Abstract
A 44-year-old Japanese woman was admitted to our hospital with fatigue and an altered liver function. She had been receiving atorvastatin treatment for 10 months. Although no jaundice was seen, the patient's serum alkaline phosphatase and γ-glutamyl transpeptidase levels were markedly elevated. Based on the results of a drug-induced lymphocyte-stimulation test, her liver disease was diagnosed as atorvastatin-induced hepatic injury. Subsequently, anti-mitochondrial antibodies (AMAs) were detected in her serum; however, a liver biopsy specimen did not show the characteristic features of primary biliary cholangitis. We herein report the detection of AMAs accompanied by drug-induced hepatic injury caused by atorvastatin.
Keywords: statin-induced hepatic injury, drug-induced lymphocyte-stimulation test, anti-mitochondrial antibody
Introduction
Primary biliary cholangitis (PBC) is an autoimmune liver disease; however, the details of the pathogenetic mechanism of the disease are still obscure (1, 2). The presence of anti-mitochondrial antibodies (AMAs) is a serum hallmark of PBC (3, 4). Although the mechanisms leading to the generation of AMAs are unclear, it has been postulated that xenobiotic-induced and/or oxidative modification of mitochondrial autoantigens is a critical step leading to the loss of tolerance (5, 6). Xenobiotics include drugs, carcinogens, environmental pollutants, food additives, hydrocarbons, and pesticides. However, in daily clinical practice, it is rare to encounter cases that suggest that these xenobiotics directly cause AMA production.
Atorvastatin (ⓇLipitor) is a member of the medication class known as statins, which are primarily used as lipid-lowering agents. Like all statins, atorvastatin works by inhibiting hydroxymethylglutaryl-coenzyme A reductase (HMGCR), an enzyme found in liver tissue that plays a key role in the production of cholesterol in the body (7). There have been reports regarding the induction of various autoimmune phenomena by statins (8, 9). However, there have been few reports about the induction of AMA production by statins.
We herein report a case in which AMAs were detected in the serum of a patient with drug-induced hepatic injury caused by atorvastatin.
Case Report
A 44-year-old Japanese woman was admitted to our hospital with fatigue and altered liver function. She had been receiving atorvastatin treatment for 10 months. Her height was 156.0 cm, and her weight was 59.0 kg, so her body mass index was 24.2. She had no significant medical history and no history of blood transfusions or alcohol intake. She had allergies to cedar pollen and house dust.
She had undergone annual medical checkups for the past 10 years, and no abnormal data, including that regarding her liver function or lipid metabolism, had been detected. However, in December 2017 her low-density lipoprotein (LDL)-cholesterol level had been found to be elevated (224 mg/dL, reference value: 70-139 mg/dL); therefore, atorvastatin (5 mg/day) therapy was started. After the treatment had been administered for 10 months, the patient's serum liver function test results appeared abnormal. Her laboratory data on admission are shown in Table 1. The patient's serum levels of alkaline phosphatase (Al-P) and γ-glutamyl transpeptidase (γ-GTP) were markedly elevated. Her total bilirubin level was normal, and her transaminase levels were slightly increased. Tests for viral markers associated with hepatitis A virus, hepatitis B virus, hepatitis C virus, hepatitis E virus, Epstein-Barr virus, cytomegalovirus, and herpes simplex virus all produced negative results. The patient's immunological data are shown in Table 2. A test for anti-nuclear antibodies (ANAs) produced a positive (low titer) result, but no anti-smooth muscle antibody (ASMA) or anti-liver-kidney microsomal antibodies were detected.
Table 1.
Laboratory Data on Admission (1).
| WBC | 7,500 | /μL | T-Bil | 0.7 | mg/dL | HDL-C | 60 | mg/dL |
| RBC | 405×104 | /μL | D-Bil | 0.5 | mg/dL | LDL-C | 125 | mg/dL |
| Hb | 12.9 | g/dL | AST | 66 | U/L | Trig | 160 | mg/dL |
| Hct | 38.9 | % | ALT | 84 | U/L | |||
| Pl | 34.5×104 | /μL | Al-P | 1,557 | U/L | HBsAg | (-) | |
| γ-GTP | 402 | U/L | HBcAb | (-) | ||||
| Neut | 54.9 | % | LDH | 181 | U/L | IgM HBcAb | (-) | |
| Baso | 2.1 | % | CK | 55 | U/L | HCVAb | (-) | |
| Eosino | 9.3 | % | T-protein | 7.8 | g/dL | IgA HEV Ab | (-) | |
| Mono | 3.0 | % | Albumin | 5.0 | g/dL | IgM EBV Ab | (-) | |
| Lymph | 30.7 | % | BUN | 16.6 | mg/dL | IgM CMV Ab | (-) | |
| Crea | 0.75 | mg/dL | ||||||
| PT | 105 | % | BS | 102 | mg/dL | CEA | 5.0 | ng/mL/L |
| HbA1c | 5.5 | % | CA19-9 | 0.5 | mU/L |
γ-GTP: γ-glutamyltransferase, Al-P: alkaline phosphatase, ALT: alanine aminotransferase, AST: aspartate aminotransferase, Baso: basophils, BS: blood sugar, BUN: blood urea nitrogen, CA19-9: carbohydrate antigen 19-9, CEA: carcinoembryonic antigen, CK: creatine kinase, CMV Ab: cytomegalovirus antibody, Crea: creatinine, D-Bil: direct bilirubin, Eosino: eosinophils, Hb: hemoglobin, EBV Ab: Epstein-Barr virus antibody, Hct: hematocrit, HbA1c: glycated hemoglobin, HDL-C: high density lipoprotein cholesterol, HBcAb: hepatitis B core antibody, HBsAg: hepatitis B surface antigen, HCV Ab: hepatitis C virus antibody, HEV Ab: hepatitis E virus antibody, IgA: immunoglobulin A, IgG: immunoglobulin G, IgM: immunoglobulin M, Lymph: lymphocytes, LDH: lactate dehydrogenase, LDL-C: low density lipoprotein cholesterol, Mono: monocytes, Neut: neutrophils, Pl: platelet count, PT: prothrombin time, RBC: red blood cell count, T-Bil: total bilirubin, Trig: triglycerides, T-protein: total protein, WBC: white blood cell count
Table 2.
Laboratory Data on Admission (2).
| IgG | 1,888 | mg/dL |
| IgA | 120 | mg/dL |
| IgM | 150 | mg/dL |
| IgE | 437 | IU/mL |
| ANA | ×40 | (Homo+Sp) |
| AMA | ||
| IF | ×40 | (+) |
| ELISA index | 42.2 | (+) |
| ASMA | (-) | |
| anti-LKM1 | (-) | |
| anti-liver cytosol | (-) |
ANA: anti-nuclear antibody, AMA: anti-mitochondrial antibody, IF: immunofluorescence, ELISA: enzyme-linked immunosorbent assay, ASMA: anti-smooth muscle antibody, anti-LKM1: anti-liver/kidney microsome 1 antibody
We examined AMAs by two methods: an indirect-immunofluorescence assay (AMA-IF) using rodent tissues as antigen and an enzyme-linked immunosorbent assay (AMA-ELISA) using three major recombinant mitochondrial proteins (PDC-E2, BCOADC-E2, and OGDC-E2) as antigens (10). Both produced positive results. The levels of all immunoglobulin subclasses, except for IgE, were within normal limits. No abnormal findings, such as bile duct obstruction, liver tumors, fatty liver changes, or pancreatic tumors, were seen on abdominal echography or abdominal computed tomography.
To confirm the presence of atorvastatin-induced hepatic injury, a drug-induced lymphocyte-stimulation test (DLST) was performed. In the DLST, lymphocytes are collected from the patient's heparinized peripheral blood and then incubated with dilutions of the suspected drug, and lymphocyte proliferation is evaluated based on the 3H-thymidine uptake (11). The stimulation index (S.I.) was found to be 2.6 (no added culture: 107 cpm, maximum response value: 278 cpm, reference value of S.I.: >1.6). A positive DLST finding was obtained for atorvastatin.
According to the criteria for drug-induced liver injury proposed by the Digestive Disease Week Japan in 2004 (DDWJ-2004) (12), the score was 11, suggesting the causality of atorvastatin was “highly probable”. Given these findings, we diagnosed her acute hepatic injury as atorvastatin-induced hepatic injury.
A liver biopsy was performed, and the specimen showed no findings of liver cell infiltration or fibrosis, except for fatty changes (Fig. 1). In addition, no chronic non-suppurative destructive cholangitis, granuloma, bile-duct epithelium changes, or bile duct loss (13), which are characteristics of PBC, were observed.
Figure 1.

Histopathological findings of the liver. No findings that were indicative of liver cell infiltration or fibrosis, except for fatty changes, were observed, and no chronic non-suppurative destructive cholangitis, granuloma, bile-duct epithelium changes, or bile duct loss was observed (Hematoxylin and Eosin staining, magnification: ×400).
The administration of 600 mg/day of ursodeoxycholic acid (UDCA) was initiated. One to three months later, the patient's serum levels of Al-P and γ-GTP had returned to within normal limits, and no elevation was observed even after UDCA treatment.
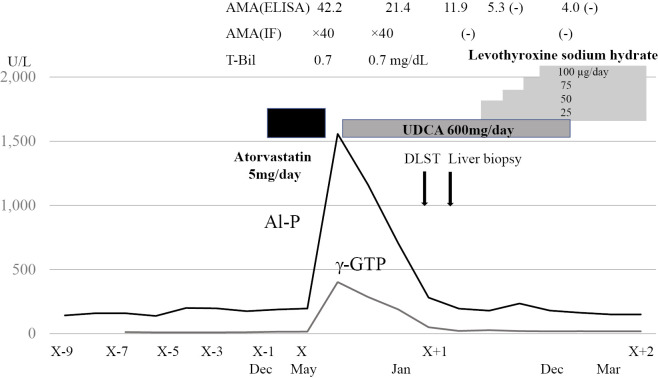
The serum AMAs disappeared 10 months after the onset of the condition. During UDCA therapy, it was newly found that the patient was complicated with Hashimoto's disease. Serum examinations revealed the following findings: thyroid-stimulating hormone (TSH): >100 μIU/mL (reference value: 0.5-5.0), free triiodothyronine (T3): <0.4 pg/mL (2.3-4.3), free thyroxine (T4): 0.08 ng/mL (0.9-1.7), anti-thyroid peroxidase antibody: 270.3 IU/mL (<16), anti-thyroglobulin antibody: 979.0 IU/mL (<28), and thyroglobulin: <0.04 ng/mL (<33.7). Levothyroxine sodium hydrate was administered, and the patient's serum TSH, free T3, and free T4 levels shifted to within the reference values. Subsequently, her serum LDL-cholesterol level also shifted to within the reference value. The patient's clinical course is shown in Fig. 2.
Figure 2.
The patient’s clinical course. AMA-ELISA: AMAs detected by ELISA using three recombinant mitochondrial proteins as antigens. AMA-IF: AMAs detected by IF using rodent tissues as antigens. AMA: anti-mitochondrial antibody, IF: immunofluorescence, ELISA: enzyme-linked immunosorbent assay, DLST: drug-induced lymphocyte-stimulation test, UDCA: ursodeoxycholic acid, T-Bil: total bilirubin, Al-P: alkaline phosphatase, γ-GTP: γ-glutamyl transpeptidase
Discussion
About 25 million people around the world are on statin therapy to lower their lipid levels and prevent adverse cardiovascular events. According to a Japanese surveillance of atorvastatin treatment, only 0.05% of the 22,921 patients that were taking atorvastatin had elevated serum levels of aspartate aminotransferase and alanine aminotransferase, and no patients had severe hepatic injuries (14). Therefore, statins are generally considered to be well-tolerated drugs because they are associated with low frequencies of severe drug-induced hepatic injury (7). In the present case, based on the results of a DLST, which is the most conclusive test for drug-induced immunoallergic reaction, in addition to the criteria for drug-induced liver injury proposed by the DDWJ-2004, we diagnosed the patient's hepatic disease as atorvastatin-induced hepatic injury. This case was categorized as acute cholestatic hepatitis, and the same condition was seen in a previous case after the reintroduction of atorvastatin (15).
In the current case, the patient's LDL-cholesterol levels remained within normal limits after the administration of levothyroxine sodium hydrate. Therefore, retrospectively, we consider that the hyper LDL-cholesterolemia seen before the onset of her liver disorder had been a symptom of her Hashimoto's disease.
Recently, the onset of autoimmune disorders related to statin therapy has gained attention (8, 9). Such autoimmune disorders include lupus erythematosus, myopathy, and autoimmune hepatitis (AIH) (16-22). The detailed mechanisms underlying these conditions are still unknown, but they are considered to be indirect effects of statins. However, in immune-mediated necrotizing myopathy, accumulating evidence suggests that statins directly trigger the condition. Namely, it has been discovered that statins upregulate the expression of HMGCR, the molecular target of statins, and overexpression of this enzyme may facilitate the presentation of highly immunogenic HMGCR peptides by human leukocyte antigen DRB1*11:01, thereby triggering autoimmune diseases (23, 24). Interestingly, anti-HMGCR antibody, a disease-specific autoantibody, was detected in these patients' sera (25, 26).
Regarding autoimmune hepatic injuries related to statin therapy, there have been some reports about statin-induced AIH (18, 19). In these cases, only ANA and ASMA were detected as inducing autoantibodies, and no statin-induced specific autoantibodies were detected. We experienced a case in which AMA appeared after 10 months of atorvastatin treatment. This period might be slightly longer than the typical period required for drug-induced hepatic injuries to occur. However, in some cases of statin-induced AIH, the onset of AIH occurred 22-48 months after the initial administration of statin therapy (27). Therefore, statin-induced hepatic autoimmunity might even occur one year after the initial administration of statins, suggesting that invasive investigations are necessary for patients on long-term lipid-lowering therapy.
To our knowledge, there are only a few reports that argue the possibility that AMAs are produced by the administration of statin and induce autoimmune liver disease (28, 29). Minha et al. reported a case of cholestatic jaundice induced by atorvastatin (28). Since AMAs were detected after acute liver injury, they pointed out the possibility of AMA induction by statin. That patient was suffering from colon and prostate carcinomas and taking doxazosin, aspirin, allopurinol, and dutasteride in addition to atorvastatin. However, since a DLST had not been performed for all of the drugs being taken, direct evidence of atorvastatin-induced hepatic injury was not obtained. Nakayama et al. reported a case of AIH and PBC overlap syndrome induced by fulvastatin. A DLST was performed and proved the presence of fulvastatin-induced hepatic injury. ANAs and AMA-ELISA were detected after fulvastatin-induced hepatic injury (29). This is thought to be the first case showing evidence of statin-induced AMAs. However, AMA-IF is not described in this report (29). Comparison with our present case, the titers of AMA-ELISA (21, index value; positive >7) were found to be slightly lower than in our patient. We previously reported that AMAs were detected in 16% of well-defined patients with AIH (30); therefore, we will carefully observe the evaluations of autoantibodies and liver biopsy findings in the present case showing new immunological reactions.
As mentioned above, the presence of AMAs is a serum hallmark of PBC (3, 4). However, it has been reported that AMAs are also found at a low frequency in sera from non-PBC patients (31). In collaborative research, we previously analyzed 69 patients (including 26 with acetyl-para-aminophenol-induced hepatic injury) with acute liver failure for three major mitochondrial autoantigens (PDC-E2, BCOADC-E2, and OGDC-E2) (32). AMAs were detected in 28 of 69 (30.6%) patients on days 1-4 after onset. The unexpectedly high frequency of AMA in patients with acute liver failure supports the notion that oxidative stress-induced liver damage might lead to AMA production.
In the current case, a liver biopsy was performed during the serum AMA-positive period. It is well recognized that, in the histological diagnosis of PBC, it sometimes not possible to obtain specific findings due to sampling errors during biopsies. In our case, a needle biopsy specimen was obtained from two locations in the liver tissue. However, we were unable to obtain pathological findings that supported a diagnosis of early-stage PBC. Furthermore, the disappearance of AMAs from the patient's serum further supports the notion that the pathogenesis of PBC requires additional factors, including genetic susceptibility.
With the increasing global demand for these agents, statin-induced autoimmunity is likely to remain a clinically relevant problem. Further molecular analyses aimed at determining the mechanisms responsible for such autoimmune disorders are needed. In addition, long-term observation is necessary in cases of acute liver injury in which AMAs are detected, as this might help clarify the pathogenesis of PBC.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Selmi C, Ichiki Y, Invernizzi P, Podda M, Gershwin ME. The enigma of primary biliary cirrhosis. Clin Rev Allergy Immunol 28: 73-81, 2005. [DOI] [PubMed] [Google Scholar]
- 2. Lleo A, Marzorati S, Anaya JM, Gershwin ME. Primary biliary cholangitis: a comprehensive overview. Hepatol Int 11: 485-499, 2017. [DOI] [PubMed] [Google Scholar]
- 3. Selmi C, Bowlus CL, Gershwin ME, Coppel RL. Primary biliary cirrhosis. Lancet 377: 1600-1609, 2011. [DOI] [PubMed] [Google Scholar]
- 4. Bowlus CL, Gershwin ME. The diagnosis of primary biliary cirrhosis. Autoimmun Rev 13: 441-444, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leung PS, Wang J, Naiyanetr P, et al. . Environment and primary biliary cirrhosis: electrophilic drugs and the induction of AMA. J Autoimmun 41: 79-86, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang J, Yang G, Dubrovsky AM, Chio J, Leung PSC. Xenobiotics and loss of tolerance in primary biliary cholangitis. World J Gastroenterol 22: 338-348, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bakker-Arkema RG, Nawrocki JW, Black DM. Safety profile of atorvastatin-treated patients with low LDL-cholesterol levels. Atherosclerosis 149: 123-129, 2000. [DOI] [PubMed] [Google Scholar]
- 8. Krattri S, Zandman-Goddard G. Statins and autoimmunity. Immunol Res 56: 348-357, 2013. [DOI] [PubMed] [Google Scholar]
- 9. Alvarado Cárdenas M, Marín Sánchez A, Lima Ruiz J. Statins and autoimmunity. Med Clin (Barc) 145: 399-403, 2015. [DOI] [PubMed] [Google Scholar]
- 10. Miyakawa H, Tanaka A, Kikuchi K, et al. . Detection of antimitochondrial autoantibodies in immunofluorescent AMA-negative patients with primary biliary cirrhosis using recombinant autoantigens. Hepatology 34: 243-248, 2001. [DOI] [PubMed] [Google Scholar]
- 11. Takikawa H, Takamori Y, Kumagi T, et al. . Assessment of 287 Japanese cases of drug induced liver injury by the diagnostic scale of the international consensus meeting. Hepatol Res 37: 192-195, 2003. [DOI] [PubMed] [Google Scholar]
- 12. Takikawa H, Onji M. A proposal of the diagnostic score of drug-induced liver injury. Hepatol Res 32: 250-251, 2005. [DOI] [PubMed] [Google Scholar]
- 13. Tsuneyama K, Baba H, Morimoto Y, Tsunematsu T, Ogawa H. Primary biliary cholangitis: Its pathological characteristics and immunopathological mechanisms. J Med Invest 64: 7-13, 2017. [DOI] [PubMed] [Google Scholar]
- 14. Teramoto T, Sakuma K, Nakao K, Yamamoto O, ALWAYS study group . Atorvastatin lipid lowering assessment survey in patients with hypercholesterolemia (ALWAYS), Final report. Ther Res 34: 445-481, 2013(in Japanese). [Google Scholar]
- 15. de Castro ML, Hermo JA, Baz A, de Luaces C, Perez R, Clofent J. Acute cholestatic hepatitis after atorvastatin reintroduction. Gastroenterol Hepatol 29: 21-24, 2006. [DOI] [PubMed] [Google Scholar]
- 16. Noël B. Lupus erythematosus and other autoimmune diseases related to statin therapy: a systematic review. J Eur Acad Dermatol Venereol 21: 17-24, 2007. [DOI] [PubMed] [Google Scholar]
- 17. Graziadei IW, Obermoser GE, Sepp NT, Erhart KH, Vogel W. Drug-induced lupus-like syndrome associated with severe autoimmune hepatitis. Lupus 12: 409-412, 2003. [DOI] [PubMed] [Google Scholar]
- 18. Moulis G, Béné J, Sommet A, Sailler L, Lapeyre-Mestre M, Montastruc JL, French Association of PharmacoVigilance Centres . Statin-induced lupus: a case/non-case study in a nationwide pharmacovigilance database. Lupus 21: 885-889, 2012. [DOI] [PubMed] [Google Scholar]
- 19. Pelli N, Setti M, Ceppa P, Toncini C, Indiveri F. Autoimmune hepatitis revealed by atorvastatin. Eur J Gastroenterol Hepatol 15: 921-924, 2003. [DOI] [PubMed] [Google Scholar]
- 20. Alla V, Abraham J, Siddiqui J, et al. . Autoimmune hepatitis triggered by statins. J Clin Gastroenterol 40: 757-761, 2006. [DOI] [PubMed] [Google Scholar]
- 21. Mammen AL. Statin-Associated Autoimmune Myopathy. N Engl J Med 374: 664-669, 2016. [DOI] [PubMed] [Google Scholar]
- 22. Nazir S, Lohani S, Tachamo N, Poudel D, Donato A. Statin-associated autoimmune myopathy: A systematic review of 100 cases. J Clin Rheumatol 23: 149-154, 2017. [DOI] [PubMed] [Google Scholar]
- 23. Mammen AL, Gaudet D, Brisson D, et al. . Increased frequency of DRB1*11:01 in anti-hydroxymethylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Care Res (Hoboken) 64: 1233-1237, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patel J, Superko HR, Martin SS, et al. . Genetic and immunologic susceptibility to statin-related myopathy. Atherosclerosis 240: 260-271, 2015. [DOI] [PubMed] [Google Scholar]
- 25. Christopher-Stine L, Casciola-Rosen LA, Hong G, et al. . A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum 62: 2757-2766, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mammen AL, Chang T, Christopher-Stine L, et al. . Autoantibodies against 3-hydroxy-3-methylgultaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 63: 713-721, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Punthakee Z, Scully LJ, Guindi MM, et al. . Liver fibrosis attributed to lipid lowering medications two cases. J Int Med 250: 249-254, 2001. [DOI] [PubMed] [Google Scholar]
- 28. Minha Sa'ar, Golzman G, Adar I, Rapoport M. Cholestatic jaundice induced by Atorvastatin: a possible association with antimitochondrial antibodies. Isr Med Assoc J 11: 440-441, 2009. [PubMed] [Google Scholar]
- 29. Nakayama S, Murashima N. Overlap syndrome of autoimmune hepatitis and primary biliary cirrhosis triggered by fluvastatin. Indian J Gastroenterol 30: 97-99, 2011. [DOI] [PubMed] [Google Scholar]
- 30. Yanagawa T, Miyakawa H, Shibata M, et al. . Immunoreacivity to pyruvate dehydrogenase complex-E2 in well-defined patients with autoimmune hepatitis: western blot analysis. Hepatol Res 26: 81-86, 2013. [DOI] [PubMed] [Google Scholar]
- 31. Klatskin G, Kantor FS. Mitochondrial antibody in primary biliary cirrhosis and other diseases. Ann Intern Med 77: 533-541, 1972. [DOI] [PubMed] [Google Scholar]
- 32. Leung PSC, Rossaro L, Davis PA, et al. . Antimitochondrial antibodies in acute liver failure: Implications for primary biliary cirrhosis. Hepatology 46: 1436-1442, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]