Abstract
We report the clinical features of a patient with hereditary transthyretin (ATTR) amyloidosis associated with a novel mutation (Y114S, p.Y134S). A 65-year-old Japanese man was admitted to our hospital after a 3-year history of progressive dyspnea on exertion. Five years previously, he presented dysesthesia in both hands caused by carpal tunnel syndrome. A genetic analysis revealed a base pair substitution of adenine to cytosine in the second codon of exon 4, residue 114, in the TTR gene (c.401A>C). The clinical characteristics were progressive cardiomyopathy with a poor vital prognosis, late onset, sporadic case, bilateral carpal tunnel syndrome, hypothyroidism, and small fiber neuropathy.
Keywords: amyloid, transthyretin, mutation, cardiomyopathy, tafamidis
Introduction
Hereditary transthyretin (ATTR) amyloidosis, a life-threatening, autosomal-dominant systemic amyloidosis that is caused by mutant transthyretin (TTR), includes the following syndromes: familial amyloid polyneuropathy (FAP), familial amyloid cardiomyopathy, and familial oculoleptomeningeal amyloidosis/cerebral amyloid angiopathy (1-4).
In addition to patients with ATTR V30M mutations who show early-onset symptoms in endemic areas, patients with ATTR V30M mutations who show late-onset symptoms and patients with non-V30M ATTR mutations are now considered to be prevalent in non-endemic areas (5). More than 140 TTR variants have been described so far, with various geographic distributions and degrees of amyloidogenicity and organ involvement (6, 7). Recently, disease modifying therapies such as liver transplantation and gene silencing to reduce the production of mutated TTR from the liver, and TTR stabilizer to reduce amyloid formation have come into clinical use in the treatment of hereditary ATTR amyloidosis (8-11). We herein describe a case of hereditary ATTR amyloidosis with cardiomyopathy that was associated with a novel mutation: ATTR Y114S (p.Y134S).
Case Report
A 65-year-old Japanese man was admitted to our hospital after a 3-year history of progressive dyspnea on exertion. Five years previously, he had had dysesthesia in both hands caused by mild carpal tunnel syndrome and the dysesthesia had improved naturally. One year earlier, he had hypothyroidism, and a thyroid biopsy revealed amyloid deposition (Congo red staining). Immunohistochemical staining was performed to identify amyloid fibril protein with specific antibodies (anti-TTR antibody, anti-amyloid A antibody, anti-kappa light chain antibody, and anti-lambda light chain antibody). The deposited amyloid reacted with anti-TTR antibody without a positive reaction to other antibodies. He had no family history of heart disorder or neuromuscular disease and no relation to endemic areas of hereditary ATTR amyloidosis.
On admission of the patient to our hospital, a physical examination revealed numbness and reduced pain and temperature sensation in both feet. The patient had decreased bilateral Achilles' tendon reflexes. The patient demonstrated no muscle weakness or atrophy, and his touch, vibration sense, joint position sense, and autonomic function were normal. A fundus examination showed no vitreous opacity.
Laboratory studies revealed a reduced TTR level (7.0 mg/dL, normal range: 22-34 mg/dL), increased serum brain natriuretic peptide level (732.4 pg/mL, normal value: <18.4 pg/mL), elevated protein in cerebrospinal fluid (81.8 mg/dL, normal range: 8-43 mg/dL), and hypothyroidism. Nerve conduction studies revealed carpal tunnel syndrome and mild sensory axonopathy of the sural nerve (Table). Anti-PGP9.5 antibody staining of a skin biopsy specimen revealed reduced intra-epidermal nerve fiber density (Figure D).
Table.
Data of Nerve Conduction Studies of This Case Examined in the Left Side. Upper or Lower Limits of Reference Values were Described in the Parenthesis.
| DL (ms) | CMAP (mV) | MCV (m/s) | F latency (ms) | SNAP (μV) | SCV (m/s) | |
|---|---|---|---|---|---|---|
| Median nerve | 5.3 (4.6) | 8.1 (3.0) | 53.8 (49.5) | 29.5 (28.2) | 5.3 (7.0) | 55.6 (47.1) |
| Ulnar nerve | 3.1 (3.8) | 5.4 (5.8) | 55.6 (49.9) | 28.2 (29.7) | 9.9 (6.9) | 53.1 (46.8) |
| Tibial nerve | 5.0 (5.7) | 8.4 (4.3) | 41.5 (41.4) | 52.5 (51.7) | ||
| Peroneal nerve | 4.9 (6.8) | 3.5 (4.0) | 43.0 (42.7) | 53.8 (53.4) | ||
| Sural nerve | 5.5 (7.4) | 57.9 (40.7) |
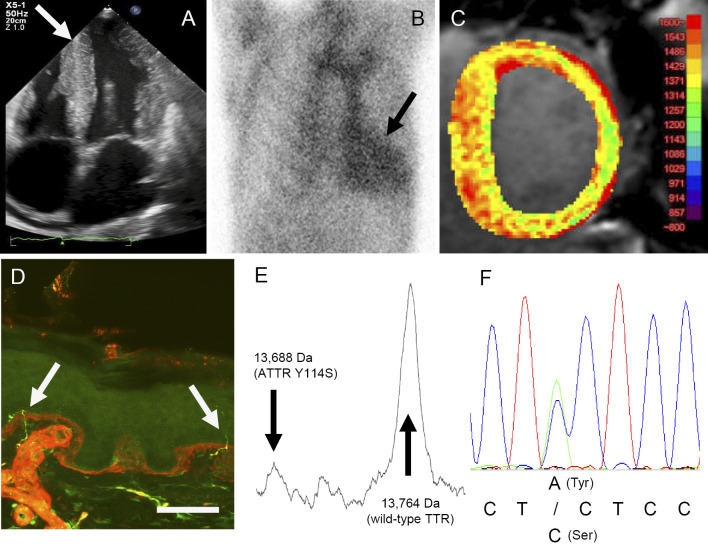
Figure.
A: Echocardiography. The arrow indicates ventricular wall thickening and a granular sparkling pattern. B: 99mTc-pyrophosphate myocardial scintigraphy. The arrow indicates a site of 99mTc-pyrophosphate accumulation, which suggests amyloid deposition in the myocardium. C: Cardiac magnetic resonance image with non-contrast T1 mapping. A markedly elevated myocardial native T1 value was obtained. D: Anti-PGP9.5 antibody staining of a skin specimen: the intra-epidermal nerve fiber (arrows) density was reduced. Scale bar=50μm. E: A MALDI-TOF MS analysis detected the variant peak of ATTR Y114S in addition to a wild-type TTR peak. F: A genetic analysis of exon 4 in the TTR gene. One base pair substitution (c.401A>C, p. TTR Y114S) was found.
An electrocardiogram indicated low voltage and first-degree atrioventricular block. Echocardiography showed concentric ventricular hypertrophy, atrial dilatation, and dystelectasis (aortic diameter: 35.1 mm, mean left atrial diameter: 50.8 mm, left ventricular end-diastolic diameter at end-diastole: 41.4 mm, interventricular septal thickness: 15.1 mm, posterior wall thickness: 15.2 mm, fractional shortening: 40%, E/e': 18.3), with a granular sparkling pattern (Figure A). 123I-MIBG myocardial scintigraphy revealed a decreased early heart to mediastinum (H/M) ratio (1.79, normal range: 2.2-4.0), a decreased late H/M ratio (1.34, normal range: 2.2-4.4), and an increased washout rate (62.7%, normal range: 0-34%), which suggested not only heart failure but also cardiac sympathetic fiber dysfunction (12). 99mTc-pyrophosphate myocardial scintigraphy showed increased 99mTc-pyrophosphate accumulation in the heart (Figure B). Cardiac magnetic resonance imaging with non-contrast T1 mapping revealed a markedly elevated myocardial native T1 value, which suggested cardiac involvement by amyloidosis (Figure C).
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) of serum TTR revealed a low peak of variant TTR with a reduced mass (by 76 Da), in addition to a markedly high wild-type TTR peak (Figure E). The coding sequence in the exons of the TTR gene was amplified by a polymerase chain reaction (PCR) using the following oligonucleotide primers: Exon 2 5`-CGTTCCTGATAATGGGATCA-3`, 5`-CAAAAAGAGGGCATCCTTCA-3`, Exon 3 5`-TCTGACTTAGTTGAGGGGAAATG-3`, 5`-AGGGAACCTTTGGTCATTCA-3`, Exon 4 5`-TGCCAGCATATTTGAGCTTTT-3`, 5`-ATGAAAAGTGCCTTTCACAGGA-3`. A genetic analysis revealed a base pair substitution of adenine (A) to cytosine (C) in the second codon of exon 4, residue 114, in the TTR gene (c.401A>C) (Figure F). This substitution resulted in heterozygosity for normal tyrosine and variant serine (ATTR Y114S, p.Y134S).
We performed a duodenal mucosa biopsy that showed ATTR amyloid deposition. Laser capture microdissection with liquid chromatography-tandem mass spectrometry of amyloid deposits revealed that TTR (25.3%) was the most abundant protein in the amyloid deposits.
Liver transplantation in patients with the non-V30M mutation or cardiomyopathy is usually less effective than that in patients with hereditary ATTR V30M amyloidosis (11). We therefore treated the patient with tafamidis to stabilize the TTR and reduce amyloid deposition (10, 13). The patient died of heart failure 5 years after the disease onset with carpal tunnel syndrome.
Discussion
Amyloid cardiomyopathy and carpal tunnel syndrome were the primary and initial clinical manifestation in this patient with the ATTR Y114S mutation, respectively. The survival time of this case was shorter in comparison to patients with ATTR V30M mutations in endemic areas (10.6 years), as well as that in non-endemic areas (7.3 years) (11, 14). In addition to wild-type ATTR amyloidosis (senile systemic amyloidosis), physicians should consider hereditary ATTR amyloidosis when elderly patients present with heart failure and carpal tunnel syndrome (15). A genetic analysis should be performed, especially for patients with polyneuropathy and hypothyroidism.
The TTR Y114C and Y114H mutation, which are mutations in the same position in TTR also produce a low concentration of a highly amyloidogenic variant TTR in the blood, as was observed in this case. The TTR Y114C mutation results in rapidly progressive cerebral amyloid angiopathy presenting with cerebral hemorrhage (16). Patients with the TTR Y114H mutation showed slowly progressive amyloidosis with carpal tunnel syndrome (17). In contrast, the TTR Y114S mutation as described here mainly showed rapidly progressive cardiomyopathy. The relationship between amino acid substitutions in TTR and the clinical phenotypes should be elucidated. Attention should be paid to the increase in total and variant TTR levels after the administration of tafamidis, which is caused by TTR stabilization, as it might contribute to the progression of ATTR amyloidosis. The serum TTR levels should be measured, the variant TTR ratio should be evaluated and clinical manifestations should be carefully followed up in patients with extremely low blood levels of TTR.
In conclusion, we reported the clinical features of a patient with hereditary ATTR Y114S amyloidosis. The clinical characteristics included progressive cardiomyopathy with a poor vital prognosis, a late onset of disease, sporadic occurrence, bilateral carpal tunnel syndrome, hypothyroidism, and mild small fiber neuropathy.
Standard protocol approval.
This study was approved by the Institutional Review Board of the Graduate School of Medical Sciences, Kumamoto University (No. 1387).
The authors state that they have no Conflict of Interest (COI).
References
- 1. Benson MD, Buxbaum JN, Eisenberg DS, et al. Amyloid nomenclature 2018: recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid 7: 1-5, 2019. [DOI] [PubMed] [Google Scholar]
- 2. Ando Y, Nakamura M, Araki S. Transthyretin-related familial amyloidotic polyneuropathy. Arch Neurol 62: 1057-1062, 2005. [DOI] [PubMed] [Google Scholar]
- 3. Sekijima Y, Ueda M, Koike H, Misawa S, Ishii T, Ando Y. Diagnosis and management of transthyretin familial amyloid polyneuropathy in Japan: red-flag symptom clusters and treatment algorithm. Orphanet J Rare Dis 13: 6, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Masuda T, Ueda M, Kitajima M, et al. Teaching neuroImages: morphology of lumbosacral dorsal root ganglia and plexus in hereditary transthyretin amyloidosis. Neurology 91: e1834-e1835, 2018. [DOI] [PubMed] [Google Scholar]
- 5. Koike H, Misu K, Ikeda S, Study Group for Hereditary Neuropathy in Japan, et al. Type I (transthyretin Met30) familial amyloid polyneuropathy in Japan: early- vs late-onset form. Arch Neurol 59: 1771-1776, 2002. [DOI] [PubMed] [Google Scholar]
- 6. Yamashita T, Ueda M, Misumi Y, et al. Genetic and clinical characteristics of hereditary transthyretin amyloidosis in endemic and non-endemic areas: experience from a single-referral center in Japan. J Neurol 265: 134-140, 2018. [DOI] [PubMed] [Google Scholar]
- 7. Yamashita T, Ueda M, Saga N, et al. Hereditary amyloidosis with cardiomyopathy caused by the novel variant transthyretin A36D. Amyloid 23: 207-208, 2016. [DOI] [PubMed] [Google Scholar]
- 8. Adams D, Gonzalez-Duarte A, O'Riordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med 379: 11-21, 2018. [DOI] [PubMed] [Google Scholar]
- 9. Benson MD, Waddington-Cruz M, Berk JL, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med 379: 22-31, 2018. [DOI] [PubMed] [Google Scholar]
- 10. Coelho T, Inês M, Conceição I, Soares M, de Carvalho M, Costa J. Natural history and survival in stage 1 Val30Met transthyretin familial amyloid polyneuropathy. Neurology 91: e1999-e2009, 2018. [DOI] [PubMed] [Google Scholar]
- 11. Yamashita T, Ando Y, Okamoto S, et al. Long-term survival after liver transplantation in patients with familial amyloid polyneuropathy. Neurology 78: 637-643, 2012. [DOI] [PubMed] [Google Scholar]
- 12. Ando Y, Obayashi K, Tanaka Y, et al. Radiolabelled meta-iodobenzylguanidine in assessment of autonomic dysfunction. Lancet 343: 984-985, 1994. [DOI] [PubMed] [Google Scholar]
- 13. Maurer MS, Schwartz JH, Gundapaneni B, ATTR-ACT Study Investigators, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 379: 1007-1016, 2018. [DOI] [PubMed] [Google Scholar]
- 14. Koike H, Tanaka F, Hashimoto R, et al. Natural history of transthyretin Val30Met familial amyloid polyneuropathy: analysis of late-onset cases from non-endemic areas. J Neurol Neurosurg Psychiatry 83: 152-158, 2012. [DOI] [PubMed] [Google Scholar]
- 15. Sekijima Y, Yazaki M, Ueda M, Koike H, Yamada M, Ando Y. First nationwide survey on systemic wild-type ATTR amyloidosis in Japan. Amyloid 25: 8-10, 2018. [DOI] [PubMed] [Google Scholar]
- 16. Yamashita T, Ando Y, Ueda M, et al. Effect of liver transplantation on transthyretin Tyr114Cys-related cerebral amyloid angiopathy. Neurology 70: 123-128, 2008. [DOI] [PubMed] [Google Scholar]
- 17. Sekijima Y, Campos RI, Hammarström P, et al. Pathological, biochemical, and biophysical characteristics of the transthyretin variant Y114H (p.Y134H) explain its very mild clinical phenotype. J Peripher Nerv Syst 20: 372-379, 2015. [DOI] [PubMed] [Google Scholar]