Abstract
Coral reefs are threatened by global warming, which disrupts the symbiosis between corals and their photosynthetic symbionts (Symbiodiniaceae), leading to mass coral bleaching. Planktonic diazotrophs or dinitrogen (N2)-fixing prokaryotes are abundant in coral lagoon waters and could be an alternative nutrient source for corals. Here we incubated untreated and bleached coral colonies of Stylophora pistillata with a 15N2-pre-labelled natural plankton assemblage containing diazotrophs. 15N2 assimilation rates in Symbiodiniaceae cells and tissues of bleached corals were 5- and 30-fold higher, respectively, than those measured in untreated corals, demonstrating that corals incorporate more nitrogen derived from planktonic diazotrophs under bleaching conditions. Bleached corals also preferentially fed on Synechococcus, nitrogen-rich picophytoplanktonic cells, instead of Prochlorococcus and picoeukaryotes, which have a lower cellular nitrogen content. By providing an alternative source of bioavailable nitrogen, both the incorporation of nitrogen derived from planktonic diazotrophs and the ingestion of Synechococcus may have profound consequences for coral bleaching recovery, especially for the many coral reef ecosystems characterized by high abundance and activity of planktonic diazotrophs.
Subject terms: Microbial communities, Stable isotope analysis
Introduction
Coral reefs are currently under threat from global warming, which disrupts the symbiosis between corals and their endosymbiotic dinoflagellates of the family Symbiodiniaceae [1], leading to mass coral bleaching [2]. When corals bleach, they lose part of their photosynthetic symbionts that provide them with nitrogen [3] and seawater warming also decreases coral nitrogen acquisition capacity [4]. Several studies have reported an increase in the consumption of mesoplankton and macroplankton by corals when exposed to thermal stress, potentially sustaining a critical supply of nutrients needed for recovery following bleaching [5–7]. The ability of corals to feed on smaller planktonic fractions, i.e., picoplankton (0.2–2 µm) and nanoplankton (2–20 µm) has also been documented [8], but the increase in the ingestion of bacteria and picoflagellates by bleached corals has only been observed in one study [9]. Among these size fractions, planktonic dinitrogen (N2)-fixing prokaryotes (subsequently referred to as planktonic diazotrophs) are very abundant in coral lagoon waters [10, 11]. They reduce atmospheric N2 into bioavailable ammonium (NH4+), providing sufficient nitrogen stocks for the development of the planktonic food web in oligotrophic waters [12]. The assimilation of nitrogen derived from planktonic diazotrophs has been recently demonstrated in corals [13]. According to [13], 15N-enrichment in corals after their incubation with 15N2-labelled natural diazotrophic assemblages could be due to three different processes: (1) direct feeding on planktonic diazotrophs digested within the coelenteron, (2) uptake of 15N-dissolved nitrogen compounds fixed by the planktonic diazotrophs and released extracellularly, and (3) ingestion of nondiazotrophic plankton enriched in 15N as a result of diazotroph-derived nitrogen transfer [14]. While some studies have demonstrated that N2 fixation by coral symbiotic diazotroph communities increases in bleached corals [15, 16], the acquisition of nitrogen derived from planktonic diazotrophic activity has never been investigated in corals facing thermal stress.
Materials & Methods
To determine if bleached corals also benefit from planktonic diazotrophs, we incubated five colonies of the branching coral S. pistillata with a 15N2-pre-labelled (24 h) natural plankton assemblage containing planktonic diazotrophs (prefiltered through a 100 µm mesh to exclude larger cells) as described in [13]. In parallel, N2 fixation within endosymbiotic diazotrophs in colonies of the same species was measured by incubating five untreated and five bleached colonies in 15N-enriched filtered seawater. Coral colonies collected in the New Caledonian lagoon were acclimated to experimental conditions for 3 weeks. They were progressively bleached over 18 days (by a gradual temperature increase up to 31 °C) or left at ambient temperature (28 °C) as a control (subsequently referred to as untreated corals, see supplementary information for details, Supplementary Fig. S1). The δ15N isotopic values were measured in symbionts, coral tissues, and plankton before and after incubation (12 h). Nitrogen assimilation rates were calculated as previously described [17]. The contribution of endosymbiotic N2 fixation was minor (see results in the supplementary information). Conversely, after the incubation with 15N2-labelled natural planktonic assemblage significant 15N-enrichments were measured in the Symbiodiniaceae of both untreated and bleached corals
Results & Discussion
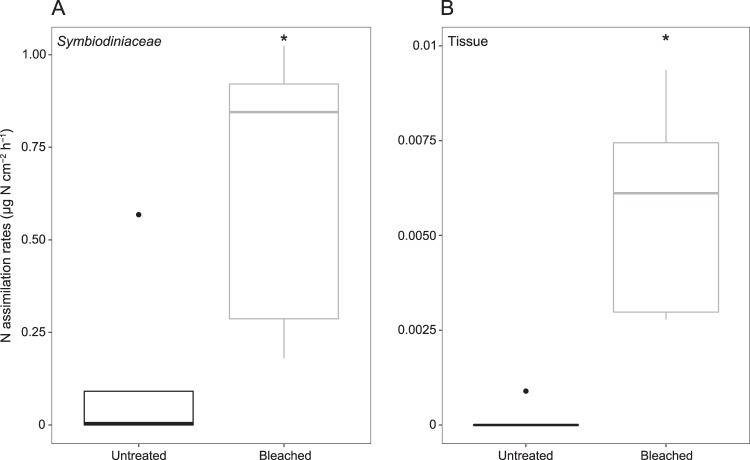
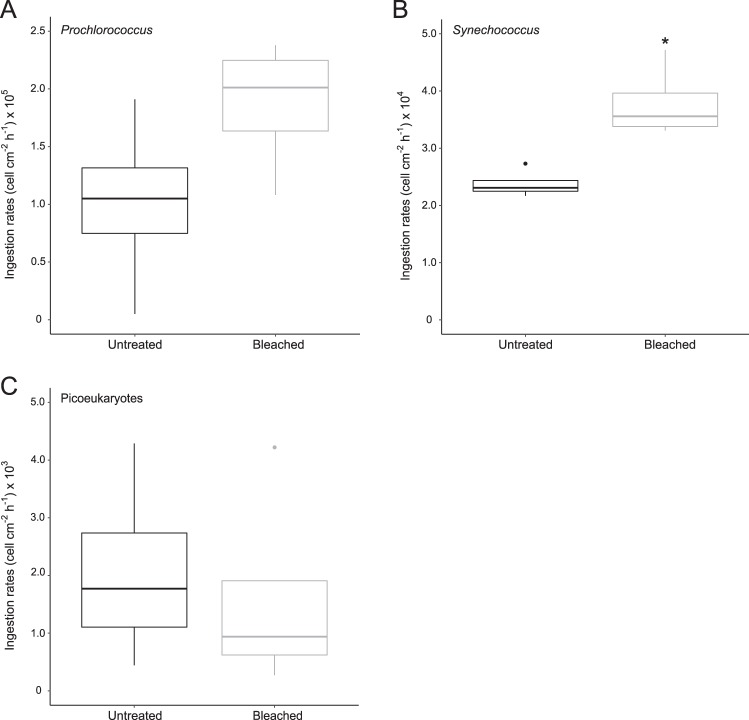
The results suggests that Symbiodiniaceae used nitrogen originating from the planktonic diazotrophs [13, 15, 18]. Nitrogen assimilation rates in Symbiodiniaceae and tissue from bleached corals increased by 5-fold (0.6512 ± 0.3890 µg N cm−2 h−1; n = 5; Mann–Whitney–Wilcoxon test, P < 0.05) and 30-fold (0.0057 ± 0.0028 µg −2 h−1; n = 5; Mann–Whitney–Wilcoxon test, P < 0.01), respectively, compared to those measured in the untreated corals (0.1330 ± 0.2465 and 0.0002 ± 0.0004 µg N cm−2 h−1) (Fig. 1, Supplementary Table 1). This demonstrates that corals could incorporate more nitrogen coming from planktonic diazotrophs under bleaching conditions than untreated corals. By providing an alternative source of bioavailable nitrogen, this increased incorporation of nitrogen derived from planktonic diazotrophs may have profound consequences for coral bleaching recovery, particularly in coral reef ecosystems characterized by high planktonic diazotroph abundance and activity. These reefs are very widespread in the Western South Pacific (e.g., New Caledonia, Papua New Guinea, and Australian Great Barrier Reef) [10, 11, 19, 20], but also in Hawaii, in the Caribbean and the Red Sea [21–23]. After 12 h of incubation, the assimilation rates were 100 times greater in Symbiodiniaceae than in coral tissues, regardless of the treatment (n = 10 for each compartment; Mann–Whitney–Wilcoxon test, P = 0.019). This observation is consistent with the results obtained by several authors (e.g., [24], [13], [25], [15, 26], [16]) who demonstrated that symbionts can immediately take up and store nitrogen-derived compounds that are then transferred to the host’s tissue. We conducted quantitative PCR assays to determine planktonic diazotroph abundances (UCYN-A1, UCYN-C, and Trichodesmium, i.e., the most important phylotypes in the lagoon [10, 27]) in the incubation medium at the beginning and at the end of incubation by targeting the nifH gene, a common biomarker for diazotrophs. These assays revealed (1) a significant abundance of diazotrophs in the incubation medium at the beginning of the experiment (UCYN-A1, UCYN-C, and Trichodesmium abundances were, respectively, 4.14 ± 5.35 102, 0.97 ± 1.26 101, and 8.63 ± 6.03 102 nifH gene copies L−1), and (2) a decrease in the abundance of UCYN-A1 (1 µm) and UCYN-C (4–8 µm) in all tanks containing corals (n = 3) compared to the controls without corals, confirming that corals fed on these two types of preys (Supplementary Table 2). While UCYN-A1 are ~1 µm in size, their association with a picoeukaryote host [28] could increase their size to 7–10 µm and thus improve their chances of being consumed by corals. Picoeukaryotes, nanoeukaryotes, and bacterial abundances were further assessed by flow cytometry at the start and end of incubations to quantify their ingestion by both bleached and untreated corals. During the 12 h of incubation, Prochlorococcus was quantitatively the major prey ingested, followed by Synechococcus and picoeukaryotes in both treatments confirming the ability of corals to feed on picoplankton [e.g., [9, 29; Supplementary Table 3]. One of the most notable results of this study is that the ingestion rates of Synechococcus were 1.6 times higher in bleached corals (3.79 ± 0.64 104 cell cm−2 h−1) than in untreated corals (2.38 ± 0.24 104 cell cm−2 h−1, Mann–Whitney–Wilcoxon test, P = 0.028; Fig.2). Until now, studies have shown that corals can regulate their heterotrophic feeding capacities on zooplankton (>50 µm) [6] and on picoflagellates and bacteria [9] in response to bleaching. For the first time, our results show that thermally stressed corals are able to increase not only their consumption of planktonic diazotrophs and plankton that likely benefited from N2 fixation, but also more specifically their ingestion of a very specific taxonomic group of picoplankton: the ubiquitous marine cyanobacterium Synechoccoccus. Surprisingly, bleached colonies of S. pistillata preferentially selected Synechococcus cells, which were not the most abundant in the medium during our incubation, but are known to be rich in nitrogen [30, 31; Supplementary Table 4] and also to benefit from nitrogen released by surrounding diazotrophs in the natural environment [12, 32]. So far, this type of selective feeding on Synechococcus cells has only been shown under controlled conditions in colonies of Porites astreoides [33]. Additional experiments are needed to determine which chemosensory cues are at the origin of this selection [34].
Fig. 1.
Nitrogen assimilation rates (µg N cm−2 h−1) in Symbiodiniaceae (a) and coral tissue (b) in untreated and bleached corals after 12 h of exposure to 15N2-enriched natural plankton assemblage (mean ± SD; n = 5 for each treatment). Horizontal line in each boxplot indicates the median and black dots represent the outlier samples. Asterisks indicate statistically significant differences
Fig. 2.
Ingestion rates (cell cm−2 h−1) of Prochlorococcus (a), Synechococcus (b), and picoeukaryotes (c) in untreated and bleached corals (mean ± SD; n = 5 for each treatment). Horizontal line in each boxplot indicates the median and black dots represent the outlier samples. The asterisk indicates statistically significant differences
Without their symbionts supplying them with nutrients [3], corals thriving within an oligotrophic environment have an urgent need for nitrogen. Our results demonstrate that, unlike in a previous study [15], bleached corals do not meet this nitrogen requirement through the activity of their endosymbiotic diazotrophs but through an external source coming from planktonic diazotrophs and plankton that benefited from N2 fixation. The amount of nitrogen coming from planktonic diazotrophs and Synechococcus for bleached corals, compared to the other nitrogen sources can be estimated (Supplementary Tables 4 and 5). S. pistillata is able to take up inorganic nitrogen (ammonium and nitrate at in situ concentrations) at a rate of 2 ng cm−2 h−1 [35–37] and also estimated that the uptake of organic nitrogen in the form of dissolved free amino acids was ca. 60 ng N cm−2 h−1 leading to a maximal uptake of total dissolved nitrogen of ca. 0.062 µg N cm−2 h−1. In our study we estimate that for the bleached corals, nitrogen coming from diazotrophic plankton and Synechococcus (0.658 µg N cm−2 h−1) brings ten times more nitrogen than what corals take up in the dissolved nitrogen pool when they still contain Symbiodiniaceae. This specific feeding also represents a non-negligible source of carbon for corals devoid of Symbiodiniaceae (Supplementary Tables 4 and 5). Studying the fate of nitrogen derived from planktonic diazotrophs within coral holobionts holds great potential to improve our understanding of nutritional interactions driving coral function and resilience in the context of climate change. Benefiting from N2 fixation could become a common strategy for coral recovery facing bleaching, as both the activity and geographical distribution of diazotrophs will likely increase with future rising sea surface temperature [38, 39].
Supplementary information
Acknowledgements
VM was the beneficiary of a PhD grant from LabEx-Corail (MACADAM project). This work was also funded by the LabEx-Corail FLAMENCO project and the EC2CO/BIOHEFECT program (TOUCAN project). We wish to thank the technical staff of the Aquarium des Lagons (Nouméa, New Caledonia) for their welcome and assistance in tank maintenance. We are especially grateful to three anonymous reviewers for critical reading and valuable comments on this manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s41396-019-0456-2) contains supplementary material, which is available to authorized users.
References
- 1.LaJeunesse TC, Parkinson JE, Gabrielson PW, Jeong HJ, Reimer JD, Voolstra CR, et al. Systematic revision of symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr Biol. 2018;28:2570–80. doi: 10.1016/j.cub.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Hoegh-Guldberg O, . Climate change coral bleaching and the future of the world’s coral reefs. Mar Freshw Res. 1999;50:839–66. [Google Scholar]
- 3.Muscatine L, D’Elia C. The uptake, retention, and release of ammonium by reef corals. Limnol Oceanogr. 1978;23:725–34.
- 4.Godinot C, Houlbrèque F, Grover R, Ferrier-Pagès C. Coral uptake of inorganic phosphorus and nitrogen negatively affected by simultaneous changes in temperature and pH. PLoS One. 2011;6:1–10. doi: 10.1371/journal.pone.0025024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palardy JE, Rodrigues LJ, Grottoli AG. The importance of zooplankton to the daily metabolic carbon requirements of healthy and bleached corals at two depths. J Exp Mar Bio Ecol. 2008;367:180–8. doi: 10.1016/j.jembe.2008.09.015. [DOI] [Google Scholar]
- 6.Grottoli AG, Rodrigues LJ, Palardy JE. Heterotrophic plasticity and resilience in bleached corals. Nature. 2006;440:1186–9. doi: 10.1038/nature04565. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg WM. Coral food, feeding, nutrition, and secretion: A Review. In: Kloc M, Kubiak J, editors. Marine Organisms as Model Systems in Biology and Medicine Results and Problems in Cell Differentiation. 65th ed. Cham: Springer; 2018. [DOI] [PubMed]
- 8.Houlbrèque, F., and Ferrier-Pagès, C. Heterotrophy in Tropical Scleractinian Corals. Biol.Rev. 2009;84:1–17. 10.1111/j.1469-185X.2008.00058.x [DOI] [PubMed]
- 9.Tremblay P, Naumann MS, Sikorski S, Grover R, Ferrier-Pagès C. Experimental assessment of organic carbon fluxes in the scleractinian coral Stylophora pistillata during a thermal and photo stress event. Mar Ecol Prog Ser. 2012;453:63–77. doi: 10.3354/meps09640. [DOI] [Google Scholar]
- 10.Turk-Kubo, K. A., Frank, I. E., Hogan, M. E., Desnues, A., Bonnet, S. and Zehr,J. P. Diazotroph community succession during the VAHINE mesocosmexperiment (New Caledonia lagoon). Biogeosciences 2015;12:7435–7452.
- 11.Messer LF, Brown MV, Furnas MJ, Carney RL, McKinnon AD, Seymour JR. Diversity and activity of diazotrophs in great barrier reef surface waters. Front Microbiol. 2017;8:1–16. doi: 10.3389/fmicb.2017.00967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonnet, S., Berthelot, H., Turk-Kubo, K., Cornet-Barthaux, V., Fawcett, S. E., Berman-Frank, I., Barani, A., Grégori, G., Dekaezemacker, J., Benavides, M. and Capone, GD. Diazotroph derived nitrogen supports diatom growth in the South West Pacific: a quantitative study using nanoSIMS. Limnol. Oceanogr. 2016.
- 13.Benavides, M., Houlbrèque, F., Camps, M., Lorrain,A., Grosso, O., and Bonnet, S. Diazotrophs: a non-negligible source of nitrogen for the tropical coral Stylophora pistillata. J Exp Biol. 2016;1–5. 10.1242/jeb.139451. [DOI] [PubMed]
- 14.Bonnet S, Berthelot H, Turk-Kubo K, Fawcett S, Rahav E, L’Helguen S, and Berman-Frank I. Dynamics of N2 fixation and fate of diazotroph-derived nitrogen in a low-nutrient, low-chlorophyll ecosystem: results from the VAHINE mesocosm experiment (New Caledonia). Biogeosciences. 2016;13:2653–2673.
- 15.Bednarz VN, Grover R, Maguer J-FF, Fine M, Ferrier-Pagès C, The M, et al. The assimilation of diazotroph-derived nitrogen by scleractinian corals depends on their metabolic status. MBio. 2017;8:1–14. doi: 10.1128/mBio.02058-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bednarz Vanessa N, Ferrier-Pagès C, Grover R, Bednarz, et al. Community and associated dinitrogen fixation within the temperate coral Oculina patagonica. Environ Microbiol. 2019;21:480–95. doi: 10.1111/1462-2920.14480. [DOI] [PubMed] [Google Scholar]
- 17.Montoya JP, Voss M, Kahler P, Capone DG. A simple, high-precision, high-sensitivity tracer assay for N2 fixation. Appl Environ Microbiol. 1996;62:986–93. doi: 10.1128/aem.62.3.986-993.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benavides M, Bednarz VN, Ferrier-Pagès C. Diazotrophs: overlooked key players within the coral symbiosis and tropical reef ecosystems? Front Mar Sci. 2017;4:10.
- 19.Messer LF, Mahaffey C, Robinson CM, Jeffries TC, Baker KG, Isaksson JB, et al. High levels of heterogeneity in diazotroph diversity and activity within a putative hotspot for marine nitrogen fixation. ISME J. 2016;10:1499–513. doi: 10.1038/ismej.2015.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonnet S, Caffin M, Berthelot H, Moutin T. Hot spot of N 2 fixation in the western tropical South Pacific pleads for a spatial decoupling between N2 fixation and denitrification. Proc Natl Acad Sci USA. 2017;114:E2800–1. doi: 10.1073/pnas.1619514114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo Y-W, Doney SC, Anderson LA, Benavides M, Berman-Frank I, Bode A, et al. Database of diazotrophs in global ocean: abundance, biomass and nitrogen fixation rates. Earth Syst Sci Data. 2012;4:47–73. doi: 10.5194/essd-4-47-2012. [DOI] [Google Scholar]
- 22.Foster RA, Paytan A, Zehr JP. Seasonality of N2 fixation and nifH gene diversity in the Gulf of Aqaba (Red Sea) Limnol Oceanogr. 2009;54:219–33. doi: 10.4319/lo.2009.54.1.0219. [DOI] [Google Scholar]
- 23.Rahav E, Bar-Zeev E, Ohayon S, Elifantz H, Belkin N, Herut B, et al. Dinitrogen fixation in aphotic oxygenated marine environments. Front Microbiol. 2013;4:1–11. doi: 10.3389/fmicb.2013.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pernice M, Meibom A, Van Den Heuvel A, Kopp C, Domart-Coulon I, Hoegh-Guldberg O, et al. A single-cell view of ammonium assimilation in coral-dinoflagellate symbiosis. ISME J. 2012;6:1314–24. doi: 10.1038/ismej.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krueger T, Bodin J, Horwitz N, Loussert-Fonta C, Sakr A, Escrig S, et al. Temperature and feeding induce tissue level changes in autotrophic and heterotrophic nutrient allocation in the coral symbiosis—a NanoSIMS study. Sci Rep. 2018;8:12710. doi: 10.1038/s41598-018-31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardini U, van Hoytema N, Bednarz VN, Rix L, Foster RA, et al. Al-Rshaidat MMDD, et al. Microbial dinitrogen fixation in coral holobionts exposed to thermal stress and bleaching. Environ Microbiol. 2016;18:2620–33. doi: 10.1111/1462-2920.13385. [DOI] [PubMed] [Google Scholar]
- 27.Henke BA, Turk-Kubo KA, Bonnet S, Zehr JP. Distributions and abundances of sublineages of the N2-fixing cyanobacterium Candidatus Atelocyanobacterium thalassa (UCYN-A) in the New Caledonian coral lagoon. Front Microbiol. 2018;9:1–14. doi: 10.3389/fmicb.2018.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson AW, Foster RA, Krupke A, Carter BJ, Musat N, Vaulot D, et al. Unicellular cyanobacterium symbiotic with a single-celled eukaryotic alga. Science (80-) 2012;337:1546–50. doi: 10.1126/science.1222700. [DOI] [PubMed] [Google Scholar]
- 29.Houlbrèque Fanny, Tambutté Eric, Allemand D, Ferrier-Pagès P. Interactions between zooplankton feeding, photosynthesis and skeletal growth in the scleractinian coral Stylophora pistillata. J Exp Biol. 2004;207:1461–9. doi: 10.1242/jeb.00911. [DOI] [PubMed] [Google Scholar]
- 30.Bertilsson S, Berglund O, Karl DM, Chisholm SW. Elemental composition of marine Prochlorococcus and Synechococcus: implications for the ecological stoichiometry of the sea. Limnol Oceanogr. 2003;48:1721–31. doi: 10.4319/lo.2003.48.5.1721. [DOI] [Google Scholar]
- 31.Jacquet S, Delesalle B, Torréton JP, Blanchot J. Response of phytoplankton communities to increased anthropogenic influences (southwestern lagoon, New Caledonia) Mar Ecol Prog Ser. 2006;320:65–78. doi: 10.3354/meps320065. [DOI] [Google Scholar]
- 32.Berthelot H, Bonnet S, Grosso O, Cornet V, Barani A. Transfer of diazotroph-derived nitrogen towards non-diazotrophic planktonic communities: a comparative study between Trichodesmium erythraeum Crocosphaera watsonii and Cyanothece sp. Biogeosciences. 2016;13:4005–21. doi: 10.5194/bg-13-4005-2016. [DOI] [Google Scholar]
- 33.McNally SP, Parsons RJ, Santoro AE, Apprill A. Multifaceted impacts of the stony coral Porites astreoides on picoplankton abundance and community composition. Limnol Oceanogr. 2017;62:217–34. doi: 10.1002/lno.10389. [DOI] [Google Scholar]
- 34.Lenhoff, H.M., and W. Heagy. 1977. Aquatic invertebrates: model systems for the study of receptor activation and evolution of receptor proteins. Annu. Rev. Pharmacol. Toxicol. 17:243–258. [DOI] [PubMed]
- 35.Grover R, Maguer JF, Reynaud-Vaganay S, Ferrier-Pagès C. Uptake of ammonium by the scleractinian coral Stylophora pistillata: effect of feeding, light, and ammonium concentrations. Limnol Oceanogr. 2002;47:782–90. doi: 10.4319/lo.2002.47.3.0782. [DOI] [Google Scholar]
- 36.Grover R, Maguer JF, Allemand D, Ferrier-Pagès C. Nitrate uptake in the scleractinian coral Stylophora pistillata. Limnol Oceanogr. 2003;48:2266–74. doi: 10.4319/lo.2003.48.6.2266. [DOI] [Google Scholar]
- 37.Hoegh-Guldberg O, Williamson J. Availability of two forms of dissolved nitrogen to the coral Pocillopora damicornis and its symbiotic zooxanthellae. Mar Biol. 1999;133:561–70. doi: 10.1007/s002270050496. [DOI] [Google Scholar]
- 38.Boyd PW, Doney SC. Modelling regional responses by marine pelagic ecosystems to global climate change. Geophys Res Lett. 2002;29:53–53(1–4). [Google Scholar]
- 39.Breitbarth E, Oschlies A, Laroche J. Physiological constraints on the global distribution of Trichodesmium—effect of temperature on diazotrophy. Biogeosciences, 2007;4:53–61
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.