Abstract
The objective of this review is to control fish bacterial diseases or infections through application of some promising novel biocontrol methods, such as probiotics, bio-encapsulated vaccines, and phage therapy, to avoid the disadvantages of traditional one that potentially affects fish and human health. Bacterial infection in intensive fish farming causes mass mortalities and the treatment of that requires the intensive use of chemicals and antibiotics. Several methods have been tried to control fish diseases including the use of antibiotics, but their haphazard use is associated with potentially negative effects as drug resistance and drug residues. The use of probiotics as biocontrol agents for aquaculture is increasing with the demand for environmental beneficial, eco-friendly alternatives for sustainable aquaculture production. The benefits of such supplements include improved food value, inhibition of pathogenic microorganisms, and increased immune response. The bio-encapsulated vaccine appears to be the most attractive method for releasing of vaccines. Several bioactive molecules which are specific for some diseases have been successfully encapsulated with nanoparticles in order to enhance their availability, bioactivity, and controlled delivery. Recently, “reverse vaccine” by using bio-informatics that aids in designing vaccines against infectious pathogens that are difficult to design, especially the intracellular bacteria. Additionally, the use of bacteriophages for biological control of pathogens in cultured fish has gained much interest. Several bacteriophages have been isolated specific to various pathogenic bacteria. Oral administration of phage cocktail is the most suitable way of application in fish, especially when large number of infected fish should be manipulated. Hence, in the following paragraphs, we will discuss some promising novel biocontrol methods that target the fish pathogens like probiotics, bio-encapsulated vaccines, and phage therapy.
Keywords: Bacteriophages, Biocontrol measures, Fish bacteria, Probiotics, Vaccination
Introduction
One-third of the world’s seafood resources come from the aquaculture industry and the sustainable aquaculture production is highly important to face future protein demands (Ravi et al., 2007). The expansion and increase in the aquaculture production have changed fish farms from traditional extensive to a semi-intensive or intensive one. This intensification is considered as the principal cause of stress which depresses the fish immunity that increases its susceptibility to bacterial infections leading to mass mortalities and decrease in fish availability with subsequent massive economic losses (Harper and Wolf, 2009).
Several methods have been tried to control fish diseases such as using chemicals. The use of chemicals and drugs has become a vital input in aquaculture for effective farming and high production. However, the use of antibiotics increased the existence of antimicrobial resistant bacteria as well as drug residues in seafood which become one of the biggest problems that motivate scientists to search for other safe and effective methods (Subharthi Pal, 2015).
Diminishing of antimicrobial drugs in aquaculture industry through using alternative methods has become a critical issue. Several bacterial vaccines, either mono or multivalent, have been successfully developed and commercialized. On the other hand, vaccination has some disadvantages such as difficulty in application, more laborers required, high cost, and impossible to develop prophylactic strategies in new diseases emerging from time to time. Therefore, scientists and aquaculturists adopted recent effective biological control methods, such as probiotics, bio-vaccination, and bacteriophage therapy to maintain a healthy and sustainable aquaculture production (Ram and Parvati, 2012).
Probiotics are cultured products or live microbial food supplements, which beneficially affect the host by improving its intestinal microbial balance. It helps in improving the water quality, aids in food digestion, and modulates the host immune responses, increasing production efficiency and reducing disease incidence (Gatlin and Peredo, 2012). Additionally, probiotics and prebiotics provide benefits to the host via the direct or indirect stimulation of the gut microbiota with different modes of action resulting from increased beneficial bacteria in the gastrointestinal tract and application of probiotics with prebiotics may result in improving health status, increasing the disease resistance and growth rate with improved gut microbial balance (Merrifield et al., 2010).
Another recent approach is the use of bacteriophages in several areas of biotechnology and medical sciences including rapid diagnosis of the bacterial disease termed as “phage typing,” prevention of bacterial disease termed as “phage vaccine,” and biocontrol or treatment as “phage therapy” (Haq et al., 2012). Application of bacteriophages therapy may provide a natural, sustainable solution to control diseases in the fish farming industry. Phage therapy may represent a viable antibiotics alternative. Also, virulent phages are natural nontoxic antimicrobials that when correctly selected and prepared do not have any risk to plant, animal, or the environment (Subharthi Pal, 2015). Due to the urgent need for the advanced measures to control fish bacterial diseases, this review will discuss some promising novel biocontrol methods that target the fish pathogens like probiotics, bio-encapsulated vaccines, and phage therapy.
Conventional control measures
Standard hygienic measures
Control of bacterial diseases in fish farms mainly depends on preventative measures by the introduction of specific-pathogen-free brood stocks, using sanitary certificates with quarantine measures, optimization of feed, improvement of water quality, and good sanitation in conjunction with good health management (FAO, 2008). It is important that effort is made to ensure that no pathogens are introduced into the farm from vehicles, visitors, staff, and equipment. If possible, incoming water should be treated for pathogens using ultra-violet radiation or ozone. This is usually only practically possible in hatcheries or land-based recirculation systems where a relatively small volume of water is being treated (Francis-Floyd, 2003).
Disinfectants
Chemicals used in aquaculture can be classified according to the purpose of use, the type of organisms, the life cycle stage for which they are used, the culture method, intensity of culture, and the type of people who are using them (Gomez-Gil et al., 2000). Disinfectants or chemicals that were and still used for prevention and control of fish bacterial diseases includes potassium permanganate 5 mg/l, 5% phenol, 1% sodium hypochlorite (low organic matter and longer contact times), iodine solutions, glutaraldehyde and formaldehyde are considered effective, and also malachite green and copper sulfate but overdoses of these compounds in aquaculture may lead to toxicity (Bornø and Colquhoun, 2009).
Antibiotics
With the increasing and intensification of fish farms, the demand for using antibiotics in high amount was very clear following the use of chemicals (Bruun et al., 2000). It is recommended not to be broadly dependent on antibiotics to face the contagious and infectious diseases in fish farms as preventative and treatment measures because it has many disadvantages, such as the expense of antibiotics, the short period of protection they offered, the need for repeated treatments in extended outbreaks of disease, the difficulties caused by resistant strains, and increased harmful residues in carcasses (Miranda and Zemelman, 2002). A further problem is the limited range of antibiotics available to treat fish (Van der Waaij and Nord, 2000).
Vaccination
Conventional vaccines are killed bacteria (bacterins) obtained from a broth culture of specific strain subjected to formalin inactivation, where bacterins include both bacterial cells and extracellular products (Soliman, 2005). With the increasing conventional vaccination problems as it mainly administered by injection (oil adjuvant vaccine), the need for booster doses stimulating only the humoral immune response and the short duration of protection that makes scientists and aquaculturists seeking for another biologically effective and eco-friendly methods for treatment and protection (Dahiya et al., 2010).
Recent biocontrol measures
Probiotics
Probiotics are defined as live or dead, or even a component of the microorganisms that acts under different modes of action and conferring beneficial effects to the host or its environment. Several probiotics have been characterized and applied in fish and a number of them are of host origin (Zorriehzahra et al., 2016).
Probiotics selection
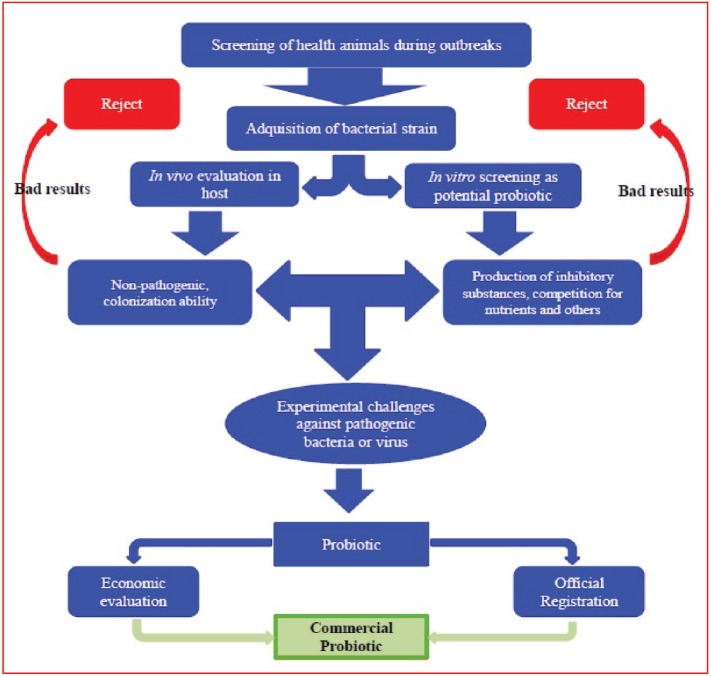
The selection of probiotics depends mainly on in vitro tests for detection of its ability to kill the pathogenic bacteria by applying agar well diffusion test to examine the releasing of some inhibitory substances. Also, applying pathogenicity or challenge test in vivo for detection of animal protection, and after official registration and economic evaluation, it can be approved as commercial products (Balcazar et al., 2006) (Fig. 1).
Fig. 1. Probiotic selection (Balcazar et al., 2006).
Probiotics application
Probiotics are present in dry form which can be administered as food supplementation or added to water or in liquid form which is ready to act. The liquid form can be added directly or mixed with food; it is faster than the dry form (Nageswara and Babu, 2006).
Antagonist of quorum sensing
Quorum sensing (QS) is defined as the regulation of gene expression in response to a communication between pathogenic bacterial cells. Many bacteria are using this system to regulate many physiological activities, and so when adding probiotics, it causes disturbance of QS which is considered a potential anti-infective approach in aquaculture (Defoirdt et al., 2004). On the other hand, halogenated furanones, which are formed by the marine red alga Delisea pulchra, have been discovered as a promising QS antagonist and by using it in adequate concentrations, it can protect rainbow trout from pathogenic vibrio (Tinh et al., 2007). Also, it was demonstrated that Lactobacillus acidophilus secretes some molecules that inhibit the QS of Escherichia coli O157 gene (Medellin-Pena et al., 2007) (Fig. 2).
Fig. 2. Mechanism of quorum sensing antagonism (Defoirdt et al., 2011).
Immunomodulation
Probiotics are considered as immunostimulant that modulates the immune response of the host against infection with increasing of leucocytes and phagocytosis. Also, it increases the lysozymes, complement, and antimicrobial peptides (Mohapatra et al., 2012).
Improving water quality (Bioremediation)
The addition of Gram-positive bacteria, such as Bacillus spp., is beneficial in improving the water quality with the conversion of organic matter into carbon dioxide in comparison to the Gram-negative bacteria, which convert a greater amount of organic matter into bacterial biomass or slime (Balcazar et al., 2006). Additionally, ammonia and nitrite toxicity can be eliminated by the application of nitrifying bacterial cultures into the fish aquaria; moreover, the temperature, pH, dissolved oxygen, NH3, and H2S in rearing water were found to be in permissible limits when probiotics were added. The improvement of water quality or the environment of fish was taking the expression of “bioremediation” (Mohapatra et al., 2012).
Prebiotics
These are non-digestible food constituents represented by mannan-oligosaccharides (MOS) which originated from cell wall components of yeast. It stimulates the intestinal health-promoting bacteria including lactobacillus and so limiting the occurrence of pathogenic bacteria in fish farms (Sohn et al., 2000). Additionally, Rodrigues-Estrada et al. (2008) stated that dietary supplementation with MOS stimulated growth, hemolytic activity, and phagocytic activity improving fish survival in a challenge study with V. anguillarum. Moreover, rainbow trout (Oncorhynchus mykiss) fed with the MOS diet had significantly improved growth performance, antibody titer, and lysozyme activity (Staykov et al., 2007).
Synbiotic
It is a combination of both prebiotics and probiotics that indicated to improve the survival and propagation of the live microbiota in the gastrointestinal tract. An individual administration of the dietary Enterococcus faecalis or MOS in salmonid fish provided a wide range of benefits regarding the immune response and survival in a challenge study with V. anguillarum. However, it was clear that synbiotic feeding (E. faecalis + MOS) yielded significantly improved results than either individual probiotic or prebiotic application (Gatlin and Peredo, 2012).
Bio vaccines
Living attenuated vaccines
This type of vaccine is not inactivated but it can be made by decreasing its virulence genetically and so, giving live vaccine induces an immune response in the host for a short period of time (Adams et al., 2008). This vaccine has great importance in aquaculture. The application of attenuated or modified live bacterial vaccine in aquaculture was started in 1990 (Sun et al., 2010). Attenuated vaccines for fish need to apply strict examination before licensing. Vaccination using an attenuated vaccine is an imitation model of infection. It significantly stimulates the cellular immune response. Also, they are capable of stimulating humoral and mucosal immunity (Clark and Cassidy-Hanley, 2005).
Encapsulated oral vaccine
Live feeds bio-encapsulated vaccine
The encapsulation needs to be applied either to prevent the escaping of antigens from the pellets or to protect it from the acidic environment in the fish stomach. Bio-encapsulated feed is particularly suitable for fish fry and by which live feeds such as Artemia, copepods, and rotifers are incorporated with the vaccine suspension and fed to fish (Lin et al., 2005). The bio-encapsulated feed then releases the vaccine into the digestive tract of the fish, which appears to be the most attractive method for releasing of vaccines. It reduces the handling of fish and consequently reduces the fish stress. It is also being suitable for mass immunization. For effective oral vaccine delivery, the antigen should not be subject to digestive hydrolysis and should be absorbed well for inducing a protective immune response (Vandenberg, 2004).
Nano-bio-encapsulated vaccine
Recent research studies have paid attention to the use of nanoparticles (NPs) as adjuvant and efficient delivery systems in fish vaccine development due to their nano size. These NPs can be grasped by cellular endocytosis mechanism which facilitates the cellular uptake of antigens and increases its presentation ability (Vinay et al., 2018).
Bacteriophages therapy
Another highly significant biological control method for pathogenic bacteria in aquaculture is the use of bacteriophages which are harmless for humans and animals and can be applied safely as therapeutic means. Phages have been used in several areas of biotechnology and medical sciences, including prevention of bacterial disease, treatment, rapid detection of disease, and biological control (Haq et al., 2012). Moreover, bacteriophages are highly specific and can only infect bacterial cells that have cell surface receptors matching those of the phage (similar to a key and lock mechanism) (Kutter and Sulakvelidze, 2004). Also, bacteriophages used for biological control of pathogens in cultured fish have gained much interest, as no drug residues are associated with such treatment (Jun et al., 2016; Silva et al., 2016). Furthermore, the use of phage for therapeutic purpose in aquaculture has potential to control diseases depending on the isolation and identification of phage, which will specifically kill the pathogens (Higuera et al., 2013).
Phage therapy dosage
Determining the proper dosage of phages is very important for effective phage therapy. A varying dosage has been reported by several studies both experimentally and in field conditions, and the phage treatment may not be cost effective if the concentration of phage required is very high. It should be focused mainly on isolating and characterizing those phages which have a high infecting ability at lower doses as well as high replication rate (Rong et al., 2014).
Modes of application of phages in aquaculture
Several modes of application for phages as therapeutic and prophylactic agents to control bacterial diseases in aquaculture have been reported, which include oral administration through the feed, intramuscular or intraperitoneal administration, anal intubation, immersion and direct release of phages in culture system (Silva et al., 2016). Moreover, each mode of application has its own advantages and disadvantages which mostly depend on nature of the pathogen, also the route of administration cannot be inclusive, for example, injecting a very small fish or shellfish is not practical. Immersion therapy with high titer bacteriophages is also difficult in large aquaria. The direct release method may depend on the aquatic habitat, the nature of infection, or phage properties (Richards, 2014). On the other hand, phage cocktails are new exciting measures in phage application as two or more phages accompanied together, or combination of phages with other therapeutics like antibiotics, lysozymes has been reported in aquaculture (Mateus et al., 2014).
Conclusion
Bacterial infection in intensive fish farming causes mass mortalities. The recent advances and future perspectives of vaccines in the aquaculture sector has new generation vaccines including recombinant, subunit, vectored, genetically engineered, DNA, peptide, and nanovaccines. Oral administration of phage cocktails is the most suitable application way when a large number of infected fish should be manipulated. Isolation and availability of phages for certain pathogens is a native matter of each country. Most of the alternative biocontrol strategies are still in the research phase.
Significance statement
According to the previous reviewed data, this study introduces the control of bacterial infection in intensive fish farming, which may be succeeded by following some recommendations as; avoid haphazard using of chemicals and antibiotics in aquaculture due to their potential negative effects as drug resistance and residues, for success of probiotic applications many factors such as the proper management of bacterial strains, necessary dosages, and ensuring of bacterial safety must be available, avoid probiotic selection with single mode of action by production of inhibitory substances because the pathogenic bacteria will probably develop resistance resembling antibiotics and due to the anxious which still remain about the toxicity of NPs and some limitations for using bacteriophages, it is preferable to use probiotics for their low limitation and widely accepted as eco-friendly alternatives.
References
- Adams A, Aoki T, Berthe C, Grisez L, Karunasagar I. Diseases in Asian Aquaculture VI. Colombo, Sri Lanka: Fish Health Section, Asian Fisheries Society; 2008. Recent technological advancements on aquatic animal health and their contributions toward reducing disease risks-a review; pp. 71–88. [Google Scholar]
- Balcazar J.L, de Blas I, Ruiz-Zarzuela I, Cunningham D, Vendrell D, Muzquiz J.L. The role of probiotics in aquaculture. Vet. Microbiol. 2006;114:173–186. doi: 10.1016/j.vetmic.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Bornø G, Colquhoun D. Classical furunculosis (in Norwegian) Fact sheet, Norwegian Veterinary Institute. 2009. Available at: https://stim.no/tjenester/fiskehelsetjenester/diagnostikk/
- Bruun M.S, Schmidt A.S, Madsen L, Dalsgaard I. Antimicrobial resistance patterns in Danish isolates of Flavobacterium psychrophilum. Aquaculture. 2000;187:201–212. [Google Scholar]
- Clark T, Cassidy-Hanley D. Recombinant subunit vaccines: potentials and constraints. Dev. Biol. 2005;121:153–163. [PubMed] [Google Scholar]
- Dahiya T.P, Kant R, Sihag R.C. Use of probiotics as an alternative method of disease control in aquaculture. Biosphere. 2010;2:52–57. [Google Scholar]
- Defoirdt T, Boon N, Boosier P, Verstraete W. Disruption of bacterial quorum sensing: an unexplored strategy to fight infections in aquaculture. Aquaculture. 2004;240:69–88. [Google Scholar]
- Defoirdt T, Sorgeloos P, Bossier P. Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr. Opin. Microbiol. 2011;14:251–258. doi: 10.1016/j.mib.2011.03.004. [DOI] [PubMed] [Google Scholar]
- FAO. Rome, Italy: Fisheries and Aquaculture Department, Food and Agriculture Organization of the United Nations; 2008. [3 January 2019]. Hygiene and fish safety. Available via http://www.fao.org/fishery/topic/12328/en . [Google Scholar]
- Francis-Floyd R. Sanitation practices for aquaculture facilities. 2003. Available via https://ufdc.ufl.edu/IR00004507/00001 .
- Gatlin D.M, Peredo A.M. Southern Regional Publication Centre; 2012. Prebiotics and probiotics: definition and application. SRAC Publication No. 4711. [Google Scholar]
- Gomez-Gil B, Roque A, Turnbull J.F. The use and selection of probiotic bacteria for use in the culture of larval aquatic organisms. Aquaculture. 2000;191:259–270. [Google Scholar]
- Haq I.U, Chaudhry W.N, Akhtar M.N, Andleeb S, Qadri I. Bacteriophages and their implications on future biotechnology: a review. Virol. J. 2012;9:1–8. doi: 10.1186/1743-422X-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Wolf J.C. Morphologic Effects of the Stress Response in Fish. ILAR J. 2009;50(4):387–396. doi: 10.1093/ilar.50.4.387. [DOI] [PubMed] [Google Scholar]
- Higuera G, Bastías R, Tsertsvadze G, Romero J, Espejo R.T. Recently discovered Vibrio anguillarum phages can protect against experimentally induced vibriosis in Atlantic salmon, Salmo salar. Aquaculture. 2013;392:128–133. [Google Scholar]
- Jun J.W, Han J.E, Tang K.F, Lightner D.V, Kim J, Seo S.W, Park S.C. Potential application of bacteriophagepVp-1: Agent combating Vibrio parahaemolyticus strains associated with acute hepatopancreatic necrosis disease (AHPND) in shrimp. Aquaculture. 2016;457:100–103. [Google Scholar]
- Kutter E, Sulakvelidze A. Boca Raton, FL: CRC Press; 2004. Bacteriophages: biology and applications; p. 528. [Google Scholar]
- Lin J.H, Yu C.C, Lin C.C, Yang H.L. An oral delivery system for recombinant subunit vaccine to fish. Dev. Biol (Basel) 2005;121:175–180. [PubMed] [Google Scholar]
- Mateus L, Costa L, Silva Y.J, Pereira C, Cunha A, Almeida A. Efficiency of phage cocktails in the inactivation of Vibrio in aquaculture. Aquaculture. 2014;424:167–173. [Google Scholar]
- Medellin-Pena M.J, Wang H, Johnson R, Griffiths M.W. Probiotic effects virulence related gene expression in Escherichia coli O157:H7. Appl. Environ. Microbiol. 2007;73:4259–4267. doi: 10.1128/AEM.00159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield D.L, Dimitroglou A, Foey A, Davies S.J, Baker R.T.M, Bøgwald J, Castex M, Ringø E. The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture. 2010;302:1–18. [Google Scholar]
- Miranda C.D, Zemelman R. Bacterial resistance to oxytetracycline in Chilean salmon farming. Aquaculture. 2002;212:31–47. [Google Scholar]
- Mohapatra S, Chakraborty T, Kumar V, De Boeck G, Mohanta K.N. Aquaculture and stress management: a review of probiotic intervention. J. Anim. Physiol. Anim. Nut. 2012;14:1–26. doi: 10.1111/j.1439-0396.2012.01301.x. [DOI] [PubMed] [Google Scholar]
- Nageswara P.V, Babu D.E. Probiotics as an alternative therapy to minimize or avoid antibiotics use in aquaculture. Fishing Chimes. 2006;26(1):112–114. [Google Scholar]
- Ram C.S, Parvati S. Probiotics: the new ecofriendly alternative measures of disease control for sustainable aquaculture. J. Fish. Aqua. Sci. 2012;7:72–103. [Google Scholar]
- Ravi A.V, Musthafa K.S, Jegathambal G, Kathiresan K, Pandian S.K. Screening and evaluation of probiotics as a biocontrol agent against pathogenic vibrio in marine aquaculture. Lett. Appl. Microbiol. 2007;45:219–223. doi: 10.1111/j.1472-765X.2007.02180.x. [DOI] [PubMed] [Google Scholar]
- Richards G.P. Bacteriophage remediation of bacterial pathogens in aquaculture: a review of the technology. Bacteriophage. 2014;4(4):e975540. doi: 10.4161/21597081.2014.975540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Estrada U, Satoh S, Haga Y, Fushimi H, Sweetman J. Studies the Effects of Mannan-oligosaccharides, Enterococcus faecalis, and Poly Hydrobutyric Acid as Immune Stimulant and Growth Promoting Ingredients in Rainbow Trout Diets. 5th World Fisheries Congress; October 20–25; Yokohama, Japan. 2008. p. 158. Abstract. [Google Scholar]
- Rong R, Lin H, Wang J, Khan M.N, Li M. Reductions of Vibrio parahaemolyticus in oysters after bacteriophage application during depuration. Aquaculture. 2014;418:171–176. [Google Scholar]
- Silva Y.J, Moreirinha C, Pereira C, Costa L, Rocha R.J, Cunha A, Gomes N.C, Calado R, Almeida A. Biological control of Aeromonas salmonicida infection in juvenile Senegalese sole (Solea senegalensis) with Phage AS-A. Aquaculture. 2016;450:225–233. [Google Scholar]
- Sohn K.S, Kim M.K, Kim J.D, Han I.K. The role of Immunostimulants in monogastric animal and fish—review. Asian-Australas J. Anim. Sci. 2000;13:1178–1187. [Google Scholar]
- Soliman W.S. Faculty of Veterinary Medicine Cairo University, Egypt: 2005. The quest for the development and efficacy of different polyvalent fish vaccine formulations against Aeromonas spp. and Pseudomonas fluorescens. Ph.D. Thesis, (Microbiol) [Google Scholar]
- Staykov Y, Spring P, Denev S, Sweetman J. Effect of amannan oligosaccharide on the growth performance and immune status of rainbow trout (Oncorhynchus mykiss) Aquacult. Int. 2007;15:153–161. [Google Scholar]
- Subharthi Pal N.A. Phage Therapy an alternate disease control in Aquaculture: a review on recent advancements. IOSR J. Agri. Vet. Sci. 2015;8(9):68–81. [Google Scholar]
- Sun Y, Liu C-S, Sun L. Isolation and analysis of the vaccine potential of an attenuated Edwardsiella tarda strain. Vaccine. 2010;28:6344–6350. doi: 10.1016/j.vaccine.2010.06.101. [DOI] [PubMed] [Google Scholar]
- Tinh N.T, Dierckens K, Sorgeloos P, Bossier P. A review of the functionality of probiotics in the larviculture food chain. Marine Biotechnol. 2007;10(1):1–12. doi: 10.1007/s10126-007-9054-9. [DOI] [PubMed] [Google Scholar]
- Van der Waaij D, Nord C.E. Development and persistence of multi-resistance to antibiotics in bacteria; an analysis and a new approach to this urgent problem. Int. J. Antimicrob. Agents. 2000;16:191–197. doi: 10.1016/s0924-8579(00)00227-2. [DOI] [PubMed] [Google Scholar]
- Vandenberg G.W. Oral vaccines for finfish: academic theory or commercial reality? Anim. Health Res. Rev. 2004;5:301–304. doi: 10.1079/ahr200488. [DOI] [PubMed] [Google Scholar]
- Vinay T.N, Bhat S, Choudhury T.G, Paria A, Jung M-H, Kallappa G.S, Jung S-J. Recent advances in application of nanoparticles in fish vaccine delivery. Rev. Fish. Sci. Aquacult. 2018;26(1):29–41. [Google Scholar]
- Zorriehzahra M.J, Delshad S.T, Adel M, Tiwari R, Karthik K, Dhama K, Lazado C.C. Probiotics as beneficial microbes in aquaculture: an update on their multiple modes of action: a review. Vet. Q. 2016;36(4):228–241. doi: 10.1080/01652176.2016.1172132. [DOI] [PubMed] [Google Scholar]