Abstract
Traumatic brain injury (TBI) accounts for approximately 16% of acute symptomatic seizures which usually occur in the first week after trauma. Children are at higher risk for post-traumatic seizures than adults. Post-traumatic seizures are a risk factor for delayed development of epilepsy. Delayed, chronic post-traumatic epilepsy is preceded by a silent period during which therapeutic interventions may arrest, revert or prevent epileptogenesis. A number of recent review articles summarize the most important features of post-traumatic seizures and epilepsy; this review will instead focus on the link between cerebrovascular permeability, epileptogenesis and ictal events after TBI. The possibility of acting on the blood-brain barrier (BBB) and the neurovascular unit to prevent, disrupt or treat post-traumatic epilepsy is also discussed. Finally, we describe the latest quest for biomarkers of epileptogenesis which may allow for a more targeted intervention.
Keywords: Peripheral markers, Blood-brain barrier, Post-traumatic epilepsy, Fluid biomarkers, Traumatic brain injury, Anti-epileptic drugs
1. Introduction
Traumatic brain injury (TBI) is a public health challenge of insufficiently recognized proportions. > 50 million people worldwide have a TBI each year, and approximately half the global population will experience one or more TBIs over their lifetime. Unlike in other acute neurological disease (e.g., brain hemorrhage or embolic stroke), significant neurological deficits may appear days, months or even years after the initiating events. For example, chronic traumatic encephalopathy (CTE) can lag decades after TBI (Smith et al., 2013). Incidents of post-traumatic seizures can occur acutely (hours to days after TBI), while chronic post-traumatic epilepsy (PTE) manifests in a more delayed fashion (months to years). Relative to no TBI, the risk of PTE has been found to be 2-fold higher after mild TBI (mTBI) and 7-fold higher after severe TBI (Christensen, 2015). There are currently no treatments to prevent PTE from occurring in these subjects. In fact, as bluntly stated in a recent review, “despite several interventions used to prevent post-traumatic epilepsy, the only proven “intervention” to date is to prevent TBI from occurring” (Christensen, 2012). Post-traumatic epilepsy stands prominently out among etiologies which are detectable and permit early preventive interventions. Thus, if at-risk patients and associated epileptogenic mechanisms can be identified, a targeted intervention may prevent PTE.
This review will first present evidence supporting a role for the blood-brain barrier (BBB) in determining post-TBI sequelae. Most of this evidence derives from animal models of PTE or imaging studies in human subjects. The few available therapeutic interventions following experimental or clinical TBI will also be presented in the context of actions on the cerebral vasculature rather than affecting parenchymal cells. Putative markers of PTE as seen from a BBB perspective will also be discussed.
1.1. Changes in neuronal excitability after TBI
To understand how TBI leads to changes in neuronal excitability one needs to underscore that post-traumatic seizures refer only to seizures which occur after TBI and are caused by TBI. Exacerbation of pre-existing seizures is not a good clinical example of post-TBI seizures. Another important preamble is the acknowledgement that the temporal relationship between the traumatic event and seizures is a key factor in the underlying mechanisms of ictogenesis. Early post-traumatic seizures (2–5% of all cases in mTBI; 10–15% in severe TBI (Langendorf et al., 2008; Temkin et al., 1990; Annegers et al., 1980a; Annegers et al., 1980b; Hauser et al., 1993)) are likely different in mechanism from late seizures. In addition, late seizures are most common after penetrating, war-related events (53% in Vietnam vets with penetrating TBI (Salazar et al., 1985)). Factors unrelated to TBI are also at play in this population; these include infection, presence of foreign material in brain parenchyma, uncontrolled bleeding, concurrent surgical therapies, anesthesia, etc. Recurrent epileptic seizures are chronic events that occur many months or years after TBI; whether these events are due to -or are a consequence of- early seizures remains unclear.
Early BBB dysfunction (BBBD) has been shown in animal models of TBI and blast injury (Tagge et al., 2018; Kawoos et al., 2016), while human data suggest similar changes to post-traumatic cerebrovascular permeability (Tomkins et al., 2011; Shlosberg et al., 2010) even after subconcussive head hits (Marchi et al., 2013). Since early sequelae of TBI include changes in neuronal excitability and firing, there remains a question as to how BBB dysfunction (BBBD) causes these neuronal changes. A few review articles have focused on asymmetric distributions of molecules and ions across the BBB; ionic changes after BBBD illustrates in-depth a few scenarios that link vascular permeability to neuronal activity (Janigro, 2012; Oby and Janigro, 2006). In general terms, the effect of BBBD on neurons is mediated by its role in controlling brain homeostasis.
It has been repeatedly demonstrated that BBBD is ictogenic (Marchi et al., 2007), and that blood biomarkers of BBBD increase at time of epileptic seizures (e.g., Fig. 6 in (Bargerstock et al., 2014)). Taken together, current evidence points to focal BBB disruption-followed by abnormal excitability patterns in the surrounding neuropil- as an initiating factor for early post-traumatic seizures. Longitudinal studies have also shown that after the initial BBBD event, the delayed onset of epileptic seizures frequently occurs in the same regions in patients as well as in animal models (Tomkins et al., 2008; Korn et al., 2005; Seiffert et al., 2004). Since BBB function is not the only mechanism of brain homeostatic control, pathological events affecting parenchymal cells produce effects comparable to loss of BBB function. For example, it was shown that post-traumatic sequelae include loss of spatial buffering of potassium ions by glial cells (D’Ambrosio et al., 1999) which is directly related to changes in long-term potentiation (LTP) reported by many after TBI (Tagge et al., 2018; D’Ambrosio et al., 1998a; Janigro et al., 1997; D’Ambrosio et al., 1998b; Vogel III et al., 2017).
Potassium (K+) regulation is key to the proper function of all excitable cells, including neurons (Misonou, 2010). K+ in the brain extracellular space (ECS) is maintained at approximately 3 mM irrespective of serum potassium levels, though serum potassium is typically around 5mM (Janigro, 2012). Intracellular K+ levels are kept high compared to the extracellular compartment (> 100 mM). There are therefore two distinct K+ gradients for potassium movement: the gradient across cell membranes and the gradient across the BBB. Physiologically, this dramatic difference in concentration allows for the rapid repolarization required of CNS neurons after depolarization. However, neurons are unable to bring about such rapid changes in K+ concentration alone. K+ homeostasis is achieved via a glial buffering system in addition to the energy-dependent neuronal mechanism (Kofuji and Newman, 2004).
Neurons contribute to K+ homeostasis through the Na+/K+ ATPase antiporter and sodium-potassium-chloride (Na+/K+/Cl−) transporters. The Na+/K+ ATPase shuttles three Na+ from the intracellular compartment and imports two K+ into the cell per cycle of the pump. The Na+/K+/Cl− transporter moves ions in a 1:1:2 ratio, respectively, and will move ions in or out of the cell in order to maintain electroneutrality (Altamirano and Russell, 1987). Glia, and in particular astroglia, play the largest role in K+ buffering by two mechanisms: K+ uptake and spatial buffering (Chen and Nicholson, 2000). In the K+ uptake mechanism, astroglia remove K+ in the ECS through a glial specific Na+/K+ ATPase, which is better suited for K+ buffering than the neuronal isotype (Sweadner, 1979; Gloor, 1997). Astrocytes also possess a Na+/K+/Cl− co-transporter (NKCC1) that has recently been implicated in potassium buffering (Kahle et al., 2009) as well as blood-brain barrier disruption after TBI (Zhang et al., 2017). In addition, blockade of NKCC1 has shown to protect from post-traumatic seizures (Wang et al., 2017a). Using these channels and co-transporters, astrocytes are able to temporarily sequester excess extracellular K+ and release it back into the extracellular space when K+ levels drop. Relevant to post-traumatic seizures is the fact that genetic variations of KCNJ10 (Kir 4.1) are associated with spontaneous seizures (Freudenthal et al., 2011) and the downregulation of this channel by traumatic brain injury (Ivens et al., 2007).
The second mechanism, spatial buffering, is a means by which astrocytes can remove K+ from areas of high concentration and release it in areas of comparably lower concentration (Chen and Nicholson, 2000). This is achieved by a syncytium of astroglia connected via gap junctions that directly link neighboring cells (Pannasch et al., 2011). In areas of high K+ concentration, K+ enters the cell primarily through potassium inward rectifier (Kir) channels, specifically Kir 4.1 (Ohno et al., 2007). Kir 4.1 channels localize at astrocyte foot processes (Higashi et al., 2001). These channels move K+ into astrocytes and are unique in having a higher conductance at negative membrane potentials (Olsen and Sontheimer, 2008). K+ entry triggers a depolarization that travels through the astroglial network resulting in net outward movement of K+. In this way, astrocytes are able to spread K+ across a large area while causing only a transient increase in local intracellular K+ concentrations (Kofuji and Newman, 2004).
The movement of water across the BBB and within the brain parenchyma follows osmotic gradients. In the brain, water moves through aquaporin channels, specifically aquaporin 1 and aquaporin 4 (AQP4). For the purposes of this discussion we will focus on the role of AQP4, as it is the primary water channel expressed on astrocytes and co-localizes with Kir 4.1 channels (Medici et al., 2011). Astrocytes express high levels of AQP4 on end-foot processes surrounding barrier capillaries; their proximity suggests a role in the regulation of water movement into and out of the brain parenchyma (Medici et al., 2011). However, AQP4 can also be found on end processes of astrocytes at synapses, suggesting an additional role in neuronal water uptake. It is interesting to note that immunocytochemical experiments do not detect the presence of the same aquaporin on neurons, indicating that astrocytes may be responsible for water homeostasis in the brain. AQP4 co-localization with Kir 4.1 channels suggests the coupling of K+ and water movement within the brain as well as across the BBB (Nielsen et al., 1997). The juxtaposition of these channels has led to the hypothesis that the astroglial syncytium may, in addition to the spatial buffering of potassium, redistribute water throughout the cortex and, in particular, perivascular areas thought to be sinks for excess water and K+ (Rash et al., 1998). Experiments have shown that an increase in extracellular K+ leads to a decrease in ECS which suggests a link between K+ and water movement (Dietzel et al., 1980). Additionally, it has been demonstrated that Kir 4.1 channels and AQP4 channels may be associated, extracellularly, by the dystrophin-glycoprotein complex (Ahn and Kunkel, 1995; Connors and Kofuji, 2002).
In addition to the aforementioned post-traumatic downregulation of potassium channels, TBI can influence potassium management by indirect mechanisms. When BBB permeability to K+ increases, the control of resting potassium levels by astrocytes becomes arduous since the source of hematic K+ likely overwhelms the capacity of glial syncytia. In addition, metabolic disruption due to decreased ATP levels will limit the amount of potassium clearance by energy dependent pumps. Finally, both hematic and CNS potassium increases in the extracellular space will attract water and cause ionic edema, a condition that will further impair homeostatic control of the brain microenvironment (see (Iffland et al., 2014)).
An additional mechanism of post-traumatic epileptogenesis has been proposed by Friedman et al. (Shlosberg et al., 2010; Weissberg et al., 2015; Frigerio et al., 2012). Under normal conditions, the BBB disallows passage of most blood-borne protein from the serum to brain. As in many instances of “barrier function”, this is not an absolute concept but rather evidence of a severely restricted diffusion. Serum albumin is the most abundant blood protein; low levels of albumin are present in CSF of normal subjects (Balslev et al., 1997). According to the aforementioned data, sudden and persistent increases of albumin in the brain parenchyma are epileptogenic. The downstream effects of albumin include inclusion in astrocytes and TGF-B signaling (Ivens et al., 2007; Cacheaux et al., 2009). The mechanism of hyperexcitability involves reduced capacity for potassium buffering, which is reminiscent of early studies relating astrocyte dysfunction and post-traumatic neuronal changes (D’Ambrosio et al., 1999; D’Ambrosio et al., 1998a; Janigro et al., 1997; D’Ambrosio et al., 1998b; McKhann et al., 1997).
1.2. TBI and antiepileptic drugs: a window on the role of the BBB?
The risk of developing post-traumatic epilepsy is correlated with the severity of brain injury and duration of loss of consciousness (LOC). In one large review of patients with head trauma who lost consciousness after impact, the relative risk of developing PTE increased 1.9-fold for those with LOC for < 30 min, 2.9-fold for those with LOC for 30 min to 24 h, and 17.2-fold for those with LOC that lasted > 1 day (Annegers and Coan, 2000) (for a recent metanalysis see (Lasry et al., 2017)). Other factors that increased the risk of late PTE include depressed skull fracture, penetrating trauma, intracranial hemorrhage, advanced age, and early post-traumatic seizures. Because of the high incidence of early post-traumatic seizures with severe TBI, patients often receive prophylactic anti-epileptic drugs (AEDs) in the first week after injury (Zimmermann et al., 2017). The efficacy of prophylaxis with AEDs suggests that at least part of the epileptogenic process depends on classic targets for antiepileptic drugs, namely sodium and calcium currents in neurons or GABA/glutamate receptors.
Relevant to this review is the question of evidence supporting an anti-seizure, anti-PTE effect of therapeutic interventions known to repair a disrupted BBB. Before this question can be answered, a definition of “BBB therapeutics” is required. Our current understanding of BBB changes after an insult to the brain suggests a number of mechanisms and therapeutic interventions.
The inflammatory hypothesis of BBB disruption implicates peripheral and parenchymal activation of immunocompetent cells and the release of peripheral and central inflammatory mediators (for a review specifically focused on inflammation, the BBB and seizures see (Marchi et al., 2014)). An important correlate of a systemic inflammatory etiology is that it allows use of traditional anti-inflammatory agents not requiring penetration across the BBB (see also below). In addition, it has been noted that traditional anti-epileptic drugs and clinical interventions have an effect not only on inflammation but also directly on the BBB (Janigro et al., 2013; Marchi et al., 2012; Marchi et al., 2009b; Granata et al., 2008) (Table 1). In fact, anti-inflammatory drugs undeniably have a beneficial effect on BBB function (Marchi et al., 2009b; Rom et al., 2016; Yuan et al., 2016; Rodriguez-Grande et al., 2017; Wang et al., 2017b). Dexamethasone and corticosteroids in general have an anti-seizure effect in experimental models and patients (Marchi et al., 2009b; Marchi et al., 2011b; Araki et al., 2006). While the effects on pediatric populations are common (Marchi et al., 2011b), response to anti-inflammatory drugs or maneuvers has been also reported in adults (see review in (Marchi et al., 2012)). As predicted by animal models (Marchi et al., 2009b), monoclonal antibody therapies designed for peripheral immune diseases are also efficacious in seizure disorders (Kenney-Jung et al., 2016; Desena et al., 2018; Sotgiu et al., 2010). It is again important to note that most of these peripherally acting drugs have little or no penetration across the BBB, and thus exert their effects directly on peripheral immunity.
The direct mechanical forces hypothesis predicts that one mechanism of post-traumatic BBBD is mediated by acceleration/deceleration forces that transmit from the skull to parenchymal microvessels (Johnson et al., 2018; Alluri et al., 1717; Shi et al., 2018). The relationship between microvascular damage and hemorrhage after TBI is not entirely understood: in particular, it is not known whether microvascular leakage is a risk factor for hemorrhage, or whether the two phenomena can occur independently.
The metabolic hypothesis is based on the idea that after moderate or severe TBI, intracranial perfusion may be reduced by a number of mechanisms; loss of energy supply or clearance of waste around the BBB may impact its function, resulting in increased permeability of endothelial cells. The permeability increase may be due to disruption of tight junctions, increased transcellular transport or a combination of the two.
Table 1.
Overlapping properties of anti-epileptic drugs or therapies and BBB protection.
| Therapeutic intervention | Classification/drug class | Accepted mechanism(s) of action | Anti-inflammatory potency | Predicted or demonstrated effects on BBB integrity | References |
|---|---|---|---|---|---|
| Fingolimod | Anti-inflammatory agents | Lymphocyte trafficking | Reduces egress from lymph nodes | Promotes repair | (Foster et al., 2009; Spampinato et al., 2015; Zinger et al., 2016) |
| Wogonin | Anti-inflammatory agents | Vascular permeability | Blocks COX2 | Promotes repair | (Chen et al., 2012) |
| Propofol | Anesthetic short-acting hypnotic agent | GABA | Inhibits NFkB | Protection/repair | (Jaaskelainen et al., 2003; Sanchez-Conde et al., 2008; Schneemilch et al., 2005) |
| Thiopental | Anesthetic short-acting hypnotic agent | GABA | Inhibits NFkB | Protection/repair | (Schneemilch et al., 2005; Roesslein et al., 2008) |
| Ketamine | Anesthetic short-acting hypnotic agent | NMDA antagonist | Inhibits NFkB and IL-1B, TNF-α surge | Protection/repair | (Beilin et al., 2007; Welters et al., 2011; Welters et al., 2010) |
| Magnesium | Electrolyte | NMDA blocker | NA | Restores NMDA receptor blockade | (Amtorp and Sorensen, 1974) |
| VNS | Device | Unknown | Nicotinic receptors | Protection/repair | (Rosas-Ballina et al., 2011; Rosas-Ballina and Tracey, 2009; Neren et al., 2016; Lee et al., 2008) |
| Ketogenic diet | Dietary regimen | Unknown | Protection/repair | (Nabbout et al., 2011; Janigro, 1999) | |
| Hypothermia | Medical management | Unknown | Inhibits NFkB | Protection/repair | (Oztas and Kaya, 1994; Polderman, 2009; Webster et al., 2009) |
| Corticosteroids | Anti-inflammatory agents | Immuno-depression | Similar to NFkB inhibition | Protection/repair | (Marchi et al., 2011a; Marchi et al., 2009a) |
| IL1 antagonists | Anti-inflammatory agents | Immuno-modulation | Block ILl-β | Protection/repair | (Kenney-Jung et al., 2016; Desena et al., 2018) |
From a vascular standpoint, at least three distinct mechanisms can be invoked as therapeutic targets for the prevention of post-traumatic epilepsy.
If early BBB dysfunction is directly involved in epileptogenesis, then drugs that improve the integrity of BBB tight junctions may be beneficial.
If BBB failure is responsible for the interictal-to-ictal transition once seizures are occurring, then drugs that improve BBB function may prevent or shorten early post-traumatic seizures or reduce seizure burden during the chronic phase.
Several lines of evidence point to multiple drug resistance to AED in PTE (Lee et al., 2008; Gupta et al., 2014). In this case the role of the BBB may be similar or identical to what seen in other pharmacoresistant epilepsies (Oby and Janigro, 2006).
2. Biomarkers of PTE
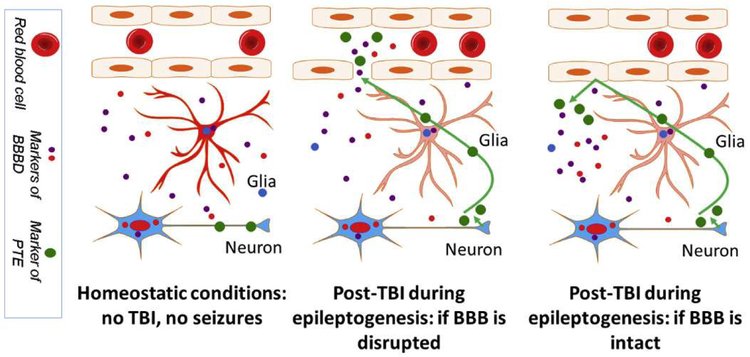
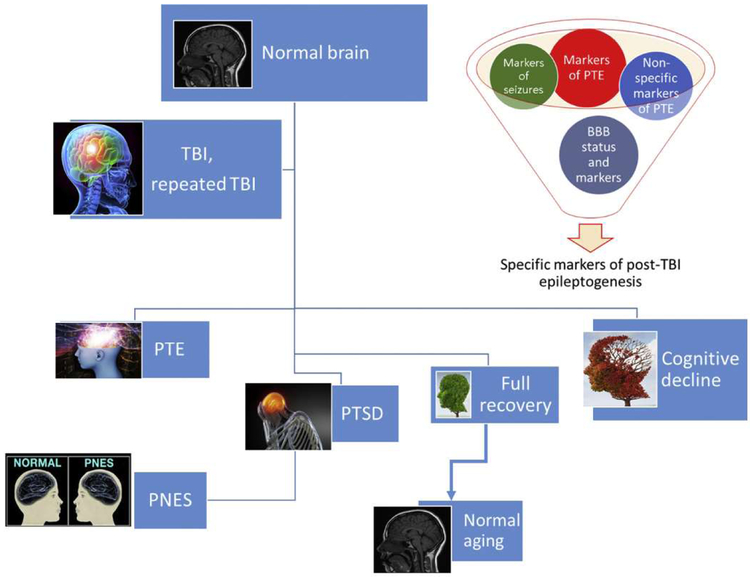
The introduction of novel AEDs in the last two decades has improved our ability to prevent seizures, however no therapy is currently available to prevent epilepsy from developing after an epileptogenic stimulus or event. We partially ascribe this to a lack of reliable bio-markers indicating individuals at risk for epilepsy, as well as a lack of biomarkers indicating already-diagnosed patients who are most likely to respond to a specific intervention. Since it is unlikely that the same mechanism is responsible for epileptogenesis in all forms of epilepsy, the underlying etiology needs to be taken into account. The use of blood or body fluid biomarkers (BBM) as an add-on to clinical findings and patient history may allow personalized, mechanism-based therapies. Due to their poor predictive values the use of BBM is very limited. In epileptic patients and TBI-related pathologies specifically, a reason for lack of predictive value for blood biomarkers is the fact that the BBB may impede markers’ passage into systemic circulation (for a more complete discussion see (Zhang et al., 2016) and (Abbott et al., 2018; Dadas and Janigro, 2018)). In many pathologies, specific markers of disease are often obscured by the presence of BBM of BBB disruption (Fig. 2). In addition, we have shown that several patient-specific elements, spanning from physiological to pathological factors, also determine BBM levels (Dadas et al., 2016). Finally, and specific for PTE, sequelae such as post-traumatic stress disorder (PTSD) or psychogenic non-epileptic seizures (PNES), a temporal relationship between markers’ appearance and last/next seizure also confounds our ability to interpret raw data derived from BBM (Figs. 1 & 2).
Fig. 2.
Mechanisms of biomarker extravasation in blood after TBI. See text.
Fig. 1.
The complex reality of post-traumatic sequelae confound the predictive properties of biomarkers. See text for details.
Seizures (Bargerstock et al., 2014; Marchi et al., 2009b) and TBI (Marchi et al., 2013; Weissberg et al., 2014) are followed by or associated with BBB disruption, providing a window to the brain “biomarker bank”. Markers of BBB dysfunction that are non-specific may carry limited significance when searching for post-traumatic sequelae such as epileptogenesis. However, understanding BBB status at a time of markers’ measurements is crucial because if the BBB is at that moment not permeable to a specific marker, a false negative interpretation may occur (Fig. 2). Since TBI increases risk of PTE, PNES, and PTSD, it may be quite difficult to establish a definitive diagnosis and a group of corresponding blood biomarkers. In a recent study on veterans from Afghanistan and Iraq (Chen et al., 2014), the authors reported that only 18% of post-TBI veterans diagnosed with seizures had PTE, while the vast majority (82%) had PNES. In a similar but larger study it was reported that PTSD (a common comorbidity of PNES (Plioplys et al., 2016)) was not a significant predictor of epilepsy, which is consistent with findings from a previous study of older veterans (Pugh et al., 2009). It is thus possible that a limited overlap exists between diagnoses of PTE and PTSD.
Between 10% and 30% of patients diagnosed with PNES also had epileptic seizures in the past or have active co-existing epileptic seizures. The patient and the witnesses are often able to describe a distinct semiology that differentiates one type of spell from the other; video electroencephalography (VEEG) is the gold-standard method for diagnosing psychogenic non-epileptic seizures, but such assessment is expensive, unavailable in many patient care centers, requires prolonged hospitalization, and is often unable to capture an actual seizure episode. A number of structural neuroimaging approaches have been used to study abnormalities associated with PNES (Sundararajan et al., 2016) but no data are available to compare blood biomarker with EEG.
The most prevalent concerns that must be addressed by post-TBI research -and, in particular, the field of biomarker research-regarding the complexity of PTE include:
Discovery of new markers of seizures that are distinct from BBB markers.
Discovery of markers to diagnose either PNES or PTE.
Validation of biomarkers indicating BBB “opening” in TBI in the subacute setting using timed contrast MRI studies. Relationship of markers with imaging and EEG findings.
Study the extent and breadth of the BBB opening after TBI.
Determine how BBB opening factors into the development of PTE (by analysis with diagnostic findings).
3. Conclusions
There are several lines of evidence that link blood-brain barrier disruption to post-traumatic epilepsy. These will likely impact the pharmacology of epileptogenesis in the pharmacodynamic space and CNS drug delivery (pharmacokinetic properties of drugs in multiple drug resistance).
References
- Abbott NJ, Pizzo ME, Preston JE, Janigro D, Thorne RG, 2018. The role of brain barriers in fluid movement in the CNS: is there a ‘glymphatic’ system? Acta Neuropathol. 135, 387–407. [DOI] [PubMed] [Google Scholar]
- Ahn AH, Kunkel LM, 1995. Syntrophin binds to an alternatively spliced exon of dystrophin. J. Cell Biol 128, 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alluri H, Shaji CA, Davis ML, Tharakan B, 1717. A mouse controlled cortical impact model of traumatic brain injury for studying blood-brain barrier dysfunctions. Methods Mol. Biol 2018, 37–52. [DOI] [PubMed] [Google Scholar]
- Altamirano AA, Russell JM, 1987. Coupled Na/K/Cl efflux, “reverse” unidirectional fluxes in squid giant axons. J. Gen. Physiol 89, 669–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amtorp O, Sorensen SC, 1974. The ontogenetic development of concentration differences for protein and ions between plasma and cerebrospinal fluid in rabbits and rats. J. Physiol 243, 387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annegers JF, Coan SP, 2000. The risks of epilepsy after traumatic brain injury. Seizure 9, 453–457. [DOI] [PubMed] [Google Scholar]
- Annegers JF, Grabow JD, Kurland LT, Laws ER, 1980a. The incidence, causes, and secular trends of head trauma in Olmsted-County, Minnesota, 1935–1974. Neurology 30, 912–919. [DOI] [PubMed] [Google Scholar]
- Annegers JF, Grabow JD, Groover RV, Laws ER, Elveback LR, Kurland LT, 1980b. Seizures after head trauma - a population study. Neurology 30, 683–689. [DOI] [PubMed] [Google Scholar]
- Araki T, Otsubo H, Makino Y, et al. , 2006. Efficacy of dexamathasone on cerebral swelling and seizures during subdural grid EEG recording in children. Epilepsia 47, 176–180. [DOI] [PubMed] [Google Scholar]
- Balslev Y, Dziegielewska KM, Mollgard K, Saunders NR, 1997. Intercellular barriers to and transcellular transfer of albumin in the fetal sheep brain. Anat. Embryol (Berl) 195, 229–236. [DOI] [PubMed] [Google Scholar]
- Bargerstock E, Puvenna V, Iffland P, et al. , 2014. Is peripheral immunity regulated by blood-brain barrier permeability changes? PLoS One 9, e101477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilin B, Rusabrov Y, Shapira Y, et al. , 2007. Low-dose ketamine affects immune responses in humans during the early postoperative period. Br. J. Anaesth 99, 522–527. [DOI] [PubMed] [Google Scholar]
- Cacheaux LP, Ivens S, David Y, et al. , 2009. Transcriptome profiling reveals TGF-beta signaling involvement in epileptogenesis. J. Neurosci 29, 8927–8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KC, Nicholson C, 2000. Spatial buffering of potassium ions in brain extracellular space. Biophys. J 78, 2776–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Hung TH, Wang YH, et al. , 2012. Wogonin improves histological and functional outcomes, and reduces activation of TLR4/NF-kappaB signaling after experimental traumatic brain injury. PLoS One 7, e30294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL, Baca CB, Choe J, Chen JW, Ayad ME, Cheng EM, 2014. Posttraumatic epilepsy in operation enduring freedom/operation Iraqi freedom veterans. Mil. Med 179, 492–496. [DOI] [PubMed] [Google Scholar]
- Christensen J, 2012. Traumatic brain injury: risks of epilepsy and implications for medicolegal assessment. Epilepsia 53 (Suppl. 4), 43–47. [DOI] [PubMed] [Google Scholar]
- Christensen J, 2015. The epidemiology of posttraumatic epilepsy. Semin. Neurol 35, 218–222. [DOI] [PubMed] [Google Scholar]
- Connors NC, Kofuji P, 2002. Dystrophin Dp71 is critical for the clustered localization of potassium channels in retinal glial cells. J. Neurosci 22, 4321–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadas A, Janigro D, 2018. The role and diagnostic significance of cellular barriers after concussive head trauma. Concussion in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadas A, Washington J, Marchi N, Janigro D, 2016. Improving the clinical management of traumatic brain injury through the pharmacokinetic modeling of peripheral blood biomarkers. Fluids Barriers CNS 13, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio R, Wenzel J, Schwartzkroin PA, McKhann GM, Janigro D, 1998a. Functional specialization and topographic segregation of hippocampal astrocytes. J. Neurosci 18, 4425–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio R, Maris DO, Grady MS, Winn HR, Janigro D, 1998b. Selective loss of hippocampal long-term potentiation, but not depression, following fluid percussion injury. Brain Res 786, 64–79. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio R, Maris DO, Grady MS, Winn HR, Janigro D, 1999. Impaired K homeostasis and altered electrophysiological properties of post-traumatic hippo-campal glia. J. Neurosci 19, 8152–8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desena AD, Do T, Schulert GS, 2018. Systemic autoinflammation with intractable epilepsy managed with interleukin-1 blockade. J. Neuroinflammation 15, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzel I, Heinemann U, Hofmeier G, Lux HD, 1980. Transient changes in the size of the extracellular space in the sensorimotor cortex of cats in relation to stimulus-induced changes in potassium concentration. Exp. Brain Res 40, 432–439. [DOI] [PubMed] [Google Scholar]
- Foster CA, Mechtcheriakova D, Storch MK, et al. , 2009. FTY720 rescue therapy in the dark agouti rat model of experimental autoimmune encephalomyelitis: expression of central nervous system genes and reversal of blood-brain-barrier damage. Brain Pathol 19, 254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenthal B, Kulaveerasingam D, Lingappa L, et al. , 2011. KCNJ10 mutations disrupt function in patients with EAST syndrome. Nephron. Physiol 119, 40–48. [DOI] [PubMed] [Google Scholar]
- Frigerio F, Frasca A, Weissberg I, et al. , 2012. Long-lasting pro-ictogenic effects induced in vivo by rat brain exposure to serum albumin in the absence of concomitant pathology. Epilepsia 53, 1887–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor SM, 1997. Relevance of Na,K-ATPase to local extracellular potassium homeostasis and modulation of synaptic transmission. FEBS Lett 412, 1–4. [DOI] [PubMed] [Google Scholar]
- Granata T, Obino L, Ragona FDI, Marchi N, Binelli S, Janigro D, 2008. Steroid treatment is effective in the treatement of status epilepticus in children. Epilepsia 1 (Suppl 0), 276. [Google Scholar]
- Gupta PK, Sayed N, Ding K, et al. , 2014. Subtypes of post-traumatic epilepsy: clinical, electrophysiological, and imaging features. J. Neurotrauma 31, 1439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser WA, Annegers JF, Kurland LT, 1993. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia 34, 453–468. [DOI] [PubMed] [Google Scholar]
- Higashi K, Fujita A, Inanobe A, et al. , 2001. An inwardly rectifying K (+) channel, Kir4.1, expressed in astrocytes surrounds synapses and blood vessels in brain. Am. J. Phys. Cell Phys 281, C922–C931. [DOI] [PubMed] [Google Scholar]
- Iffland P, Grant GG, Janigro D, 2014. Mechanisms of cerebral edema leading to early seizures after traumatic brain injury In: Lo EH (Ed.), Vascular Mechanisms in CNS Trauma, pp. 29–36. [Google Scholar]
- Ivens S, Kaufer D, Flores LP, et al. , 2007. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain 130, 535–547. [DOI] [PubMed] [Google Scholar]
- Jaaskelainen SK, Kaisti K, Suni L, Hinkka S, Scheinin H, 2003. Sevoflurane is epileptogenic in healthy subjects at surgical levels of anesthesia. Neurology 61, 1073–1078. [DOI] [PubMed] [Google Scholar]
- Janigro D, 1999. Blood-brain barrier, ion homeostatis and epilepsy: possible implications towards the understanding of ketogenic diet mechanisms. Epilepsy Res 37, 223–232. [DOI] [PubMed] [Google Scholar]
- Janigro D, 2012. Are you in or out? Leukocyte, ion, and neurotransmitter permeability across the epileptic blood-brain barrier. Epilepsia 53 (Suppl. 1), 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janigro D, Gasparini S, D’Ambrosio R, McKhann G, DiFrancesco D, 1997. Reduction of K+ uptake in glia prevents long-term depression maintenance and causes epileptiform activity. J. Neurosci 17, 2813–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janigro D, Iffland PH, Marchi N, Granata T, 2013. A role for inflammation in status epilepticus is revealed by a review of current therapeutic approaches. Epilepsia 54, 30–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Weber MT, Xiao R, et al. , 2018. Mechanical disruption of the blood-brain barrier following experimental concussion. Acta Neuropathol 135 (5), 711–726 (May). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle KT, Simard JM, Staley KJ, Nahed BV, Jones PS, Sun D, 2009. Molecular mechanisms of ischemic cerebral edema: role of electroneutral ion transport. Physiology (Bethesda) 24, 257–265. [DOI] [PubMed] [Google Scholar]
- Kawoos U, Gu M, Lankasky J, McCarron RM, Chavko M, 2016. Effects of exposure to blast overpressure on intracranial pressure and blood-brain barrier permeability in a rat model. PLoS One 11, e0167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney-Jung DL, Vezzani A, Kahoud RJ, et al. , 2016. Febrile infection-related epilepsy syndrome treated with anakinra. Ann. Neurol 80, 939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P, Newman EA, 2004. Potassium buffering in the central nervous system. Neuroscience 129, 1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn A, Golan H, Melamed I, Pascual-Marqui R, Friedman A, 2005. Focal cortical dysfunction and blood-brain barrier disruption in patients with Postconcussion syndrome. J. Clin. Neurophysiol 22, 1–9. [DOI] [PubMed] [Google Scholar]
- Langendorf FG, Pedley TA, Temkin NR, 2008. Posttraumatic seizures In: Engel G, Pedley TA (Eds.), Epilepsy: A Comprehensive Textbook. Lippincott Williams and Wilkins, Philadelphia, PA, pp. 2537–2542. [Google Scholar]
- Lasry O, Liu EY, Powell GA, Ruel-Laliberte J, Marcoux J, Buckeridge DL, 2017. Epidemiology of recurrent traumatic brain injury in the general population: a systematic review. Neurology 89, 2198–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HO, Koh EJ, Oh YM, Park SS, Kwon KH, Choi HY, 2008. Effect of vagus nerve stimulation in post-traumatic epilepsy and failed epilepsy surgery: preliminary report. J. Korean Neurosurg. Soc 44, 196–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Angelov L, Masaryk T, et al. , 2007. Seizure-promoting effect of blood-brain barrier disruption. Epilepsia 48 (4), 732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Fan Q, Ghosh C, et al. , 2009a. Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol. Dis 33, 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Fan QY, Ghosh C, et al. , 2009b. Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol. Dis 33, 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Granata T, Freri E, et al. , 2011a. Efficacy of anti-inflammatory therapy in a model of acute seizures and in a population of pediatric drug resistant epileptics. PLoS One 6, e18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Granata T, Freri E, et al. , 2011b. Efficacy of anti-inflammatory therapy in a model of acute seizures and in a population of pediatric drug resistant epileptics. PLoS One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Granata T, Alexopoulos A, Janigro D, 2012. The blood-brain barrier hypothesis in drug resistant epilepsy. Brain 135, e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Bazarian JJ, Puvenna V, et al. , 2013. Consequences of repeated blood-brain barrier disruption in football players. PLoS One 8, e56805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Granata T, Janigro D, 2014. Inflammatory pathways of seizure disorders. Trends Neurosci 37, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, D’Ambrosio R, Janigro D, 1997. Heterogeneity of astrocyte resting membrane potentials revealed by whole cell and gramicidin-perforated patch recordings from cultured neocortical and hippocampal slice astrocytes. J. Neurosci 17, 6850–6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici V, Frassoni C, Tassi L, Spreafico R, Garbelli R, 2011. Aquaporin 4 expression in control and epileptic human cerebral cortex. Brain Res 1367, 330–339. [DOI] [PubMed] [Google Scholar]
- Misonou H, 2010. Homeostatic regulation of neuronal excitability by K (+) channels normal and diseased brains. Neuroscientist 16, 51–64. [DOI] [PubMed] [Google Scholar]
- Nabbout R, Vezzani A, Dulac O, Chiron C, 2011. Acute encephalopathy with inflammation-mediated status epilepticus. Lancet Neurol 10, 99–108. [DOI] [PubMed] [Google Scholar]
- Neren D, Johnson MD, Legon W, Bachour SP, Ling G, Divani AA, 2016. Vagus nerve stimulation and other neuromodulation methods for treatment of traumatic brain injury. Neurocrit Care 24, 308–319. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP, 1997. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neurosci 17, 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oby E, Janigro D, 2006. The blood-brain barrier and epilepsy. Epilepsia 47, 1761–1774. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Hibino H, Lossin C, Inanobe A, Kurachi Y, 2007. Inhibition of astroglial Kir4.1 channels by selective serotonin reuptake inhibitors. Brain Res 1178, 44–51. [DOI] [PubMed] [Google Scholar]
- Olsen ML, Sontheimer H, 2008. Functional implications for Kir4.1 channels in glial biology: from K+ buffering to cell differentiation. J. Neurochem 107, 589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztas B, Kaya M, 1994. The effect of profound hypothermia on blood-brain barrier permeability dining pentylenetetrazol-induced seizures. Epilepsy Res 19, 221–227. [DOI] [PubMed] [Google Scholar]
- Pannasch U, Vargova L, Reingruber J, et al. , 2011. Astroglial networks scale synaptic activity and plasticity. Proc. Natl. Acad. Sci. U. S. A 108, 8467–8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plioplys S, Doss J, Siddarth P, et al. , 2016. Risk factors for comorbid psychopathology in youth with psychogenic nonepileptic seizures. Seizure 38, 32–37. [DOI] [PubMed] [Google Scholar]
- Polderman KH, 2009. Mechanisms of action, physiological effects, and complications of hypothermia. Crit. Care Med 37, S186–S202. [DOI] [PubMed] [Google Scholar]
- Pugh MJ, Knoefel JE, Mortensen EM, Amuan ME, Berlowitz DR, Van Cott AC, 2009. New-onset epilepsy risk factors in older veterans. J. Am. Geriatr. Soc 57, 237–242. [DOI] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Hudson CS, Agre P, Nielsen S, 1998. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc. Natl. Acad. Sci. U. S. A 95, 11981–11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Grande B, Ichkova A, Lemarchant S, Badaut J, 2017. Early to long-term alterations of CNS barriers after traumatic brain injury: considerations for drug development. AAPS J 19, 1615–1625. [DOI] [PubMed] [Google Scholar]
- Roesslein M, Schibilsky D, Muller L, et al. , 2008. Thiopental protects human T lymphocytes from apoptosis in vitro via the expression of heat shock protein 70. J. Pharmacol. Exp. Ther 325, 217–225. [DOI] [PubMed] [Google Scholar]
- Rom S, Zuluaga-Ramirez V, Reichenbach NL, et al. , 2016. PARP inhibition in leukocytes diminishes inflammation via effects on integrins/cytoskeleton and protects the blood-brain barrier. J. Neuroinflammation 13, 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Tracey KJ, 2009. Cholinergic control of inflammation. J. Intern. Med 265, 663–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Olofsson PS, Ochani M, et al. , 2011. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar AM, Jabbari B, Vance SC, Grafman J, Amin D, Dillon JD, 1985. Epilepsy after penetrating head-injury. 1. Clinical correlates - a report of the Vietnam head-injury study. Neurology 35, 1406–1414. [DOI] [PubMed] [Google Scholar]
- Sanchez-Conde P, Rodriguez-Lopez JM, Nicolas JL, et al. , 2008. The comparative abilities of propofol and sevoflurane to modulate inflammation and oxidative stress in the kidney after aortic cross-clamping. Anesth. Analg 106, 371–378 table. [DOI] [PubMed] [Google Scholar]
- Schneemilch CE, Hachenberg T, Ansorge S, Ittenson A, Bank U, 2005. Effects of different anaesthetic agents on immune cell function in vitro. Eur. J. Anaesthesiol 22, 616–623. [DOI] [PubMed] [Google Scholar]
- Seiffert E, Dreier JP, Ivens S, et al. , 2004. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J. Neurosci 24, 7829–7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi ZS, Duckwiler GR, Jahan R, et al. , 2018. Early blood-brain barrier disruption after mechanical thrombectomy in acute ischemic stroke. J. Neuroimaging 28 (3), 283–288 (May). [DOI] [PubMed] [Google Scholar]
- Shlosberg D, Benifla M, Kaufer D, Friedman A, 2010. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev. Neurol 6, 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Johnson VE, Stewart W, 2013. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev. Neurol 9, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotgiu S, Murrighile MR, Constantin G, 2010. Treatment of refractory epilepsy with natalizumab in a patient with multiple sclerosis. Case report BMC Neurol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampinato SF, Obermeier B, Cotleur A, et al. , 2015. Sphingosine 1 phosphate at the blood brain barrier: can the modulation of S1P receptor 1 influence the response of endothelial cells and astrocytes to inflammatory stimuli? PLoS One 10, e0133392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundararajan T, Tesar GE, Jimenez XF, 2016. Biomarkers in the diagnosis and study of psychogenic nonepileptic seizures: a systematic review. Seizure 35, 11–22. [DOI] [PubMed] [Google Scholar]
- Sweadner KJ, 1979. Two molecular forms of (Na+ + K+)-stimulated ATPase in brain. Separation, and difference in affinity for strophanthidin. J. Biol. Chem 254, 6060–6067. [PubMed] [Google Scholar]
- Tagge CA, Fisher AM, Minaeva OV, et al. , 2018. Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model. Brain 141, 422–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin NR, Dikmen SS, Wilensky AJ, Keihm J, Chabal S, Winn HR, 1990. A randomized, double-blind-study of phenytoin for the prevention of posttraumatic seizures. N. Engl. J. Med 323, 497–502. [DOI] [PubMed] [Google Scholar]
- Tomkins O, Shelef I, Kaizerman I, et al. , 2008. Blood-brain barrier disruption in post-traumatic epilepsy. J. Neurol. Neurosurg. Psychiatry 79, 774–777. [DOI] [PubMed] [Google Scholar]
- Tomkins O, Feintuch A, Benifla M, Cohen A, Friedman A, Shelef I, 2011. Blood-brain barrier breakdown following traumatic brain injury: a possible role in post-traumatic epilepsy. Cardiovasc. Psychiatry Neurol 2011, 765923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel HI EW, Rwema SH, Meaney DF, Bass CR, Morrison B III, 2017. Primary blast injury depressed hippocampal long-term potentiation through disruption of synaptic proteins. J. Neurotrauma 34, 1063–1073. [DOI] [PubMed] [Google Scholar]
- Wang F, Wang X, Shapiro LA, et al. , 2017a. NKCC1 up-regulation contributes to early post-traumatic seizures and increased post-traumatic seizure susceptibility. Brain Struct. Funct 222, 1543–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Zhu G, Qian P, Zhu T, 2017b. Tetramethylpyrazine reduces blood-brain barrier permeability associated with enhancement of peripheral cholinergic anti-inflammatory effects for treating traumatic brain injury. Exp. Ther. Med 14, 2392–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster CM, Kelly S, Koike MA, Chock VY, Giffard RG., Yenari MA, 2009. Inflammation and NFkappaB activation is decreased by hypothermia following global cerebral ischemia. Neurobiol. Dis 33, 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissberg I, Veksler R, Kamintsky L, et al. , 2014. Imaging blood-brain barrier dysfunction in football players. JAMA Neurol 71, 1453–1455. [DOI] [PubMed] [Google Scholar]
- Weissberg I, Wood L, Kamintsky L, et al. , 2015. Albumin induces excitatory synaptogenesis through astrocytic TGF-beta/ALK5 signaling in a model of acquired epilepsy following blood-brain barrier dysfunction. Neurobiol. Dis 78, 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welters ID, Hafer G, Menzebach A, et al. , 2010. Ketamine inhibits transcription factors activator protein 1 and nuclear factor-kappaB, interieukin-8 production, as well as CD11b and CD16 expression: studies in human leukocytes and leukocytic cell lines. Anesth. Analg 110, 934–941. [DOI] [PubMed] [Google Scholar]
- Welters ID, Feurer MK, Preiss V, et al. , 2011. Continuous S-(+)-ketamine administration during elective coronary artery bypass graft surgery attenuates pro-inflammatory cytokine response during and after cardiopulmonary bypass. Br. J. Anaesth 106, 172–179. [DOI] [PubMed] [Google Scholar]
- Yuan J, Wang A, He Y, et al. , 2016. Cordycepin attenuates traumatic brain injury-induced impairments of blood-brain barrier integrity in rats. Brain Res. Bull 127, 171–176. [DOI] [PubMed] [Google Scholar]
- Zhang J, Puvenna V, Janigro D, 2016. Biomarkers of Traumatic Brain Injury and Their Relationship to Pathology. [PubMed] [Google Scholar]
- Zhang J, Pu H, Zhang H, et al. , 2017. Inhibition of Na(+)-K(+)-2Cl(−) cotransporter attenuates blood-brain-barrier disruption in a mouse model of traumatic brain injury. Neurochem. Int 111, 23–31. [DOI] [PubMed] [Google Scholar]
- Zimmermann LL, Martin RM, Girgis F, 2017. Treatment options for posttraumatic epilepsy. Curr. Opin. Neurol 30, 580–586. [DOI] [PubMed] [Google Scholar]
- Zinger A, Latham SL, Combes V, et al. , 2016. Plasma levels of endothelial and B-cell-derived microparticles are restored by fingolimod treatment in multiple sclerosis patients. Mult. Scler 22, 1883–1887. [DOI] [PubMed] [Google Scholar]