Abstract
Since the pioneering work of Penfield and his colleagues in the 1930s, the somatosensory cortex, which is located on the postcentral gyrus, has been known for its central role in processing sensory information from various parts of the body. More recently, a converging body of literature has shown that the somatosensory cortex also plays an important role in each stage of emotional processing, including identification of emotional significance in a stimulus, generation of emotional states, and regulation of emotion. Importantly, studies conducted in individuals suffering from mental disorders associated with abnormal emotional regulation, such as major depression, bipolar disorder, schizophrenia, post-traumatic stress disorder, anxiety and panic disorders, specific phobia, obesity, and obsessive-compulsive disorder, have found structural and functional changes in the somatosensory cortex. Common observations in the somatosensory cortices of individuals with mood disorders include alterations in gray matter volume, cortical thickness, abnormal functional connectivity with other brain regions, and changes in metabolic rates. These findings support the hypothesis that the somatosensory cortex may be a treatment target for certain mental disorders. In this review, we discuss the anatomy, connectivity, and functions of the somatosensory cortex, with a focus on its role in emotional regulation.
Keywords: Somatosensory cortex, emotional regulation, mental disorders
Anatomy
The somatosensory cortex is divided into two regions: the primary somatosensory cortex (SI) and the secondary somatosensory cortex (SII).1
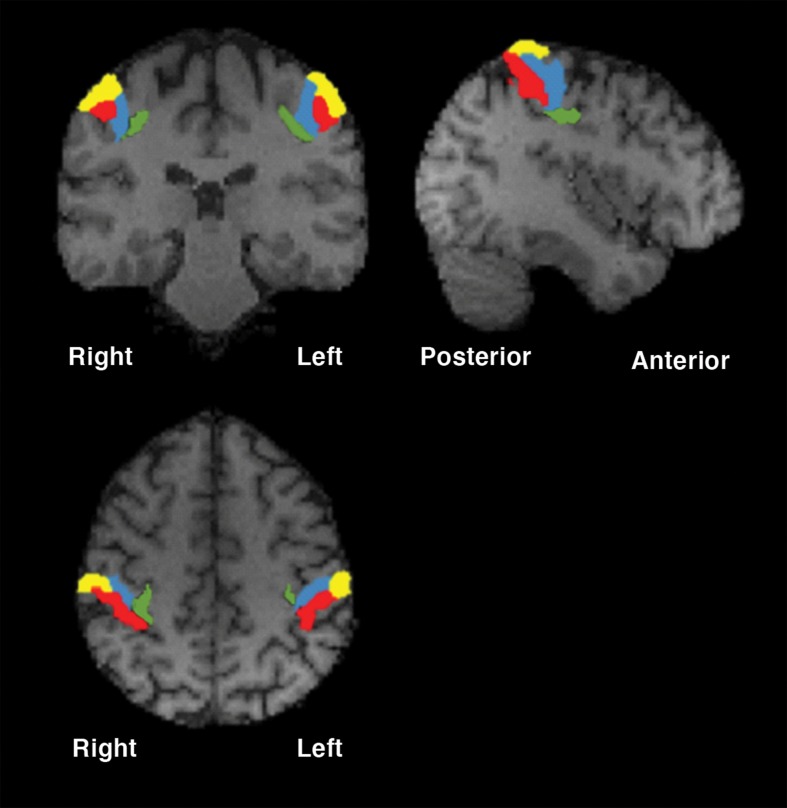
SI is located on the postcentral gyrus, running parallel to the central sulcus.2 This region corresponds to Brodmann areas 3, 2, and 1, with area 3 being further divided into areas 3a and 3b3,4 (Figure 1). The gray matter thickness of the postcentral gyrus, as determined by cortical morphometry, is approximately 1.8 mm, which varies sharply in contrast to the neighboring precentral gyrus (premotor cortex), which has an average thickness of approximately 2.7 mm.5 The location and borders of SI and the regions within it can be identified by analyzing the histological changes that occur from one area to the next.6 An in vitro quantitative receptor autoradiography analysis of various neurotransmitter binding sites across SI, such as muscarinic (m1 and m2) receptors, α1-adrenoceptors, serotonin receptors (5-HT1A, 5-HT1D, 5-HT1E, and 5-HT2A), L-glutamate receptors and gamma-amino butyric acid (GABA) receptors (specifically GABAA receptors) revealed that most receptor densities are located lower in deeper cortical layers and exhibit changes in density patterns at the borders defined by the cytoarchitecture.7
Figure 1. Anatomical subregions of the somatosensory cortex (Montreal Neurological Institute [MNI] coordinates: x = -42, y = -28, z = 47). Yellow = Brodmann area 1; red = Brodmann area 2; blue = Brodmann area 3a; green = Brodmann area 3b.
Area 3a is located on the region of the postcentral gyrus immediately posterior to the central sulcus, and borders on the caudal edge of the primary motor cortex (M1; Brodmann area 4).4,7 In contrast with M1, the tissue in area 3a does not contain giant pyramidal cells, and cells in 3a are present at a higher density in cortical layers II-VI than in M1.7 Further, granular cells in area 3a are present at lower densities than other areas of SI, with cellular density in this region reaching its lowest levels in cortical layer V.6,7 In 3a, layer V contains numerous pyramidal cells of varying sizes, some of which are very large.7,8 In areas 3a and 3b, there appears to be a high density of m1, m2, 5-HT2A, and GABAA receptors and a moderate density of L-glutamate receptors. Area 3b is located along the postcentral gyrus dorsal to 3a,3 and it is composed of a dense cluster of small, granular cells from layer IV of the cortex, as well as a high density of layer VI cells.6,8 Area 1 is located posterior to 3b.3 Layers II-VI of the cortex in area 1 are less dense than that of area 3b.6,7 A hallmark feature of area 1 are the extended pyramidal cells present in cortical layer III, which decrease in size in more caudal areas of the postcentral gyrus.6,7 In this area, there is a lower density of m1, m2, GABAA, and 5-HT2A receptors than in area 3, while L-glutamate receptors are present at a higher density.7
Area 2 occupies the remaining portion of the postcentral gyrus, posterior to area 1,3 and contains a dense network of cells from layer IV of the cortex.6,8,9 Similar to area 1, there is a low density of m2 receptors in area 2, and a high density of L-glutamate receptors.7 Area 2 contains higher densities of GABAA, m1, and 5-HT2A receptors than area 1.7 It is important to note that the exact locations and sizes of each area of SI vary within individuals, and borders between each region are often described as “wide transition zones” rather than definite borders,6,9 allowing for inter-individual anatomical (and possibly functional) variability.
SII corresponds to Brodmann areas 40 and 43.4 This region is located in the parietal operculum and can be divided into four regions; OP1, OP2, OP3, and OP4.10 Each of these regions is separated from neighboring regions by changes in cytoarchitecture, which appear to be consistent across both hemispheres of the brain.10 The most caudal area of SII is OP1, which is included in the inferior parietal locations of Brodmann area 40.10 OP1 is located in the superficial region of the parietal operculum, immediately ventral to the inferior parietal cortex. In OP1, cortical layer II contains granular cells that spread into upper layer III. The size of pyramidal cells in layer III increases in deeper regions. Layer IV of OP1 is characterized by a layer of granular cells that are distinct from the low density of medium-sized pyramids in layer V. Layer VI contains pyramidal cells that are larger in size and density than the cells in layer V of OP1.10
OP2 is also included in the inferior part of Brodmann area 40 and is located deep within the Sylvian fissure.10 OP2 borders on the ventral edge of OP1 and on the dorsal edge of the granular insular cortex (Ig). Along with OP1, OP2 extends into the retroinsular area of the Sylvian fissure, but does not extend as far as its caudal end. As in OP1, the granular cells in layer II of OP2 spread into cortical layer III. However, unlike OP1, the pyramidal cells in layer III of OP2 are mostly small in size, with a uniform density throughout the layer. Layer IV of OP2 contains a very high density of cells, which separates it from layers III and V. Layer V of OP2 is mostly composed of small pyramidal cells.10
OP3 is contained within Brodmann area 43 and, like OP2, is located within the Sylvian fissure, although its area spreads over the majority of the parietal operculum.10 It is located rostral to OP2, with its ventral end bordering on the Ig and its dorsal side bordering on both OP1 and OP4. Similar to OP1 and OP2, cells of layer II extend into layer III, which is composed of a small number of large pyramidal cells. The granular cells of layer IV are sparse, similar to layer V, in which small pyramidal cells are present at low densities.10
The most rostral area of SII is OP4, which is also included in Brodmann area 43.10 OP4 is present immediately rostral to OP1 on the superficial area of the parietal operculum. There are some individual differences with respect to the borders of OP4, as it appears to border with Brodmann areas 3a, 3b, 2, and 1 inconsistently across different brains, although in most brains OP4 borders on area 1. In OP4, the superficial portion of layer III is composed of many small pyramidal cells. Cell density increases from layer IV to layer V. Medium pyramids at a high density make up the superficial portion of layer V, and cells in layer VI extend into the white matter.10
Connectivity
The somatosensory cortex has numerous connections between its sub-regions, as well as with other areas of the brain.3 Within SI, axons from area 3a are mainly connected with area 2, while axons from area 3b are highly connected with areas 1 and 2.3 Some of the fibers within SI travel to the spinal cord, where they continue to descend through the white matter via the lateral corticospinal tract.1 Before entering the spinal cord, this tract traverses the pons and decussates to the contralateral side in the medulla oblongata.11 SI also projects to adjacent brain areas through U-shaped fibers.11 For example, area 2 is connected with Brodmann areas 5 and 7, and axons from these areas eventually end up in the motor and premotor cortices (Brodmann areas 4 and 6, respectively) in the frontal lobe.3,4 Additionally, each of areas 3a, 3b, 1, and 2 connect with SII.3 From SII, some of these fibers make up the trigeminal nerve, which travels through the pons via the sensory root.1 Some fibers of the trigeminal system, such as A-fibers, decussate more rostrally and terminate in the principal sensory nucleus of the trigeminal nerve. Some of the axons continue descending and decussate more caudally, where they synapse in the spinal trigeminal nucleus. Other axons from SII decussate even more caudally and terminate in the cuneate nucleus of the medulla oblongata.1
SI and SII both project to, and receive projections from, the ventral posterior nucleus of the thalamus.1 This nucleus can be divided into medial and lateral nuclei, both of which send efferent neurons to SI, particularly to area 3b.3,11 The ventral posterior medial nucleus is connected with SII, as it receives neurons from the trigeminal tract and contains the principal sensory nucleus of the trigeminal nerve.1,11 Trigeminal neurons also travel back to SI from the ventral posterior medial nucleus.1
Areas SI and SII are also connected reciprocally with the insula.12 Studies in non-human primates have demonstrated that neurons from the ventral portion of SI synapse in SII and then arrive in the insula.12,13 In particular, projections from SII terminate in the Ig and the dysgranular insular area (Id).12,13 Neurons from Ig and Id then project to the amygdala.12 Id projects more heavily to the amygdala than Ig, with projections reaching the lateral, central and basal nuclei of the amygdala.13 Meanwhile, most projections from Ig to the amygdala terminate dorsally in the lateral nucleus.13 Additionally, some neurons from SII connect directly to the amygdala, primarily to the lateral nucleus of the amygdala.14 Some portions of SII also project to other areas of the limbic system, such as the hippocampus.3 It is interesting to note that there are many more descending pathways from the somatosensory cortex than ascending ones.3 It is likely that these efferent neurons regulate the ascending afferent information before it reaches the somatosensory cortex.3
Evidently, the brain is a highly interconnected structure, with regions of the somatosensory cortex connecting with numerous cortical and subcortical areas. It is these numerous connections that allow the somatosensory cortex to have various functions,3 including the generation of bodily representations and tactile attention, sensory motor integration, the processing of painful stimuli, the generation of empathy and emotion, and emotion regulation. Each of these functions is described in greater detail below.
Functions
Representations of the body
The most well-known role of the somatosensory cortex is the processing of sensory information from various parts of the body. Results from functional mapping studies suggest that the body is completely represented in both SI and SII.2,15-17 Each of areas 3a, 3b, 1, and 2 contain full body representations, resulting in a total of four representations in SI.2 Penfield & Boldrey18 were able to map areas of the somatosensory cortex that were associated with sensory processing for different parts of the body by using electrical stimulation. This representation is known as the sensory homunculus. In general, the face is represented most laterally in SI, with the fingers, hands, arms, torso, legs, feet, and toes represented in progressively more medial locations.1,3,8 Interestingly, the cortical surface area of SI dedicated to processing each part of the body is proportional to the density of sensory receptors in the given extremity.3 Thus, areas with a higher density of sensory receptors are processed by larger cortical areas, and larger areas of the body do not necessarily occupy more space in SI.3 The variations in the types of afferents received and efferents projected from the various regions of SI likely contribute to their differential functions.3,7 For example, information from muscle spindle stimulation is sent to area 3a, which mainly processes information regarding body position and movement. The main information sent to area 3b comes from cutaneous receptors, so the majority of cells in this area are dedicated to processing and responding to cutaneous stimuli. Action potentials from deep pressure receptors are received in area 2, which is involved in processing tactile and proprioceptive information.3,7
SII contains additional representations of the body.16,17 Similar to SI, topographic representations of lower extremities, such as the foot and toes, are medial to upper extremities, such as the hand and face.15 The map of the body in SII may be less precise than that of SI, since regions of activation for separate body parts overlapped in functional magnetic resonance imaging (fMRI) mapping studies.15,17 It is interesting to note that unilateral stimulation of most limbs resulted in activity in both the contralateral and ipsilateral SII hemispheres.15,17 This suggests that neurons in SII have large, bilateral receptive fields.15 As mentioned previously, SII can be divided into regions OP1, OP2, OP3, and OP4.10 Each of these regions contain representations of the extremities and appear to have distinct functions.16 Of these regions, OP1 is thought to be of particular importance for bilateral processing of somatosensory information. This region is likely important for the perception of somatosensory information and its integration with information from other cortical areas. It has also been proposed that OP1 may contribute to tactile discrimination and working memory.16
Tactile attention
Positron emission tomography (PET) studies indicate that, in addition to processing somatosensory information, the somatosensory cortex is involved in modulating tactile attention.19 Both SI and SII areas appear to be involved in tactile attention tasks, although SII is thought to have a larger role than SI. This is evidenced by PET and fMRI studies that observed increased blood flow in SI and SII during tactile attention tasks, with greater increases seen in SII than SI. Results from Burton et al.19 suggest that SI only becomes active when the tactile information being processed is of utmost importance. If another modality (e.g., counting or verbalization) contributes more relevant information, then activity in SI will be suppressed. Based on the same study, it appears as though SII is involved in directing tactile attention for many stimulus features, such as duration and texture.19
Sensorimotor integration
Another important function of the somatosensory cortex is integration of somatosensory information with motor information.20 As mentioned previously, the somatosensory cortex contributes axons to the corticospinal tract and is heavily connected with the motor cortex, both of which are primarily dedicated to motor processing.1,3 The connections between SI and the primary motor cortex are essential for motor function, since they are involved in generating a conscious awareness of motion.20
SII is also involved in motor function.16 In particular, OP4 is thought to be involved in perception of motor information and in processes such as object manipulation through its connections with SI and the motor cortex.16
Processing of painful stimuli
The somatosensory cortex not only contributes to sensorimotor integration, but is also involved in processing pain.21 A study using magnetoencephalography demonstrates that activity in the somatosensory cortex increases with nociceptive stimulus intensity and subjective pain ratings. Interestingly, SI and SII respond differently to painful stimulation. SI is likely to be involved in perception of the intensity of pain, since its activity increases exponentially as stimulus intensity increases. Unlike SI, activity in SII is minimal when stimulus intensity is low, but rapidly increases for higher intensity stimuli. Based on this information, along with supporting information from non-human primate studies, it is likely that the role of SII is related to identifying nociceptive stimuli and directing attention towards the source of the pain.21
Empathy
The somatosensory cortex is involved in the emotional reaction to pain by contributing to empathetic responses that result from viewing pain inflicted on others.22 Using somatosensory-evoked potentials recorded from subjects who watched pain being inflicted on another individual, Bufalari et al.22 showed that such observation elicits activity in various regions of SI, likely corresponding to Brodmann areas 3b and either area 1 or 2. The activity in SI appears to increase as the intensity of the painful stimulus observed increases, and decreases when nonpainful stimulation is observed. It is suggested that when the actions of others are viewed, the sensations of the actor are processed in the observer’s somatosensory system and may affect the observer’s somatosensation. While there are certainly other brain areas associated with processing empathy, it is likely that SI also plays an important role.22
Emotion
The role of the somatosensory cortex in processing emotion is not limited to its contribution in the generation of empathy.23,24 It has been suggested that the somatosensory cortex makes numerous contributions to emotional regulation. One such example is given in a lesion study by Adolphs et al.,23 which demonstrated that recognizing emotion is dependent on the right somatosensory cortex. In this study, lesions in SII resulted in impaired ability to recognize emotion.23 Another example can be found in a PET imaging study, which demonstrated that activity in SII contributes to the self-generation of emotions.24 For example, activity increased in SII when the subjects reflected on positive situations that resulted in happiness. Alternatively, thinking about situations that resulted in sadness caused a decrease in SII activity. The activation patterns of SII in either hemisphere differ for each emotion, since activity was seen only in the right hemisphere for emotions like happiness and fear, and activity decreased in both hemispheres for emotions like sadness and anger. These results suggest that emotion generation is partly dependent on somatotopic representations of the body, which are generated in the somatosensory cortex.24 In addition to emotion generation, an emerging body of literature suggests that the somatosensory cortex may also play a role in emotion regulation. This is supported by research on both healthy subjects and individuals with mood disorders, in whom emotional dysregulation is a clinical hallmark.
Emotion regulation
The generation and regulation of emotion is a multi-step process involving multiple brain areas; one of which is the somatosensory cortex.25,26 According to the model proposed by Phillips et al.,25 the first step of emotional processing requires evaluation of a stimulus to determine whether or not it contains emotional significance. The next step is to generate the appropriate emotion, which requires input from various systems throughout the body. Finally, this emotional state and the resulting behavior must be regulated.25 Studies involving cortical changes in mood disorders have implied that the somatosensory cortex plays a role in emotion regulation. More specifically, it has been shown that the somatosensory cortex is involved in each stage of emotion processing, which indicates a central role of the somatosensory cortex in emotion regulation.12,14,19,22-24,27-41 For instance, Adolphs et al.23 demonstrated that the somatosensory cortex plays a role in emotion recognition. In this study, participants with lesions in the right somatosensory cortex had impaired ability to rate the intensity of emotions, as well as to recognize, name and categorize emotions. These results indicate that the somatosensory cortex is involved in the first stage of emotion processing, since it appears to be necessary for identifying emotions.23 One potential mechanism through which the somatosensory cortex may be involved in such identification is through its connection to the amygdala,14 considering the well-established role of the amygdala in emotion regulation and the fact that the somatosensory cortex is connected with the amygdala, both directly and indirectly through the insula.12,14 Cunningham & Zelazo27 suggested that emotional significance is determined by the re-evaluation of stimuli through neural processing between the amygdala and neighboring regions. In this context, early evaluations are conducted through amygdala interaction between the thalamus and limbic regions; later evaluations are then analyzed through connections between the amygdala and somatosensory cortex.27 This highlights the role of the somatosensory cortex in higher order re-evaluation of stimuli to establish emotional salience.
In order to determine emotional salience, an appropriate affective state must first be generated.25 Numerous articles suggest that the somatosensory cortex is involved in this stage of emotion processing.24,28-35 As previously mentioned, Damasio et al.24 observed activity changes in the somatosensory cortex while subjects reflected on various experiences that resulted in different emotional states. This suggests that the somatosensory cortex is likely to be involved in generating emotional responses such as sadness, happiness, and anger.24 The results of Damasio et al. are supported by a functional imaging study by Critchley et al.,28 which demonstrates somatosensory cortex activation in tasks requiring interoceptive attention. This activity suggests that the somatosensory cortex may be involved in awareness of the internal state of the body,28 consistent with a generally accepted theory that states that generation of emotional states requires awareness of the state of the internal and external systems of the body. Since SI and SII contain somatotopic representations of the body, and this study also implicates them in interoceptive attention, it is reasonable to suggest that activity in the somatosensory cortex also contributes to emotional feeling states.28 Functional imaging by Straube & Miltner29 indicates high levels of activation in the somatosensory cortex when attention is focused on emotional state, which provides further evidence for the idea that the somatosensory cortex is involved in emotion processing. Additionally, it has been shown that pain-related activity in the somatosensory cortex interacts with emotional stimuli.30 In their recent functional imaging study, Orenius et al.30 observed differential activation of SII in response to pain stimuli when exposed to positive emotional stimuli vs. negative emotional stimuli. SII was active bilaterally in conditions involving pain and a positive emotional stimulus, while SII was active in the left hemisphere only in negative emotion and pain stimulus conditions. These results could be interpreted to mean that pain-related activity in SII is modulated by emotional state, or that activity in SII contributes to the generation of emotional states. Either way, it is evident that the somatosensory cortex is involved in emotion processing in some way.30
Further suggestions for the role of the somatosensory cortex in the generation of emotional states comes from a meta-analysis by Satpute et al.31 The authors found that the somatosensory cortex was engaged during affective experiences that were driven by inputs from visual, gustatory, olfactory, auditory, and somatosensory sensory modalities. This suggests that the somatosensory cortex may not be involved in the generation of all types of emotion. Rather, cortical activity may be dependent on the sensory qualities of the emotional stimulus, suggesting that the somatosensory cortex may be mainly involved in somatosensory-related emotion generation.31 Evidently, there is compelling evidence to suggest that the somatosensory cortex contributes to the production of emotions related to somatosensory stimuli, and potentially to emotions related to other sensory qualities as well.
The involvement of the somatosensory cortex in processing fear and generating fear memory has also been studied, although not as extensively. Damasio et al.24 found activity in the right SII when subjects reflected on fearful memories, suggesting that SII may be involved in fear processing. Further, Liddell et al.32 observed activity in the somatosensory- related cortices of the left hemisphere in response to fearful stimuli that were not consciously perceived. These results suggest a role for the somatosensory cortex in unconscious fear perception, which may be involved in a rapid processing pathway that responds to potential threats in the environment.32 Additionally, classical fear conditioning experiments can be used to evaluate the involvement of the somatosensory cortex in emotional learning, specifically in the generation of fear memory.33-35 In an fMRI study, individuals with a history of psychopathy did not demonstrate activation in the SII area and had difficulty associating noxious stimulation with fearful emotions.33 Although the researchers in this study attributed the somatosensory activation to pain processing, it is possible that activity in the somatosensory cortex is also involved in processing and/or generating fear.33 In fact, Wei et al.34 provide evidence suggesting that the somatosensory cortex is involved in such fear conditioning and memory. Specifically, they studied a transcription factor, cyclic adenosine monophosphate-responsive element binding protein (CREB), which contributes to long term fear memory upon upregulation.34 This transcription factor is activated by phosphorylation, and increased levels of phosphorylated CREB (pCREB) were found in SI and other cortical areas in response to fear conditioning trials. When activators of the CREB pathway were knocked out, mice did not show activation in the somatosensory cortex and had decreased fear memory.34 These results suggest that the somatosensory cortex is involved in pCREB-dependent fear conditioning, leading to fear memory.34 However, Tang et al.35 present somewhat opposing results, finding that stimulation of SI did not result in the production of fear memory in mice, while stimulation of the anterior cingulate cortex (ACC) did.35 In conciliating these seemingly contradictory results, it is possible that the somatosensory cortex contributes to fear processing and the production of fear memory, but activity in this area alone is insufficient to produce fear memory.34,35 Based on these studies, it is plausible that the somatosensory cortex contributes to processing and/or generation of fearful emotions, although further research in this area is required to clarify this hypothesis.
Once an emotional state is generated, it is then regulated to ensure its appropriateness for the social context.25 Strategies used to regulate emotions include attentional deployment, situational selection, and controlled generation.36,37 Attentional deployment involves directing one’s attention to a specific aspect of the emotion-provoking stimulus or situation.36,37 Several lines of evidence suggest that the somatosensory cortex is involved in this emotion regulation strategy, since activation of this cortical area has been observed in various studies of attention.19,22,25,31,36-40 For example, Burton et al.19 observed a role for the somatosensory cortex in modulating tactile attention. As previously mentioned, completion of tactile attention tasks resulted in increased blood flow in the somatosensory cortex, particularly in SII.19 Based on these results, it is reasonable to suggest that increased activity in SII contributes to increased attention towards tactile stimuli, which could be useful in in emotion regulation strategies.19,36 In such strategies, the activation of SII may result in generation of somatosensory-related emotional states, rather than emotional states that could have arisen from other sensory qualities of the stimulus.31 Similarly, functional imaging reveals SII activity during selective attention tasks.38 This study provides further support for the idea that activation of the somatosensory cortex could contribute to emotional regulation by directing attention towards specific aspects of a situation.38 Additionally, results from a magnetoencephalography study show that increasing attention results in stronger responses in the somatosensory cortex, especially in SII.39 The results of this study led the researchers to hypothesize that representations in SII correspond only to what has been actively selected in the environment, rather than an accurate somatotopic representation of the body.39 Evidently, the somatosensory cortex (SII in particular) is involved in directing attention, and it is reasonable to suggest that this cortical area could contribute to emotional regulation involving attentional strategies.
Situational selection is another emotion regulation strategy.36 This process requires a person to decide which situations they allow themselves to be a part of, including where and with whom to spend time.36 It is possible that the somatosensory cortex plays a role in this regulation strategy through its connection with the anterior insula.40 The anterior insula contributes to the social emotions experienced upon interaction with others.40 These emotions are thought to depend upon the social environment in which interactions occur, with the somatosensory cortex contributing to experiences of pain and empathy.22,40 Therefore, it could be concluded that the connections between the somatosensory cortex and the anterior insula may contribute to awareness of social context, allowing individuals to select people, places and things to surround themselves with in order to regulate their affective state.36,40
Finally, the controlled generation strategy suggests that expectations can be used to modulate one’s neural activity and their resulting affective state.37 It is plausible that the somatosensory cortex could be involved in this emotion regulation strategy, since it has been found that representations within SI can be altered based on top-down factors such as focus of attention and expectation.41 For example, it was found in one study of SI that the location of the representation of digit 2, as well as the angle between this representation and that of other digits, varied based on whether attention was focused on a local or global scale. This suggests that the maps within SI are not static, and that they can be manipulated by attention and other top-down processing strategies.41 It is, therefore, reasonable to suggest that the somatosensory cortex may contribute to controlled generation by altering cortical representations of a situation or stimulus, leading to a different affective state.37,41
Discussion
Analysis of the current literature suggests that the somatosensory cortex may play a role in in each stage of emotion processing, beginning with its involvement in the recognition of emotions.23 Additionally, the somatosensory cortex is heavily connected to the amygdala and insula, both of which have also been implicated in emotion recognition.14 Current literature also suggests a role for the somatosensory cortex in the generation of emotional states, since SII activity differs with reflection on various types of emotional situations.24 The somatosensory cortex has been shown to have a role in interoceptive attention, which may contribute to the generation of affective states, and activity increases when attention is focused on an emotional state.28,29 It is also plausible that activity in the somatosensory cortex contributes to the generation of fear, although results in the literature are somewhat contradictory in this area.24,32-35 Finally, the somatosensory cortex is likely involved in regulation of emotion through various strategies.19,38-41 The somatosensory cortex may contribute to attentional deployment through its involvement in various types of attention, such as tactile attention and selective attention.19,38,39 Moreover, this region likely contributes to situational selection through its connection with the anterior insula, which contributes to the control of social emotions experienced upon interaction with others.40 Finally, the somatosensory cortex may regulate emotions via controlled generation, in which neural activity is modulated to regulate the resulting affective state, since SI representations have been shown to vary based on factors such as attention and expectation.25,41 Notably, the role of the somatosensory cortex in emotion processing may also be related to the numerous neurotransmitter binding sites that are present throughout this region.7,42-44 As previously mentioned, the borders of the sub-regions within SI are marked by varying densities of neurotransmitter receptors, such as muscarinic receptors, α-adrenoceptors, serotonin receptors, L-glutamate receptors, and GABA receptors.7
Clinical implications
Given the emerging role of the somatosensory cortex in emotion regulation, perhaps it is not surprising that alterations in the structure and function of this brain region have also been found in numerous psychiatric illnesses, including anxiety disorders, major depressive disorder (MDD), schizophrenia, bipolar disorder, posttraumatic stress disorder (PTSD), specific phobia, obesity, and obsessive-compulsive disorder (OCD).45-61
A common alteration seen in the somatosensory cortex in mood disorders is changes in gray matter volume.45-49 For example, one study found increased right SI gray matter volume in patients with comorbid anxiety and depressive disorders.45 Another study found greater gray matter volume in the somatosensory cortices of schizophrenia patients than MDD patients.46 Similarly, reductions in the overall surface area of the somatosensory cortex have been observed in adolescents with MDD.47 These observations are supported by measurements of decreased cortical thickness in the postcentral gyri of individuals with early onset MDD.48 Similar changes have also been observed in bipolar disorder, where a reduction in gray matter volume in the left somatosensory cortex has been observed.49
Numerous studies have also analyzed the functional connectivity of the somatosensory cortex with other brain areas in mood and anxiety disorders.49-54 For instance, increased functional connectivity between the somatosensory cortex and both the thalamus and left dorsal anterior cingulate cortex was observed in patients with panic disorder.50 These results provide further evidence for the idea that the somatosensory cortex may be involved in interoceptive attention related to the generation and regulation of emotional state, since the thalamus and anterior cingulate cortex have been implicated in interoceptive attention and self-awareness, respectively.28,50 Increases in functional connectivity between the somatosensory cortex and thalamus have also been observed in schizophrenia, as well as between the somatosensory cortex and insular cortex in patients with bipolar disorder.49,51 Women with comorbid bipolar and premenstrual dysphoric disorder displayed decreased functional connectivity between the left somatosensory cortex and left hippocampus compared to women with premenstrual dysphoric disorder alone.53 Lower functional connectivity was also observed between the left SI and areas such as the left superior frontal gyrus, the right middle frontal gyrus, and the left mid cingulum in individuals with MDD.54
Functional imaging studies have also implicated the somatosensory cortex in disorders such as PTSD, specific phobia, obesity, OCD, and schizophrenia.55-61 In a task requiring reaction to auditory tones, SI activity levels were higher in patients with PTSD than controls, whose activity was primarily in the midbrain.55 Higher activity levels in the somatosensory cortex have also been noted in study participants with specific phobia.56 In this study, provoking subjects with phobic stimuli resulted in higher regional blood flow in the somatosensory cortex compared to their resting, control values.56 This suggests that greater sensory processing in the somatosensory cortex may contribute to the progression of PTSD and simple phobias.55,56
Abnormalities in the somatosensory cortex have also been observed in obese individuals.57,58 In one study, a higher rate of glucose metabolism was measured bilaterally in Brodmann area 1 of obese subjects than lean controls.57 These results are supported by a more recent fMRI study that observed increased activity in the somatosensory cortex in obese subjects when anticipating food consumption.58 They suggested that the increased activity in the somatosensory cortex may be associated with a heightened awareness of food palatability that leads to increased appetite in obesity.57,58 Based on the increased somatosensory activity in PTSD and simple phobias, it is conceivable that heightened activity in the somatosensory cortex may contribute to difficulties in emotion regulation and/or lower self-control, leading to the overeating that often accompanies obesity.57,58
An interesting opposite trend has been observed in OCD studies, in which less activation of the somatosensory cortex has been observed.59,60 In one study, most individuals with OCD exhibited decreased regional cerebral blood flow in Brodmann areas 1, 2, 3 and 40 as obsessive-compulsive symptoms increased.59 These results are consistent with another study showing a decrease in resting state cerebral glucose metabolic rates in the sensorimotor areas of OCD subjects compared to healthy controls.60 Together, these studies suggest that activity in the somatosensory cortex may help suppress OCD symptoms,59,60 given that a decrease in activity is associated with worsening of obsessive compulsive symptoms.59,60
Lower activity levels in the somatosensory cortex have also been observed in individuals with schizophrenia,61 with unmedicated subjects showing lower regional glucose metabolism bilaterally in the somatosensory cortex than those on medication. In the same study, glucose metabolism in the left somatosensory cortex of individuals with schizophrenia was lower than that of healthy controls upon stimulation of the right forearm. Treatment with neuroleptics increased activity levels in the somatosensory cortices compared to those found in healthy controls.61 These results suggest that decreased activity in the somatosensory cortex may also contribute to the pathogenesis of schizophrenia.61
Conclusion
In conclusion, the somatosensory cortex can be divided into the primary and secondary somatosensory cortices (SI and SII, respectively).1 These regions can be further subdivided into sub-regions based on differences in cytoarchitecture and neurotransmitter binding sites.6,7 Each region is highly connected to other areas of the brain, allowing the somatosensory cortex to have numerous functions, including representation of the body, tactile attention, sensorimotor integration, and the processing of painful stimuli, empathy, and emotion.2,15-17,19-26 Current literature suggests a role for the somatosensory cortex in each stage of emotion processing, including identification of the emotional significance of a stimulus, generating an appropriate affective state in response to the stimulus, and regulating the resulting emotional state.2,15-17,19-26 This review highlights the importance of the somatosensory cortex as a critical region in emotion regulation and consequently the pathophysiology of several psychiatric disorders, including anxiety and panic disorder, depression, schizophrenia, bipolar disorder, PTSD, simple phobias, obesity, and OCD.45-61 Importantly, these studies suggest that the somatosensory cortex may be a specific target for treatment. Further research using neuromodulation, such as transcranial magnetic stimulation or deep brain stimulation, in this particular brain region may enhance our understanding and potentially reveal new treatment options for conditions in which emotion dysregulation is a central feature.
Disclosure
The authors report no conflicts of interest.
Acknowledgements
This study was supported by the Ontario Mental Health Foundation (Type-A grant to BNF) and the JP Bickell Foundation (Medical Research grant to BNF).
Footnotes
How to cite this article: Kropf E, Syan SK, Minuzzi L, Frey BN. From anatomy to function: the role of the somatosensory cortex in emotional regulation. Braz J Psychiatry. 2019;41:261-269. http://dx.doi.org/10.1590/1516-4446-2018-0183
References
- 1.Nieuwenhuys R, Voogd J, van Huijzen C. The human central nervous system: a synopsis and atlas. 3rd ed. Berlin: Springer-Verlag; 1988. [Google Scholar]
- 2.Kaas JH, Randall JN, Sur M, Lin CS, Merzenich MM. Multiple representations of the body within the primary somatosensory cortex of primates. Science. 1979;204:521–3. doi: 10.1126/science.107591. [DOI] [PubMed] [Google Scholar]
- 3.Purves D, Augustine GJ, Fitzpatrick D, Hall WC, LaMantia A-S, White LE. Neuroscience. 5th ed. Sunderland: Sinauer Associates; 2012. [Google Scholar]
- 4.Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig: Johann Ambrosius Barth; 1909. [Google Scholar]
- 5.Meyer JR, Roychowdhury S, Russell EJ, Callahan C, Gitelman D, Mesulam MM. Location of the central sulcus via cortical thickness of the precentral and postcentral gyri on MR. AJNR Am J Neuroradiol. 1996;17:1699–706. [PMC free article] [PubMed] [Google Scholar]
- 6.Geyer S, Schormann T, Mohlberg H, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. Part 2. Spatial normalization to standard anatomical space. Neuroimage. 2000;11:684–96. doi: 10.1006/nimg.2000.0548. [DOI] [PubMed] [Google Scholar]
- 7.Geyer S, Schleiches A, Zilles K. The somatosensory cortex of human: cytoarchitecture and regional distributions of receptor-binding sites. Neuroimage. 1997;6:27–45. doi: 10.1006/nimg.1997.0271. [DOI] [PubMed] [Google Scholar]
- 8.Paxinos G, Mai J. The human nervous system. Cambridge: Academic Press; 1990. [Google Scholar]
- 9.Grefkes C, Geyer S, Schormann T, Roland P, Zilles K. Human somatosensory area 2: observer-independent cytoarchitectonic mapping, interindividual variability, and population map. Neuroimage. 2001;14:617–31. doi: 10.1006/nimg.2001.0858. [DOI] [PubMed] [Google Scholar]
- 10.Eickhoff SB, Schleicher A, Zilles K, Amunts K. The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb Cortex. 2006;16:254–67. doi: 10.1093/cercor/bhi105. [DOI] [PubMed] [Google Scholar]
- 11.Catani M, Thiebaut de Schotten M. Atlas of Human Brain Connections. New York: Oxford University; 2012. [Google Scholar]
- 12.Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–44. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 13.McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 14.Höistad M, Barbas H. Sequence of information processing for emotions through pathways linking temporal and insular cortices with the amygdala. Neuroimage. 2008;40:1016–33. doi: 10.1016/j.neuroimage.2007.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Disbrow E, Roberts T, Krubitzer L. Somatotopic organization of cortical fields in the lateral sulcus of Homo sapiens: evidence for SII and PV. J Comp Neurol. 2000;418:1–21. doi: 10.1002/(sici)1096-9861(20000228)418:1<1::aid-cne1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 16.Eickhoff SB, Jbabdi S, Caspers S, Laird AR, Fox PT, Zilles K, et al. Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. J Neurosci. 2010;30:6409–21. doi: 10.1523/JNEUROSCI.5664-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruben J, Schwiemann J, Deuchert M, Meyer R, Krause T, Curio G, et al. Somatotopic organization of human secondary somatosensory cortex. Cereb Cortex. 2001;11:463–73. doi: 10.1093/cercor/11.5.463. [DOI] [PubMed] [Google Scholar]
- 18.Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- 19.Burton H, Abend NS, MacLeod AM, Sinclair RJ, Snyder AZ, Raichle ME. Tactile attention tasks enhance activation in somatosensory regions of parietal cortex: a positron emission tomography study. Cereb Cortex. 1999;9:662–74. doi: 10.1093/cercor/9.7.662. [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci. 2013;16:1662–70. doi: 10.1038/nn.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Timmermann L, Ploner M, Haucke K, Schmitz F, Baltissen R, Schnitzler A. Differential coding of pain intensity in the human primary and secondary somatosensory cortex. J Neurophysiol. 2001;86:1499–503. doi: 10.1152/jn.2001.86.3.1499. [DOI] [PubMed] [Google Scholar]
- 22.Bufalari I, Aprile T, Avenanti A, Di Russo F, Aglioti SM. Empathy for pain and touch in the human somatosensory cortex. Cereb Cortex. 2007;17:2553–61. doi: 10.1093/cercor/bhl161. [DOI] [PubMed] [Google Scholar]
- 23.Adolphs R, Damasio H, Tranel D, Cooper G, Damasio AR. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. J Neurosci. 2000;20:2683–90. doi: 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- 25.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–28. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 26.Dolan RJ. Emotion, cognition, and behavior. Science. 2002;298:1191–4. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham WA, Zelazo PD. Attitudes and evaluations: a social cognitive neuroscience perspective. Trends Cogn Sci. 2007;11:97–104. doi: 10.1016/j.tics.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 29.Straube T, Miltner WH. Attention to aversive emotion and specific activation of the right insula and right somatosensory cortex. Neuroimage. 2011;54:2534–8. doi: 10.1016/j.neuroimage.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Orenius TI, Raij TT, Nuortimo A, Näätänen P, Lipsanen J, Karlsson H. The interaction of emotion and pain in the insula and secondary somatosensory cortex. Neuroscience. 2017;349:185–94. doi: 10.1016/j.neuroscience.2017.02.047. [DOI] [PubMed] [Google Scholar]
- 31.Satpute AB, Kang J, Bickart KC, Yardley H, Wager TD, Barrett LF. Involvement of sensory regions in affective experience: a meta-analysis. Front Psychol. 2015;6:1860. doi: 10.3389/fpsyg.2015.01860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, et al. A direct brainstem-amygdala-cortical “alarm” system for subliminal signals of fear. Neuroimage. 2005;24:235–43. doi: 10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, Grodd W, et al. Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Arch Gen Psychiatry. 2005;62:799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- 34.Wei F, Qiu CS, Liauw J, Robinson DA, Ho N, Chatila T, et al. Calcium-calmodulin-dependent protein kinase IV is required for fear memory. Nat Neurosci. 2002;5:573–9. doi: 10.1038/nn0602-855. [DOI] [PubMed] [Google Scholar]
- 35.Tang J, Ko S, Ding HK, Qiu CS, Calejesan AA, Zhuo M. Pavlovian fear memory induced by activation in the anterior cingulate cortex. Mol Pain. 2005;1:6. doi: 10.1186/1744-8069-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gross J. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–91. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- 37.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Backes WH, Mess WH, van Kranen-Mastenbroek V, Reulen JP. Somatosensory cortex responses to median nerve stimulation: fMRI effects of current amplitude and selective attention. Clin Neurophysiol. 2000;111:1738–44. doi: 10.1016/s1388-2457(00)00420-x. [DOI] [PubMed] [Google Scholar]
- 39.Mima T, Nagamine T, Nakamura K, Shibasaki H. Attention modulates both primary and second somatosensory cortical activities in humans: a magnetoencephalographic study. J Neurophysiol. 1998;80:2215–21. doi: 10.1152/jn.1998.80.4.2215. [DOI] [PubMed] [Google Scholar]
- 40.Lamm C, Singer T. The role of anterior insular cortex in social emotions. Brain Struct Funct. 2010;214:579–91. doi: 10.1007/s00429-010-0251-3. [DOI] [PubMed] [Google Scholar]
- 41.Braun C, Haug M, Wiech K, Birbaumer N, Elbert T, Roberts LE. Functional organization of primary somatosensory cortex depends on the focus of attention. Neuroimage. 2002;17:1451–8. doi: 10.1006/nimg.2002.1277. [DOI] [PubMed] [Google Scholar]
- 42.Yoo JH, Lee SY, Loh HH, Ho IK, Jang CG. Altered emotional behaviors and the expression of 5-HT1A and M1 muscarinic receptors in micro-opioid receptor knockout mice. Synapse. 2004;54:72–82. doi: 10.1002/syn.20067. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Z, Gadotti VM, Chen L, Souza IA, Stemkowski PL, Zamponi GW. Role of prelimbic GABAergic circuits in sensory and emotional aspects of neuropathic pain. Cell Rep. 2015;12:752–9. doi: 10.1016/j.celrep.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Cortese BM, Phan KL. The role of glutamate in anxiety and related disorders. CNS Spectr. 2005;10:820–30. doi: 10.1017/s1092852900010427. [DOI] [PubMed] [Google Scholar]
- 45.Qi H, Ning Y, Li J, Guo S, Chi M, Gao M, et al. Gray matter volume abnormalities in depressive patients with and without anxiety disorders. Medicine (Baltimore) 2014;93:e345. doi: 10.1097/MD.0000000000000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koutsouleris N, Meisenzahl EM, Borgwardt S, Riecher-Rössler A, Frodl T, Kambeitz J, et al. Individualized differential diagnosis of schizophrenia and mood disorders using neuroanatomical biomarkers. Brain. 2015;138:2059–73. doi: 10.1093/brain/awv111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmaal L, Hibar DP, Sämann PG, Hall GB, Baune BT, Jahanshad N, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 2017;22:900–9. doi: 10.1038/mp.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Truong W, Minuzzi L, Soares CN, Frey BN, Evans AC, MacQueen GM, et al. Changes in cortical thickness across the lifespan in major depressive disorder. Psychiatry Res. 2013;214:204–11. doi: 10.1016/j.pscychresns.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Minuzzi L, Syan SK, Smith M, Hall A, Hall GBC, Frey BN. Structural and functional changes in the somatosensory cortex in euthymic females with bipolar disorder. Aust N Z J Psychiatry. 2018;52:1075–83. doi: 10.1177/0004867417746001. [DOI] [PubMed] [Google Scholar]
- 50.Cui H, Zhang J, Liu Y, Li Q, Li H, Zhang L, et al. Differential alterations of resting-state functional connectivity in generalized anxiety disorder and panic disorder. Hum Brain Mapp. 2016;37:1459–73. doi: 10.1002/hbm.23113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169:1092–9. doi: 10.1176/appi.ajp.2012.12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brady RO, Jr, Masters GA, Mathew IT, Margolis A, Cohen BM, Öngür D, et al. State dependent cortico-amygdala circuit dysfunction in bipolar disorder. J Affect Disord. 2016;201:79–87. doi: 10.1016/j.jad.2016.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Syan SK, Minuzzi L, Smith M, Costescu D, Allega OR, Hall GBC, et al. Brain structure and function in women with comorbid bipolar and premenstrual dysphoric disorder. Front Psychiatry. 2018;8:301. doi: 10.3389/fpsyt.2017.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tadayonnejad R, Yang S, Kumar A, Ajilore O. Clinical, cognitive, and functional connectivity correlations of resting-state intrinsic brain activity alterations in unmedicated depression. J Affect Disord. 2015;172:241–50. doi: 10.1016/j.jad.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bryant RA, Felmingham KL, Kemp AH, Barton M, Peduto AS, Rennie C, et al. Neural networks of information processing in posttraumatic stress disorder: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:111–8. doi: 10.1016/j.biopsych.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 56.Rauch SL, Savage CR, Alpert NM, Miguel EC, Baer L, Breiter HC, et al. A positron emission tomographic study of simple phobic symptom provocation. Arch Gen Psychiatry. 1995;52:20–8. doi: 10.1001/archpsyc.1995.03950130020003. [DOI] [PubMed] [Google Scholar]
- 57.Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;23:39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- 58.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. Food and drug reward: overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci. 2012;11:1–24. doi: 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- 59.McGuire PK, Bench CJ, Frith CD, Marks IM, Frackowiak RS, Dolan RJ. Functional anatomy of obsessive compulsive phenomena. Br J Psychiatry. 1994;164:459–68. doi: 10.1192/bjp.164.4.459. [DOI] [PubMed] [Google Scholar]
- 60.Martinot JL, Allilaire JF, Mazoyer BM, Hantouche E, Huret JD, Legaut-Demare F, et al. Obsessive-compulsive disorder: a clinical, neuropsychological and positron emission tomography study. Acta Psychiatr Scand. 1990;82:233–42. doi: 10.1111/j.1600-0447.1990.tb03059.x. [DOI] [PubMed] [Google Scholar]
- 61.Buchsbaum MS, Wu JC, DeLisi LE, Holcomb HH, Hazlett E, Cooper-Langston K, et al. Positron emission tomography studies of basal ganglia and somatosensory cortex neuroleptic drug effects: differences between normal controls and schizophrenic patients. Biol Psychiatry. 1987;22:479–94. doi: 10.1016/0006-3223(87)90170-3. [DOI] [PubMed] [Google Scholar]