Abstract
Thyroglobulin (TG) gene mutations cause thyroid dyshormonogenesis, which is typically associated with a congenital goiter. We herein report the case of a 64-year-old man with congenital primary hypothyroidism who had a normal-sized thyroid gland on levothyroxine replacement. He had short stature (−3.1 standard deviations) and mild intellectual impairment. Thyroid autoantibodies were all negative, and the serum TG levels were undetectable. Eventually, he was found to have the novel homozygous nonsense mutation p.K1374* in the TG gene. The possibility of TG mutation should be considered for patients with congenital primary hypothyroidism and a very low serum TG level, regardless of the thyroid size.
Keywords: thyroglobulin, gene mutation, congenital hypothyroidism, goiter
Introduction
Mutations in the thyroglobulin (TG) gene cause defective thyroid hormone synthesis, which usually results in congenital primary hypothyroidism (1-3). Patients with TG gene mutations typically develop a large goiter (dyshormonogenetic goiter), and a very low level of serum TG is a useful diagnostic marker for the presence of a TG mutation in patients with congenital primary hypothyroidism.
We herein report an adult case of childhood-diagnosed primary hypothyroidism associated with a novel homozygous nonsense mutation (p.K1374*) in TG. In contrast to typical cases of dyshormonogenetic goiter, his thyroid gland was not enlarged on adequate levothyroxine (LT4) replacement.
Methods
Serum levels of free triiodothyronine (T3), free thyroxine (T4), and thyrotropin (TSH) were measured using commercial immunoassays (Roche Diagnostics, Tokyo, Japan). Serum anti-thyroid peroxidase (TPO) antibody, anti-TG antibody, and TG were measured using commercial electro-chemiluminescence immunoassays (ECLusysⓇ Anti-TPO, ECLusysⓇ Anti-Tg, and ECLusysⓇ Tg; Roche Diagnostics). Serum TSH receptor (TSHR) antibody was determined by a first-generation assay (TRAb Cosmic III; Cosmic Corporation, Tokyo, Japan). The thyroid volume was estimated by ultrasound, and we adopted the reference range of the thyroid volume (4.8-17.8 mL) reported by Suzuki et al. (4). The TSHR gene was analyzed for all coding sequences (exons 1-10) (5). A sequencing analysis of the TG gene in exons 1-48 was performed as previously reported (1). Genetic testing of the TSHR and TG genes was performed with the patient's consent.
Case Report
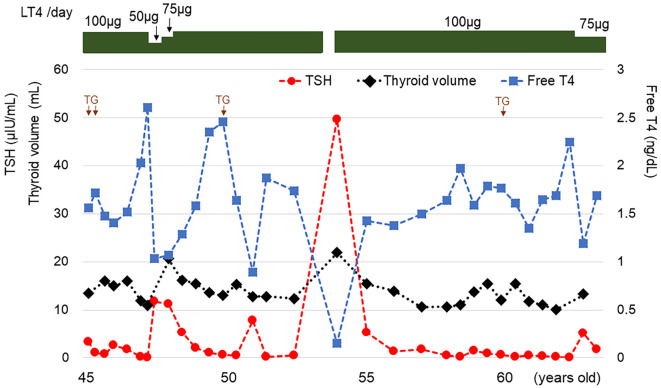
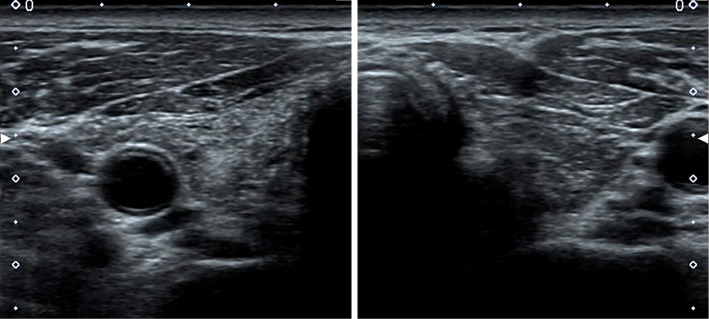
A 45-year-old man visited our clinic to continue thyroid hormone replacement therapy of LT4 at a dose of 100 μg/day (Fig. 1). He had begun taking LT4 at seven years of age when he had first been diagnosed with primary hypothyroidism. There was no consanguineous marriage or thyroid disorder in his family history. He had mild intellectual impairment without hearing problems. He was 153 cm tall (−3.1 standard deviations) and weighed 65 kg. His blood pressure was 138/96 mmHg, and his pulse was regular at 85 beats/min. The thyroid gland was not palpable. The heart, lungs, and abdomen were unremarkable on a physical examination. Under treatment with 100 μg/day LT4, the serum levels of free T3, free T4, and TSH were 3.68 pg/mL (reference range, 2.20-4.30 pg/mL), 1.56 ng/dL (0.90-1.80 ng/dL), and 3.31 μIU/mL (0.220-3.30 μIU/mL), respectively. Serum antibodies against TPO, TG, and TSHR were 10.3 IU/mL (reference range, <16 IU/mL), 14.7 IU/mL (<28 IU/mL), and <0.1% (<10.0%), respectively. The serum TG level was <0.10 ng/mL (reference range, <25.0 ng/mL). Ultrasonography revealed a normal-sized thyroid with diffusely heterogeneous echogenicity (estimated thyroid volume, 13 mL) (Fig. 2).
Figure 1.
Course of the thyroid function and thyroid volume. TG: measurement of serum thyroglobulin level (each measured value, <0.10 ng/mL).
Figure 2.
Ultrasonogram of the thyroid (transverse section). The thyroid size is normal. The echogenicity of the thyroid parenchyma is increased diffusely, showing heterogeneous echogenicity. Granular hyperechoic lesions are scattered throughout the thyroid gland.
The patient continued LT4 replacement therapy. Nine months after the reduction of the LT4 dosage to 50-75 μg/day at 48 years of age, the serum levels of free T4 and TSH were 1.07 ng/dL and 11.24 μIU/mL, respectively. At that time, the thyroid volume slightly increased to 21 mL. Two months after the discontinuation of LT4 against medical advice at 54 years of age, the patient's serum levels of free T3 and free T4 were markedly reduced to 0.66 pg/mL and 0.15 ng/dL, respectively, whereas the serum TSH level had increased to 49.77 μIU/mL. The thyroid was slightly enlarged (estimated volume, 22 mL). The patient is currently still being treated with 75 μg/day LT4; at 64 years of age, the estimated thyroid volume was 13 mL, and the serum levels of free T3, free T4, and TSH were 3.10 pg/mL, 1.69 ng/dL, and 1.700 μIU/mL, respectively. He does not have any nodular lesions in the thyroid gland according to ultrasonographic follow-up.
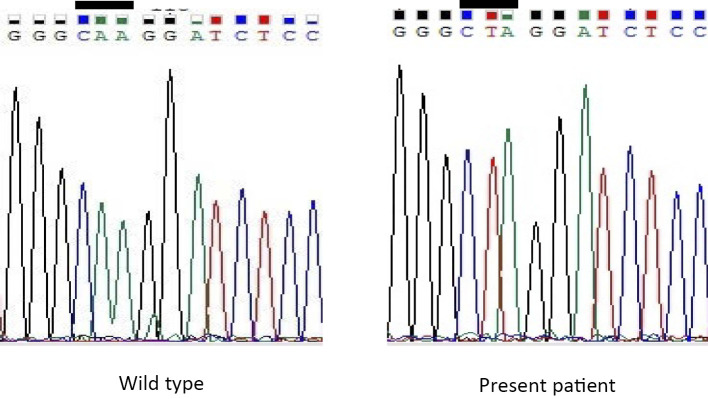
The patient had childhood-diagnosed primary hypothyroidism, and serum thyroid autoantibodies were all negative. His thyroid gland was not enlarged on adequate LT4 replacement. Since a germline TSHR mutation prevents the growth of the thyroid due to resistance to TSH, we first suspected the presence of a TSHR mutation and therefore performed genetic testing of the TSHR gene. However, no TSHR mutation was identified. Despite performing 4 different time measurements, the serum TG levels were all undetectable (simultaneous serum TSH levels 3.31, 0.99, 0.06, and 0.583 μIU/mL) without anti-TG antibodies. Therefore, genetic testing of the TG gene was performed next. A homozygous A to T transition (c.4177A>T) was identified, which resulted in a nonsense mutation at codon 1374 (p.K1374*) (Fig. 3). This finding led to a diagnosis of primary hypothyroidism associated with a homozygous TG mutation at p.K1374*.
Figure 3.
Sequencing chromatograms of the thyroglobulin (TG) gene. A homozygous adenine to thymine transition at position 4177 was identified in the present patient (bar).
Discussion
The present patient had already been taking LT4 at the time of his first visit to our clinic, but he discontinued the treatment at 54 years of age, which led to the onset of apparent primary hypothyroidism within 2 months of the treatment discontinuation. He had been diagnosed with primary hypothyroidism at the age of 7, although the actual onset timing of his hypothyroidism is unknown. Primary newborn screening for congenital hypothyroidism has been adopted by most countries around the world, although mass screening for congenital hypothyroidism was not initiated in Japan until 1979, so the present patient did not undergo mass screening for congenital hypothyroidism at birth. However, he was suspected of having congenital primary hypothyroidism because of the lack of evidence of thyroidal autoimmunity, his short stature, and his intellectual impairment.
Regarding the etiology of hypothyroidism in the present patient, undetectable serum TG levels functioned as a clue for detecting the novel homozygous TG mutation. In general, a very low or undetectable serum TG level in patients without anti-TG antibodies suggests thyroid dysgenesis, resistance to TSH, or a TG mutation (6). The serum TG levels were all undetectable without anti-TG antibodies in the present patient. Unfortunately, the TSH-stimulated serum TG level during LT4 withdrawal could not be determined.
Thyroid dyshormonogenesis due to a TG mutation, which is one of the causes of congenital primary hypothyroidism, has an estimated prevalence of approximately 1:67,000 in Japan (2) and 1:100,000 in China (6). Although most known TG pathogenic variants typically cause congenital primary hypothyroidism, the actual clinical presentation varies from euthyroid to mild or severe hypothyroidism (1-3, 7). In the presence of a sufficient iodine supply, some patients with TG mutations can compensate for the impaired hormonogenesis and generate sufficient thyroid hormones (8). To date, a total of 117 deleterious mutations have been identified in the human TG gene (9). TG mutations are inherited in an autosomal recessive manner, and affected individuals are either homozygous or compound heterozygous. The nonsense mutation p.K1374* identified in the TG gene of the present patient is novel. The TG protein is composed of four structural and functional regions: N-terminal regions (regions I, II, and III) and a C-terminal region (ACHE-like domain) (10). Lysine 1374 is located in the hinge region (exon 20) between regions I and II.
Most TG mutations are associated with characteristic goiter (dyshormonogenetic goiter), which is very large with an elastic and soft consistency (2, 3). The goitrous phenotype can be attributed to long-term TSH stimulation and the accumulation of misfolded proteins in the endoplasmic reticulum of the thyrocytes (1, 11). The thyroid gland of the present patient was not enlarged under adequate LT4 replacement therapy during the follow-up period. There was slight temporary enlargement of the thyroid gland after the dosage reduction and discontinuation of LT4, indicating some effect of LT4 treatment on the thyroid gland. However, TG mutation-associated goiters are often remarkably large and display gradual, continuous growth (11). In the present patient, not only long-term LT4 replacement therapy but also other factors may have prevented the progressive enlargement of the thyroid.
Several TG mutations associated with congenital primary hypothyroidism and normal-sized or hypoplastic thyroid have been reported (6, 12-14) (Table). Despite involving the same TG mutations, the phenotype of the goiter differs among cases (14). The cause of such phenotypic differences in the goiter remains unclear. The majority of cases with normal-sized or hypoplastic thyroid were found to have congenital primary hypothyroidism in the neonatal period. Although patients with a TG synthesis defect generally present with a congenital goiter or goiter appearing shortly after birth, the absence of an obvious goiter may be partly ascribed to the early detection and treatment of congenital hypothyroidism by neonatal screening in those reported cases (15).
Table.
Previously Reported Cases of Homozygous or Compound Heterozygous TG Mutations without an Enlarged Thyroid.
| Case No. | Gene No. | Ethnic | Sex | TG mutation | Diagnosis of hypothyroidism | Last evaluation on LT4 | [Reference] | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (year) | Thyroid size | Age (year) | Thyroid size | |||||||
| 1 | 1 | Taiwanese | F | c.1348delT | neonatal | NA | 3 | no goiter | [12] | |
| 2 | 2 | Taiwanese | F | p.Q1765*/ g.IVS3+2T>G |
neonatal | NA | 6 | no goiter | ||
| 3 | 3 | Taiwanese | F | p.R432*/c.1348delT | neonatal | no goiter | NA | NA | ||
| 4 | 4 | Taiwanese | M | g.IVS3+2T>G/ c.6047delA |
neonatal | no goiter | NA | NA | ||
| 5 | 5 | French | F | c.3788-3789insT/ g.IVS19+3_+4delAT |
neonatal | enlarged right lobe normal left lobe |
3 | NA | [13] | |
| 6 | 6 | Turkish | F | p.Q630* | neonatal | upper limit of normal | NA | NA | [14] | |
| [6] | Turkish | M | p.Q630* | neonatal | enlarged thyroid | NA | NA | |||
| 7 | 7 | Turkish | F | p.W637* | 14 y | hypoplasia | NA | NA | ||
| 8 | Turkish | F | p.W637* | 15 y | hypoplasia | NA | NA | |||
| 9 | 8 | Chinese | F | c.274+2T>G | neonatal | normal | 7.3 | normal | [6] | |
| 10 | Chinese | M | c.274+2T>G | neonatal | normal | 5 | normal | |||
| 11 | Chinese | M | c.274+2T>G | neonatal | NA | 3.7 | normal | |||
| 12 | Chinese | M | c.274+2T>G | neonatal | enlarged thyroid | 1.5 | normal | |||
| 13 | 9 | Chinese | F | c.6391delTTGT | neonatal | normal | 2.5 | normal | ||
| 14 | 10 | Chinese | F | c.6782+1delG | neonatal | enlarged thyroid | 5 | normal | ||
| 15 | Chinese | F | c.6782+1delG | neonatal | normal | 2.5 | normal | |||
| 16 | 11 | Chinese | M | p.P1012L | neonatal | normal | 5.5 | normal | ||
| 17 | 12 | Chinese | M | p.T1111R/p.R2585W | neonatal | NA | 2.7 | normal | ||
| 18 | 13 | Chinese | M | p.C1051*/p.P1012L | neonatal | normal | 2.0 | normal | ||
| 19 | 14 | Japanese | M | p.K1374* | 7 y | NA | 64 | normal | present case | |
Cases 6 and [6], and Cases 7 and 8 are siblings.
NA: not available
Mutations in TPO, SLC26A4/PDS, and DUOX2 are major causes of dyshormonogenetic goiter. Recently, normal-sized or hypoplastic thyroid gland in congenital primary hypothyroidism associated with mutations of TPO, SLC26A4/PDS, or DUOX2 has been reported (16-18). Since those TPO or SLC26A4/PDS gene mutations are found not only in goitrous patients but also in non-goitrous patients (16, 17), a second additional genetic or epigenetic hit in the thyroid tissue is assumed to contribute to the small (or at least not obviously enlarged) thyroid (16). The present patient might have had some genetic or epigenetic factors that inhibited the development of a typical goitrous phenotype.
Tg mutations have also been described in animals, such as Afrikander cattle, Dutch goats (mixed Saanen and dwarf goats), cog/cog mice, WIC-rdw rats, and Wistar Hannover GALAS rats (9). All animal models of congenital primary hypothyroidism due to a Tg mutation, except for WIC-rdw rats, develop goitrogenesis. WIC-rdw rats, by contrast, present with a hypoplastic thyroid gland despite elevated circulating TSH and reduced serum thyroid hormone levels, suggesting that the mutant proteins in the WIC-rdw rat may be cytotoxic for thyroid growth and proliferation (19, 20). The influence of the novel p.K1374* mutation on thyrocytes remains to be elucidated.
Many adult patients with TG mutation present with nodular thyroid hyperplasia (3). A high incidence of thyroid cancer is also a characteristic of long-standing goiters with TG mutations, and TG mutations are associated with the development of thyroid cancer (21). At present, our patient has no nodular lesions in the thyroid gland according to ultrasonographic follow-up.
Molecular genetic TG studies are usually performed for goitrous patients with very low serum TG levels. However, in patients with congenital primary hypothyroidism and a very low serum TG level, TG mutations should be considered, regardless of the thyroid size and lack of a typical goitrous phenotype.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Hishinuma A, Takamatsu J, Ohyama Y, et al. Two novel cysteine substitutions (C1263R and C1995S) of thyroglobulin cause a defect in intracellular transport of thyroglobulin in patients with congenital goiter and the variant type of adenomatous goiter. J Clin Endocrinol Metab 84: 1438-1444, 1999. [DOI] [PubMed] [Google Scholar]
- 2. Hishinuma A, Fukata S, Nishiyama S, et al. Haplotype analysis reveals founder effects of thyroglobulin gene mutations C1058R and C1977S in Japan. J Clin Endocrinol Metab 91: 3100-3104, 2006. [DOI] [PubMed] [Google Scholar]
- 3. Targovnik HM, Citterio CE, Rivolta CM. Thyroglobulin gene mutations in congenital hypothyroidism. Horm Res Paediatr 75: 311-321, 2011. [DOI] [PubMed] [Google Scholar]
- 4. Suzuki S, Midorikawa S, Matsuzuka T, et al. Prevalence and characterization of thyroid hemiagenesis in Japan: The Fukushima health management survey. Thyroid 27: 1011-1016, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shibayama K, Ohyama Y, Hishinuma A, et al. Subclinical hypothyroidism caused by a mutation of the thyrotropin receptor gene. Pediatr Int 47: 105-108, 2005. [DOI] [PubMed] [Google Scholar]
- 6. Hu X, Chen R, Fu C, et al. Thyroglobulin gene mutations in Chinese patients with congenital hypothyroidism. Mol Cell Endocrinol 423: 60-66, 2016. [DOI] [PubMed] [Google Scholar]
- 7. Hishinuma A, Kasai K, Masawa N, et al. Missense mutation (C1263R) in the thyroglobulin gene causes congenital goiter with mild hypothyroidism by impaired intracellular transport. Endocr J 45: 315-327, 1998. [DOI] [PubMed] [Google Scholar]
- 8. Pardo V, Vono-Toniolo J, Rubio IG, et al. The p.A2215D thyroglobulin gene mutation leads to deficient synthesis and secretion of the mutated protein and congenital hypothyroidism with wide phenotype variation. J Clin Endocrinol Metab 94: 2938-2944, 2009. [DOI] [PubMed] [Google Scholar]
- 9. Targovnik HM, Citterio CE, Siffo S, Rivolta CM. Advances and perspectives in genetics of congenital thyroid disorders associated with thyroglobulin gene mutations. Peertechz J Biol Res Dev 1: 62-70, 2016. [Google Scholar]
- 10. Targovnik HM. Thyroglobulin structure, function, and biosynthesis. In: Werner and Ingbar's The Thyroid. A Fundamental and Clinical Text. 10th ed Braverman LE, Cooper DS, Eds. Lippincott Williams and Wilkins, Philadelphia, 2013: 74-92. [Google Scholar]
- 11. Vono-Toniolo J, Rivolta CM, Targovnik HM, Medeiros-Neto G, Kopp P. Naturally occurring mutations in the thyroglobulin gene. Thyroid 15: 1021-1033, 2005. [DOI] [PubMed] [Google Scholar]
- 12. Niu DM, Hsu JH, Chong KW, et al. Six new mutations of the thyroglobulin gene discovered in Taiwanese children presenting with thyroid dyshormonogenesis. J Clin Endocrinol Metab 94: 5045-5052, 2009. [DOI] [PubMed] [Google Scholar]
- 13. Targovnik HM, Edouard T, Varela V, et al. Two novel mutations in the thyroglobulin gene as cause of congenital hypothyroidism: identification a cryptic donor splice site in the exon 19. Mol Cell Endocrinol 348: 313-321, 2012. [DOI] [PubMed] [Google Scholar]
- 14. Cangul H, Boelaert K, Dogan M, et al. Novel truncating thyroglobulin gene mutations associated with congenital hypothyroidism. Endocrine 45: 206-212, 2014. [DOI] [PubMed] [Google Scholar]
- 15. Targovnik HM, Esperante SA, Rivolta CM. Genetics and phenomics of hypothyroidism and goiter due to thyroglobulin mutations. Mol Cell Endocrinol 322: 44-55, 2010. [DOI] [PubMed] [Google Scholar]
- 16. Stoupa A, Chaabane R, Guériouz M, et al. Thyroid hypoplasia in congenital hypothyroidism associated with thyroid peroxidase mutations. Thyroid 28: 941-944, 2018. [DOI] [PubMed] [Google Scholar]
- 17. Kühnen P, Turan S, Fröhler S, et al. Identification of PENDRIN (SLC26A4) mutations in patients with congenital hypothyroidism and “apparent” thyroid dysgenesis. J Clin Endocrinol Metab 99: E169-E176, 2014. [DOI] [PubMed] [Google Scholar]
- 18. Srichomkwun P, Takamatsu J, Nickerson DA, Bamshad MJ, Chong JX, Refetoff S. DUOX2 gene mutation manifesting as resistance to thyrotropin phenotype. Thyroid 27: 129-131, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hishinuma A, Furudate S, Oh-Ishi M, Nagakubo N, Namatame T, Ieiri T. A novel missense mutation (G2320R) of the thyroglobulin causes hypothyroidism in rdw rats. Endocrinology 141: 4050-4055, 2000. [DOI] [PubMed] [Google Scholar]
- 20. Kim PS, Ding M, Menon S, et al. A missense mutation G2320R in the thyroglobulin gene causes non-goitrous congenital primary hypothyroidism in the WIC-rdw rat. Mol Endocrinol 14: 1944-1953, 2000. [DOI] [PubMed] [Google Scholar]
- 21. Hishinuma A, Fukata S, Kakudo K, Murata Y, Ieiri T. High incidence of thyroid cancer in long-standing goiters with thyroglobulin mutations. Thyroid 15: 1079-1084, 2005. [DOI] [PubMed] [Google Scholar]