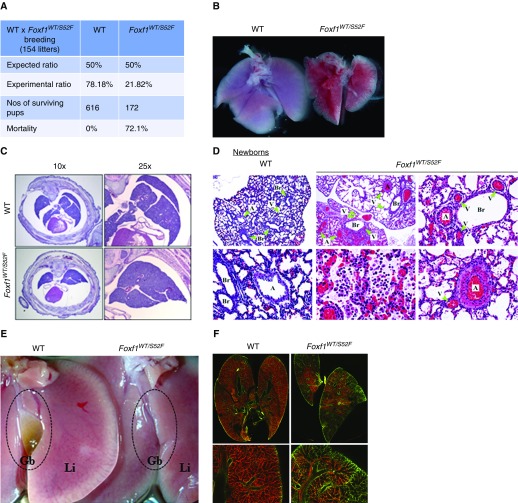
Mortality and Lung Abnormalities in Foxf1WT/S52F Mice
To test the importance of FOXF1-STAT3 interactions in vivo, we used CRISPR/Cas9 genome-editing to generate a Foxf1WT/S52F knock-in mouse line (Figure E4). Homozygous Foxf1S52F/S52F mice were early embryonic lethal, whereas heterozygous Foxf1WT/S52F pups exhibited a 72% mortality rate after birth (Figure 2A). Although the weight of Foxf1WT/S52F embryos was unaltered, surviving Foxf1WT/S52F mice had a progressive decrease of body weight during the postnatal period (see Figure E5). Lungs of Foxf1WT/S52F newborn mice were smaller with fusion of the right lobes (Figures 2B and 2C). Histologic evaluation of Foxf1WT/S52F newborn lungs showed pulmonary inflammation, hemorrhage, and hypertrophy of pulmonary arteries (Figure 2D). In Foxf1WT/S52F newborns and embryos, multiple veins were seen close to airways and arteries (Figures 2D and E6), a distinctive feature of misalignment of pulmonary veins seen in patients with ACDMPV (1). In surviving Foxf1WT/S52F mice, alveolar simplification was observed along with accumulation of fibrin in alveolar regions and pulmonary bronchioles (Figure E7) consistent with persistent vascular leak and hemorrhage. Similar to clinical findings in ACDMPV (1), gallbladders of Foxf1WT/S52F mice were either absent (Figure 2E) or underdeveloped (Figure E8).
Figure 2.
Heterozygous S52F-Foxf1+/− mutation causes increased mortality and pulmonary hypoplasia. (A) Table shows the survival data of Foxf1WT/S52F pups (n = 618) from 154 litters after breeding of Foxf1WT/S52F males and wild-type females. Survival was assessed 1 month after birth. (B) Image shows pulmonary hypoplasia and diffuse hemorrhage in a Foxf1WT/S52F newborn. (C) Hematoxylin and eosin–stained sections of E18.5 embryos show fusion of lung lobes in Foxf1WT/S52F mice. (D) Hematoxylin and eosin staining shows lung inflammation, hemorrhage, hypertrophy of pulmonary arteries, and misalignment of pulmonary veins in Foxf1WT/S52F newborn mice. Green arrowheads show veins, arteries, and bronchioles. (E) Image shows absence of gallbladder in adult Foxf1WT/S52F mouse. (F) P2 pups were intravenously injected with isolectin B4. Perfused lung vasculature was imaged using confocal microscopy (red). Green shows autofluorescence. Magnification: top left and middle panels in D, ×50; bottom middle panel in D, ×400; remaining panels in D, ×200; top panels in F, ×10; bottom panels in F, ×50. A = artery; Br = bronchiole; Gb = gallbladder; Li = liver; V = vein; WT = wild type.
Confocal imaging of whole-lung vasculature perfused with Isolectin B4 and immunostaining of paraffin sections for endomucin indicated the loss of microvascular network in the peripheral lung of Foxf1WT/S52F embryos (Figures 2F and E9). Immunostaining for endothelial markers PECAM-1 and FLK1, both transcriptional targets of FOXF1 (17), was decreased in FOXF1 mutants (Figures 3A and E9A). Pecam1 and Flk1 mRNAs and proteins were reduced in Foxf1WT/S52F lungs as shown by qRT-PCR and immunoblot (Figure 3B). Consistent with the loss of vasculature in Foxf1WT/S52F lungs, proliferation of endothelial cells was reduced as shown by BrdU incorporation and immunostaining for Ki-67 (Figures 3C and 3D). Location and abundance of pulmonary epithelial and smooth muscle cells were unaltered in Foxf1WT/S52F mice, and lymphatic vessels were present (Figure E10). There were no obvious histologic abnormalities in the trachea, heart, kidney, and intestine (Figure E11). Thus, heterozygous S52F Foxf1 mutation caused lung hypoplasia, ACDMPV, arterial hypertrophy, pulmonary hemorrhage, and gallbladder abnormalities, all key histopathologic features of ACDMPV in humans.
Figure 3.
Decreased endothelial cell proliferation and STAT3 signaling in Foxf1WT/S52F mice. (A) PECAM1 and FLK1 staining was decreased in lungs of E15.5 Foxf1WT/S52F embryos. Magnification ×200, inserts ×400. (B) Protein and mRNA of Flk1 and Pecam1 were reduced in lungs from E15.5 Foxf1WT/S52F mice as shown by Western blot (top) and qRT-PCR (bottom) (n = 5 embryos in each group were used for qRT-PCR). (C) Decreased pulmonary endothelial cell proliferation in the Foxf1WT/S52F mice is shown using Ki-67 and BrdU immunostaining. Magnification ×400, inserts ×1,600. (D) Graphical representation of cell proliferation by Ki-67 and BrdU staining. Percentage of Ki-67-positive and BrdU-positive cells was counted in 10 random microscope fields (n = 3–6 mice in each group). (E and F) Immunoblots (top) and qRT-PCR (bottom) show gene expression in lungs of Foxf1WT/S52F and Foxf1+/− E18.5 embryos. mRNA was normalized to β-actin mRNA (n = 5 embryos in each group were used for qRT-PCR). *P < 0.05. WT = wild type.
Decreased STAT3 in Foxf1WT/S52F Lung Tissue
Because STAT3 induces endothelial proliferation (36), we examined STAT3 in Foxf1WT/S52F lungs. Total STAT3, phospho-STAT3 (Tyr705), and Stat3 mRNA were decreased in Foxf1WT/S52F lungs compared with wild-type (WT) littermates (Figure 3E). Expression of STAT3 targets genes, such as Cyclin D1 and Bax (37), was decreased in Foxf1WT/S52F lungs (Figure 3E), indicating diminished STAT3 signaling. Similar results were obtained using lungs of mice containing a heterozygous null Foxf1 mutation (Foxf1+/−) (Figure 3F). Increased mortality, gallbladder defects, and reduced numbers of endothelial cells were previously observed in Foxf1+/− mice (12, 13). Next, we examined STAT3 phosphorylation and expression of FLK1 and Cyclin D1 in biopsy tissue from patients with ACDMPV. Immunostaining for Cyclin D1 and pSTAT3 was decreased, coinciding with diminished endothelial FLK1 staining and decreased endothelial cell proliferation (Figure 4A). Thus, the S52F Foxf1 mutation or the loss of one Foxf1 allele reduces STAT3 signaling in developing mouse and human lungs.
Figure 4.
FOXF1 stimulates STAT3 expression. (A) Immunostaining shows decreased pSTAT3, Ki-67, FLK1, and Cyclin D1 in human alveolar capillary dysplasia with misalignment of pulmonary veins (ACDMPV) compared with donor lungs (n = 3). Sections show ACDMPV lung associated with A231fs FOXF1 mutation. Green lines show arteries. Black arrowheads show Ki-67+ and Cyclin D1+ cells. Magnification: left, ×200; remaining panels; ×400, inserts ×1,000. A = artery; Br = bronchiole; V = vein. (B) Immunoblots show decreased FOXF1, phospho-STAT3 (Y705), and total STAT3 in FOXF1-depleted MFLM-91 U endothelial cells. FOXF1 levels were decreased by siRNA (siFoxf1). (C) FOXF1 depletion decreases Foxf1, Flk1, Pdgfb, Pecam1, Stat3, Bax, Bcl2, Mmp2, Mmp9, and Ccnd1 mRNAs. Transfection of WT FOXF1 (FOXF1-OE) but not S52F FOXF1 increased gene expression in FOXF1-depleted MFLM-91 U cells. mRNAs were normalized to β-actin (n = 5 cell cultures in each group), *P < 0.05. H&E = hematoxylin and eosin; UTR = untranslated region.
Inhibition of FOXF1 Decreases STAT3 In Vitro
The effect of Foxf1 inhibition on STAT3 was examined in fetal mouse lung endothelial cells (MFLM-91 U) in vitro. Foxf1-specific siRNA decreased STAT3 phosphorylation (Y705) and total STAT3 levels in nuclei and cytoplasm of transfected cells (Figure 4B). Nuclear STAT3 immunostaining was decreased in FOXF1-depleted cells after stimulation with IL-6 (Figures E12A and E12B), an upstream activator of STAT3 (37). Stat3 mRNA was decreased in FOXF1-depleted cells and was reversed by expression of exogenous HF-FOXF1, an isoform resistant to Foxf1 siRNA (Figure 4C). ChIP-seq analysis identified three FOXF1-binding regions in the first intron of the mouse Stat3 gene (Figure E12C). The FOXF1-binding regions had activating H3K4me1 methylation marks and lacked repressive H3K9me3 and H3K27me3 marks (Figure E12C), indicating that FOXF1 bound to transcriptionally active enhancer regions. Thus, FOXF1 directly activates Stat3 gene expression.
Expression of STAT3 target genes (Ccnd1, Mmp9, Mmp2, Bcl2, and Bax) was decreased in the FOXF1-depleted cells (Figure 4C), a finding consistent with reduced STAT3 protein and mRNA. Expression of exogenous HF-FOXF1 in FOXF1-deficient cells increased Stat3 mRNA and restored both STAT3 and FOXF1 target genes, whereas the S52F FOXF1 mutant was ineffective (Figure 4C). Interestingly, expression of exogenous STAT3 selectively increased proliferation-specific mRNAs (Ccnd1, Ccnb1, and c-Myc) in FOXF1-deficient cells but did not change other targets (Figure E13A). Likewise, knockdown of STAT3 decreased Ccnd1, Ccnb1, and c-Myc mRNAs, which were not rescued by exogenous FOXF1 (Figure E13B). Altogether, these in vitro data demonstrate that FOXF1 regulates expression of Ccnd1, Ccnb1, and c-Myc via STAT3.
Genome-wide ChIP-seq analysis demonstrated that 72% of FOXF1 endothelial target genes overlapped with those of STAT3 (Figure E14). Many of these common genes, such as Ccnd1, c-Myc, and Pecam1, had multiple DNA-regulatory elements bound by both FOXF1 and STAT3 (Figures E14B and E15). Mmp2, Brd4, and Bax represented a subset of commonly regulated genes, with FOXF1 and STAT3 binding to distinct DNA regions (Figures E14B and E15). Altogether, our data indicate that FOXF1 induces Stat3 gene transcription and cooperates with STAT3 protein to regulate expression of downstream target genes.
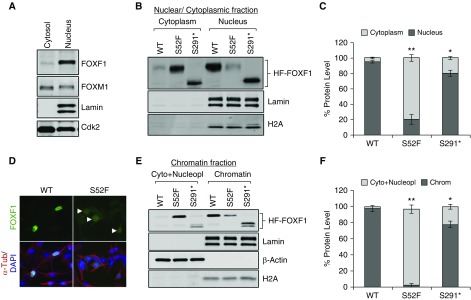
Decreased Nuclear Localization and Chromatin Binding of S52F-FOXF1
Because the S52F FOXF1 was transcriptionally inactive (Figure 1F), we used nuclear-cytoplasmic fractionation to examine nuclear localization and chromatin binding of S52F FOXF1. Endogenous FOXF1 was enriched in the nuclear compartment of MFLM-91 U cells (Figure 5A). After stable expression, approximately 95% of exogenous HF-FOXF1 was present in the nuclear fraction (Figures 5B and 5C), consistent with efficient translocation of HF-FOXF1 to the nuclei. In contrast, the nuclear fraction of the S52F FOXF1 was decreased to 20% as shown by Western blot and immunostaining (Figures 5B–5D).
Figure 5.
S52F-FOXF1 exhibits diminished nuclear localization and decreased binding to chromatin. (A) Immunoblot shows the nucleocytoplasmic distribution of endogenous FOXF1 in MFLM-91 U endothelial cells. Lamin A/C was used to assess nuclear purification. (B and C) Immunoblots show the nucleocytoplasmic distribution of exogenous HF-WT, HF-S52F, and HF-S291* FOXF1 proteins after stable transfection into MFLM-91 U cells. Data were quantified using densitometry (n = 3 immunoblots). (D) Immunofluorescent images show the subcellular distribution of HF-tagged FOXF1 and S52F-FOXF1 proteins in MFLM-91 U cells. Arrowheads show decreased FOXF1 staining in cell nuclei. Magnification ×400. (E and F) Immunoblots show the chromatin association of WT HF-tagged FOXF1 and mutant FOXF1 proteins. Lamin A/C, H2A served as loading controls for the chromatin fractions (n = 3 immunoblots). *P < 0.05 and **P < 0.01. WT = wild type.
Next, we purified the chromatin fractions, to identify proteins that bound to DNA with high affinity. WT FOXF1 was found in the chromatin-bound fraction (Figures 5E and 5F). In contrast, S52F FOXF1 was enriched in the cytoplasm and nucleoplasmic fraction (Figures 5E and 5F), a finding consistent with reduced transcriptional activity of the S52F mutant. S291* FOXF1 was capable of translocation into cell nuclei (Figures 5B and 5C) and bound to chromatin (Figures 5E and 5F). To locate the position of S52 in three dimensions, we generated a three-dimensional model of the forkhead DNA binding domain using a resolved structure of human FOXK2 as a template (38). The model predicts that S52 is located between DNA-binding helix 3 (H3) and the second wing (W2) of the forkhead domain (Figure E16, top). The S52F substitution impedes FOXF1-DNA interaction because of steric constrains (Figure E16, bottom), consistent with the loss of chromatin binding and transcriptional activity of the S52F-FOXF1 mutant.
Nanoparticle-mediated Delivery of STAT3 Restores Endothelial Proliferation and Stimulates Angiogenesis in Foxf1WT/S52F Mutant Lungs
STAT3 stimulates proliferation of endothelial cells in vitro and in vivo (36). Because STAT3 was reduced in Foxf1-deficient mice (Figures 3E and 3F) and ACDMPV lungs (Figure 4A) we tested whether restoring STAT3 signaling in Foxf1WT/S52F newborns would enhance pulmonary endothelial proliferation and angiogenesis. To deliver Stat3 cDNA, we used cationic PEI nanoparticles that were capable of delivering DNA plasmids and shRNAs in vivo (39). PEI were used for DNA delivery in phase 2 clinical trials in patients with bladder (NCT00595088) and ovarian cancers (NCT01118052). To improve the efficiency of the in vivo targeting, we used the EDC/NHS conjugation strategy to create a novel formulation of PEI nanoparticles, PEI 600-MA5.0, which was stabilized with cholesterol and PEG2K-OA (Figure E17) (35). Fluorescently labeled PEI 600-MA5.0 nanoparticles were used to deliver a single dose of CMV-Stat3 plasmid into the facial vein of newborn pups. After gene delivery, nanoparticles were detected by fluorescence-activated cell sorter analysis in 88% of lung endothelial and 57% of mesenchymal cells (Figures 6A and E18A). Efficient targeting of endothelial cells in the lung tissue was confirmed by CMV-GFP reporter encapsulated into PEI 600-MA5.0 nanoparticles (Figures E18B and E18C). Nanoparticles were ineffective in targeting hematopoietic and epithelial cells in the lung tissue (Figure 6A).
Figure 6.
Nanoparticle-mediated delivery of STAT3 restores endothelial cell proliferation and angiogenesis in Foxf1WT/S52F newborn mice. (A) Fluorescence-activated cell sorter analysis gating strategy for the hematopoietic (a), endothelial (b), epithelial (c), and lineage negative cells (d) with histograms highlighting respective cell-selective targeting (n = 3 mice). (B and C) Immunoblots show the levels of STAT3, pSTAT3, FLK-1, PECAM-1, and PDGFb in lung extracts after nanoparticle-mediated delivery of CMV-STAT3 via facial vein. CMV-empty was used as a control. Nanoparticle/DNA complexes were injected at P2 and mice were harvested at P7. Images were quantified using densitometry (n = 3–6 mice per group). *P < 0.05. (D) qRT-PCR shows the expression of Flk1 and Pecam1 mRNAs in P7 lungs after nanoparticle-mediated delivery of CMV-STAT3 (n = 3–6 mice in each group). (E) Images show the Ki-67 (arrowheads), isolectin B4, and endomucin staining of P7 lungs after nanoparticle-mediated delivery of STAT3. Magnification ×400. (F) Percentage of Ki-67–positive endothelial cells was determined using 10 random Ki-67–stained lung images (n = 3–6 mice in each group). (G) Quantification of endomucin staining was performed using ImageJ software in 10 random images from three to six mouse lungs in each group. **P < 0.01. CMV = cytomegalovirus; NS = not significant; WT = wild type.
Stat3 cDNA increased total STAT3 protein and STAT3 phosphorylation in Foxf1WT/S52F lungs as shown by Western blot (Figures 6B and 6C and Table E5). After Stat3 delivery, lung angiogenesis was improved as evidenced by increased mRNA and protein levels of endothelial markers PECAM1, FLK1, and PDGFb (Figures 6B–6D, and Tables E5 and E6), enhanced binding of endothelial cells to isolectin B4 (Figure 6E), increased immunostaining for endomucin (Figures 6E and 6G and Table E7), and elevated numbers of Ki-67–positive endothelial cells in Foxf1WT/S52F lungs (Figures 6E and 6F and Table E8). Stat3 cDNA decreased lung inflammation and improved alveogenesis in Foxf1WT/S52F mice (Figure E19). Hematoxylin–eosin staining and endomucin were unaltered in the heart, liver, and kidney after nanoparticle delivery of Stat3 (Figure E20). Altogether, nanoparticle delivery of STAT3 improves neonatal lung angiogenesis in Foxf1WT/S52F mice.
Discussion
STAT3 is a key transcriptional regulator of cellular proliferation during embryonic development, carcinogenesis, and organ injury and repair (37, 40). STAT3 is phosphorylated on tyrosine 705 in response to various cytokines and growth factors; translocates to the cell nucleus; and regulates transcription of numerous genes critical for cellular proliferation, migration, and survival (41, 42). Stat3−/− mice die in utero because of gastrulation defects (43). An important contribution of the present study is that FOXF1 activates STAT3 signaling in the developing lung. Because STAT3 stimulated cellular proliferation in various in vivo models (40, 44), decreased STAT3 signaling can contribute to reduced endothelial proliferation and loss of pulmonary microvasculature in Foxf1WT/S52F mutant lungs. Endothelial deletion of Stat3 did not cause ACDMPV in mice, possibly because of compensatory effects from other STATs (45). Our study showed that nanoparticle-mediated delivery of Stat3 cDNA increased endothelial proliferation and improved lung vascularity in Foxf1WT/S52F mice, suggesting that STAT3 can overcome proliferative defects caused by the S52F Foxf1 mutation, possibly through increased expression of cell cycle regulatory genes. Consistent with this concept, overexpression of Stat3 in FOXF1-deficient endothelial cells in vitro increased expression of Ccnd1, Ccnb1, and c-Myc.
FOXF1 activated STAT3 at the transcriptional level, as supported by reduced Stat3 mRNA in Foxf1-deficient cells and Foxf1WT/S52F lungs. FOXF1 directly bound to DNA regulatory elements located in the promoter and the first intron of Stat3 gene. Because these regulatory elements contained activating H3K4me1 histone methylation marks and lacked repressive H3K9me3 and H3K27me3, FOXF1 seems to be a transcriptional activator of the Stat3 gene. In addition to regulating Stat3 gene expression, FOXF1 directly bound to STAT3 protein. FOXF1–STAT3 protein–protein interactions can be important for regulation of gene expression because FOXF1 and STAT3 were found to bind to the same enhancers. S52F Foxf1 mutation inhibited expression of both FOXF1 and STAT3 transcriptional targets, which can directly contribute to decreased endothelial proliferation and reduced angiogenesis (Figure 7). Because FOXF1 stimulates tumor cell proliferation in rhabdomyosarcomas and gastrointestinal stromal tumors (9, 46), FOXF1 transcriptional activities or FOXF1 interactions with STAT3 may be important for growth and progression of these cancers.
Figure 7.
Schematic diagram shows the proposed molecular mechanisms whereby FOXF1 regulates STAT3 signaling and pulmonary angiogenesis. FOXF1 induces transcription of Stat3 gene leading to increased expression of cell cycle regulatory genes (Ccnb1, Ccnd1, and c-Myc). FOXF1 physically binds the STAT3 protein and these protein-protein interactions are dependent on Serine 52 of FOXF1. Increased STAT3 signaling leads to activation of endothelial cell proliferation and increased angiogenesis. FOXF1 regulates other proangiogenic genes independently of STAT3. S52 = Serine 52.
In the current study, we found that mice heterozygous for S52F Foxf1, a mutation found in patients with ACDMPV (4), exhibited alveolar capillary dysplasia, malpositioning of pulmonary veins, lung hypoplasia, and gallbladder defects but lacked histologic abnormalities in the heart, kidney, and intestinal tract that are common for ACDMPV (4). A patient with ACDMPV carrying the S52F FOXF1 mutation survived for 51 days and had malrotation of the intestine, hydronephrosis, and hydroureter in addition to respiratory insufficiency (4).Although the respiratory phenotype is severe and rapidly fatal in most infants with ACDMPV (4), the phenotypes of Foxf1WT/S52F and Foxf1+/− mice are less severe, highlighting important differences between mice and humans. Interestingly, in contrast to Foxf1WT/S52F mice misalignment of pulmonary veins was not reported for Foxf1+/− mice. Increased severity of the S52F Foxf1 as compared with the Foxf1 null allele may be related to its interference with normal function of WT FOXF1 protein. The S52F FOXF1 may interact with various coactivators/corepressors and limit their availability for interactions with WT FOXF1, therefore altering its function.
In summary, we developed a clinically relevant model of ACDMPV by introducing the S52F FOXF1 mutation into murine Foxf1 gene. Similarly to ACDMPV in humans, the knock-in of S52F Foxf1 mutation reduced lung angiogenesis and increased mortality after birth. We used Foxf1WT/S52F mutant mice to demonstrate that nanoparticle-mediated STAT3 gene delivery stimulates neonatal pulmonary angiogenesis. Although these findings may be specific to S52F FOXF1 mutation, our results suggest that nanoparticle-mediated STAT3 gene delivery is a promising strategy to treat at least a subset of ACDMPV cases associated with decreased STAT3 signaling. Because activating STAT3 mutations have been implicated in a variety of human diseases (47–50), the potential for adverse, dose-related effects of STAT3 gene therapy should be carefully considered.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Dr. Kofron for help with confocal microscopy and Dr. Lin for help with statistical analyses.
Footnotes
Supported by NIH Grants HL84151, HL141174, and HL123490 (V.V.K.), HL137203 (P. Stankiewicz), and HL132849 (T.V.K.); and NORD grants (A. Pradhan and P. Szafranski).
Author Contributions: A. Pradhan, J.A.W., D.S., T.V.K., and V.V.K. designed the study. A. Pradhan, A.D., V.U., C.B., G.W., Y.Z., and Y.-C.H. conducted experiments. A. Porollo conducted bioinformatic analyses. V.U., P. Szafranski, P. Stankiewicz, and R.X. conducted human alveolar capillary dysplasia with misalignment of pulmonary veins studies. A. Pradhan and V.V.K. wrote the manuscript with input from all authors.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201810-1897OC on June 14, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bishop NB, Stankiewicz P, Steinhorn RH. Alveolar capillary dysplasia. Am J Respir Crit Care Med. 2011;184:172–179. doi: 10.1164/rccm.201010-1697CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Towe CT, White FV, Grady RM, Sweet SC, Eghtesady P, Wegner DJ, et al. Infants with atypical presentations of alveolar capillary dysplasia with misalignment of the pulmonary veins who underwent bilateral lung transplantation. J Pediatr. 2018;194:158–164. doi: 10.1016/j.jpeds.2017.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stankiewicz P, Sen P, Bhatt SS, Storer M, Xia Z, Bejjani BA, et al. Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations Am J Hum Genet 200984780–791.[Published erratum appears in Am J Hum Genet 85:537.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sen P, Yang Y, Navarro C, Silva I, Szafranski P, Kolodziejska KE, et al. Novel FOXF1 mutations in sporadic and familial cases of alveolar capillary dysplasia with misaligned pulmonary veins imply a role for its DNA binding domain. Hum Mutat. 2013;34:801–811. doi: 10.1002/humu.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garabedian MJ, Wallerstein D, Medina N, Byrne J, Wallerstein RJ. Prenatal diagnosis of cystic hygroma related to a deletion of 16q24.1 with haploinsufficiency of FOXF1 and FOXC2 genes. Case Rep Genet. 2012;2012:490408. doi: 10.1155/2012/490408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dello Russo P, Franzoni A, Baldan F, Puppin C, De Maglio G, Pittini C, et al. A 16q deletion involving FOXF1 enhancer is associated to pulmonary capillary hemangiomatosis. BMC Med Genet. 2015;16:94. doi: 10.1186/s12881-015-0241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilger AC, Halbritter J, Pennimpede T, van der Ven A, Sarma G, Braun DA, et al. Targeted resequencing of 29 candidate genes and mouse expression studies implicate ZIC3 and FOXF1 in human VATER/VACTERL association. Hum Mutat. 2015;36:1150–1154. doi: 10.1002/humu.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai Y, Bolte C, Le T, Goda C, Xu Y, Kalin TV, et al. FOXF1 maintains endothelial barrier function and prevents edema after lung injury. Sci Signal. 2016;9:ra40. doi: 10.1126/scisignal.aad1899. [DOI] [PubMed] [Google Scholar]
- 9.Milewski D, Pradhan A, Wang X, Cai Y, Le T, Turpin B, et al. FoxF1 and FoxF2 transcription factors synergistically promote rhabdomyosarcoma carcinogenesis by repressing transcription of p21Cip1 CDK inhibitor. Oncogene. 2017;36:850–862. doi: 10.1038/onc.2016.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolte C, Whitsett JA, Kalin TV, Kalinichenko VV. Transcription factors regulating embryonic development of pulmonary vasculature. Adv Anat Embryol Cell Biol. 2018;228:1–20. doi: 10.1007/978-3-319-68483-3_1. [DOI] [PubMed] [Google Scholar]
- 11.Mahlapuu M, Ormestad M, Enerbäck S, Carlsson P. The forkhead transcription factor Foxf1 is required for differentiation of extra-embryonic and lateral plate mesoderm. Development. 2001;128:155–166. doi: 10.1242/dev.128.2.155. [DOI] [PubMed] [Google Scholar]
- 12.Kalinichenko VV, Lim L, Stolz DB, Shin B, Rausa FM, Clark J, et al. Defects in pulmonary vasculature and perinatal lung hemorrhage in mice heterozygous null for the Forkhead Box f1 transcription factor. Dev Biol. 2001;235:489–506. doi: 10.1006/dbio.2001.0322. [DOI] [PubMed] [Google Scholar]
- 13.Kalinichenko VV, Zhou Y, Shin B, Stolz DB, Watkins SC, Whitsett JA, et al. Wild-type levels of the mouse Forkhead Box f1 gene are essential for lung repair. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1253–L1265. doi: 10.1152/ajplung.00463.2001. [DOI] [PubMed] [Google Scholar]
- 14.Sen P, Dharmadhikari AV, Majewski T, Mohammad MA, Kalin TV, Zabielska J, et al. Comparative analyses of lung transcriptomes in patients with alveolar capillary dysplasia with misalignment of pulmonary veins and in foxf1 heterozygous knockout mice. PLoS One. 2014;9:e94390. doi: 10.1371/journal.pone.0094390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalinichenko VV, Zhou Y, Bhattacharyya D, Kim W, Shin B, Bambal K, et al. Haploinsufficiency of the mouse Forkhead Box f1 gene causes defects in gall bladder development. J Biol Chem. 2002;277:12369–12374. doi: 10.1074/jbc.M112162200. [DOI] [PubMed] [Google Scholar]
- 16.Kalinichenko VV, Gusarova GA, Shin B, Costa RH. The forkhead box F1 transcription factor is expressed in brain and head mesenchyme during mouse embryonic development. Gene Expr Patterns. 2003;3:153–158. doi: 10.1016/s1567-133x(03)00010-3. [DOI] [PubMed] [Google Scholar]
- 17.Ren X, Ustiyan V, Pradhan A, Cai Y, Havrilak JA, Bolte CS, et al. FOXF1 transcription factor is required for formation of embryonic vasculature by regulating VEGF signaling in endothelial cells. Circ Res. 2014;115:709–720. doi: 10.1161/CIRCRESAHA.115.304382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pradhan A, Ustiyan V, Bolte C, Zhang Y, Porollo A, Hu YC, et al. S52F point mutation in the DNA-binding domain of FoxF1 causes ACD/MPV phenotype and impairment in STAT3 signaling [abstract] Am J Respir Crit Care Med. 2017;195:A4933. [Google Scholar]
- 19.Pradhan A, Ustiyan V, Dunn A, Bolte C, Zhang Y, Porollo A, et al. Endothelium in COPD and Group 3 PH session. Nanoparticle-mediated delivery of STAT3 stimulates lung angiogenesis in mouse model of alveolar capillary dysplasia. Presented at the Grover Conference. September 6–10, 2017, Sedalia, CO. [Google Scholar]
- 20.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prothro SL, Plosa E, Markham M, Szafranski P, Stankiewicz P, Killen SA. Prenatal diagnosis of alveolar capillary dysplasia with misalignment of pulmonary veins. J Pediatr. 2016;170:317–318. doi: 10.1016/j.jpeds.2015.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pradhan A, Ustiyan V, Zhang Y, Kalin TV, Kalinichenko VV. Forkhead transcription factor FoxF1 interacts with Fanconi anemia protein complexes to promote DNA damage response. Oncotarget. 2016;7:1912–1926. doi: 10.18632/oncotarget.6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolte C, Ren X, Tomley T, Ustiyan V, Pradhan A, Hoggatt A, et al. Forkhead box F2 regulation of platelet-derived growth factor and myocardin/serum response factor signaling is essential for intestinal development. J Biol Chem. 2015;290:7563–7575. doi: 10.1074/jbc.M114.609487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim IM, Zhou Y, Ramakrishna S, Hughes DE, Solway J, Costa RH, et al. Functional characterization of evolutionarily conserved DNA regions in forkhead box f1 gene locus. J Biol Chem. 2005;280:37908–37916. doi: 10.1074/jbc.M506531200. [DOI] [PubMed] [Google Scholar]
- 25.Cheng XH, Black M, Ustiyan V, Le T, Fulford L, Sridharan A, et al. SPDEF inhibits prostate carcinogenesis by disrupting a positive feedback loop in regulation of the Foxm1 oncogene. PLoS Genet. 2014;10:e1004656. doi: 10.1371/journal.pgen.1004656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balli D, Ren X, Chou FS, Cross E, Zhang Y, Kalinichenko VV, et al. Foxm1 transcription factor is required for macrophage migration during lung inflammation and tumor formation. Oncogene. 2012;31:3875–3888. doi: 10.1038/onc.2011.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang IC, Snyder J, Zhang Y, Lander J, Nakafuku Y, Lin J, et al. Foxm1 mediates cross talk between Kras/mitogen-activated protein kinase and canonical Wnt pathways during development of respiratory epithelium. Mol Cell Biol. 2012;32:3838–3850. doi: 10.1128/MCB.00355-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang IC, Zhang Y, Snyder J, Sutherland MJ, Burhans MS, Shannon JM, et al. Increased expression of FoxM1 transcription factor in respiratory epithelium inhibits lung sacculation and causes Clara cell hyperplasia. Dev Biol. 2010;347:301–314. doi: 10.1016/j.ydbio.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Bhattacharyya D, Dennewitz MB, Kalinichenko VV, Zhou Y, Lepe R, et al. Rapid hepatocyte nuclear translocation of the Forkhead Box M1B (FoxM1B) transcription factor caused a transient increase in size of regenerating transgenic hepatocytes. Gene Expr. 2003;11:149–162. doi: 10.3727/000000003108749044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren X, Zhang Y, Snyder J, Cross ER, Shah TA, Kalin TV, et al. Forkhead box M1 transcription factor is required for macrophage recruitment during liver repair. Mol Cell Biol. 2010;30:5381–5393. doi: 10.1128/MCB.00876-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ustiyan V, Wert SE, Ikegami M, Wang IC, Kalin TV, Whitsett JA, et al. Foxm1 transcription factor is critical for proliferation and differentiation of Clara cells during development of conducting airways. Dev Biol. 2012;370:198–212. doi: 10.1016/j.ydbio.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolte C, Zhang Y, Wang IC, Kalin TV, Molkentin JD, Kalinichenko VV. Expression of Foxm1 transcription factor in cardiomyocytes is required for myocardial development. PLoS One. 2011;6:e22217. doi: 10.1371/journal.pone.0022217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akeson AL, Wetzel B, Thompson FY, Brooks SK, Paradis H, Gendron RL, et al. Embryonic vasculogenesis by endothelial precursor cells derived from lung mesenchyme. Dev Dyn. 2000;217:11–23. doi: 10.1002/(SICI)1097-0177(200001)217:1<11::AID-DVDY2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 34.Bolte C, Flood HM, Ren X, Jagannathan S, Barski A, Kalin TV, et al. FOXF1 transcription factor promotes lung regeneration after partial pneumonectomy. Sci Rep. 2017;7:10690. doi: 10.1038/s41598-017-11175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunn AW, Kalinichenko VV, Shi D. Highly efficient in vivo targeting of the pulmonary endothelium using novel modifications of polyethylenimine: an importance of charge. Adv Healthc Mater. 2018;7:e1800876. doi: 10.1002/adhm.201800876. [DOI] [PubMed] [Google Scholar]
- 36.Ou H, Li Y, Kang M. Activation of miR-21 by STAT3 induces proliferation and suppresses apoptosis in nasopharyngeal carcinoma by targeting PTEN gene. PLoS One. 2014;9:e109929. doi: 10.1371/journal.pone.0109929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiong A, Yang Z, Shen Y, Zhou J, Shen Q. Transcription factor STAT3 as a novel molecular target for cancer prevention. Cancers (Basel) 2014;6:926–957. doi: 10.3390/cancers6020926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai KL, Huang CY, Chang CH, Sun YJ, Chuang WJ, Hsiao CD. Crystal structure of the human FOXK1a-DNA complex and its implications on the diverse binding specificity of winged helix/forkhead proteins. J Biol Chem. 2006;281:17400–17409. doi: 10.1074/jbc.M600478200. [DOI] [PubMed] [Google Scholar]
- 39.Xia T, Kovochich M, Liong M, Meng H, Kabehie S, George S, et al. Polyethyleneimine coating enhances the cellular uptake of mesoporous silica nanoparticles and allows safe delivery of siRNA and DNA constructs. ACS Nano. 2009;3:3273–3286. doi: 10.1021/nn900918w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dutzmann J, Daniel JM, Bauersachs J, Hilfiker-Kleiner D, Sedding DG. Emerging translational approaches to target STAT3 signalling and its impact on vascular disease. Cardiovasc Res. 2015;106:365–374. doi: 10.1093/cvr/cvv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeda K, Akira S. Multi-functional roles of Stat3 revealed by conditional gene targeting. Arch Immunol Ther Exp (Warsz) 2001;49:279–283. [PubMed] [Google Scholar]
- 42.Akira S, Nishio Y, Inoue M, Wang XJ, Wei S, Matsusaka T, et al. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994;77:63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 43.Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, et al. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci USA. 1997;94:3801–3804. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grandis JR, Drenning SD, Zeng Q, Watkins SC, Melhem MF, Endo S, et al. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci USA. 2000;97:4227–4232. doi: 10.1073/pnas.97.8.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welte T, Zhang SS, Wang T, Zhang Z, Hesslein DG, Yin Z, et al. STAT3 deletion during hematopoiesis causes Crohn’s disease-like pathogenesis and lethality: a critical role of STAT3 in innate immunity. Proc Natl Acad Sci USA. 2003;100:1879–1884. doi: 10.1073/pnas.0237137100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ran L, Chen Y, Sher J, Wong EWP, Murphy D, Zhang JQ, et al. FOXF1 defines the core-regulatory circuitry in gastrointestinal stromal tumor. Cancer Discov. 2018;8:234–251. doi: 10.1158/2159-8290.CD-17-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saarimäki-Vire J, Balboa D, Russell MA, Saarikettu J, Kinnunen M, Keskitalo S, et al. An activating STAT3 mutation causes neonatal diabetes through premature induction of pancreatic differentiation. Cell Reports. 2017;19:281–294. doi: 10.1016/j.celrep.2017.03.055. [DOI] [PubMed] [Google Scholar]
- 48.Flanagan SE, Haapaniemi E, Russell MA, Caswell R, Allen HL, De Franco E, et al. Activating germline mutations in STAT3 cause early-onset multi-organ autoimmune disease. Nat Genet. 2014;46:812–814. doi: 10.1038/ng.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fabre A, Marchal S, Forbes LR, Vogel TP, Barlogis V, Triolo V, et al. STAT3 gain of function: a new kid on the block in interstitial lung diseases. Am J Respir Crit Care Med. 2018;197:e22–e23. doi: 10.1164/rccm.201707-1500IM. [DOI] [PubMed] [Google Scholar]
- 50.Soon E, Crosby A, Southwood M, Yang P, Tajsic T, Toshner M, et al. Bone morphogenetic protein receptor type II deficiency and increased inflammatory cytokine production: a gateway to pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;192:859–872. doi: 10.1164/rccm.201408-1509OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.