Abstract
Kynurenine 3-monooxygenase (KMO) is an essential enzyme of the kynurenine pathway, converting kynurenine into 3-hydroxykynurenine. Inhibition of KMO increases kynurenine, resulting in elevated levels of kynurenic acid (KYNA), an endogenous N-methyl-d-aspartate and α*7-nicotinic receptor antagonist. The concentration of KYNA is elevated in the brain of patients with schizophrenia, possibly as a result of a reduced KMO activity. In the present study, using in vivo single cell recording techniques, we investigated the electrophysiological characteristics of ventral tegmental area dopamine (VTA DA) neurons and their response to antipsychotic drugs in a KMO knock-out (K/O) mouse model. KMO K/O mice exhibited a marked increase in spontaneous VTA DA neuron activity as compared to wild-type (WT) mice. Furthermore, VTA DA neurons showed clear-cut, yet qualitatively opposite, responses to the antipsychotic drugs haloperidol and clozapine in the two genotypes. The anti-inflammatory drug parecoxib successfully lowered the firing activity of VTA DA neurons in KMO K/O, but not in WT mice. Minocycline, an antibiotic and anti-inflammatory drug, produced no effect in this regard. Taken together, the present data further support the usefulness of KMO K/O mice for studying distinct aspects of the pathophysiology and pharmacological treatment of psychiatric disorders such as schizophrenia.
Keywords: Kynurenic acid, Electrophysiology, Dopamine, Schizophrenia, NMDA receptor
1. Introduction
The kynurenine pathway, the major route of tryptophan degradation in mammals, has attracted substantial interest with regard to the pathophysiology and treatment of major neurological and psychiatric diseases (Erhardt et al., 2009, 2017b; Schwarcz et al., 2012). The first and rate-limiting step in the pathway, catalyzed by indoleamine-2,3-dioxygenase (IDO) and tryptophan-2,3-dioxygenase (TDO), yields N-formylkynurenine, which is degraded further to form kynurenine. Kynurenine can then be metabolized by 3 different enzymes: kynureninase, which produces anthranilic acid, kynurenine 3-monooxygenase (KMO), leading to 3-hydroxykynurenine and several downstream metabolites including the N-methyl-d-aspartate (NMDA) receptor agonist quinolinic acid (QUIN), and kynurenine aminotransferase (KAT), which forms kynurenic acid (KYNA), a competitive antagonist of the obligatory glycine site of the N-methyl-d-aspartate (NMDA) receptor and a non-competitive inhibitor of the α7* nicotinic acetylcholine receptor (Schwarcz et al., 2012; Stone et al., 2013).
As both IDO and TDO are activated as a result of immune activation, the kynurenine pathway is strongly influenced by the immune system (Erhardt et al., 2017b; Guillemin et al., 2001; Schwarcz et al., 2012; Schwieler et al., 2015; Sellgren et al., 2016). This makes the kynurenine pathway of great interest in psychiatric disorders such as schizophrenia where a low-grade inflammation has been hypothesized to give rise to symptoms, possibly through increased activity of the kynurenine pathway and, specifically, an elevation of brain KYNA (Erhardt et al., 2017b). Accordingly, increased central levels of KYNA are found in individuals with schizophrenia as well as in bipolar disorder patients with a history of psychosis (Erhardt et al., 2001a; Linderholm et al., 2012; Nilsson et al., 2005; Olsson et al., 2010, 2012; Sathyasaikumar et al., 2011; Schwarcz et al., 2001; Sellgren et al., 2016). In line with a link to a compromised immune system, these patients also have increased levels of the pro-inflammatory cytokines IL-1β and IL-6 in the cerebrospinal fluid (CSF) (Schwieler et al., 2015; Sellgren et al., 2016; Söderlund et al., 2009, 2011).
An involvement of the kynurenine pathway in the pathophysiology of schizophrenia is also supported by experimental studies in rats, which demonstrated that elevated levels of brain KYNA disrupt prepulse inhibition and auditory sensory gating in rats (Erhardt et al., 2004; Shepard et al., 2003) and impair cognitive functions such as spatial working memory, attentional processing, contextual learning, social interaction and reward circuitry (Alexander et al., 2012; Chess and Bucci, 2006; Chess et al., 2007, 2009; DeAngeli et al., 2014; Trecartin and Bucci, 2011). Furthermore, electrophysiological studies show that brain KYNA plays an important role in regulating the activity of midbrain dopamine (DA) neurons. Thus, elevated levels of brain KYNA cause an increased firing rate and burst firing activity of these neurons, while lowered levels, conversely, lead to a reduction in firing activity (Erhardt and Engberg, 2002; Erhardt et al., 2001b, 2003; Linderholm et al., 2007, 2016; Nilsson et al., 2006; Schwieler and Erhardt, 2003; Schwieler et al., 2006).
Interestingly, the KMO gene has been linked to the levels of KYNA (Holtze et al., 2012; Lavebratt et al., 2014), and genetic variations in the KMO gene, as well as reduced KMO expression and activity in the brain, are associated with schizophrenia and psychotic features in bipolar type 1 disorder (Aoyama et al., 2006; Ekelund et al., 2004; Holtze et al., 2011; Lavebratt et al., 2014; Sathyasaikumar et al., 2011; Wonodi et al., 2011). By shifting kynurenine metabolism toward enhanced KYNA formation, which may in turn result in increased NMDA receptor inhibition and psychotomimetic effects (Javitt and Zukin, 1991; Moghaddam and Krystal, 2012), KMO hypofunction may therefore play a pathophysiologically significant role in these diseases.
Mice with a targeted genomic deletion of KMO (“KMO K/O mice”) have elevated brain KYNA levels (Giorgini et al., 2013) and offer an opportunity for testing this hypothesis in greater detail. We reported recently that these animals show impaired hippocampus-dependent contextual memory, deficits in social interaction and anxiety-like behavior (Erhardt et al., 2017a). Here, we used these mice to investigate a possible link between hyperphysiological levels of brain KYNA and the spontaneous activity of ventral tegmental area (VTA) DA cells, and to examine the response of these cells to currently used antipsychotic drugs and other relevant pharmacological agents.
2. Methods
2.1. Animals
Adult KMO K/O and wild-type (WT) mice were bred on a FVB/N background, as previously described (Giorgini et al., 2013), and experiments were performed on 3–4 month-old male mice weighing approximately 30 g.
The mice were group housed with free access to water and food. Environmental conditions were checked daily and maintained under constant temperature (25 °C) and 40%–60% humidity in a room with a regulated 12 h light/dark cycle (lights on at 06:00). Experiments were approved by and performed in accordance with the guidelines of the Ethical Committee of Northern Stockholm, Sweden, and all efforts were made to minimize the number of animals used and to optimize their well-being.
2.2. Surgery
Mice were anesthetized by chloral hydrate (400 mg/kg, i.p.) and mounted in a conventional stereotaxic frame (Stoelting) so that the skull was set in a horizontal plane, with the nose secured using a clamp at the front of the frame. Throughout the experiments, the depth of anesthesia was monitored by hind paw pinching and, whenever necessary, maintained by additional i.p. injections of chloral hydrate. The body temperature of the animals was monitored and maintained at 37 °C by means of a thermostatic heating pad. An incision was made along the midline to expose the skull surface. A ~2 mm wide burr hole was made with its center located above the VTA, approximately 3.1 mm posterior to bregma and 0.5 mm lateral to the midline.
2.3. Extracellular single unit recording
Following surgery, a glass electrode (filled with 0.5 M sodium acetate and saturated with Chicago Sky Blue) was lowered by means of a hydraulic drive (David Kopf Instruments, Tujunga, CA, USA) just above the region of VTA, according to stereotaxic coordinates from the mouse brain atlas of Franklin and Paxinos (2008). Electrodes were prepared by pulling glass capillaries (Harvard Apparatus) in a heated vertical electrode puller (Narishige, Japan) at an effect of ~15 A. The tip was carefully broken under a microscope giving an opening of about 1–2 μm and an in vitro impedance between 6 and 8 MΩ, measured at 135 Hz in 0.9% saline. Single unit potentials were passed through a high input-impedance amplifier and filters. The impulses were discriminated from background noise based on their amplitude and fed into a computer, and simultaneously displayed on a digital storage oscilloscope, monitored on an audio monitor and on a strip chart recorder (Gould). All DA neurons were found 4.0–4.7 mm underneath the brain surface and fulfilled the neurophysiological characteristics (i.e. triphasic action potentials with a duration more than 2.0 ms, basal firing rates between 1 and 10 Hz and frequent occurrence of burst firing including progressively decreasing spike amplitude) previously described for DA neurons in the mouse and rat VTA (Grace and Bunney, 1984a, 1984b; Sanghera et al., 1984; Ungless and Grace, 2012). To further confirm that recordings had been made exclusively from DA neurons, the inhibitory action of a single dose of amphetamine (6 mg/kg, i.v.) was verified at the end of the experiments when the experiments allowed.
When determining the number of spontaneously active DA neurons by track recording, an electrode was lowered 3.2 mm posterior to bregma and 0.5 mm lateral to the midline. The electrode was subsequently moved in a predetermined grid pattern with each grid separated by 100 μm, 3.2–2.8 mm posterior to bregma and 0.4–0.6 mm lateral to the midline. A minimum of 3 tracks was assessed for each animal. For these recordings, the electrode impedance was always between 6.5 and 7.5 MΩ. The mean number of DA cells found in each track as well as their firing characteristics was assessed. All recorded VTA DA neurons were found 4.0–4.7 mm from the brain surface and fulfilled the neurophysiological characteristics described above. Whenever possible, track recordings were followed by pharmacological experiments in order to reduce the number of animals used.
For acute pharmacological experiments, a tail vein cannula was inserted for i.v. administration of drugs. Haloperidol was given i.v. in four doses (0.05, 0.05, 0.1, and 0.2 mg/kg), for a total accumulated dose of 0.4 mg/kg. Clozapine was administered i.v. in five doses (0.625, 0.625, 1.25, 2.5 and 5.0 mg/kg), for a total accumulated dose of 10.0 mg/kg. Parecoxib and minocycline was given i.v. as a single dose of 25 mg/kg and 50 mg/kg, respectively. The DA cells were monitored for 45 min. For pharmacological experiments, recordings were made from the first viable cell found. Following the cessation of the electrophysiological experiments, the mice were euthanized by an overdose of chloral hydrate and decapitated. The brains were then removed and stored at −70°C until analysis of kynurenine and KYNA levels with high-performance liquid chromatography (HPLC).
2.4. Data analysis
The distribution of spikes was analyzed on line utilizing a Spike II software program. In order to avoid artifacts in the sampling procedure, the program was set to ignore time intervals below 20 ms. The onset of a burst was determined as an inter-spike interval shorter than 80 ms and the termination of a burst by the next interval longer than 160 ms (Grace and Bunney, 1984a, 1984b). Cells were considered to be bursting if at least one inter-spike time interval of 100 recorded spikes was below 80 ms. The intervals were analyzed with regard to the number of bursts that occurred during a sampling period of 100–500 spikes along with a calculation of the percentage of spikes fired in bursts.
2.5. HPLC analysis
Brains were homogenized in 0.4 M perchloric acid (containing 0.1% sodium metabisulfite, 0.05% EDTA) using a disperser (Ultra-Turrax®, IKA, Stauffen, Germany). For analysis of kynurenine and KYNA, an isocratic reversed-phase HPLC system was used, including a Shimadzu LC-10AD dual piston, high pressure liquid delivery pump (Shimadzu Corporation, Kyoto, Japan), and a ReproSil-Pur C18 column (4 × 150 mm, Dr. Maisch GmbH, Ammerbuch, Germany). A mobile phase of sodium acetate (50 mM, pH 6.2, adjusted with acetic acid) and 7% acetonitrile was pumped through the reversed-phase column at a flow rate of 0.5 ml/min, and 20 μl samples were manually injected using a Rheodyne 7725i injector (Rheodyne, Cotati, CA, USA). Zinc acetate (0.5 M, not pH adjusted) was delivered post column by a syringe pump (P-500, Pharmacia, Uppsala, Sweden) at a flow rate of 10 ml/h. Kynurenine was detected using a Shimadzu SPD-10A UV detector (Shimadzu Corporation), coupled before the delivery of zinc, at an absorption wavelength of 360 nm. KYNA was measured with a Jasco FP-2020 Plus fluorescence detector (Jasco Ltd, Hachioji City, Japan) and excitation and emission wavelengths of 344 nm and 398, respectively. The signals from the detectors were transferred to a computer for analysis utilizing Datalys Azur (Grenoble, France). The retention times of kynurenine and KYNA were about 4 min and 7 min, respectively.
2.6. Statistics
Statistically significant differences in change of firing rate were determined by Mann-Whitney U test with Bonferroni correction. Differences in percent of action potentials fired in bursts were determined by Wilcoxon signed rank test. For analysis of brain levels of kynurenine and KYNA, a Kruskal-Wallis test followed by Dunn’s multiple comparison test was used to determine differences within groups and Mann-Whitney U test between groups. All data are expressed as the mean ± SEM. Significance was assumed for all values with p < 0.05.
2.7. Drugs and chemicals
The following drugs were used: Chloral hydrate, clozapine, minocycline (Sigma, St. Louis, MO, USA), haloperidol (Janssen Pharmaceutical, Beerse, Belgium), parecoxib (Pharmacia, Buckinghamshire, UK). The drugs were pH-adjusted using acetic acid and sodium bicarbonate where needed. Vehicle was pH-adjusted to match the corresponding drug.
The chemicals used were: zinc acetate (Sigma), sodium acetate (Riedel-de Haen, Germany), perchloric acid (Kebo Lab, Stockholm, Sweden) and acetonitrile (Labasco, Partille, Sweden).
3. Results
3.1. Levels of kynurenine and KYNA are elevated in the brain of KMO K/O mice
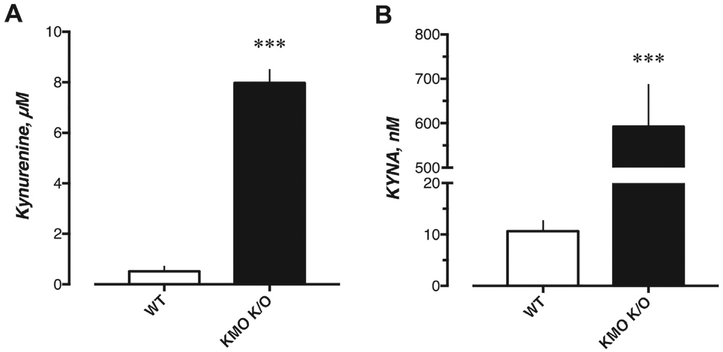
KMO K/O mice showed a substantial elevation of brain kynurenine and KYNA levels (8.0 ± 0.5 μM and 592 ± 94 nM, respectively, n = 9) compared to WT mice (0.5 ± 0.2 μM and 11 ± 2 nM, respectively, n = 7; U = 0 and U = 0, p = 0.0002 and p = 0.0002 for kynurenine and KYNA, respectively) (Fig. 1).
Fig. 1.
Whole brain levels of A) kynurenine and B) KYNA in untreated WT (n = 9) and KMO K/O (n = 7) mice. Bars represent the mean ± SEM, ***p < 0.001 vs. WT, Mann–Whitney U test.
3.2. Track recordings reveal increased VTA DA activity in KMO K/O mice
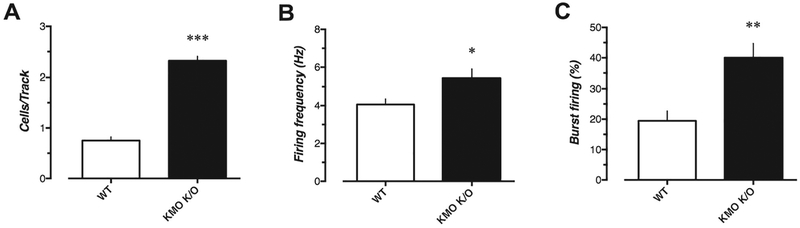
WT mice (n = 7, 37 cells) showed an average of 0.75 ± 0.07 spontaneously active DA neurons per electrode track, with a mean firing rate of 4.1 ± 0.30 Hz, and 19.5 ± 3.2% of the action potentials fired in bursts (Fig. 2). However, KMO K/O mice (n = 6 mice, 44 cells) exhibited significantly more spontaneously active DA neurons per electrode track (2.3 ± 0.09; U = 0, p = 0.0006). In addition, the DA neurons recorded in KMO K/O mice had significantly higher average firing rate (5.5 ± 0.45 Hz; U = 611.5, p = 0.0366) and percentage of action potentials fired in bursts (40.0 ± 4.7; U = 476.5, p = 0.0012) as compared to WT mice.
Fig. 2.
Basal VTA DA cell activity in WT mice (n = 7 mice, 37 cells) compared to KMO K/O mice (n = 6, 44 cells). A) Numbers of spontaneously active cells found per electrode track, B) firing rate (Hz) and C) percent of action potentials fired in bursts. Bars represent the mean ± SEM. ***p < 0.001, **p < 0.01, *p < 0.05 vs. WT, Mann–Whitney U test.
3.3. Haloperidol causes increased firing and depolarization block of VTA DA neurons in KMO K/O but not in WT mice
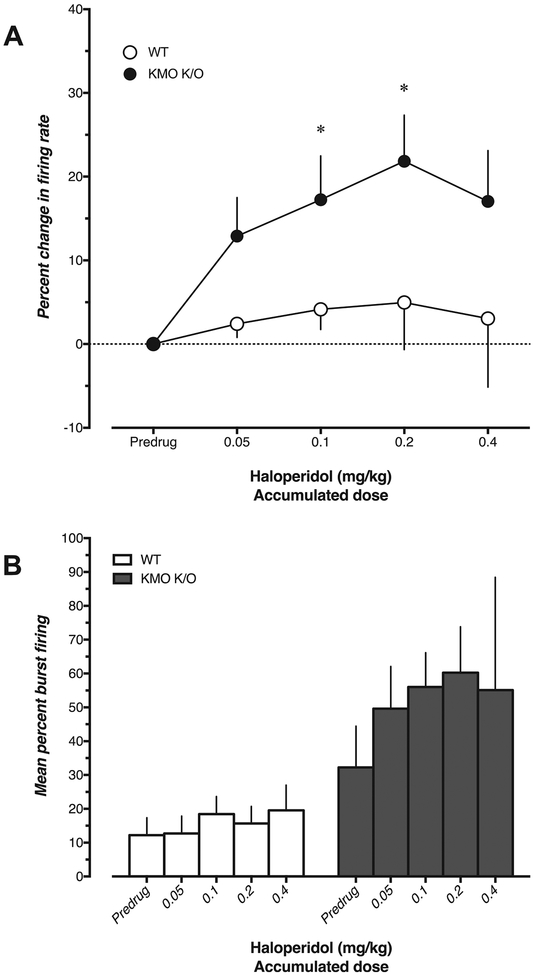
In WT mice (n = 6), acute administration of the typical antipsychotic haloperidol (0.05–0.4 mg/kg, i.v.) did not cause any change in either firing rate (U = 6, 6, 18, 12, p = 0.104, 0.104, >0.99, 0.71 at doses 0.05, 0.1, 0.2, 0.4 mg/kg, respectively) or percentage of action potentials fired in bursts of VTA DA neurons (W = 5, 13, 1, 9, p = 0.687, 0.219, >0.99, 0.437 at doses 0.05, 0.1, 0.2, 0.4 mg/kg, respectively) (Fig. 3). In contrast, haloperidol produced a dose-dependent increase in firing rate of VTA DA neurons in KMO K/O mice (n = 2–6; U = 6, 0, 0, 0, p = 0.104, 0.019, 0.0238, 0.714 at doses 0.05, 0.1, 0.2, 0.4 mg/kg, respectively) together with a trend towards an increase of burst firing activity (W = 19, 10, 6, p = 0.0625, 0.125, 0.25 at doses 0.05, 0.1, 0.2 mg/kg, respectively; no values acquired dose 0.4 mg/kg due to low n). Additionally, acute administration of haloperidol (0.1–0.4 mg/kg) induced depolarization block, reflected by a sudden loss of spikes followed by an unstable recovery within one minute, in 4 VTA DA neurons from KMO K/O mice. This phenomenon was not observed in WT mice. There was no significant difference in change in firing rate between the two genotypes (U = 8, 3, 2, 3, p = 0.264, 0.133, 0.190, 0.857 at doses 0.05, 0.1, 0.2, 0.4 mg/kg, respectively). Vehicle injection did not cause any observable changes in firing rate or burst firing (n = 3, data not shown).
Fig. 3.
Effect of haloperidol (0.05–0.4 mg/kg, i.v.) on A) firing rate and B) percent burst firing of VTA DA neurons in WT (n = 6) and KMO K/O (n = 2–6) mice. In A, the percent change was compared to the corresponding pre-drug value (*p < 0.05; Mann–Whitney U test) and between WT and KMO K/O mice at corresponding doses (no significance, Mann-Whiney U test). In B, percent burst firing was compared to the pre-drug value within subjects (no significance, Wilcoxon matched pairs sign rank test). Each value is the mean ± SEM.
3.4. Clozapine causes different effects on VTA DA neurons in WT and KMO K/O mice
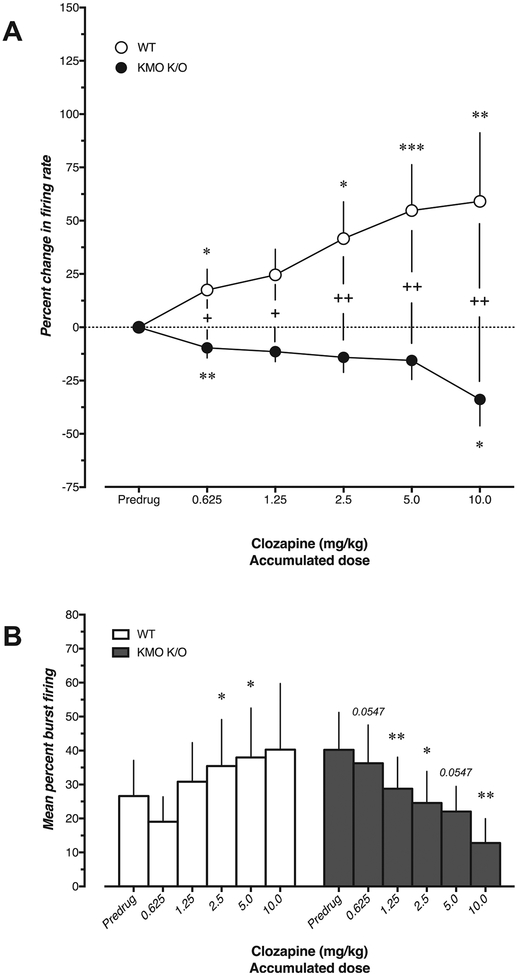
In WT mice (n = 4–8), acute administration of the atypical antipsychotic clozapine (0.625–10.0 mg/kg, i.v.) produced a marked dose-dependent increase in firing rate (U = 8, 24, 8, 0, 0, p = 0.029, >0.99, 0.029, 0.0006, 0.004 at doses 0.625, 1.25, 2.5, 5.0, 10.0 mg/kg, respectively) and percent spikes fired in bursts of VTA DA neurons (W = 1, 15, 21, 21, 10, p = >0.99, 0.156, 0.0312, 0.0312, 0.125 at doses 0.625, 1.25, 2.5, 5.0, 10.0 mg/kg, respectively) (Fig. 4). In contrast, clozapine produced a dose-dependent decrease in firing rate of VTA DA neurons in KMO K/O mice (n = 8–9; U = 9, 18, 18, 36, 9, p = 0.0058, 0.0858, 0.0858, >0.99, 0.0112 at doses 0.625, 1.25, 2.5, 5.0, 10.0 mg/kg, respectively), accompanied by a strong reduction of burst firing activity (W = −33, −43, −39, −33, −36, p = 0.0547, 0.0078, 0.0195, 0.0547, 0.0078 at doses 0.625, 1.25, 2.5, 5.0, 10.0 mg/kg, respectively). In addition, there was a strong difference in change in firing rate between the two genotypes (U = 8, 7, 5, 4, 0, p = 0.023, 0.0158, 0.0066, 0.0042, 0.008 at doses 0.625, 1.25, 2.5, 5.0, 10.0 mg/kg, respectively).
Fig. 4.
Effect of clozapine (0.625–10.0 mg/kg, i.v.) on A) firing rate and B) burst firing of VTA DA neurons in WT (n = 4–8) and KMO K/O (n = 8–9) mice. In A, the percent change was compared to corresponding pre-drug values (***p < 0.001, **p < 0.01, *p < 0.05, Mann–Whitney U test) and between WT and KMO K/O mice at corresponding doses (++p < 0.01, +p < 0.05, Mann–Whitney U test). In B, burst firing was compared to the pre-drug value within subjects (**p < 0.01, *p < 0.05, Wilcoxon matched pairs sign rank test). Each value is the mean ± SEM.
Vehicle injection did not cause any observable changes in firing rate or burst firing activity (n = 3, data not shown).
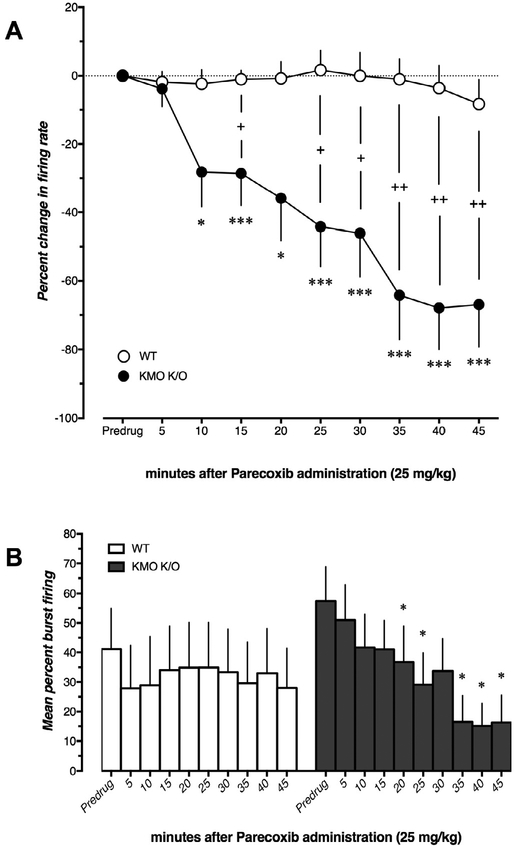
3.5. Parecoxib decreases VTA DA firing activity in KMO K/O but not in WT mice
In WT mice (n = 6–7), administration of the selective COX-2 inhibitor and anti-inflammatory drug parecoxib (25 mg/kg, i.v.) did not alter firing rate (U = 7, 14, 14, 14, 21, 14, 14, 14, 14, p = 0.197, >0.99, 0.667, 0.667, >0.99, 0.667, 0.667, 0.667, 0.667 for timepoints 5–45 min, respectively) or burst firing activity of VTA DA neurons (W = 0, 5, 3, −1, −1, −9, −10, −6, −13, p = >0.99, 0.625, 0.844, >0.99, >0.99, 0.312, 0.125, 0.375, 0.125 for timepoints 5–45 min, respectively) within 45 min after administration (Fig. 5). In contrast, parecoxib caused a robust decrease in both firing rate (U = 26.5, 8, 0, 8, 0, 0, 0, 0, 0, p = >0.99, 0.0168, 0.0004, 0.0338, 0.0004, 0.0006, 0.0006, 0.0006, 0.0006 for timepoints 5–45 min, respectively) and burst firing (W = −9, −28, −24, −28, −26, −19, −21, −21, −21, p = 0.437, 0.0547, 0.109, 0.0156, 0.0312, 0.0625, 0.0312, 0.0312, 0.0312 for timepoints 5–45 min, respectively) of VTA DA neurons in KMO K/O mice (n = 6–8). Notably, 2 out of 8 VTA DA neurons in KMO K/O mice completely ceased firing during the 45-min recording. There was robust difference in change in firing rate between the two genotypes (U = 17, 7, 4, 7, 3, 2, 0, 1, 1, p = >0.99, 0.131, 0.016, 0.103, 0.0164, 0.0174, 0.0044, 0.0086, 0.0086 for timepoints 5–45 min, respectively).
Fig. 5.
Effect of parecoxib (25 mg/kg, i.v.), administered over 45 min, on A) firing rate and B) burst firing of VTA DA neurons in WT (n = 6–7) and KMO K/O (n = 6–8) mice. In A, the percent change in firing rate at different timepoints was compared to the corresponding value before the administration of parecoxib (***p < 0.001, *p < 0.05, Mann–Whitney U test). The change in firing rate between WT and KMO K/O mice was compared at corresponding timepoints (++p < 0.01, +p < 0.05, Mann–Whitney U test). In B, burst firing was compared within subjects to the basal value before parecoxib administration (*p < 0.05, Wilcoxon matched pairs sign rank test). Each value is the mean ± SEM.
Vehicle injections did not affect the firing of VTA DA neurons in control FVB/N mice although a modest increase of firing rate in KMO K/O mice was seen after 45 min (data not shown).
3.6. Minocycline does not affect VTA DA firing in either WT or KMO K/O mice
Minocycline (50 mg/kg, i.v.), an antibiotic and anti-inflammatory drug, failed to produce any significant changes in either firing rate or percentage of action potentials fired in bursts of VTA DA neurons in either WT (n = 4) or KMO K/O mice (n = 4) during the 45-min recording period (data not shown; Mann-Whitney U test for firing rate and Wilcoxon signed rank test for burst firing).
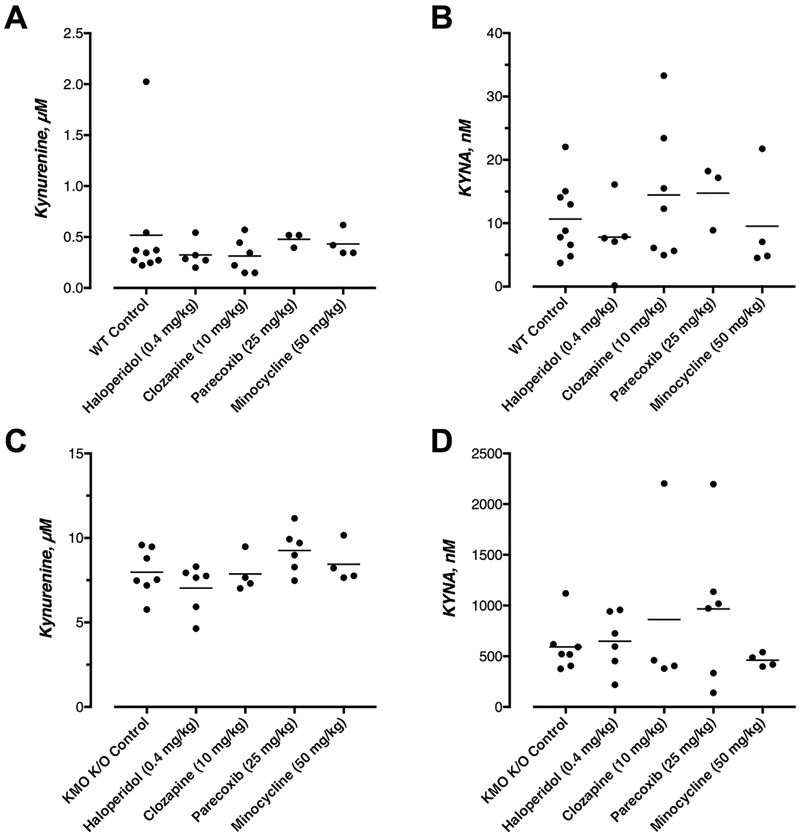
3.7. Kynurenine and KYNA levels after pharmacological treatment with antipsychotics
Treatment with any of the pharmacological agents used in the present study did not significantly affect the levels of brain kynurenine or KYNA in the brain of WT (H = 4.486 and 3.529, respectively) (Fig. 6A and B) or KMO K/O mice (H = 6.384 and 1.666, respectively) (Fig. 6C and D).
Fig. 6.
Pharmacological treatments do not affect whole brain levels of kynurenine and KYNA in WT (A, B) and KMO K/O (C, D) mice. Animals were euthanized 5 min and 50 min following completed administration of haloperidol/clozapine and parecoxib/minocycline respectively. Individual and mean values are indicated. No significant within-group differences were seen (Dunn’s multiple comparison test).
4. Discussion
The results of the present study show that mice with a targeted deletion of KMO display enhanced activity of VTA DA neurons compared to WT mice, and, in line with previous studies, increased brain kynurenine and KYNA levels. The activation of firing complements our previous data showing an increase in spontaneous firing of VTA DA neurons following pharmacological manipulation of the kynurenine pathway to increase endogenous KYNA, e.g. administration of the KMO inhibitor PNU 156561A or kynurenine, KYNA’s immediate precursor (Erhardt and Engberg, 2002; Erhardt et al., 2001b; Schwieler et al., 2006). Notably, the various abnormal electrophysiological phenomena in KMO K/O mice described here are unlikely to be related to the very modest (~20%) reduction in the brain levels of the kynurenine pathway metabolite and NMDA receptor agonist QUIN in KMO K/O mice (Giorgini et al., 2013).
The present results are in agreement with previous studies showing that systemic administration of kynurenine or NMDA receptor antagonists is associated with an increased activity of VTA DA neurons (French, 1994; French et al., 1993; Murase et al., 1993). This activation is thought to be induced indirectly by inhibition of afferent GABAergic projections from the prefrontal cortex and/or subcortical areas (Carr and Sesack, 2000; Kalivas et al., 1993; Patton et al., 2013; Phillipson, 1979; Sesack et al., 1989), or by local interneurons within the VTA (Tan et al., 2012; van Zessen et al., 2012; see Erhardt and Engberg, 2002; Erhardt et al., 2002). Of note in this context, submicromolar concentrations of KYNA antagonize α*7-nicotinic receptor function in the prefrontal cortex, resulting in the inhibition of GABAergic neurons (Flores-Barrera et al., 2017). Although the effect of KYNA on the α*7-nicotinergic receptor does not seem to contribute to the regulation of VTA DA firing (Linderholm et al., 2016), this action appears important for terminal control of DA release, where acute local administration of nanomolar concentrations of KYNA reduces DA in the rat striatum (Rassoulpour et al., 2005). Thus, KYNA appears to have two distinct and opposite actions on DA neurotransmission, namely an excitatory effect on VTA DA firing induced by blockade of the glycine site of the NMDA receptor of GABAergic afferents and a reduction in DA release caused by antagonism of α*7-nicotinic receptors on dopaminergic nerve terminals.
In WT mice, the antipsychotic drug haloperidol, administered in low doses, caused a modest, non-significant increase in the firing rate of VTA DA neurons. This is not entirely in agreement with observations in rats where administration of haloperidol is associated with an increased firing of VTA DA neurons (Gessa et al., 2000; Schwieler and Erhardt, 2003; Tung et al., 1991). In KMO K/O mice, however, haloperidol generated a robust activation in firing of VTA DA neurons, where 4 out of 6 neurons went into a depolarization block. This pronounced excitatory action of haloperidol is similar to the effect observed in rats with elevated levels of endogenous brain KYNA due to pharmacological inhibition of KMO (Schwieler and Erhardt, 2003). The increased sensitivity to haloperidol seen in KMO/KO mice may therefore be causally related to the fact that VTA DA neurons are driven into a hyperexcitatory state when two critical regulatory feed-back mechanisms are dysfunctional, i.e., GABAergic inhibition by KYNA (Linderholm et al., 2016) and somatodendritic D2 receptor inhibition by haloperidol (Pucak and Grace, 1994, 1996).
In contrast to the effects of haloperidol, the atypical antipsychotic drug clozapine induced opposite effects on VTA DA neurons in the two genotypes. Thus, clozapine administration caused a dose-dependent excitation of VTA DA firing in WT mice, whereas KMO K/O mice showed a dose-dependent inhibition in firing. As clozapine is a relatively weak D2 receptor antagonist (Mukherjee et al., 2001), and as the more potent D2 receptor antagonist haloperidol was essentially ineffective in WT mice (see above), we suggest that the divergent effects of clozapine in WT and KMO K/O mice may be unrelated to D2 receptors. The qualitatively disparate effects of clozapine in WT and KMO K/O mice observed in the present study may rather be traced to the obligatory glycine site of the NMDA receptor, which is competitively blocked by KYNA (cf. Introduction). Indeed, clozapine is suggested to act as a partial agonist at the glycine site of the NMDA receptor (Schwieler et al., 2008). Thus, fluctuations in brain KYNA levels substantially – and bi-directionally – will affect the response of VTA DA neurons to clozapine. Normally, this site is largely occupied by endogenous glycine or D-serine (Billups and Attwell, 2003), allowing clozapine’s inhibitory action on this receptor to prevail (Schwieler et al., 2008) and leading to increased firing of VTA DA neurons in WT mice. However, when the glycine site is largely blocked by the elevated concentrations of KYNA, as might be the case in KMO K/O mice, the agonistic action of clozapine on the NMDA/glycine complex should prevail (Millan, 2005), leading to an inhibition of DA firing.
Treatment with COX-2 inhibitors has been proposed as a novel strategy to treat schizophrenia as an add-on therapy to conventional antipsychotic treatment (Müller et al., 2013). As COX-2 inhibitors inhibit IDO, one of the initial enzymes of the kynurenine pathway (Basu et al., 2006; Lee et al., 2009), these compounds might act by lowering the amount of available kynurenine and, in turn, the production of KYNA. Indeed, we showed previously that parecoxib lowers brain KYNA levels and firing of VTA DA neurons in rats, and that these effects can be counteracted by pretreatment with l-kynurenine (Schwieler et al., 2006). In the present study, a single dose of parecoxib reduced the firing rate and burst activity of VTA DA neurons in KMO K/O mice but did not produce any observable changes in firing in WT mice. This genotypic diversity therefore does not appear to be directly related to brain KYNA levels, since parecoxib did not influence brain KYNA levels in either WT or mutant mice. The mechanism underlying this interesting, functionally relevant phenomenon is currently unclear and ought to be explored in future studies.
An increasing number of clinical studies suggests that the antibiotic drug minocycline has beneficial effects in the treatment of schizophrenia (Miyaoka et al., 2008; Solmi et al., 2017). The drug is commonly believed to inhibit microglial activation, a reduction in pro-inflammatory cytokines, and an associated decrease in IDO activity (O’Connor et al., 2009; Rojewska et al., 2014). In our experiments, minocycline failed to alter brain KYNA levels and did not affect the firing of VTA DA neurons in either WT or KMO K/O mice. In light of, the apparent absence of microglial activation and immune system abnormalities in our experimental animals (no differences in the brain levels of several cytokines, i.e. IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12, INF-γ and TNF-α, between WT and KMO K/O mice; unpublished observations from our laboratory), these results were not surprising. A possible effect of minocycline on the control of midbrain DA neurons by KYNA or, conceivably by other metabolites of the kynurenine pathway, e.g. QUIN, should therefore be reexamined in immunologically challenged animals.
5. Conclusion
Our electrophysiological experiments revealed pronounced differences in the firing characteristics of VTA DA neurons of WT and KMO K/O mice. Although not directly tested in the present study, these differences are proposed to be causally related to the greatly elevated levels of endogenous KYNA in the brain of the mutant animals. Although careful additional studies are clearly needed in this regard, this major change in cerebral kynurenine pathway metabolism may also be causally related to the differential responses of VTA DA neurons in the two genotypes to certain antipsychotic drugs. Taken together with our recent study describing their distinct, schizophrenia-like behavioral impairments (Erhardt et al., 2017a), KMO K/O mice may therefore not only be a valuable experimental tool to investigate the pathophysiology of schizophrenia but also an attractive model for studying the effects and mechanism of action of antipsychotic drugs.
Acknowledgements
This work was supported by grants from the Swedish Medical Research Council S. Erhardt (2013–2838), the Swedish Brain Foundation, Åhlén-stiftelsen, and grant P50-MH103222 from the U.S. National Institutes of Health. No funding sources had any role in the study design, in the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication. The authors would like to thank Dr. Paul Shepard for kindly providing his laboratory for our initial electrophysiological experiments.
Footnotes
Declarations of interest
None.
References
- Alexander KS, Wu HQ, Schwarcz R, Bruno JP, 2012. Acute elevations of brain kynurenic acid impair cognitive flexibility: normalization by the alpha7 positive modulator galantamine. Psychopharmacology (Berlin) 220, 627–637. 10.1007/s00213-011-2539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama N, Takahashi N, Saito S, Maeno N, Ishihara R, Ji X, Miura H, Ikeda M, Suzuki T, Kitajima T, Yamanouchi Y, Kinoshita Y, Yoshida K, Iwata N, Inada T, Ozaki N, 2006. Association study between kynurenine 3-monooxygenase gene and schizophrenia in the Japanese population. Gene Brain Behav 5, 364–368. 10.1111/j.1601-183X.2006.00231.x. [DOI] [PubMed] [Google Scholar]
- Basu GD, Tinder TL, Bradley JM, Tu T, Hattrup CL, Pockaj BA, Mukherjee P, 2006. Cyclooxygenase-2 inhibitor enhances the efficacy of a breast cancer vaccine: role of Ido. J. Immunol 177, 2391–2402. [DOI] [PubMed] [Google Scholar]
- Billups D, Attwell D, 2003. Active release of glycine or D-serine saturates the glycine site of NMDA receptors at the cerebellar mossy fibre to granule cell synapse. Eur. J. Neurosci 18, 2975–2980. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR, 2000. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J. Neurosci 20, 3864–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess AC, Bucci DJ, 2006. Increased concentration of cerebral kynurenic acid alters stimulus processing and conditioned responding. Behav. Brain Res 170, 326–332. 10.1016/j.bbr.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Chess AC, Simoni MK, Alling TE, Bucci DJ, 2007. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr. Bull 33, 797–804. 10.1093/schbul/sbl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess AC, Landers AM, Bucci DJ, 2009. L-kynurenine treatment alters contextual fear conditioning and context discrimination but not cue-specific fear conditioning. Behav. Brain Res 201, 325–331. 10.1016/j.bbr.2009.03.013. [DOI] [PubMed] [Google Scholar]
- DeAngeli NE, Todd TP, Chang SE, Yeh HH, Yeh PW, Bucci DJ, 2014. Exposure to kynurenic acid during adolescence increases sign-tracking and impairs longterm potentiation in adulthood. Front. Behav. Neurosci 8 (451) 10.3389/fnbeh.2014.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekelund J, Hennah W, Hiekkalinna T, Parker A, Meyer J, Lonnqvist J, Peltonen L, 2004. Replication of 1q42 linkage in Finnish schizophrenia pedigrees. Mol. Psychiatr 9, 1037–1041. 10.1038/sj.mp.4001536. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G, 2001a. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci. Lett 313, 96–98. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Oberg H, Mathe JM, Engberg G, 2001b. Pharmacological elevation of endogenous kynurenic acid levels activates nigral dopamine neurons. Amino Acids 20, 353–362. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Engberg G, 2002. Increased phasic activity of dopaminergic neurones in the rat ventral tegmental area following pharmacologically elevated levels of endogenous kynurenic acid. Acta Physiol. Scand 175, 45–53. 10.1046/j.1365-201X.2002.00962.x. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Mathe JM, Chergui K, Engberg G, Svensson TH, 2002. GABA(B) receptor-mediated modulation of the firing pattern of ventral tegmental area dopamine neurons in vivo. Naunyn-Schmiedeberg’s Arch. Pharmacol 365, 173–180. 10.1007/s00210-001-0519-5. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Schwieler L, Engberg G, 2003. Kynurenic acid and schizophrenia. Adv. Exp. Med. Biol 527, 155–165. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Schwieler L, Emanuelsson C, Geyer M, 2004. Endogenous kynurenic acid disrupts prepulse inhibition. Biol. Psychiatr 56, 255–260. 10.1016/j.biopsych.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Olsson SK, Engberg G, 2009. Pharmacological manipulation of kynurenic acid: potential in the treatment of psychiatric disorders. CNS Drugs 23, 91–101. 10.2165/00023210-200923020-00001. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Pocivavsek A, Repici M, Liu XC, Imbeault S, Maddison DC, Thomas MAR, Smalley JL, Larsson MK, Muchowski PJ, Giorgini F, Schwarcz R, 2017a. Adaptive and behavioral changes in kynurenine 3-monooxygenase knockout mice: relevance to psychotic disorders. Biol. Psychiatr 82, 756–765. 10.1016/j.biopsych.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt S, Schwieler L, Imbeault S, Engberg G, 2017b. The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology 112, 297–306. 10.1016/j.neuropharm.2016.05.020. [DOI] [PubMed] [Google Scholar]
- Flores-Barrera E, Thomases DR, Cass DK, Bhandari A, Schwarcz R, Bruno JP, Tseng KY, 2017. Preferential disruption of prefrontal GABAergic function by nanomolar concentrations of the alpha7nACh negative modulator kynurenic acid. J. Neurosci 37, 7921–7929. 10.1523/JNEUROSCI.0932-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G, 2008. The Mouse Brain in Stereotaxic Coordinates, third ed Elsevier Academic Press, San Diego. [Google Scholar]
- French ED, Mura A, Wang T, 1993. MK-801, phencyclidine (PCP), and PCP-like drugs increase burst firing in rat A10 dopamine neurons: comparison to competitive NMDA antagonists. Synapse 13, 108–116. 10.1002/syn.890130203. [DOI] [PubMed] [Google Scholar]
- French ED, 1994. Phencyclidine and the midbrain dopamine system: electrophysiology and behavior. Neurotoxicol. Teratol 16, 355–362. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Devoto P, Diana M, Flore G, Melis M, Pistis M, 2000. Dissociation of haloperidol, clozapine, and olanzapine effects on electrical activity of mesocortical dopamine neurons and dopamine release in the prefrontal cortex. Neuropsychopharmacology 22, 642–649. 10.1016/S0893-133X(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Giorgini F, Huang SY, Sathyasaikumar KV, Notarangelo FM, Thomas MA, Tararina M, Wu HQ, Schwarcz R, Muchowski PJ, 2013. Targeted deletion of kynurenine 3-monooxygenase in mice: a new tool for studying kynurenine pathway metabolism in periphery and brain. J. Biol. Chem 288, 36554–36566. 10.1074/jbc.M113.503813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS, 1984a. The control of firing pattern in nigral dopamine neurons: burst firing. J. Neurosci 4, 2877–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS, 1984b. The control of firing pattern in nigral dopamine neurons: single spike firing. J. Neurosci 4, 2866–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin GJ, Kerr SJ, Smythe GA, Smith DG, Kapoor V, Armati PJ, Croitoru J, Brew BJ, 2001. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J. Neurochem 78, 842–853. [DOI] [PubMed] [Google Scholar]
- Holtze M, Saetre P, Erhardt S, Schwieler L, Werge T, Hansen T, Nielsen J, Djurovic S, Melle I, Andreassen OA, Hall H, Terenius L, Agartz I, Engberg G, Jonsson EG, Schalling M, 2011. Kynurenine 3-monooxygenase (KMO) polymorphisms in schizophrenia: an association study. Schizophr. Res 127, 270–272. 10.1016/j.schres.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Holtze M, Saetre P, Engberg G, Schwieler L, Werge T, Andreassen OA, Hall H, Terenius L, Agartz I, Jonsson EG, Schalling M, Erhardt S, 2012. Kynurenine 3-monooxygenase polymorphisms: relevance for kynurenic acid synthesis in patients with schizophrenia and healthy controls. J. Psychiatry Neurosci 37, 53–57. 10.1503/jpn.100175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR, 1991. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatr 148, 1301–1308. 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Churchill L, Klitenick MA, 1993. GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience 57, 1047–1060. [DOI] [PubMed] [Google Scholar]
- Lavebratt C, Olsson S, Backlund L, Frisen L, Sellgren C, Priebe L, Nikamo P, Traskman-Bendz L, Cichon S, Vawter MP, Osby U, Engberg G, Landen M, Erhardt S, Schalling M, 2014. The KMO allele encoding Arg452 is associated with psychotic features in bipolar disorder type 1, and with increased CSF KYNA level and reduced KMO expression. Mol. Psychiatr 19, 334–341. 10.1038/mp.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Choi HK, Lee KJ, Jung JY, Hur GY, Jung KH, Kim JH, Shin C, Shim JJ, In KH, Kang KH, Yoo SH, 2009. The immune tolerance of cancer is mediated by Ido that is inhibited by COX-2 inhibitors through regulatory T cells. J. Immunother 32, 22–28. 10.1097/CJI.0b013e31818ac2f7. [DOI] [PubMed] [Google Scholar]
- Linderholm KR, Andersson A, Olsson S, Olsson E, Snodgrass R, Engberg G, Erhardt S, 2007. Activation of rat ventral tegmental area dopamine neurons by endogenous kynurenic acid: a pharmacological analysis. Neuropharmacology 53, 918–924. 10.1016/j.neuropharm.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Linderholm KR, Skogh E, Olsson SK, Dahl ML, Holtze M, Engberg G, Samuelsson M, Erhardt S, 2012. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr. Bull 38, 426–432. 10.1093/schbul/sbq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linderholm KR, Alm MT, Larsson MK, Olsson SK, Goiny M, Hajos M, Erhardt S, Engberg G, 2016. Inhibition of kynurenine aminotransferase II reduces activity of midbrain dopamine neurons. Neuropharmacology 102, 42–47. 10.1016/j.neuropharm.2015.10.028. [DOI] [PubMed] [Google Scholar]
- Millan MJ, 2005. N-Methyl-D-aspartate receptors as a target for improved antipsychotic agents: novel insights and clinical perspectives. Psychopharmacology (Berlin) 179, 30–53. 10.1007/s00213-005-2199-1. [DOI] [PubMed] [Google Scholar]
- Miyaoka T, Yasukawa R, Yasuda H, Hayashida M, Inagaki T, Horiguchi J, 2008. Minocycline as adjunctive therapy for schizophrenia: an open-label study. Clin. Neuropharmacol 31, 287–292. 10.1097/WNF.0b013e3181593d45. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Krystal JH, 2012. Capturing the angel in “angel dust”: twenty years of translational neuroscience studies of NMDA receptor antagonists in animals and humans. Schizophr. Bull 38, 942–949. 10.1093/schbul/sbs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J, Christian BT, Narayanan TK, Shi B, Mantil J, 2001. Evaluation of dopamine D-2 receptor occupancy by clozapine, risperidone, and haloperidol in vivo in the rodent and nonhuman primate brain using 18F-fallypride. Neuropsychopharmacology 25, 476–488. 10.1016/S0893-133X(01)00251-2. [DOI] [PubMed] [Google Scholar]
- Müller N, Myint AM, Krause D, Weidinger E, Schwarz MJ, 2013. Anti-inflammatory treatment in schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 42, 146–153. 10.1016/j.pnpbp.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Murase S, Mathe JM, Grenhoff J, Svensson TH, 1993. Effects of dizocilpine (MK-801) on rat midbrain dopamine cell activity: differential actions on firing pattern related to anatomical localization. J. Neural Transm. Gen. Sect 91, 13–25. [DOI] [PubMed] [Google Scholar]
- Nilsson LK, Linderholm KR, Engberg G, Paulson L, Blennow K, Lindstrom LH, Nordin C, Karanti A, Persson P, Erhardt S, 2005. Elevated levels of kynurenic acid in the cerebrospinal fluid of male patients with schizophrenia. Schizophr. Res 80, 315–322. 10.1016/j.schres.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Nilsson LK, Linderholm KR, Erhardt S, 2006. Subchronic treatment with kynurenine and probenecid: effects on prepulse inhibition and firing of midbrain dopamine neurons. J. Neural. Transm 113, 557–571. 10.1007/s00702-005-0343-z. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R, 2009. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatr 14, 511–522. 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson SK, Samuelsson M, Saetre P, Lindstrom L, Jonsson EG, Nordin C, Engberg G, Erhardt S, Landen M, 2010. Elevated levels of kynurenic acid in the cerebrospinal fluid of patients with bipolar disorder. J. Psychiatry Neurosci 35, 195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson SK, Sellgren C, Engberg G, Landen M, Erhardt S, 2012. Cerebrospinal fluid kynurenic acid is associated with manic and psychotic features in patients with bipolar I disorder. Bipolar Disord 14, 719–726. 10.1111/bdi.12009. [DOI] [PubMed] [Google Scholar]
- Patton MH, Bizup BT, Grace AA, 2013. The infralimbic cortex bidirectionally modulates mesolimbic dopamine neuron activity via distinct neural pathways. J. Neurosci 33, 16865–16873. 10.1523/JNEUROSCI.2449-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson OT, 1979. Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J. Comp. Neurol 187, 117–143. 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- Pucak ML, Grace AA, 1994. Evidence that systemically administered dopamine antagonists activate dopamine neuron firing primarily by blockade of somatodendritic autoreceptors. J. Pharmacol. Exp. Therapeut 271, 1181–1192. [PubMed] [Google Scholar]
- Pucak ML, Grace AA, 1996. Effects of haloperidol on the activity and membrane physiology of substantia nigra dopamine neurons recorded in vitro. Brain Res 713, 44–52. [DOI] [PubMed] [Google Scholar]
- Rassoulpour A, Wu HQ, Ferre S, Schwarcz R, 2005. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. J. Neurochem 93, 762–765. 10.1111/j.1471-4159.2005.03134.x. [DOI] [PubMed] [Google Scholar]
- Rojewska E, Makuch W, Przewlocka B, Mika J, 2014. Minocycline prevents dynorphin-induced neurotoxicity during neuropathic pain in rats. Neuropharmacology 86, 301–310. 10.1016/j.neuropharm.2014.08.00. [DOI] [PubMed] [Google Scholar]
- Sanghera MK, Trulson ME, German DC, 1984. Electrophysiological properties of mouse dopamine neurons: in vivo and in vitro studies. Neuroscience 12, 793–801. [DOI] [PubMed] [Google Scholar]
- Sathyasaikumar KV, Stachowski EK, Wonodi I, Roberts RC, Rassoulpour A, McMahon RP, Schwarcz R, 2011. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr. Bull 37, 1147–1156. 10.1093/schbul/sbq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC, 2001. Increased cortical kynurenate content in schizophrenia. Biol. Psychiatr 50, 521–530. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ, 2012. Kynurenines in the mammalian brain: when physiology meets pathology. Nat. Rev. Neurosci 13, 465–477. 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwieler L, Erhardt S, 2003. Inhibitory action of clozapine on rat ventral tegmental area dopamine neurons following increased levels of endogenous kynurenic acid. Neuropsychopharmacology 28, 1770–1777. 10.1038/sj.npp.1300255. [DOI] [PubMed] [Google Scholar]
- Schwieler L, Erhardt S, Nilsson L, Linderholm K, Engberg G, 2006. Effects of COX-1 and COX-2 inhibitors on the firing of rat midbrain dopaminergic neurons–possible involvement of endogenous kynurenic acid. Synapse 59, 290–298. 10.1002/syn.20241. [DOI] [PubMed] [Google Scholar]
- Schwieler L, Linderholm KR, Nilsson-Todd LK, Erhardt S, Engberg G, 2008. Clozapine interacts with the glycine site of the NMDA receptor: electrophysiological studies of dopamine neurons in the rat ventral tegmental area. Life Sci 83, 170–175. 10.1016/j.lfs.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Schwieler L, Larsson MK, Skogh E, Kegel ME, Orhan F, Abdelmoaty S, Finn A, Bhat M, Samuelsson M, Lundberg K, Dahl ML, Sellgren C, Schuppe-Koistinen I, Svensson C, Erhardt S, Engberg G, 2015. Increased levels of IL-6 in the cerebrospinal fluid of patients with chronic schizophrenia–significance for activation of the kynurenine pathway. J. Psychiatry Neurosci 40, 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellgren CM, Kegel ME, Bergen SE, Ekman CJ, Olsson S, Larsson M, Vawter MP, Backlund L, Sullivan PF, Sklar P, Smoller JW, Magnusson PK, Hultman CM, Walther-Jallow L, Svensson CI, Lichtenstein P, Schalling M, Engberg G, Erhardt S, Landen M, 2016. A genome-wide association study of kynurenic acid in cerebrospinal fluid: implications for psychosis and cognitive impairment in bipolar disorder. Mol. Psychiatr 21, 1342–1350. 10.1038/mp.2015.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS, 1989. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J. Comp. Neurol 290, 213–242. 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shepard PD, Joy B, Clerkin L, Schwarcz R, 2003. Micromolar brain levels of kynurenic acid are associated with a disruption of auditory sensory gating in the rat. Neuropsychopharmacology 28, 1454–1462. 10.1038/sj.npp.1300188. [DOI] [PubMed] [Google Scholar]
- Söderlund J, Schroder J, Nordin C, Samuelsson M, Walther-Jallow L, Karlsson H, Erhardt S, Engberg G, 2009. Activation of brain interleukin-1beta in schizophrenia. Mol. Psychiatr 14, 1069–1071. 10.1038/mp.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlund J, Olsson SK, Samuelsson M, Walther-Jallow L, Johansson C, Erhardt S, Landen M, Engberg G, 2011. Elevation of cerebrospinal fluid interleukin-1ss in bipolar disorder. J. Psychiatry Neurosci 36,114–118. 10.1503/jpn.100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solmi M, Veronese N, Thapa N, Facchini S, Stubbs B, Fornaro M, Carvalho AF, Correll CU, 2017. Systematic review and meta-analysis of the efficacy and safety of minocycline in schizophrenia. CNS Spectr 1–12 10.1017/S1092852916000638. [DOI] [PubMed] [Google Scholar]
- Stone TW, Stoy N, Darlington LG, 2013. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol. Sci 34, 136–143. 10.1016/j.tips.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouebe G, Deisseroth K, Tye KM, Luscher C, 2012. GABA neurons of the VTA drive conditioned place aversion. Neuron 73, 1173–1183. 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trecartin KV, Bucci DJ, 2011. Administration of kynurenine during adolescence, but not during adulthood, impairs social behavior in rats. Schizophr. Res 133, 156–158. 10.1016/j.schres.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung CS, Grenhoff J, Svensson TH, 1991. Kynurenate blocks the acute effects of haloperidol on midbrain dopamine neurons recorded in vivo. J. Neural Transm. Gen. Sect 84, 53–64. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Grace AA, 2012. Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci 35, 422–430. 10.1016/j.tins.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD, 2012. Activation of VTA GABA neurons disrupts reward consumption. Neuron 73, 1184–1194. 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonodi I, Stine OC, Sathyasaikumar KV, Roberts RC, Mitchell BD, Hong LE, Kajii Y, Thaker GK, Schwarcz R, 2011. Downregulated kynurenine 3-monooxygenase gene expression and enzyme activity in schizophrenia and genetic association with schizophrenia endophenotypes. Arch. Gen. Psychiatr 68, 665–674. 10.1001/archgenpsychiatry.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]