Abstract
IN BRIEF The use of long-acting basal insulin analogs is a recommended strategy in older people with diabetes because of their lower risk of hypoglycemia compared to intermediate-acting insulins. In this article, we review the results from recent clinical trials of second-generation basal insulin preparations. We conclude that, although these preparations have improved the management of insulin-requiring older people with type 2 diabetes, there is a need for additional and more specific studies to address the complexities of hyperglycemia management in this population.
Glucose management in older people with type 2 diabetes presents many challenges because individuals’ age, health, and psychological condition, and the availability of social support, can all influence their ability to safely manage their blood glucose levels (Table 1). Diabetes in older patients can be metabolically distinct from diabetes in younger people (1,2). Important differences include altered glucose metabolism resulting from changes in insulin production, secretion, and disposal; fasting glucose production; and glucose counterregulatory responses (1,3). Furthermore, age-related decline in renal function and changes in hepatic drug metabolism in older adults limit the choice of antihyperglycemic medications and may increase the risk of hypoglycemia (4). The heterogeneous health and functional status of older adults with diabetes further complicates diabetes management. This population includes many people with recently diagnosed type 2 diabetes who are relatively fit and well and have few comorbidities and little functional impairment, yet many have long-standing type 2 diabetes, multiple comorbidities, cognitive impairment, and functional disability.
TABLE 1.
Summary of Potential Challenges in Managing Type 2 Diabetes in Older Adults
| Age-Related Factors | Health and Psychological Factors | Socioeconomic Factors |
|---|---|---|
|
|
|
Glucose control in older patients needs to account for the progression of type 2 diabetes over time. Because of age-related decline in β-cell function, maintaining appropriate glycemic targets often necessitates escalation of drug doses and addition of other antihyperglycemic agents (5). Therapies that stimulate endogenous insulin secretion from β-cells, such as sulfonylureas or glucagon-like peptide 1 receptor agonists, will therefore become less effective over time. Medications such as metformin, thiazolidinediones, and sodium–glucose cotransporter 2 inhibitors may help to address some of the vicious cycles contributing to hyperglycemia but do not directly address the effects of aging on β-cells (6). As a result, a significant proportion of older patients will require insulin therapy to achieve glycemic targets. Recently, the second-generation basal insulin formulations insulin degludec (IDeg) (available in 100- and 200- units/mL formulations) and insulin glargine 300 units/mL (Gla-300) have been approved by the European Medicines Agency and U.S. Food and Drug Administration. These formulations have flatter, more stable pharmacodynamic profiles and longer durations of action than the first-generation basal insulin analogs insulin glargine 100 units/mL (Gla-100) and insulin detemir 100 units/mL (IDet) (7,8), resulting in similar overall glycemic control, but with a lower risk of hypoglycemia, which may be particularly advantageous to older patients.
Despite the high prevalence of diabetes in older adults, current pharmacologic management of hyperglycemia in this demographic is primarily based on data extrapolated from subgroup and post-hoc analyses of large clinical trials of the general adult population, which often exclude adults of very old age (e.g., ≥75 years) and those with complex health and psycho-socioeconomic issues (9). In this article, we review specific issues related to the management of hyperglycemia in older patients with type 2 diabetes and discuss recent data from studies in older patients using first- and second-generation basal insulin analogs.
Specific Considerations in Older Patients
Comorbidities are common in older adults with type 2 diabetes; most have at least one chronic comorbid condition, and ∼40% have at least three such conditions (10). Particular attention should be given to conditions that may lead to functional impairment and difficulty with self-care, such as arthritis, emphysema, and chronic pain; mental health issues such as depression (9,10); and end-organ dysfunction such as advanced heart failure and renal insufficiency (10). Important comorbidities such as hypertension should be managed alongside type 2 diabetes (11). In fact, some older adults may have greater reductions in morbidity and mortality by controlling other comorbidities, rather than by focusing on glycemic control alone (11).
Functional and cognitive impairment are important factors that can interfere with patients’ ability to manage their diabetes. This can increase the risk of poor adherence, medication errors, hypoglycemia, and hyperglycemia (12). Older adults with functional impairment may have difficulty performing diabetes self-care tasks (9). For such patients, diabetes treatment regimens should be simplified (9). In the case of insulin therapy, simplification may include the use of once-daily injections of basal insulin analogs rather than multiple daily injections. Moreover, the use of insulin pens rather than vials and syringes should be encouraged because these have been shown to improve persistence and adherence in older patients, with a significantly reduced risk of hypoglycemia (13). Diabetes management difficulties associated with functional impairment may be ameliorated by strong social support, improved access to care, and trained health care professionals in assisted-living or long-term nursing home settings. The availability of these types of support should be taken into consideration when deciding on a diabetes management plan (12).
In addition, older adults with type 2 diabetes who have cognitive impairment or dementia are at high risk of morbidity (e.g., hypoglycemia and its complications) and mortality; the glycemic target and insulin regimen for these patients should be carefully considered to avoid hypoglycemia. The relationship between cognitive impairment and hypoglycemia appears to be bidirectional (14). In a large meta-analysis, the risk of dementia in patients who experienced hypoglycemia significantly increased (pooled odds ratio [OR] 1.68, 95% CI 1.45–1.95), as did the risk of hypoglycemia in patients with dementia (pooled OR 1.61, 95% CI 1.24–2.06) (14). Thus, patients with type 2 diabetes and cognitive impairment could become subject to a vicious cycle of ever-worsening cognitive decline and more frequent hypoglycemia (14). Such patients may therefore be better served by a strategy that focuses not solely on strict glycemic control, but also on reducing the risk of hypoglycemia.
Hypoglycemia is associated with confusion, falls, and cardiac arrhythmias and with long-term complications such as cognitive impairment and dementia (15). In older adults, preventing hypoglycemia as much as possible should be the primary consideration when deciding on antihyperglycemic therapy. Strategies that set higher glycemic targets when necessary, particularly for frail patients and those with multiple comorbidities, have been published (2,9,11,16); these strategies include careful step-wise titration of insulin and the avoidance of hypoglycemia risk related to polypharmacy.
Recognizing hypoglycemia in older people with type 2 diabetes can be challenging because many of the symptoms of hypoglycemia are nonspecific and may also be associated with other age-related problems. These include dizziness and visual disturbances, agitation and confusion, fatigue and weakness, and simply feeling unwell (15). Education of patients and caregivers regarding hypoglycemia, its symptoms, and its management is crucial, particularly for patients initiating basal insulin analogs.
Hypoglycemia unawareness, defined as the onset of neurologic symptoms before the appearance of common autonomic symptoms or the inability to sense a significant decrease in blood glucose levels, is common in older patients with diabetes (17). People with this problem are at an increased risk of severe recurrent hypoglycemia and associated sequelae (17). Older adults who live alone or have a poor support system are at particular risk. In these individuals, the recommendation to transiently elevate the glycemic target range to avoid hypoglycemia (e.g., moving from a target fasting plasma glucose [FPG] range of 70–120 mg/dL to 100–150 mg/dL) has resulted in some improvement in early recognition of falling plasma glucose and fewer neuroglycopenic symptoms (18–20). A history of hypoglycemia, and in particular recurrent episodes, is a key factor that should raise the suspicion of hypoglycemia unawareness (17).
Continuous glucose monitoring (CGM) may be useful in combating hypoglycemia risk in older adults, although clinical experience with CGM in these patients is limited. A recent international consensus recommends the use of CGM in insulin treated patients with type 2 diabetes who are not achieving glucose targets, particularly for those experiencing problematic hypoglycemia (21). In older adults, CGM has been shown to detect higher numbers of both hyperglycemia and hypoglycemia events, particularly nocturnal hypoglycemia, compared to self-monitored plasma glucose (SMPG) (22). Such information could guide treatment decisions to reduce hypoglycemia risk and potentially identify patients with hypoglycemia unawareness. CGM systems that allow remote viewing of data by caregivers and physicians may be particularly valuable for vulnerable adults.
Real-time CGM systems can also give warnings if blood glucose is trending toward hypoglycemia or hyperglycemia (21). However, a careful rescue process must be put in place to respond during such real-time warnings because older patients might not be able to efficiently act alone under these circumstances.
There are also issues such as the need to calibrate some CGM systems before use, problems associated with applying and wearing sensors, and the cost and availability of systems (21), all of which may limit the use of CGM in older adults. The availability of new-generation CGM devices that do not require calibration might help to overcome some of these limitations, especially if they are covered by patients’ existing insurance.
Selection of a Treatment Regimen: The Role of Basal Insulin Analogs for Older Adults in Different Clinical Settings
Because older adults with type 2 diabetes have considerable variability in their living arrangements, self-care abilities, and social support, the care setting and how it might affect diabetes management are important considerations when deciding on an insulin regimen (23). For example, such patients may live at home independently, receive sporadic care from people who are not medically trained, or live at a 24-hour skilled nursing care facility. For older adults living in the community, once-daily first- or second-generation basal insulin analog injection therapy is a reasonable option when initiating insulin treatment (11,24). The goal is to lower glucose levels at a constant rate throughout the day, with minimal fluctuations. Simplifying treatment by using basal insulin analogs alone and avoiding the additional use of regular and rapid-acting insulin is recommended when feasible (11,16).
A single-dose regimen of basal insulin analog can have an important role in managing hyperglycemia among patients in the post-acute and long-term care (LTC) settings. One-fourth to one-third of residents in LTC have diabetes (23), and treatment of these residents needs to be tailored according to the facility staff’s ability to administer medications and monitor for hypoglycemia and hyperglycemia. For example, would the staff know to withhold premeal short-acting insulin if a resident is not going to eat a meal? Can the staff recognize symptoms of hypoglycemia among residents who are cognitively impaired? Hence, a sliding-scale insulin regimen should be avoided given its complexity with regard to monitoring and the variability of glucose-lowering activities (23,25–27).
There is a lack of well-designed clinical studies on optimal glucose management among patients near the end of life. The optimal glucose range varies according to the patient’s stage of illness, ability to eat and drink normally, presence of hypoglycemia, nutritional status, and type of treatment, but recommended target ranges are 108–270 mg/dL (6–15 mmol/L) (28). Simplified treatment and testing regimens are recommended for diabetes management in these patients to reduce the burden of care and risk of adverse events or medication errors (23). A basal insulin analog with or without oral antidiabetic drugs may reduce hypoglycemia risk and simplify the regimen. A strategy of once-daily plasma glucose monitoring and once-daily administration of a basal insulin analog has also been proposed for palliative care patients (29).
An intensive educational review, hypoglycemia education, and nutritional re-evaluation are important steps when starting or changing any insulin regimen in an older patient with diabetes. This process often requires the involvement of third parties such as family members, caregivers, or care facility providers. At times, patients can implement a new insulin regimen themselves. The choice of and access to a specific insulin agent will likely be affected by financial challenges, insurance coverage, and ethnic and cultural differences. Unfortunately, there are limited clinical data regarding older patients with type 2 diabetes on which to base choices for antihyperglycemic agents. Most recommendations for the management of older patients with diabetes have been made based on post-hoc or pooled analyses of larger trials, with some expert opinions included. Nevertheless, there are recent data that may guide treatment choices with the use of basal insulin analogs in these individuals.
Recent Studies of First- and Second-Generation Basal Insulin Analogs in Older Patients
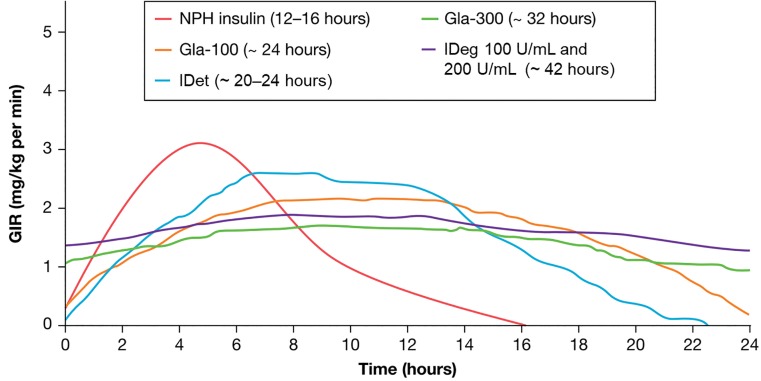
Basal insulin analogs have more consistent, longer, and more stable action profiles, as well as steady and reliable levels of circulating insulin over a 24-hour period compared to earlier formulations such as NPH insulin (30) (Figure 1); these improvements result in a lower risk of hypoglycemia, particularly nocturnal hypoglycemia (5), and the ability to dose once daily. Worldwide, four basal insulin analogs have been approved for the management of type 2 diabetes: first-generation Gla-100 and IDet and second-generation IDeg and Gla-300 (Figure 1). In clinical trials, second-generation basal insulin analogs have been shown to provide similar overall glycemic control, but with lower rates of hypoglycemia compared to first-generation formulations (7,8). In the following section, we briefly review the data from clinical studies that included older adults for each of these basal insulin analogs (Tables 2 and 3).
FIGURE 1.
Pharmacokinetic profiles of first- and second-generation basal insulin analogs. GIR, glucose infusion rate. Adapted from ref. 30.
TABLE 2.
Hypoglycemia in Studies of First-Generation Basal Insulin Analogs in Older Patients With Type 2 Diabetes
| Reference | Type of Study | Age-Group, years | n | Glycemic Target | Hypoglycemia, %* | ||
|---|---|---|---|---|---|---|---|
| Overall | Nocturnal | Severe | |||||
| Insulin glargine 100 units/mL | |||||||
| Pandya et al. (32) | Pooled analysis of 24-week data from nine prospective, open-label, multicenter, phase 3/4 clinical trials | ˂65 | 2,263 | FPG ≤100 mg/dL | 23.7† | 8.7† | 0.8‡,§ |
| ≥65 | 675 | FPG ≤100 mg/dL | 27.3† | 7.2† | 2.2‡,§ | ||
| Owens et al. (33) | Pooled analysis of 16 prospective, randomized, treat-to-target clinical trials | ˂65 | 2,411 | FPG ˂100 mg/dL | 47.6|| | 19.9|| | 2.1¶ |
| ≥65 | 777 | FPG ˂100 mg/dL | 47.0|| | 17.8|| | 2.1¶ | ||
| Chien et al. (34) | Retrospective, registry analysis | ˂65 | 40 | No set goal†† | 15.0 | NR | 2.5 |
| ≥65 | 32 | No set goal†† | 9.4 | NR | 0 | ||
| Insulin detemir | |||||||
| Bhargava et al. (37) | Post-hoc subanalysis of a randomized, open-label, phase 4 trial | ˂65 | 1,915 | No set goal†† | NR | 4.4# | 0.3¶ (day), 0.3¶ (nocturnal) |
| ≥65 | 897 | No set goal†† | NR | 5.3# | 0.5¶ (day), 0.4¶ (nocturnal) |
||
| Karnieli et al. (38) | Subanalysis of an observational study | ˂75 | 14,873 | No set goal†† | NR | NR | 0.005 per patient-year |
| ≥75 | 2,398 | No set goal†† | NR | NR | 0.007 per patient-year | ||
| Malek et al. (39) | Subanalysis of observational study | ˃40–65 | 10,967 | No set goal†† | 4.7# | 1.7# | 0** |
| ˃65 | 2,807 | A1C ˂7.5% | 6.6# | 2.3# | 0** | ||
NR, not reported.
No significant difference reported between age-groups within treatment groups unless otherwise stated.
Blood glucose ˂50 mg/dL.
Events requiring assistance and blood glucose ˂36 mg/dL or prompt recovery after oral carbohydrate, intravenous glucose, or glucagon administration.
P = 0.023. ||Blood glucose ≤70 mg/dL.
Events requiring assistance of another person.
Changes in A1C and/or FPG were measured in these studies, but no analysis was performed against a pre-defined target level.
Symptoms of hypoglycemia that resolved with oral carbohydrate intake, glucagon, or intravenous glucose, or any symptomatic or asymptomatic blood glucose ˂56 mg/dL.
Severe central nervous system symptoms consistent with hypoglycemia, during which the patient was unable to self-treat and had one of the following characteristics: plasma glucose ˂56 mg/dL or reversal of symptoms after either food intake, glucagon, or intravenous glucose administration.
TABLE 3.
Hypoglycemia in Studies of Second-Generation Basal Insulin Analogs in Older Patients With Type 2 Diabetes
| Reference | Type of Study | Age-Group, years | n | Glycemic Target | Hypoglycemia, %* | ||
|---|---|---|---|---|---|---|---|
| Overall | Nocturnal | Severe | |||||
| IDeg | |||||||
| Sorli et al. (40) | Pooled analysis of phase 3 clinical trials | ≥65 | IDeg: 589 | FPG ˂90 mg/dL | 58.7† | 21.2† | 2.9‡ |
| Gla-100: 265 | FPG ˂90 mg/dL | 58.7† | 25.4† | 4.2‡ | |||
| Gla-300 | |||||||
| Munshi et al. (45) | Pooled analysis of two phase 3 clinical trials | ˂55 | Gla-300: 273 | A1C ˂7.0 or ˂7.5% | 55.5§ | NR | NR |
| Gla-100: 280 | A1C ˂7 or ˂7.5% | 62.7§ | NR | NR | |||
| ≥55 to ˂60 | Gla-300: 162 | A1C ˂7 or ˂7.5% | 57.5§ | NR | NR | ||
| Gla-100: 181 | A1C ˂7 or ˂7.5% | 70.5§ | NR | NR | |||
| ≥60 to ˂65 | Gla-300: 201 | A1C ˂7 or ˂7.5% | 60.4§ | NR | NR | ||
| Gla-100: 163 | A1C ˂7 or ˂7.5% | 69.7§ | NR | NR | |||
| ≥65 | Gla-300: 199 | A1C ˂7 or ˂7.5% | 63.6§ | NR | NR | ||
| Gla-100: 211 | A1C ˂7 or ˂7.5% | 70.5§ | NR | NR | |||
| Yale et al. (46) | Pooled analysis of three phase 3 clinical trials | ≥65 | Gla-300: 325 | A1C ˂7% | 71.6§ | 31.8§ | NR |
| Gla-100: 329 | A1C ˂7% | 77.4§ | 44.9§ | NR | |||
| Ritzel et al. (47) | International, randomized, open-label, phase 3 clinical trial | All (≥65) | Gla-300: 508 | A1C ˂7.5% | 32.9§ | 12.0§ | 6.3|| |
| Gla-100: 506 | A1C ˂7.5% | 34.7§ | 12.9§ | 8.7|| | |||
| ≥75 | Gla-300: 135 | A1C ˂7.5% | 24.4§ | 10.4§ | 1.5|| | ||
| Gla-100: 106 | A1C ˂7.5% | 34.0§ | 12.3§ | 10.4|| | |||
NR, not reported.
No significant difference reported between age-groups within treatment groups unless otherwise stated.
Severe episodes or blood glucose ˂56 mg/dL.
Events requiring assistance of another person.
Blood glucose ≤70 mg/dL.
Blood glucose ≤54 mg/dL.
Gla-100
The safety and efficacy of Gla-100 has been established in numerous clinical studies across a wide spectrum of patients with type 2 diabetes (5), including older patients. Treatment with Gla-100 has been shown to result in greater reductions in A1C and less hypoglycemia compared to NPH or premixed insulin in older adults (31). Overall, data suggest that older age does not affect either glycemic control or hypoglycemia risk with Gla-100, with similar or greater reductions in A1C. Similar incidence rates of overall, nocturnal, and severe hypoglycemia have been reported in large analyses of patient-level data for patients aged ˂65 years versus those ≥65 years (32,33). Real-world registry data support findings from clinical trials, showing similar glycemic efficacy and low rates of hypoglycemia in both age-groups (34).
IDet
IDet and Gla-100 have comparable efficacy in patients with type 2 diabetes, with a similar incidence of hypoglycemia. However, twice-daily dosing of IDet is commonly needed to achieve glycemic targets (35,36), and injection-site reactions are more common for IDet compared to Gla-100 (36); IDet may therefore be less tolerable or convenient for older patients. Higher doses of IDet compared to Gla-100 are generally required, and although weight gain may be lower than with Gla-100 (gain of 0.6–3.0 kg across studies with IDet vs. 1.4–3.9 kg with Gla-100), this result is reported primarily in patients receiving once-daily doses (5). Three subgroup analyses of real-world studies in patients with type 2 diabetes reported no difference in improvements in glycemic control after initiation or switch to once-daily IDet, or during dose titration, in older patients (37–39). In terms of hypoglycemia risk, results from these studies are mixed, with some studies reporting a higher rate of severe hypoglycemia in older patients (Table 2).
IDeg
In a meta-analysis of data from patients aged ≥65 years included in the IDeg phase 3 trials, there was a 24% lower estimated rate of overall confirmed hypoglycemia and a 36% lower rate of nocturnal confirmed hypoglycemia with IDeg compared to Gla-100 over the study period; glycemic outcomes were not reported (40). The prescribing information for IDeg states that there were no differences in effectiveness in subgroup analyses of pivotal clinical trials comparing subjects ˃65 years of age to younger subjects (41).
The long duration of action (˃42 hours) of IDeg has raised the possibility of extending the dosing period from daily to thrice weekly. This might be of particular interest for patients who are unable or hesitant to perform self-injection and who lack daily assistance with injection from caregivers (42). Whether thrice-weekly IDeg might be a viable option for older patients in different settings with less strict glycemic targets remains to be thoroughly evaluated; the approved dosing schedule for IDeg in all patients is once daily.
Gla-300
Gla-300 has a more stable and prolonged duration of action than Gla-100, lasting up to 36 hours (43,44). A pooled analysis of data from the EDITION phase 3 clinical trials reported similar outcomes in both younger and older patients (45). Across all age-groups, A1C reductions from baseline and proportions of patients who achieved their glycemic target (≤7.5%) were similar for Gla-300 and Gla-100 at 6 and 12 months (45). In patients aged ≥65 years, hypoglycemia incidence was lower in those treated with Gla-300 compared to Gla-100 at any time of day or at night (45,46) (Table 3). In another pooled analysis of the EDITION studies in patients aged ≥65 years, a greater number of patients treated with Gla-300 than Gla-100 reached A1C targets overall and without hypoglycemia (46). Preliminary data from the DELIVER 3 retrospective cohort study of older patients in routine practice showed that switching to Gla-300 compared to other basal insulin analogs led to comparable changes in A1C, but patients who switched to Gla-300 were 57% less likely to have hypoglycemia at the 6-month follow-up (OR 0.432, 95% CI 0.307–0.607, P ˂0.0001) (47). After adjusting for baseline characteristics, hypoglycemia event rates were also significantly lower in the Gla-300 cohort versus other basal insulins (least squares mean difference –4.94 events/100 patient-months, P = 0.0002).
Most recently, the phase 3b SENIOR study was the first prospectively designed clinical trial to specifically compare the efficacy and safety of basal insulin analogs (Gla-300 and Gla-100) in older people (≥65 years of age) with type 2 diabetes (n = 1,014) (47). Overall, Gla-300 was effective in older people with type 2 diabetes, with a good safety profile, resulting in comparable reductions in A1C (–0.89 vs. –0.91%) with Gla-100 at week 26 and lower rates of documented symptomatic hypoglycemia (48) (Table 3). A1C reductions in the SENIOR study were consistent with those reported in the EDITION studies. The incidence of confirmed (≤70 mg/dL [≤3.9 mmol/L]) and/or severe hypoglycemia was lower than expected, possibly related to the higher glycemic treatment target set in SENIOR compared with the EDITION trials. Interestingly, reduced hypoglycemia risk for Gla-300 versus Gla-100 was most pronounced in participants ≥75 years of age, with consistently lower annualized event rates and proportion of patients with documented symptomatic hypoglycemia in the subpopulation ≥75 years of age (48).
Treatment Guidelines for Older Patients
Less than a decade ago, the American Diabetes Association (ADA) and the European Association for the Study of Diabetes recommended the achievement and maintenance of near normoglycemia (A1C ˂7.0%) for all patients. It has since been recognized that this goal is inappropriate or impractical for some patients (49). More specific guidelines that consider elements such as life expectancy, comorbidities, vascular complications, function, cognition, psycho-socioeconomic status, and overall health status have now been produced (2,11,50). The Society for Post-Acute and Long-Term Care Medicine has published guidelines for diabetes management for patients in the post-acute and LTC settings (27), and the ADA has also developed a position paper for specific patient populations in skilled nursing facilities and LTC settings (23). This includes recommendations for those receiving palliative care or at the end of life.
Glycemic Targets and Avoidance of Hypoglycemia
In recommendations developed by the ADA and the American Geriatrics Society, A1C targets for older patients vary based on patient characteristics (9). In general, healthy older patients can be managed using goals and treatment strategies similar to those used for younger adults, provided they agree (2,11,50). Older patients with a complicated medical history or with impaired cognition or function and frail patients with a reduced life expectancy are less likely to benefit from long-term reduction in microvascular complications and are more likely to experience hypoglycemia associated with tight glycemic control; therefore, in these cases, glycemic goals should be relaxed (Table 4). Hyperglycemia should not be disregarded in older patients; glycemic goals should be set, at minimum, to avoid acute complications such as dehydration, poor wound healing, incontinence, polyuria, nocturia, and hyperglycemic hyperosmolar coma. A1C targets ˃8.5% are generally not recommended (11).
TABLE 4.
Recommendations for A1C Targets in Older People With Type 2 Diabetes
| Guidelines/Position Statement | Patient Characteristics | A1C Goal, % |
|---|---|---|
| American Diabetes Association (11) Society for Post-Acute and Long-Term Care Medicine (27) |
Healthy (few coexisting chronic illnesses, intact cognitive and functional status) | ˂7.5* |
| Complex/intermediate health (multiple coexisting chronic illnesses† or two or more instrumental ADL impairments or mild to moderate cognitive impairment) | ˂8.0* | |
| Very complex/poor health (LTC or end-stage chronic illnesses‡ or moderate-to-severe cognitive impairment or two or more ADL dependencies) | ˂8.5*,§ | |
| American Diabetes Association (23) | Community-dwelling patients at skilled nursing facility for short-term rehabilitation | Avoid relying on A1C because of recent acute illness; follow current glucose trends |
| Patients residing in LTC facility | ˂8.5 | |
| Patients at end of life | No role for A1C | |
| International Diabetes Federation (50) | Functionally independent (no important impairments in ADL; no or minimal caregiver support; may have other medical comorbidities that may influence diabetes care) | 7.0–7.5 |
| Functionally dependent | 7.0–8.0 | |
| Frail/dementia | Up to 8.5 | |
| End of life care | None; avoid symptomatic hyperglycemia and minimize hypoglycemia | |
| Canadian Diabetes Association (2) | Healthy elderly | As for younger patients (˂7.0) |
| Frail elderly | ≤8.5% | |
| Diabetes Care Program of Nova Scotia and the Palliative and Therapeutic Harmonization Program (16) | Frail older adults | Maintain A1C ≥8% rather than below a specific level |
| European Diabetes Working Party for Older People (25) | Healthy (no other major comorbidities) | 7.0–7.5 |
| Frail (dependent; multisystem disease; care home residency, including those with dementia) | 7.6–8.5 |
A lower A1C goal may be set for an individual if achievable without recurrent or severe hypoglycemia or undue treatment burden.
Coexisting chronic illnesses are conditions serious enough to require medications or lifestyle management and may include arthritis, cancer, congestive heart failure, depression, emphysema, falls, hypertension, incontinence, stage 3 or worse chronic kidney disease, myocardial infarction, and stroke. “Multiple” means three or more illnesses, but many patients may have five or more illnesses.
The presence of a single end-stage chronic illness, such as stage 3–4 congestive heart failure or oxygen-dependent lung disease, chronic kidney disease requiring dialysis, or uncontrolled metastatic cancer, may cause significant symptoms or impairment of functional status and significantly reduce life expectancy.
A1C = 8.5% equates to an estimated average glucose of 200 mg/dL (11.1 mmol/L). Looser A1C targets ˃8.5% are not recommended because they may expose patients to more frequent higher glucose values and acute risks from glycosuria, dehydration, hyperglycemic hyperosmolar syndrome, and poor wound healing.
Although relaxing A1C targets in vulnerable older patients appears logical, there is some evidence to suggest that higher A1C alone may not be associated with a reduced risk of hypoglycemia. Studies in older patients with type 2 diabetes and dementia have shown that even those with higher A1C levels may experience severe hypoglycemia (51,52). The reason behind this is unclear; higher A1C targets were probably pre-set for patients with characteristics that predispose them to a greater risk of hypoglycemia. Other factors that are common in older adults, such as recent illness, anemia, blood transfusions, and blood dyscrasia, may interfere with A1C levels (23). Recently, a study of 65 patients with a mean age of 76 years treated with insulin-based therapy found that A1C levels were not associated with hypoglycemia risk and that the presence of cognitive dysfunction, depression, or difficulty performing activities of daily living (ADL) did not correlate with hypoglycemia (52). Such data suggest that the focus of treatment in older patients, particularly frail patients or those with dementia, should be on reducing the risk of hypoglycemia rather than targeting a specific A1C goal. The results also indicate that a review of SMPG values and trends may be more important than A1C levels when adjusting treatment.
Summary
Older people with type 2 diabetes represent a highly heterogeneous population with a wide spectrum of health conditions, cognition, functional status, socioeconomic support, and residential settings. There is an urgent need for more clinical research inclusive of older populations of different health and functional statuses.
Glycemic targets and treatment regimens should be individualized after careful consideration of each patient’s ability to follow a complicated diabetes regimen. In patients with multiple comorbidities, cognitive issues, or functional disabilities, or those with a reduced life expectancy, intensive glucose management and stringent glycemic targets are likely to be of secondary importance compared to preventing the chronic and acute complications associated with hypoglycemia.
The newer, second-generation basal insulin analogs IDeg and Gla-300 have been shown to provide similar levels of glycemic control to first-generation basal insulin analogs but with a lower risk of hypoglycemia. Although data for older patients are currently limited, recent data on Gla-300 suggest that these benefits are also seen in older patients. The clinical data presented here show that basal insulin analogs are effective and safer than other forms of insulin in older adults. However, these data are limited because most are derived from subanalyses of an older population from a larger study or are pooled from a number of randomized controlled trials that were usually completed within a short timeframe (e.g., 24 weeks) and were performed in individuals who were in good health and without cognitive impairment.
Given the differences between clinical trial and real-world populations, the long-term effects of these insulins in older adults managed in routine practice remain uncertain. Based on the increasing age and survival rates of older adults with type 2 diabetes, dedicated studies (including head-to-head comparisons) are warranted to assess the use of basal insulin analogs in older individuals with regard to safety, tolerability, and clinical outcomes and with a particular interest in hypoglycemia events and other consequences.
Acknowledgments
Acknowledgments
The authors thank Jeffrey Halter, MD, for his contributions to this article. The authors received writing/editorial support in the preparation of this manuscript from Nicholas Patterson, PhD, of Excerpta Medica.
Funding
Writing/editorial support for this manuscript was funded by Sanofi.
Duality of Interest
E.C. has received research funds from AstraZeneca and VeroScience and is on speakers bureaus for AstraZeneca, Bi-Lilly Alliance, Janssen, and Sanofi. P.G.L. is on the research advisory committee for the T1D Exchange and Unitio. N.P. is on Sanofi’s speakers bureau. No other potential conflicts of interest relevant to this article were reported.
Author Contributions
All authors were involved in the concept and design of the manuscript, analysis and interpretation of the literature, and critical review of the manuscript drafts, and all authors provided final approval of the version to be submitted. E.C. is the guarantor of this work and, as such takes responsibility for the integrity and accuracy of the review.
References
- 1.Meneilly GS, Elahi D. Metabolic alterations in middle-aged and elderly lean patients with type 2 diabetes. Diabetes Care 2005;2:1498–1499 [DOI] [PubMed] [Google Scholar]
- 2.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Diabetes in the elderly. Can J Diabetes 2013;37(Suppl. 1):S184–S190 [DOI] [PubMed] [Google Scholar]
- 3.Kalyani RR, Egan JM. Diabetes and altered glucose metabolism with aging. Endocrinol Metab Clin North Am 2013;42:333–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gates BJ, Walker KM. Physiological changes in older adults and their effect on diabetes treatment. Diabetes Spectr 2014;27:20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hedrington MS, Pulliam L, Davis SN. Basal insulin treatment in type 2 diabetes. Diabetes Technol Ther 2011;13(Suppl. 1):S33–S42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee PG, Halter JB. The pathophysiology of hyperglycemia in older adults: clinical considerations. Diabetes Care 2017;40:444–452 [DOI] [PubMed] [Google Scholar]
- 7.Lau IT, Lee KF, So WY, Tan K, Yeung VTF. Insulin glargine 300 U/mL for basal insulin therapy in type 1 and type 2 diabetes mellitus. Diabetes Metab Syndr Obes 2017;10:273–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vora J, Cariou B, Evans M, et al. . Clinical use of insulin degludec. Diabetes Res Clin Pract 2015;109:19–31 [DOI] [PubMed] [Google Scholar]
- 9.Kirkman MS, Briscoe VJ, Clark N, et al. ; Consensus Development Conference on Diabetes and Older Adults. Diabetes in older adults: a consensus report. J Am Geriatr Soc 2012;60:2342–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care 2006;29:725–731 [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association 1. Improving care and promoting health in populations: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl. 1):S7–S12 [DOI] [PubMed] [Google Scholar]
- 12.Valencia WM, Florez H. Pharmacological treatment of diabetes in older people. Diabetes Obes Metab 2014;16:1192–1203 [DOI] [PubMed] [Google Scholar]
- 13.Miao R, Wei W, Lin J, Xie L, Baser O. Does device make any difference? A real-world retrospective study of insulin treatment among elderly patients with type 2 diabetes. J Diabetes Sci Technol 2014;8:150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattishent K, Loke YK. Bi-directional interaction between hypoglycaemia and cognitive impairment in elderly patients treated with glucose-lowering agents: a systematic review and meta-analysis. Diabetes Obes Metab 2016;18:135–141 [DOI] [PubMed] [Google Scholar]
- 15.Abdelhafiz AH, Rodríguez-Mañas L, Morley JE, Sinclair AJ. Hypoglycemia in older people: a less well recognized risk factor for frailty. Aging Dis 2015;6:156–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mallery LH, Ransom T, Steeves B, Cook B, Dunbar P, Moorhouse P. Evidence-informed guidelines for treating frail older adults with type 2 diabetes: from the Diabetes Care Program of Nova Scotia (DCPNS) and the Palliative and Therapeutic Harmonization (PATH) program. J Am Med Dir Assoc 2013;14:801–818 [DOI] [PubMed] [Google Scholar]
- 17.Martín-Timón I, Del Cañizo-Gómez FJ. Mechanisms of hypoglycemia unawareness and implications in diabetic patients. World J Diabetes 2015;6:912–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fanelli CG, Epifano L, Rambotti AM, et al. . Meticulous prevention of hypoglycemia normalizes the glycemic thresholds and magnitude of most of neuroendocrine responses to, symptoms of, and cognitive function during hypoglycemia in intensively treated patients with short-term IDDM. Diabetes 1993;42:1683–1689 [DOI] [PubMed] [Google Scholar]
- 19.Fanelli C, Pampanelli S, Epifano L, et al. . Long-term recovery from unawareness, deficient counterregulation and lack of cognitive dysfunction during hypoglycaemia, following institution of rational, intensive insulin therapy in IDDM. Diabetologia 1994;37:1265–1276 [DOI] [PubMed] [Google Scholar]
- 20.Cryer PE. Elimination of hypoglycemia from the lives of people affected by diabetes. Diabetes 2011;60:24–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danne T, Nimri R, Battelino T, et al. . International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pazos-Couselo M, García-López JM, González-Rodríguez M, et al. . High incidence of hypoglycemia in stable insulin-treated type 2 diabetes mellitus: continuous glucose monitoring vs. self-monitored blood glucose: observational prospective study. Can J Diabetes 2015;39:428–433 [DOI] [PubMed] [Google Scholar]
- 23.Munshi MN, Florez H, Huang ES, et al. . Management of diabetes in long-term care and skilled nursing facilities: a position statement of the American Diabetes Association. Diabetes Care 2016;39:308–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munshi MN, Slyne C, Segal AR, Saul N, Lyons C, Weinger K. Simplification of insulin regimen in older adults and risk of hypoglycemia. JAMA Intern Med 2016;176:1023–1025 [DOI] [PubMed] [Google Scholar]
- 25.Sinclair AJ, Paolisso G, Castro M, Bourdel-Marchasson I, Gadsby R, Rodriguez Mañas L; European Diabetes Working Party for Older People. European Diabetes Working Party for Older People 2011 clinical guidelines for type 2 diabetes mellitus: executive summary. Diabetes Metab 2011;37(Suppl. 3):S27–S38 [DOI] [PubMed] [Google Scholar]
- 26.Sinclair A, Morley JE, Rodriguez-Mañas L, et al. . Diabetes mellitus in older people: position statement on behalf of the International Association of Gerontology and Geriatrics (IAGG), the European Diabetes Working Party for Older People (EDWPOP), and the International Task Force of Experts in Diabetes. J Am Med Dir Assoc 2012;13:497–502 [DOI] [PubMed] [Google Scholar]
- 27.Society for Post-Acute and Long-Term Care Medicine Diabetes management in the post-acute and long-term care setting. Available from www.paltc.org/sites/default/files/sam/Diabetes%20Management.pdf. Accessed 31 July 2018
- 28.Diabetes UK. End of life in diabetes care: clinical care recommendations. Available from www.diabetes.org.uk/resources-s3/2018-03/EoL_Guidance_2018_Final.pdf. Accessed 31 July 2018
- 29.Zylicz Z. Management of diabetes mellitus in terminally ill cancer patients. Adv Pall Med 2010;9:99–102 [Google Scholar]
- 30.Mathieu C, Gillard P, Benhalima K. Insulin analogues in type 1 diabetes mellitus: getting better all the time. Nat Rev Endocrinol 2017;13:385–399 [DOI] [PubMed] [Google Scholar]
- 31.Lee P, Chang A, Blaum C, Vlajnic A, Gao L, Halter J. Comparison of safety and efficacy of insulin glargine and neutral protamine Hagedorn insulin in older adults with type 2 diabetes mellitus: results from a pooled analysis. J Am Geriatr Soc 2012;60:51–59 [DOI] [PubMed] [Google Scholar]
- 32.Pandya N, DiGenio A, Gao L, Patel M. Efficacy and safety of insulin glargine compared to other interventions in younger and older adults: a pooled analysis of nine open-label, randomized controlled trials in patients with type 2 diabetes. Drugs Aging 2013;30:429–438 [DOI] [PubMed] [Google Scholar]
- 33.Owens DR, Bolli GB, Charbonnel B, et al. . Effects of age, gender, and body mass index on efficacy and hypoglycaemia outcomes across treat-to-target trials with insulin glargine 100 U/mL added to oral antidiabetes agents in type 2 diabetes. Diabetes Obes Metab 2017;19:1546–1554 [DOI] [PubMed] [Google Scholar]
- 34.Chien M-N, Lee C-C, Liu S-C, et al. . Basal insulin initiation in elderly patients with type 2 diabetes in Taiwan: a comparison with younger patients. Int J Gerontol 2015;9:142–145 [Google Scholar]
- 35.Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia 2008;51:408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swinnen SG, Simon AC, Holleman F, Hoekstra JB, Devries JH. Insulin detemir versus insulin glargine for type 2 diabetes mellitus. Cochrane Database Syst Rev 2011;7:CD006383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhargava A, Chan V, Kimball ES, Oyer DS. Effects of age on glycemic control in patients with type 2 diabetes treated with insulin detemir: a post-hoc analysis of the PREDICTIVE 303 Study. Drugs Aging 2016;33:135–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karnieli E, Baeres FM, Dzida G, et al.; SOLVE Study Group . Observational study of once-daily insulin detemir in people with type 2 diabetes aged 75 years or older: a sub-analysis of data from the Study of Once daily LeVEmir (SOLVE). Drugs Aging 2013;30:167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malek R, Gonzalez-Galvez G, El Naggar N, Shah S, Prusty V, Litwak L. Safety and effectiveness of insulin detemir in different age-groups in the A1chieve study. Diabetes Ther 2013;4:77–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sorli C, Warren M, Oyer D, Mersebach H, Johansen T, Gough SC. Elderly patients with diabetes experience a lower rate of nocturnal hypoglycaemia with insulin degludec than with insulin glargine: a meta-analysis of phase IIIa trials. Drugs Aging 2013;30:1009–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Novo Nordisk Tresiba (insulin degludec injection) package insert. Plainsboro, N.J, Novo Nordisk, 2018 [Google Scholar]
- 42.Nagai Y, Murakami M, Igarashi K, et al. . Efficacy and safety of thrice-weekly insulin degludec in elderly patients with type 2 diabetes assessed by continuous glucose monitoring. Endocr J 2016;63:1099–1106 [DOI] [PubMed] [Google Scholar]
- 43.Becker RH, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 units · mL−1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 units · mL−1. Diabetes Care 2015;38:637–643 [DOI] [PubMed] [Google Scholar]
- 44.Shiramoto M, Eto T, Irie S, et al. . Single-dose new insulin glargine 300 U/ml provides prolonged, stable glycaemic control in Japanese and European people with type 1 diabetes. Diabetes Obes Metab 2015;17:254–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munshi MN, Gill J, Chao J, Nikonova EV, Patel M. Insulin glargine 300 U/mL is associated with less weight gain while maintaining glycemic control and low risk of hypoglycemia compared with insulin glargine 100 U/mL in an aging population with type 2 diabetes. Endocr Pract 2018;24:143–149 [DOI] [PubMed] [Google Scholar]
- 46.Yale J-F, Aroda VR, Charbonnel B, et al. . Older people with type 2 diabetes: glycemic control and hypoglycemia risk with new insulin glargine 300 U/mL. Diabetes 2015;64(Suppl. 1):A252 [Google Scholar]
- 47.Ritzel R, Harris SB, Baron H, et al. . A randomized controlled trial comparing efficacy and safety of insulin glargine 300 units/mL versus 100 units/mL in older people with type 2 diabetes: results from the SENIOR study. Diabetes Care 2018;41:1672–1680 [DOI] [PubMed] [Google Scholar]
- 48.Zhou FL, Ye F, Gupta V, et al. . Older adults with type 2 diabetes (type 2 diabetes) experience less hypoglycemia when switching to insulin glargine 300 U/mL (Gla-300) vs. other basal insulins (DELIVER 3 Study). Diabetes 2017;66(Suppl. 1):A256 [Google Scholar]
- 49.Nathan DM, Buse JB, Davidson MB, et al. ; American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.International Diabetes Federation Global guideline for managing older people with type 2 diabetes. Available from www.idf.org/e-library/guidelines/78-global-guideline-for-managing-older-people-with-type-2-diabetes.html. Accessed 31 July 2018
- 51.Abbatecola AM, Bo M, Barbagallo M, et al. . Severe hypoglycemia is associated with antidiabetic oral treatment compared with insulin analogs in nursing home patients with type 2 diabetes and dementia: results from the DIMORA study. J Am Med Dir Assoc 2015;16:349.e7–e12 [DOI] [PubMed] [Google Scholar]
- 52.Munshi MN, Slyne C, Segal AR, Saul N, Lyons C, Weinger K. Liberating A1C goals in older adults may not protect against the risk of hypoglycemia. J Diabetes Complications 2017;31:1197–1199 [DOI] [PubMed] [Google Scholar]