Abstract
Von Hippel-Lindau (VHL) disease is a hereditary cancer syndrome characterized by poor survival. The effect of the involvement of each organ on survival remains unclear. Our study aimed to study the effect of the involvement of each organ on survival in VHL disease patients. We retrospectively analyzed 336 patients from 125 families. The onset age was compared between different groups using Mann-Whitney U test and Kruskal-Wallis test. Univariate and multivariate time-dependent Cox regression analyses were conducted to evaluate how survival was influenced by the involvement of each organ. The median survival time for VHL disease patients was 66 years. The onset age was earlier in the central nervous system (CNS) group than in the abdominal group. The involvement of central nervous system hemangioblastoma (CHB) and retinal hemangioblastoma (RA) were independent risk factors for overall survival. The involvement of renal cell carcinoma (RCC) was not a significant risk factor for overall survival. Only RA was a risk factor for CHB-specific survival. This study analyzed the relationship between organ involvement and survival of VHL patients. This may help guide future genetic counseling and clinical decision-making.
Keywords: von Hippel-Lindau disease, risk factor, genetic cancer syndrome, prognosis, survival
Introduction
Von Hippel-Lindau (VHL) disease (MIM 193300) is an autosomal dominant tumor syndrome caused by germline mutations in the tumor suppressor gene VHL located on 3p25. The incidence is ~1 in 36,000–40,000 live births and has high penetrance (1–3). Benign and malignant tumors including central nervous system hemangioblastoma (CHB), retinal hemangioblastoma (RA), renal cell carcinoma (RCC), pheochromocytoma (PHEO), pancreatic lesions, including pancreatic cystic lesion, and pancreatic neuroendocrine tumors (PCL/PNET), endolymphatic sac tumor (ELST) and papillary cystadenomas of the epididymis or broad ligament are the main manifestations of this disease (4–11). Both intra- and interfamilial phenotypic heterogeneity can be seen in VHL disease patients (12, 13).
Many studies indicated that the survival of VHL disease patients was worse than the general population. A study in 1990 showed that the median survival was 49 years (14). A recent study reported the estimated life expectancy for male and female patients was 67 and 60 years, respectively (15). In 2012, a study reported that the median life expectancy for VHL disease patients was 52 years (16). Previously reported risk factors for survival of VHL disease patients included early birth year, fewer monitoring visits, female gender, positive family history, early disease onset, truncating mutation type and RCC larger than 3 cm (15, 17, 18). However, no previous research has studied the effects of the involvement of each organ in a large patient group.
The effect of the involvement of different organs on survival have been studied in other systemic diseases. For example, uterine involvement indicates worse survival in patients with diffuse large B-cell lymphoma than in those with the involvement of other organs (19). Yuda et al. reported that pericardial effusion and multiple organ involvement were predictors of mortality in patients with systemic light chain amyloidosis (20). Although some previous studies established genotype-phenotype correlations in VHL disease: patients with missense mutations are more likely to develop pheochromocytoma, and patients with truncating mutations are more likely to develop RCC and CHB (21–23). The existing genotype-phenotype correlations can not explain other complex phenotypes. Hence it's vital to study the prognosis of VHL disease from an organ involvement perspective (23, 24).
In this study, we aimed to study the overall survival and CHB-specific survival in a large VHL disease patient group that includes asymptomatic mutation carriers and to investigate the influence of CHB, RCC, RA, PCL/PNET and PHEO, in order to improve genetic counseling and clinical treatment strategies for VHL disease patients.
Materials and Methods
Patients and Samples
We recruited all the patient information stored in the database of VHL disease patients at Peking University First Hospital. The diagnosis was established when the patient was found to carry a germline VHL gene mutation or fulfilled the previously described clinical criteria (25, 26). At least one patient was diagnosed by VHL mutation test in each family, except those refused the test. In total, 376 patients from 134 families were diagnosed. Clinical data including sex, family history, mutation type, onset age of each organ and the cause of death were obtained by reviewing medical records or interviewing family members. Forty patients were excluded from the study due to unclear data. A total of 336 patients from 125 families were finally enrolled in this cohort, in which 298 of the patients had at least one VHL disease-related lesion and 38 patients were asymptomatic mutation carriers.
We recorded the survival status of the 336 patients from birth to death or to the end of follow-up in June 2017. The median follow-up time was 37 years/person (range 1–75 years), with a total of 12,966 person-years.
Genetic Analysis
A QIAamp DNA Blood Mini Kit (QIAGEN, Germany) was used to extract genomic DNA from peripheral blood of suspected patients. Three coding exons and their flanking intronic regions were amplified by PCR using primers described previously (27). Missense mutations, splicing mutations, and small indels were detected by direct sequencing. A multiplex ligation-dependent probe amplification kit (MLPA, P016-C2, MRC-Holland, Amsterdam) was used to detect large deletions. Large exon deletions were confirmed by real-time quantitative PCR with the primers described by Ebenazer et al. (28). Patients were divided into a missense mutation group (n = 162) and a truncating mutation group (n = 174) based on the criteria described previously (18).
Statistical Analysis
Patient demographics and basic clinical data were analyzed using descriptive statistics. The onset age of lesions in different organs was compared using Kruskal-Wallis test; pairwise comparisons were conducted, and the P value was determined after Bonferroni adjustment. The onset age in the CNS group and abdominal group was compared using Mann-Whitney U test.
Because follow-up was performed from birth, patients before disease onset and after disease onset have different risks for survival. This situation does not fit the requirements of the proportional hazard model, making Cox regression analysis inappropriate. Instead, the involvement of every organ was treated as a time-dependent covariate in the Cox regression model. Univariate and multivariate Cox regression analyses were used to assess the effects of CHB, RCC, RA, PCL/PNET, and PHEO on overall survival. We then used univariate and multivariate Cox regression analyses to determine the effect of RCC, RA, PCL/PNET, and PHEO on CHB-specific survival because CHB is the most common cause of death in VHL disease patients.
Statistical analysis was performed using SPSS 22.0 and GraphPad Prism software (version 6). P < 0.05 was considered statistically significant. For pairwise comparisons, Bonferroni's adjustment was conducted.
Ethics Statement
This study was approved by the Institutional Review Board of Peking University First Hospital (Beijing, China), and written consent was obtained from all patients.
Results
Clinical Characteristics of VHL Disease Patients
CHB was the most common lesion in our study, and more than half of the patients had CHB lesions (62.2%). PCL/PNET was the second most common lesion (46.4%). Nearly two-thirds of the deceased patients died of CHB (66.2%). The prevalence of CHB and RCC in this study was generally in line with the results of previous studies (Supplementary Table 1). The median years of age were ≤ 30 years at the onset of CHB and RA, and were >30 years at the onset of RCC, PHEO and PCL/PNET (Table 1). We combined CHB and RA into a central nervous system (CNS) group, and RCC, PHEO and PCL/PNET into an abdominal group. The distribution of the onset age in the two groups is shown in Figure 1. The median years of age at the onset of tumors in abdominal and CNS groups were 34 and 29 years, respectively. Mann-Whitney U test indicated that the onset age was significantly younger in CNS group than in abdominal group (P < 0.001).
Table 1.
Age at the onset of VHL-related lesions.
| Lesion | Mean ± SD | Median (range) |
|---|---|---|
| CHB | 31.4 ± 11.0 | 30 (12–66) |
| RCC | 38.2 ± 11.3 | 36.5 (18–69) |
| RA | 30.3 ± 13.3 | 28.5 (11–66) |
| PCL/PNET | 36.3 ± 11.9 | 34 (12–67) |
| PHEO | 35.0 ± 13.6 | 36 (8–62) |
Figure 1.
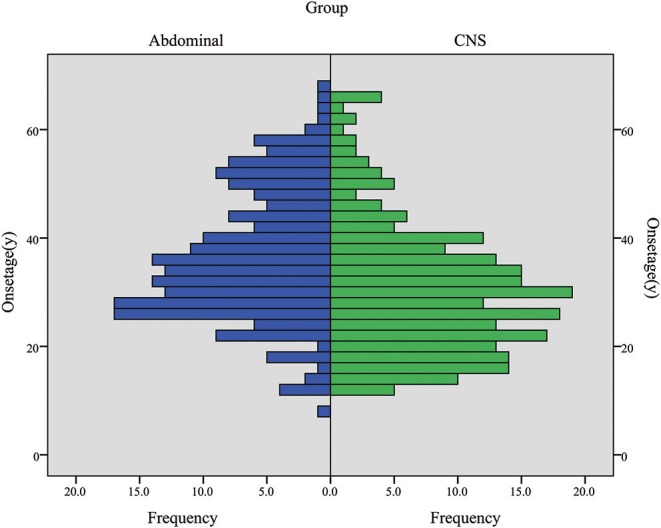
The onset age frequency in CNS group (including CHB & RA patients) and abdominal group (including RCC, PHEO & PCL/PNET patients).
Overall Survival and CHB-Specific Survival in VHL Disease Patients
The overall survival and CHB-specific survival are shown in Figures 2A,B. The median survival time for VHL disease patients was 66 years. Together, CHB and RCC accounted for 95.6% of all death cases. Nearly two thirds of the patients died of CHB-related conditions and more than one-third of the patients died of RCC. Among 209 patients with CHB, 45 patients died of CHB, 10 patients died of RCC. Among 138 patients with RCC, 16 patients died of RCC, 4 patients died of CHB. Among 86 patients with both lesions, CHB and RCC contributed equally to deceased patients, both cause 8 deaths. CHB was the more common cause of death than RCC in our study (Table 2).
Figure 2.
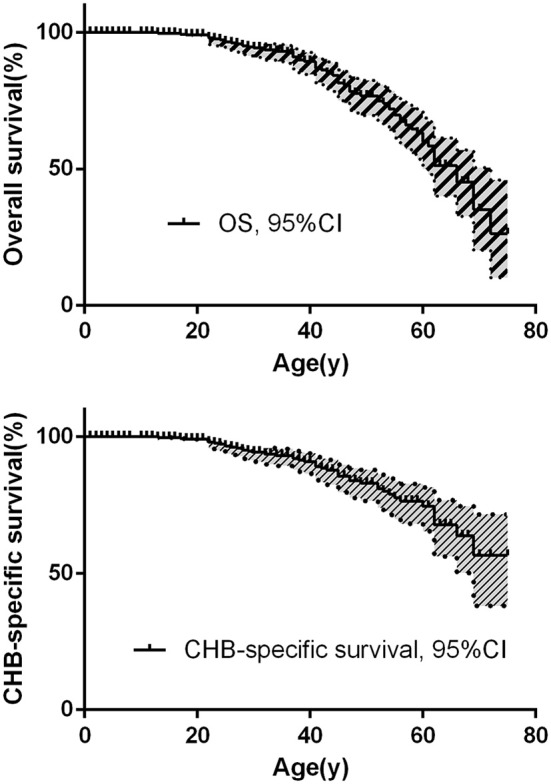
(A) The overall survival of VHL patients. (B) CHB-specific survival of VHL patients.
Table 2.
Baseline clinical characteristics of VHL disease patients.
| Patient (n) | Patient (n) | ||
|---|---|---|---|
| Overall | 336 | CHB involvement | |
| Sex | Yes | 209 (62.2%) | |
| Male | 160 (47.6%) | No | 127 (37.8%) |
| Female | 176 (52.4%) | RCC involvement | |
| Family history | Yes | 138 (41.1%) | |
| Yes | 265 (78.9%) | No | 198 (58.9%) |
| No | 71 (21.1%) | RA involvement | |
| Mutation type | Yes | 80 (23.8%) | |
| Missense | 162 (48.2%) | No | 256 (76.2%) |
| Truncating | 174 (51.8%) | PCL/PNET involvement | |
| First onset organ | Yes | 156 (46.4%) | |
| CHB | 157 (46.7%) | No | 180 (53.6%) |
| RCC | 52 (15.5%) | PHEO involvement | |
| RA | 37 (11.0%) | Yes | 46 (13.7%) |
| PCL/PNET | 16 (4.8%) | No | 290 (86.3%) |
| PHEO | 19 (5.7%) | Total number of death | 68 |
| Two or more organs* | 17 (5.1%) | CHB | 45 (66.2%) |
| Mutation carriers | 38(11.3%) | RCC | 20 (29.4%) |
| PHEO | 2 (2.9%) | ||
| Unrelated to VHL | 1 (1.5%) |
Two or more organs involved at disease onset.
Univariate and Multivariate Cox Regression Analyses for Overall Survival
In univariate analysis, CHB (HR 2.583, 95% CI 1.545–4.318, P = 0.001) and RA (HR 2.832, 95% CI 1.581–5.071, P = 0.001) were associated with poorer overall survival. RCC did not significantly affect the overall survival (HR 1.533, 95% CI 0.835–2.814, P = 0.168). Multivariate Cox regression analysis showed similar results. CHB (HR 2.426, 95% CI 1.433–4.106, P = 0.001) and RA (HR 2.440, 95% CI 1.337–4.455, P = 0.004) were predictors of poorer overall survival. Again, the effect of RCC on overall survival was not statistically significant (HR 1.554, 95% CI 0.747–3.233, P = 0.239; Table 3).
Table 3.
Univariate and multivariate Cox regression analyses for overall survival.
| Involvement | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| CHB (Yes vs. No) | 2.583 (1.545–4.318) |
0.001 | 2.426 (1.433–4.106) |
0.001 |
| RCC (Yes vs. No) | 1.533 (0.835–2.814) |
0.168 | 1.554 (0.747–3.233) |
0.239 |
| RA (Yes vs. No) | 2.832 (1.581–5.071) |
0.001 | 2.440 (1.337–4.455) |
0.004 |
| PCL/PNET (Yes vs. No) | 0.954 (0.476–1.913) |
0.894 | 0.534 (0.232–1.228) |
0.140 |
| PHEO (Yes vs. No) | 0.586 (0.182–1.886) |
0.370 | 0.814 (0.250–2.649) |
0.733 |
HR, hazard ratio; CI, confidence interval. Y, yes. N, no.
Bold values are statistically significant (P < 0.05). The involvement of each organ was treated as a time-dependent covariate.
Effects of Different Organ Involvement on CHB-Specific Survival
Because CHB accounted for nearly two-thirds of the death (66.2%, 45 of 68) and CHB was a significant risk factor for overall survival in both univariate and multivariate Cox regression analyses, we further studied the effect of other organ involvement on CHB-specific survival. The involvement of every organ was treated as a time-dependent covariate.
In univariate Cox regression analysis, only RA was a risk factor for CHB-specific survival (HR 2.080, 95% CI 0.962–4.495) but the P value was not significant (P = 0.063). In the multivariate analysis, RA was a significant risk factor for CHB-specific survival (HR 2.210, 95% CI 1.005–4.862, P = 0.049; Table 4).
Table 4.
Univariate and multivariate time-dependent Cox regression analyses of the effect of different organ involvement on CHB-specific survival.
| Involvement | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | |
| RCC (Y vs. N) | 0.576 (0.200–1.661) |
0.308 | 0.523 (0.152–1.797) |
0.303 |
| RA (Y vs. N) | 2.080 (0.962–4.495) |
0.063 | 2.210 (1.005–4.862) |
0.049 |
| PCL/PNET (Y vs. N) | 0.769 (0.294–2.010) |
0.592 | 1.003 (0.334–3.015) |
0.995 |
| PHEO (Y vs. N) | 0.043 (0.001–9.138) |
0.250 | 0.001 (0.001–9.458) |
0.974 |
HR, hazard ratio; CI, confidence interval. Y, yes. N, no.
Bold values are statistically significant (P < 0.05).
The involvement of each organ was treated as a time-dependent covariate.
The Effect of RCC Size on Patient Survival
Our database contained only 51 patients with a complete natural history of RCC. We further categorized these patients into 2 groups: the first group, RCC diameter of <3 cm throughout their natural history (n = 15), and the second group, RCC diameter of >3 cm in the period of their natural history (n = 36). All the patients in the first group survived, Three patients in the second group died by the end of follow-up; one died of a CHB-related condition, and the other two died of metastatic RCC with the RCC diameters of 7 and 8.5 cm at the time of diagnosis.
Discussion
In this study, CHB and RCC were still the leading causes of deaths in VHL patients, together caused 95.6% of all deaths. We analyzed the effect of every involved organ on overall survival and CHB-specific survival of VHL disease patients. CHB and RA were independent risk factors for overall survival. Surprisingly, RCC was not a significant risk factor for overall survival. RA was the only risk factor for CHB-specific survival. In the past decades, the cause of death in VHL disease patients has shifted greatly. In studies on VHL disease published in the early years, CHB and RCC contributed approximately equally to the death of patients; in some studies, the number of death due to RCC was even twice more than that due to CHB (29). In recent studies, however, the proportion of CHB-related death was significantly higher than that of RCC-related death (Supplementary Table 1). Such discrepancy can partially explained by the development of advanced imaging technologies and treatment strategies including targeted therapy, minimally invasive surgery and active surveillance for RCCs, contributing to the early diagnosis and effective treatment before metastasis of RCCs. On the other hand, the pharmacological and surgical treatment for CHB are required to be further improved.
Although RCC in VHL disease is usually considered to be less malignant and less likely to metastasize and to have higher cancer-specific survival compared with sporadic clear cell RCC (30), RCC is still an important cause of death in VHL patients. Most deaths are due to tumor metastasis and tumor-related complications. Surprisingly in this study, the effect of RCC on overall survival was not significant. A Korean study including 24 VHL disease patients published in 2009 showed similar results (31). Previous studies have demonstrated that the RCC diameter of >3 cm is a risk factor for metastasis and overall survival (17, 32). In the VHL disease patient database we analyzed in this study, among the 51 patients with complete natural history of RCC tumor size, only 3 patients died during followup, rendering it impossible to perform survival analysis. Jilg et al. reported that the mean tumor diameter in patients without metastases was significantly smaller than that in patients with metastases (33). Duffey et al. reported in 2004 that patients with RCCs smaller than 3 cm were at a low risk of distant metastasis and recommended 3 cm as the cutoff value for surgical intervention (34). RCC is still an important cause of death in VHL disease patients. Tumor size and growth rate may be of greater significance for prognosis. Larger studies with longer follow-up periods are needed to study the relationship between survival and RCCs.
Although CHB is considered to be a benign tumor, it can cause severe neurological defects, such as hydrocephalus, herniation, and brain stem compression, and in rare cases intracranial hemorrhage (14, 29). Perioperative/postoperative complications can also lead to death in CHB patients. The frequency of CHB involvement in study sample may affect survival analysis. 62.2% (209 of 336) patients had CHB in our study, which is below the average number of patients with CHB in the studies listed in Supplementary Table 1. CHB accounted for 66.2% of all death patients in this cohort, which is higher than 6 of the 7 studies listed in Supplementary Table 1. This may be caused by birthyear of patients. In a Danish study published in 2016, CHB-related death rate rise from 42% (birth year 1901–1955, 18 of 50) to 83% (birth year 1956–2010, 5 of 6) as birth year increases, with a total CHB-related death rate of 51%. The author attributed such discrepancy to progress in diagnosis and treatment of RCC (15). However, in our study, 83.3% (280 of 336) patients were born after 1955, only 16.7% (56 of 336)patients were born before 1955. And CHB-related death rate was 60.1% for patient born before 1955, but increased to 70.1 and 75% for patients born during 1955–1975 and after 1975. RA causes blindness; however in our study, RA was a significant risk factor for overall survival and CHB-specific survival. Retina embryologically originates from brain, and both CHB and RA share the same histopathological structures (7). The oncogenesis of hemangioblastoma is related to the dysregulation of HIF pathway leading to the increase of vascular endothelial growth factor and erythropoietin (35, 36). Franke et al. reported that deletion mutations in VHL gene had a higher risk of CHB and RA (37). Thus, we hypothesize that RA may reflect a greater burden of CHB, leading to unfavorable survival of VHL disease patients.
Hemangioblastoma is a significant risk factor for survival. Another possible explanation for this is the earlier onset age of this tumor. Our previous research revealed that early onset age was a risk factor for overall survival and VHL disease-specific survival (18). The median age at the onset of CHB and RA were 28.5 and 30 years, respectively, being the earliest among all VHL-related lesions. When CHB and RA were combined as a CNS group, this group also had earlier onset age than abdominal group. We conducted a Kruskal-Wallis test to compare the onset age of the lesions in 5 organs, and P < 0.001 suggests a significant difference. We further made pairwise comparisons and the P value was valuated after Bonferroni adjustment, and significant differences of age onset were found between RA-PCL/PNET, RA-RCC, CHB-PCL/PNET, and CHB-RCC (Supplementary Table 2). Therefore, although hemangioblastoma is considered to be a benign tumor, its early onset may worsen the survival of the VHL disease patients.
Several previous studies that only included symptomatic patients may have biased results that overestimate the rate of involved organs and the effect of involved organs on patient survival (31, 38). We first used the time-dependent Cox regression analysis to evaluate the effect of every involved organ on the survival of VHL disease patients. In a previous study, overall survival was calculated from disease diagnosis to death; however asymptomatic mutation carriers can develop tumors later and do not fit the requirements of the proportional hazard model (17).
This large retrospective study focused on different organ involvement on survival in VHL disease patients from a clinical perspective. The life expectancy of VHL disease patients was 66 years. The presence of CHB and RA were the independent risk factors for overall survival. RCC was not a significant risk factor for overall survival. RA was a risk factor for CHB-specific survival. Patients with RA should have careful active surveillance due to the higher risk of death from CHB. Future larger studies with longer follow-up periods are needed to study the effect of RCC on survival of VHL patients. These findings may help guide future genetic counseling and clinical decision making.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Peking University First Hospital (Beijing, China). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
KG and LC were responsible for the concept and design of the study. BZ and JW dealt with the clinical data. SL and XP performed the statistical work. BH and JZho grafted the manuscript. KM and JZha provides the figures and tables. All authors revised the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Li Xue-ying in the Statistical Department of Peking University First Hospital for her assistance with the statistical analyses, and Bu Ding-Fang, Medical Experiment Center, Peking University First Hospital, for his technical assistance.
Glossary
Abbreviations
- CHB
central nervous system hemangioblastoma
- RA
retinal hemangioblastoma
- RCC
renal cell carcinoma
- PHEO
pheochromocytoma
- PCL/PNET
pancreatic cystic lesion and pancreatic neuroendocrine tumors.
Footnotes
Funding. This work was supported by The Fundamental Research Funds of the Central Universities (grant BMU2018JI002).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.01037/full#supplementary-material
References
- 1.Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. (1993) 260:1317–20. 10.1126/science.8493574 [DOI] [PubMed] [Google Scholar]
- 2.Lonser RR, Kim HJ, Butman JA, Vortmeyer AO, Choo DI, Oldfield EH. Tumors of the endolymphatic sac in von Hippel-Lindau disease. N Engl J. Med. (2004) 350:2481–6. 10.1056/NEJMoa040666 [DOI] [PubMed] [Google Scholar]
- 3.Maher ER, Neumann HP, Richard S. von Hippel-Lindau disease: a clinical and scientific review. Eur J Hum Genet. (2011) 19:617–23. 10.1038/ejhg.2010.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filling-Katz MR, Choyke PL, Oldfield E, Charnas L, Patronas NJ, Glenn GM, et al. Central nervous system involvement in Von Hippel-Lindau disease. Neurology. (1991) 41:41–6. 10.1212/WNL.41.1.41 [DOI] [PubMed] [Google Scholar]
- 5.Neumann HP, Wiestler OD. Clustering of features and genetics of von Hippel-Lindau syndrome. Lancet. (1991) 338:258. 10.1016/0140-6736(91)90401-A [DOI] [PubMed] [Google Scholar]
- 6.Walther MM, Linehan WM. Von Hippel-Lindau disease and pheochromocytoma. JAMA. (1996) 275:839–40. 10.1001/jama.275.11.839 [DOI] [PubMed] [Google Scholar]
- 7.Webster AR, Maher ER, Moore AT. Clinical characteristics of ocular angiomatosis in von Hippel-Lindau disease and correlation with germline mutation. Arch Ophthalmol. (1999) 117:371–8. 10.1001/archopht.117.3.371 [DOI] [PubMed] [Google Scholar]
- 8.Dollfus H, Massin P, Taupin P, Nemeth C, Amara S, Giraud S, et al. Retinal hemangioblastoma in von Hippel-Lindau disease: a clinical and molecular study. Invest Ophthalmol Vis Sci. (2002) 43:3067–74. Available online at: https://iovs.arvojournals.org/article.aspx?articleid=2162471 [PubMed] [Google Scholar]
- 9.Lonser RR, Weil RJ, Wanebo JE, DeVroom HL, Oldfield EH. Surgical management of spinal cord hemangioblastomas in patients with von Hippel-Lindau disease. J Neurosurg. (2003) 98:106–16. 10.3171/jns.2003.98.1.0106 [DOI] [PubMed] [Google Scholar]
- 10.Wanebo JE, Lonser RR, Glenn GM, Oldfield EH. The natural history of hemangioblastomas of the central nervous system in patients with von Hippel-Lindau disease. J Neurosurg. (2003) 98:82–94. 10.3171/jns.2003.98.1.0082 [DOI] [PubMed] [Google Scholar]
- 11.Weil RJ, Lonser RR, DeVroom HL, Wanebo JE, Oldfield EH. Surgical management of brainstem hemangioblastomas in patients with von Hippel-Lindau disease. J Neurosurg. (2003) 98:95–105. 10.3171/jns.2003.98.1.0095 [DOI] [PubMed] [Google Scholar]
- 12.Chauveau D, Duvic C, Chretien Y, Paraf F, Droz D, Melki P, et al. Renal involvement in von Hippel-Lindau disease. Kidney Int. (1996) 50:944–51. 10.1038/ki.1996.395 [DOI] [PubMed] [Google Scholar]
- 13.Webster AR, Richards FM, MacRonald FE, Moore AT, Maher ER. An analysis of phenotypic variation in the familial cancer syndrome von Hippel-Lindau disease: evidence for modifier effects. Am J Hum Genet. (1998) 63:1025–35. 10.1086/302037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maher ER, Yates JR, Harries R, Benjamin C, Harris R, Moore AT, et al. Clinical features and natural history of von Hippel-Lindau disease. Q J Med. (1990) 77:1151–63. 10.1093/qjmed/77.2.1151 [DOI] [PubMed] [Google Scholar]
- 15.Binderup ML, Jensen AM, Budtz-Jorgensen E, Bisgaard ML. Survival and causes of death in patients with von Hippel-Lindau disease. J Med Genet. (2017) 54:11–8. 10.1136/jmedgenet-2016-104058 [DOI] [PubMed] [Google Scholar]
- 16.Wilding A, Ingham SL, Lalloo F, Clancy T, Huson SM, Moran A, et al. Life expectancy in hereditary cancer predisposing diseases: an observational study. J Med Genet. (2012) 49:264–9. 10.1136/jmedgenet-2011-100562 [DOI] [PubMed] [Google Scholar]
- 17.Kwon T, Jeong IG, Pak S, You D, Song C, Hong JH, et al. Renal tumor size is an independent prognostic factor for overall survival in von Hippel-Lindau disease. J Cancer Res Clin Oncol. (2014) 140:1171–7. 10.1007/s00432-014-1654-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang JY, Peng SH, Li T, Ning XH, Liu SJ, Hong BA, et al. Risk factors for survival in patients with von Hippel-Lindau disease. J Med Genet. (2018) 55:322–8. 10.1136/jmedgenet-2017-104995 [DOI] [PubMed] [Google Scholar]
- 19.El-Galaly TC, Cheah CY, Hutchings M, Mikhaeel NG, Savage KJ, Sehn LH, et al. Uterine, but not ovarian, female reproductive organ involvement at presentation by diffuse large B-cell lymphoma is associated with poor outcomes and a high frequency of secondary CNS involvement. Br J Haematol. (2016) 175:876–83. 10.1111/bjh.14325 [DOI] [PubMed] [Google Scholar]
- 20.Yuda S, Hayashi T, Yasui K, Muranaka A, Ohnishi H, Hashimoto A, et al. Pericardial effusion and multiple organ involvement are independent predictors of mortality in patients with systemic light chain amyloidosis. Intern Med. (2015) 54:1833–40. 10.2169/internalmedicine.54.3500 [DOI] [PubMed] [Google Scholar]
- 21.Gallou C, Chauveau D, Richard S, Joly D, Giraud S, Olschwang S, et al. Genotype-phenotype correlation in von Hippel-Lindau families with renal lesions. Hum Mutat. (2004) 24:215–24. 10.1002/humu.20082 [DOI] [PubMed] [Google Scholar]
- 22.Ong KR, Woodward ER, Killick P, Lim C, Macdonald F, Maher ER. Genotype-phenotype correlations in von Hippel-Lindau disease. Hum Mutat. (2007) 28:143–9. 10.1002/humu.20385 [DOI] [PubMed] [Google Scholar]
- 23.Peng S, Shepard MJ, Wang J, Li T, Ning X, Cai L, et al. Genotype-phenotype correlations in Chinese von Hippel-Lindau disease patients. Oncotarget. (2017) 8:38456–65. 10.18632/oncotarget.16594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu SJ, Wang JY, Peng SH, Li T, Ning XH, Hong BA, et al. Genotype and phenotype correlation in von Hippel-Lindau disease based on alteration of the HIF-α binding site in VHL protein. Genet Med. (2018) 20:1266–73. 10.1038/gim.2017.261 [DOI] [PubMed] [Google Scholar]
- 25.Ning XH, Zhang N, Li T, Wu PJ, Wang X, Li XY, et al. Telomere shortening is associated with genetic anticipation in Chinese Von Hippel-Lindau disease families. Cancer Res. (2014) 74:3802–9. 10.1158/0008-5472.CAN-14-0024 [DOI] [PubMed] [Google Scholar]
- 26.Chittiboina P, Lonser RR. Von Hippel-Lindau disease. Handb Clin Neurol. (2015) 132:139–56. 10.1016/B978-0-444-62702-5.00010-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu P, Zhang N, Wang X, Ning X, Li T, Bu D, et al. Family history of von Hippel-Lindau disease was uncommon in Chinese patients: suggesting the higher frequency of de novo mutations in VHL gene in these patients. J Hum Genet. (2012) 57:238–43. 10.1038/jhg.2012.10 [DOI] [PubMed] [Google Scholar]
- 28.Ebenazer A, Rajaratnam S, Pai R. Detection of large deletions in the VHL gene using a Real-Time PCR with SYBR Green. Fam Cancer. (2013) 12:519–24. 10.1007/s10689-013-9606-2 [DOI] [PubMed] [Google Scholar]
- 29.Lamiell JM, Salazar FG, Hsia YE. von Hippel-Lindau disease affecting 43 members of a single kindred. Medicine. (1989) 68:1–29. 10.1097/00005792-198901000-00001 [DOI] [PubMed] [Google Scholar]
- 30.Neumann HP, Bender BU, Berger DP, Laubenberger J, Schultze-Seemann W, Wetterauer U, et al. Prevalence, morphology and biology of renal cell carcinoma in von Hippel-Lindau disease compared to sporadic renal cell carcinoma. J Urol. (1998) 160:1248–54. 10.1016/S0022-5347(01)62509-6 [DOI] [PubMed] [Google Scholar]
- 31.Kim WT, Ham WS, Ju HJ, Lee JS, Lee JS, Choi YD. Clinical characteristics of renal cell carcinoma in Korean patients with von Hippel-Lindau disease compared to sporadic bilateral or multifocal renal cell carcinoma. J Korean Med Sci. (2009) 24:1145–9. 10.3346/jkms.2009.24.6.1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, et al. von Hippel-Lindau disease. Lancet. (2003) 361:2059–67. 10.1016/S0140-6736(03)13643-4 [DOI] [PubMed] [Google Scholar]
- 33.Jilg CA, Neumann HP, Glasker S, Schafer O, Leiber C, Ardelt PU, et al. Nephron sparing surgery in von Hippel-Lindau associated renal cell carcinoma; clinicopathological long-term follow-up. Fam Cancer. (2012) 11:387–94. 10.1007/s10689-012-9525-7 [DOI] [PubMed] [Google Scholar]
- 34.Duffey BG, Choyke PL, Glenn G, Grubb RL, Venzon D, Linehan WM, et al. The relationship between renal tumor size and metastases in patients with von Hippel-Lindau disease. J Urol. (2004) 172:63–5. 10.1097/01.ju.0000132127.79974.3f [DOI] [PubMed] [Google Scholar]
- 35.Wizigmann-Voos S, Breier G, Risau W, Plate KH. Up-regulation of vascular endothelial growth factor and its receptors in von Hippel-Lindau disease-associated and sporadic hemangioblastomas. Cancer Res. (1995) 55:1358–64. [PubMed] [Google Scholar]
- 36.Krieg M, Marti HH, Plate KH. Coexpression of erythropoietin and vascular endothelial growth factor in nervous system tumors associated with von Hippel-Lindau tumor suppressor gene loss of function. Blood. (1998) 92:3388–93. [PubMed] [Google Scholar]
- 37.Franke G, Bausch B, Hoffmann MM, Cybulla M, Wilhelm C, Kohlhase J, et al. Alu-Alu recombination underlies the vast majority of large VHL germline deletions: molecular characterization and genotype-phenotype correlations in VHL patients. Hum Mutat. (2009) 30:776–86. 10.1002/humu.20948 [DOI] [PubMed] [Google Scholar]
- 38.Niemela M, Lemeta S, Summanen P, Bohling T, Sainio M, Kere J, et al. Long-term prognosis of haemangioblastoma of the CNS: impact of von Hippel-Lindau disease. Acta Neurochir. (1999) 141:1147–56. 10.1007/s007010050412 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author


