Abstract
Along with the increase in consumption of raw animal meat, the prevalence of food poisoning is increasing. A 67-year-old Japanese man had eaten raw venison 4 hours prior to the beginning of vomiting. Many white cysts were discovered in the venison, with numerous bradyzoites being detected after the cysts were punctured. The presence of the Sarcocystis spp. 18S rRNA gene was detected by polymerase chain reaction, and Sarcocystis truncata was isolated from the venison. Sarcocystis truncata has not previously been identified in sika deer (Cervus nippon) in Japan. This is the first report of possible Sarcocystis truncata-induced food poisoning following consumption of venison.
Keywords: Sarcocystis truncata, food poisoning, venison
Introduction
The habit of consuming raw animal meat is common in some countries. As this practice increases, the prevalence of food poisoning or paragonimiasis is also increasing. With recent increases in the population of sika deer (Cervus nippon) (1) and the increasing popularity of wild game cuisine, the incidence of food poisoning or paragonimiasis following ingestion of raw venison has been increasing in Japan. A variety of zoonotic pathogens may be found in wild game meat, including venison (2). Sarcocystis is one such pathogen, living as a parasite in the muscles and digestive tracts of domestic animals (3).
Aoki et al. isolated S. sybillensis and S. wapiti from venison that had caused food poisoning and revealed by immunohistochemical staining that both had a protein that reacted with an antibody against a 15-kDa protein from S. fayeri (4). It is widely known that Sarcocystis spp. are a causative pathogen of food poisoning from horse meat. However, Sarcocystis infection of venison has rarely been reported.
This is the first report describing a case of food poisoning possibly caused by S. truncata in venison.
Case Report
A 67-year-old Japanese man, suffering from abdominal pain, vomiting, watery diarrhea, and fever, visited a hospital by ambulance. He had experienced no episodes of cough, sputum, or trauma in the immediate past, and his symptoms had occurred suddenly an hour before he presented to the hospital. The patient had no history of operation. Despite suffering from hepatitis C virus infection, he had never been hospitalized for hepatic diseases. He lived in a rural area surrounded by forests and had a habit of consuming 700 mL of beer a day.
The patient had eaten raw venison 4 hours before the start of vomiting. The deer had been hunted 8 hours prior to consumption and was stored at room temperature. Upon admission, the patient was febrile with a temperature of 37.3℃, blood pressure of 108/83 mmHg, and a pulse rate of 70/min. Vomiting and watery diarrhea continued in the emergency room. The results of a physical examination were normal, except for a remarkable increase in intestinal peristaltic sounds. The laboratory examination results are shown in Table. The serum levels of creatinine, urea acid, and total protein were found to be elevated, whereas the serum sodium level was decreased.
Table.
Laboratory Data on Admission.
| Hematological analysis | Biochemistry | ||||
| WBC | 8,700 | /μL | AST | 54 | U/L |
| Neutro | 78.4 | % | ALT | 31 | IU/L |
| Lym | 17.2 | % | γ-GTP | 41 | IU/L |
| Eosino | 0.3 | % | TP | 8.2 | g/dL |
| Mono | 3.9 | % | Alb | 4.4 | g/dL |
| Baso | 0.2 | % | LDH | 276 | IU/L |
| RBC | 465 | ×104/μL | ALP | 305 | IU/L |
| Hb | 14.0 | g/dL | CK | 161 | IU/L |
| Ht | 42.3 | % | CHE | 185 | IU/L |
| PLT | 18.3 | ×104/μL | TB | 0.6 | mg/dL |
| CRP | 0.05 | mg/dL | |||
| Immunology | PG | 114 | mg/dL | ||
| HBs-Antigen | negative | Cr | 1.23 | mg/dL | |
| HBs-Antibody | neagtive | BUN | 19.7 | mg/dL | |
| HCV-Antibody | positive | UA | 10.7 | mg/dL | |
| Na | 142 | mEq/L | |||
| K | 3.3 | mEq/L | |||
| Cl | 104 | mEq/L | |||
Neutro: neutrocyte, Lym: lymphocyte, Eosino: eosinocyte, Mono: monocyte, RBC: red blood cell, Ht: hematocrit, PLT: platelet, HB: hepatitis B virus, HCV: hepatitis C virus, AST: aspartate transaminase, ALT: alanine transaminase, γ-GTP: γ-glutamyl transpeptidase, TP: total protein, Alb: albumin, LDH: lactate dehydrogenase, ALP: alkaline phosphatase, CK: creatine kinase, CHE: cholinesterase, TB: total bilirubin, CRP: C-reactive protein, PG: plasma glucose, Cr: creatinine, BUN: blood urea nitrogen, UA: uric acid, Na: Natrium, K: Kalium, Cl: chlorine
The stool culture was negative, with no enteric pathogens, ova, or parasites detected. Shigella and other enteritis pathogens were also absent. The differential diagnosis of Salmonella, Escherichia coli, hepatitis E virus, Shigella, Enterobacteriaceae, Leptospira, Listeria, and Campylobacter was considered. However, with the exception of Escherichia coli, infection symptoms from these pathogens would be expected to present ≥6 hours after infection. Besides this patient, two other people who had eaten the same venison displayed identical symptoms, and food poisoning was diagnosed in both cases. Considering the possibility of Sarcocystis infection, we asked the public health center to examine the venison for pathogens.
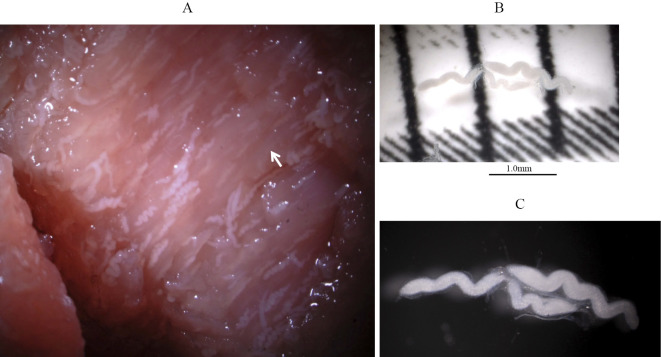
An examination by stereoscopic microscopy revealed the presence of white cysts in the venison muscle tissue (Fig. 1A). The cysts were 1 to 2 mm long and approximately 0.1 mm wide (Fig. 1B and C). Many bradyzoites were detected after puncturing the cysts (Fig. 2). The presence of the Sarcocystis spp. 18S rRNA gene was detected by polymerase chain reaction, and Sarcocystis truncata was isolated from the venison. We extracted proteins from the cysts isolated from the venison and determined their amino acid sequences, identifying a toxic 15-kDa protein (Fig. 3). No cysts were detected in the venison liver tissue.
Figure 1.
Sarcocystis truncata in venison muscle tissue (A), cysts of Sarcocystis truncata isolated from the venison causing food poisoning (B, C).
Figure 2.
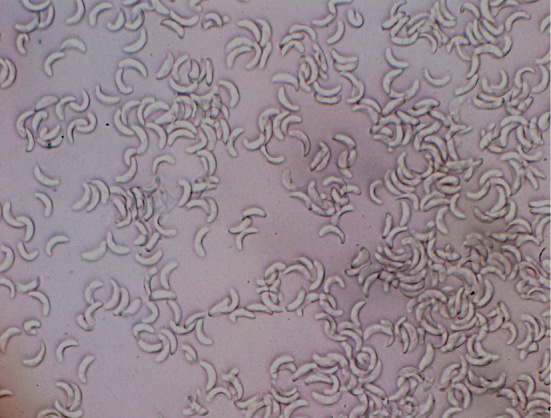
Bradyzoites of Sarcocystis truncata isolated from the venison causing food poisoning.
Figure 3.
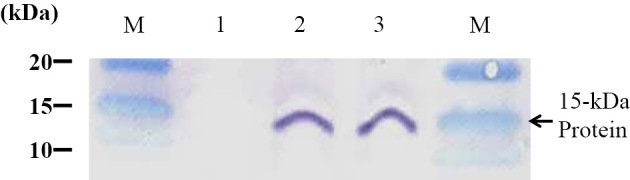
Immunoblotting pattern of the 15-kDa protein in Sarcosystis spp. M: Molecular weight marker, 1: control (uninfected horse meat), 2: horse meat infected with Sarcocystis, 3: venison infected with Sarcocystis truncata (our case).
No antibiotics were administered. Sufficient infusion was performed. On day 3, the patient improved clinically and became afebrile. He was therefore discharged.
Discussion
Sarcocystis is a zoonotic pathogen that lives as a parasite in the muscles and digestive tracts of domestic animals (3). Carnivorous animals act as final hosts for these parasites and excrete Sarcocystis sporocysts in their feces. In herbivorous animals, which are its intermediate hosts, Sarcocystis species are present within cysts in the musculature, containing proliferating bradyzoites.
Many species of Sarcocystis have already been identified in other animals, including S. cruzi, S. hirsuta, and S. bovihominis in cattle; S. miescheriana, S. porcifelis, and S. suihominis in pigs; and S. tenella and S. mihoensis in sheep (3, 5-10). Among these, S. bovihominis and S. suihominis are known to be transmitted to humans, which are their final host (11), and cause a type of intestinal sarcocystosis after infection (12).
Infection with Sarcocystis in humans can result in a type of intestinal sarcocystosis (12). After eating raw beef containing the S. hominis cysts, patients in Spain experienced nausea, vomiting, abdominal discomfort, abdominal pain, and frequent diarrhea. Sporocysts of S. hominis were detected in a specimen of the patients' diarrheal feces, confirming the diagnosis (13).
In the present case, the patient developed symptoms within 4 hours of ingestion, with recovery after 24 hours. Besides this patient, two people who had eaten the same venison showed similar symptoms. Given the duration, rapidity of onset, and clinical symptoms, we suspected food poisoning. However, multiple stool cultures on enriched and selective media gave negative results.
Recently, S. fayeri in horses (13) and Sarcocystis spp. in deer (4) have been reported as new food poisoning agents that induce gastrointestinal symptoms, such as nausea, vomiting, and diarrhea, within 24 hours of infection, similar to this case. We therefore suspected the food poisoning to have been caused by Sarcocystis spp. Using polymerase chain reaction, the presence of the Sarcocystis spp. 18S rRNA gene was detected. Furthermore, Sarcocystis truncata was isolated from the venison.
To date, S. sybillensis, S. wapiti, and S. hofmanni have been identified in Japanese sika deer (C. nippon) (4, 14). S. truncata has been identified in white-tailed sea eagle (15) and red deer (C. elaphus) in Norway (16) but never in sika deer (C. nippon) in Japan. In 2018, S. truncata was identified in sika deer farmed in Lithuania (17). It is therefore unsurprising that S. truncata would be found in these animals in Japan.
Since 1969, the toxicity of Sarcocystis has been studied experimentally. Rabbits injected with Sarcocystis cysts developed rapid respiration, depression, and paralysis and eventually died (18). The protein extracts of S. gigantea are toxic to rats and mice (19, 20). A toxic protein fraction has also been isolated from S. cruzi cysts in beef (21). This protein had a molecular mass of approximately 15-kDa; however, precise studies on this toxic protein have not been performed.
A related 15-kDa protein produced by Sarcocystis spp. present in horsemeat evoked enteropathogenic changes in an animal model (22). Kamata et al. found that, following intravenous administration, a similar protein from S. fayeri induced diarrhea and lethal toxicity in rabbits and that it also exhibited enterotoxicity in an ileal loop test (13). This toxin showed homology with the actin-depolymerizing factor from Toxoplasma gondii and Eimeria tenella. Irikura et al. showed that a recombinant version of the same 15-kDa protein induced enterotoxicity in a rabbit ileal loop test (23). These findings showed that the 15-kDa protein from S. fayeri originating from horse meat was a diarrheal toxin functioning as a food poisoning agent in humans. We suspected that one or more toxins originating from S. truncata are able to induce pathological changes similar to those caused by S. cruzi and confirmed this by isolating a toxic 15-kDa protein from a strain isolated from the patient in the present case. It is reasonable to suspect that this protein was the cause of the gastrointestinal symptoms in this patient.
The cysts of Sarcocystis species lose viability following rapid freezing at -20℃, heating at 70℃ for 1 minutes, and in 2.0% salt for 1 day (14). Therefore, adequate freezing and heating are needed for the cooking and storage of venison in order to reduce the risk of food poisoning. The 15-kDa protein might be a useful indicator for determining the possibility of food poisoning induced by Sarcocystis spp. Further studies will be necessary to reveal whether or not the 15-kDa protein is responsible for the enterotoxicity induced by Sarcocystis detected in venison.
Some people prefer to serve their meat rare or raw, allowing consumers to inadvertently ingest live Sarcocystis cysts. Sarcocystis cysts in meat are therefore a risk factor for public health. To prevent food poisoning caused by venison, it is essential to understand how to inactivate the responsible toxin during storage. In S. fayeri, this can be accomplished by freezing (22). In the present case, the cause of food poisoning was failure to treat the venison at the proper temperature.
This is the first report to describe a case of food poisoning presumably caused by S. truncata from venison. While examining patients suffering from venison-related food poisoning, the possibility of infection by Sarcocystis spp. should be kept in mind.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors would like to acknowledge the significant contribution of Akiko Yamazaki (Cooperative Department of Veterinary Medicine, Faculty of Agriculture, Iwate University, Japan) in providing the 15-kDa protein antibody.
References
- 1. Ministry of the Environment :[The population estimation of nihon deer and wild boar by statistical method] [Internet]. [cited 2018 Feb 22]. Available from : http://www.env.go.jp/press/files/jp/26914.pdf
- 2. Ruiz-Fons F. A review of the current status of relevant zoonotic pathogens in wild swine (Sus scrofa) populations: changes modulating the risk of transmission to humans. Transbound Emerg Dis 64: 68-88, 2017. [DOI] [PubMed] [Google Scholar]
- 3. Markus MB. Sarcocystis and sarcocystosis in domestic animals and man. Adv Vet Sci Comp Med 22: 159-193, 1978. [PubMed] [Google Scholar]
- 4. Aoki K, Ishikawa K, Hayashi K, et al. An outbreak of suspected food poisoning related to deer meat containing Sarcocystis cysts. Jpn J Food Microbiol 30: 28-32, 2013(in Japanese, Abstract in English). [Google Scholar]
- 5. Ashford RW. The fox, Vulpes vulpes, as a final host for Sarcocystis of sheep. Ann Trop Med Parasitol 71: 29-34, 1977. [DOI] [PubMed] [Google Scholar]
- 6. Dubey JP, Streitel RH, Stromberg PC, Toussant MJ. Sarcocystis fayeri sp. n. from the horse. J Parasitol 63: 443-447, 1977. [PubMed] [Google Scholar]
- 7. Fayer R, Esposito DH, Dubey JP. Human infections with Sarcocystis species. Clin Microbiol Rev 28: 295-311, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saito M, Shibata Y, Kobayashi T, Kobayashi M, Kubo M, Itagaki H. Ultrastructure of the cyst wall of Sarcocystis species with canine final host in Japan. J Vet Med Sci 58: 861-867, 1996. [DOI] [PubMed] [Google Scholar]
- 9. Saito M, Shibata Y, Kubo M, Itagaki H. Sarcocystis mihoensis n. sp. from sheep in Japan. J Vet Med Sci 59: 103-106, 1997. [DOI] [PubMed] [Google Scholar]
- 10. Saito M, Shibata Y, Ohno A, Kubo M, Shimura K, Itagaki H. Sarcocystis suihominis detected for the first time from pigs in Japan. J Vet Med Sci 60: 307-309, 1998. [DOI] [PubMed] [Google Scholar]
- 11. Guo M, Dubey JP, Hill D, et al. Prevalence and risk factors for Toxoplasma gondii infection in meat animals and meat products destined for human consumption. J Food Prot 78: 457-476, 2015. [DOI] [PubMed] [Google Scholar]
- 12. Fayer R. Sarcocystis spp. in human infections. Clin Microbiol Rev 17: 894-902, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kamata Y, Saito M, Irikura D, et al. A toxin isolated from Sarcocystis fayeri in raw horsemeat may be responsible for food poisoning. J Food Prot 77: 814-819, 2014. [DOI] [PubMed] [Google Scholar]
- 14. Honda M, Sawaya M, Taira K, et al. Effects of temperature, pH and curing on the viability of Sarcocystis, a Japanese sika deer (Cervus Nippon centralis) parasite, and the inactivation of their diarrheal toxin. J Vet Med Sci 80: 1337-1344, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gjerde B, Vikøren T, Hamnes IS. Molecular identification of Sarcocystis halieti n. sp., Sarcocystis lari and Sarcocystis truncata in the intestine of a white-tailed sea eagle (Haliaeetus albicilla) in Norway. Int J Parasitol Parasites Wildl 7: 1-11, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gjerde B. Sarcocystis species in red deer revisited: with a re-description of two known species as Sarcocystis elongata n. sp. and Sarcocystis truncata n. sp. based on mitochondrial cox1 sequences. Parasitology 141: 441-452, 2014. [DOI] [PubMed] [Google Scholar]
- 17. Rudaitytė-Lukošienė E, Prakas P, Butkauskas D, Kutkienė L, Vepštaitė-Monstavičė I, Servienė E. Morphological and molecular identification of Sarcocystis spp. from the sika deer (Cervus nippon), including two new species Sarcocystis frondea and Sarcocystis nipponi. Parasitol Res 117: 1305-1315, 2018. [DOI] [PubMed] [Google Scholar]
- 18. Mandour AM. Studies on the toxicity of Sarcocystis. J Med Microbiol 2: 361-363, 1969. [DOI] [PubMed] [Google Scholar]
- 19. Al-Hyali NS, Aljawady MA, Mohammad-Fakhri MA. The influence of some physio-chemical properties of sarcotoxin in rats. J Anim Vet Adv 9: 302-305, 2010. [Google Scholar]
- 20. Al-Hyali NS, Khalil LY, Aljawady MA. Sarcotoxin effect on leukocytic finding and phagocytic activity in mice. J Anim Vet Adv 8: 2395-2398, 2009. [Google Scholar]
- 21. Saito M, Taguchi K, Shibata Y, Kobayashi T, Shimura K, Itagaki H. Toxicity and properties of the extract from Sarcocystis cruzi cysts. J Vet Med Sci 57: 1049-1051, 1995. [DOI] [PubMed] [Google Scholar]
- 22. Harada S, Furukawa M, Tokuoka E, et al. Control of toxicity of Sarcocystis fayeri in horsemeat by freezing treatment and prevention of food poisoning. Shokuhin Eiseigaku Zasshi (J Food Hyg Soc Japan) 54: 198-203, 2013(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 23. Irikura D, Saito M, Sugita-Konishi Y, et al. Characterization of Sarcocystis fayeri's actin-depolymerizing factor as a toxin that causes diarrhea. Genes Cells 22: 825-835, 2017. [DOI] [PubMed] [Google Scholar]